Hepatitis B virus (HBV) infection is one of the major risk factors in the development of hepatocellular carcinoma (HCC) worldwide. The therapy for HBV-related hepatocellular carcinoma (HCC) remains a major challenge in medicine considering the low response rate (usually below 10%) to current chemotherapy and the high relapsing rate (up to or beyond 50%) after surgery (1). Whether antivirals or interferon therapy for chronic HBV infection will offer a reduction or prevention of HCC development remains to be clarified (2). There are several reasons to explain the difficulty or failure to treat HCC. One reason may lie in the inherent nature of the liver which is the organ responsible for the metabolism of chemicals and drugs. The second reason is the heterogenecity of HCCs and complex mechanisms of HBV tumorigenesis which make the choice of therapies difficult (3). Although current advances in cancer genomics have tried to identify the potential HCC biomarkers and molecular classification of HCC is proposed (4), their application to clinical therapy remains to be verified.
Due to the difficulty of therapy for HCCs, many efforts have currently turned to prevent HCC development during the long course of chronic HBV infection. In the paper published in PNAS, Sitia et al. (5) reported the anti-platelet therapy to prevent HCC in a mouse model of chronic HBV infection. Although the anti-platelet agents aspirin (Asp) and clopidogrel (Clo) have been used for the chemoprevention of cancer (6), the pathogenesis underlying the antiplatelet chemoprevention in this paper is entirely novel. Their idea comes from the observation of platelet aggregation in sites of tissue damage and that platelet depletion ameliorates disease severity by reducing the accumulation of hepatic virus-specific CD8+ T cells in a transgenic mice model reconstituted with HBsAg-primed wild type immune system. This animal model has been demonstrated to develop HCC through the chronic immune-mediated necroinflammatory and regenerative process (7). This animal model will explain part of the HBV tumorigenesis in human, especially for the HCC development at the high HBV replicative status. Whether antiplatelet therapy will trigger other growth-related pathway or play other anti-tumor effects unrelated to its impact on CD8-mediated immunopathology remains to be clarified. Apparently, the authors appear to emphasize the liver regeneration after necroinflammatory injuries as the major neoplastic process in this animal model. However, the experimental model may not well cover the transactivating role of HBV oncoproteins in tumorigenesis.
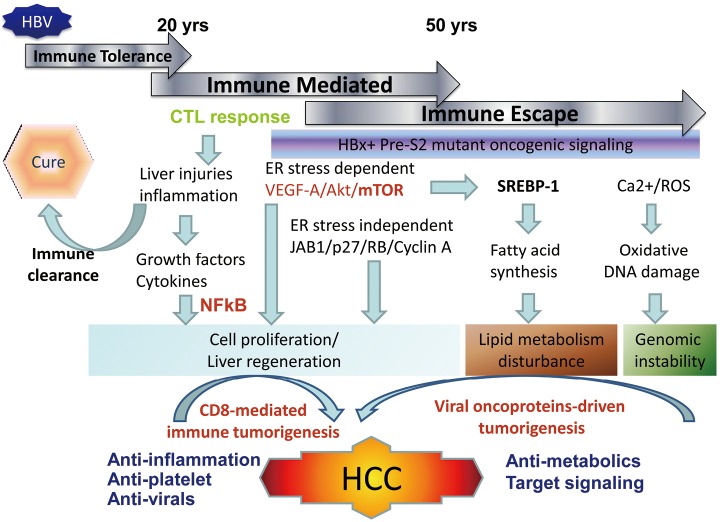
HBV tumorigenesis may include host factors associated anti-viral immune response and viral factors associated with insertional mutagenesis and expression of viral oncoproteins (8). The host factor-driven tumorigenesis may prevail at the early stage of HBV infection associated with CD8-mediated immune response, resulting in inflammation, liver injuries, liver fibrosis and regeneration, and finally HCC development as described by the same group in their previous reports (9). In this scenario, the anti-platelet therapy as reported by Sitia et al. will provide the candidate agents for prevention of HCC development at the high replicative stage of chronic HBV infection. Both Asp and Clo are suitable for preventive therapy considering the long-term usage and low toxicity in patients with chronic HBV infection. However, the viral oncoproteins-driven HBV tumorigenesis usually develops HCCs at the low or non-replicative phase of chronic HBV infection (Figure 1). The hepatocytes harboring the mutated viral oncoproteins escape from the immune attack and series of biologic events are initiated by viral oncoproteins to develop HCC (10). In this case, the immune-mediated antiplatelet therapy will be less effective for the prevention of HCC and other preventive measures should be searched for. Both the recognized HBV oncoproteins HBx and pre-S2 deletion mutants have been found to induce fatty livers through the induction of endoplasmic reticulum (ER) stress, inflammation, dysregulated lipid metabolism, and activation of NFκB and mTOR signaling by viral oncoproteins (11,12). Lipid metabolism is disturbed at the advanced stage of chronic liver disease including chronic HBV and HCV infection, and carries a significantly increased risk of HCC development (13,14). Therefore, ameliorating lipid metabolism may provide an alternative target for preventing HCC development. Several agents have been tried for cancer prevention, including agonists of peroxisome proliferator activated receptors (PPAR), unsaturated fatty acids, and extracts of natural products (15).
