Abstract