Figure 1.
Natural course of chronic HBV infection and HBV tumorigenesis. Chronic HBV infection starts from the immune tolerance phase at the young childhood period. The leakage or loss of immune tolerance will lead to the immune-mediated CD8 cytotoxic phase at the adolescent age and result in chronic liver injuries, inflammations and liver regeneration. The sustained, untoward clinical consequences is the development of liver cirrhosis and tumorigenesis. In the late stage or low replicative phase of HBV infection, a series of oncogenic signal pathways that were activated by the mutated HBx and pre-S mutants which escape from immune attack and retined in the endoplasmic reticulum (ER) of ground glass hepatocytes. The oncoprotins-driven signals may lead to abnormal lipid metabolics and genomic instability, finally developing HCCs. Based on the pathogenesis of these two models and consequence of chronic HBV infection, different strategies of chemoprevention approaches to prevent HCC development or therapy can then be adopted
To clarify the underlying pathogenesis and mechanism of HBV tumorigenesis is critical not only for the preventive therapy for high risk chronic HBV infection patients but can also identify the driven signaling or early biologic events for developing target therapy for HCCs. The development of HCC is a highly heterogeneous process that involves host immune mediated phase in the early infection stage and immune escape phase in the late infection stage. Chronic liver injury and the attendant inflammatory and regenerative responses create CD8-mediated tumorigenesis that provides a scenario of hepatocarcinogenesis. As the disease progresses, viral proteins escape immune attack via mutation and initiate a series of viral oncoproteins-driven tumorigenesis pathways which could contribute to 50% of the HCC development in humans (Figure 1). As mentioned above, human HBV-related HCCs are complicated and we may need personalized therapy which targets at pathways specific for the patients or the driving signals specific for each tumor in the future.
Acknowledgements
This project is supported by grants from National Science Council and National Health Research Institutes (Dr. Su IJ).
Disclosure: The authors declare no conflict of interest.
References
- 1.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg 2003;237:536-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon H, Lok AS. Does antiviral therapy prevent hepatocellular carcinoma? Antivir Ther 2011;16:787-95 [DOI] [PubMed] [Google Scholar]
- 3.Fransvea E, Paradiso A, Antonaci S, et al. HCC heterogeneity: molecular pathogenesis and clinical implications. Cell Oncol 2009;31:227-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 2009;69:7385-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 2012;109:E2165-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5:164-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisari FV, Klopchin K, Moriyama T, et al. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989;59:1145-56 [DOI] [PubMed] [Google Scholar]
- 8.Kremsdorf D, Soussan P, Paterlini-Brechot P, et al. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene 2006;25:3823-33 [DOI] [PubMed] [Google Scholar]
- 9.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995;13:29-60 [DOI] [PubMed] [Google Scholar]
- 10.Tan A, Yeh SH, Liu CJ, et al. Viral hepatocarcinogenesis: from infection to cancer. Liver Int 2008;28:175-88 [DOI] [PubMed] [Google Scholar]
- 11.Brown AJ. Viral hepatitis and fatty liver disease: how an unwelcome guest makes pâté of the host. Biochem J 2008;416:e15-7 [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Teng CF, Wu HC, et al. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology 2009;49:1962-71 [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Nilsson-Ehle P, Xu N.Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis 2006;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer 2009;115:5651-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer 2012;12:181-95 [DOI] [PMC free article] [PubMed] [Google Scholar]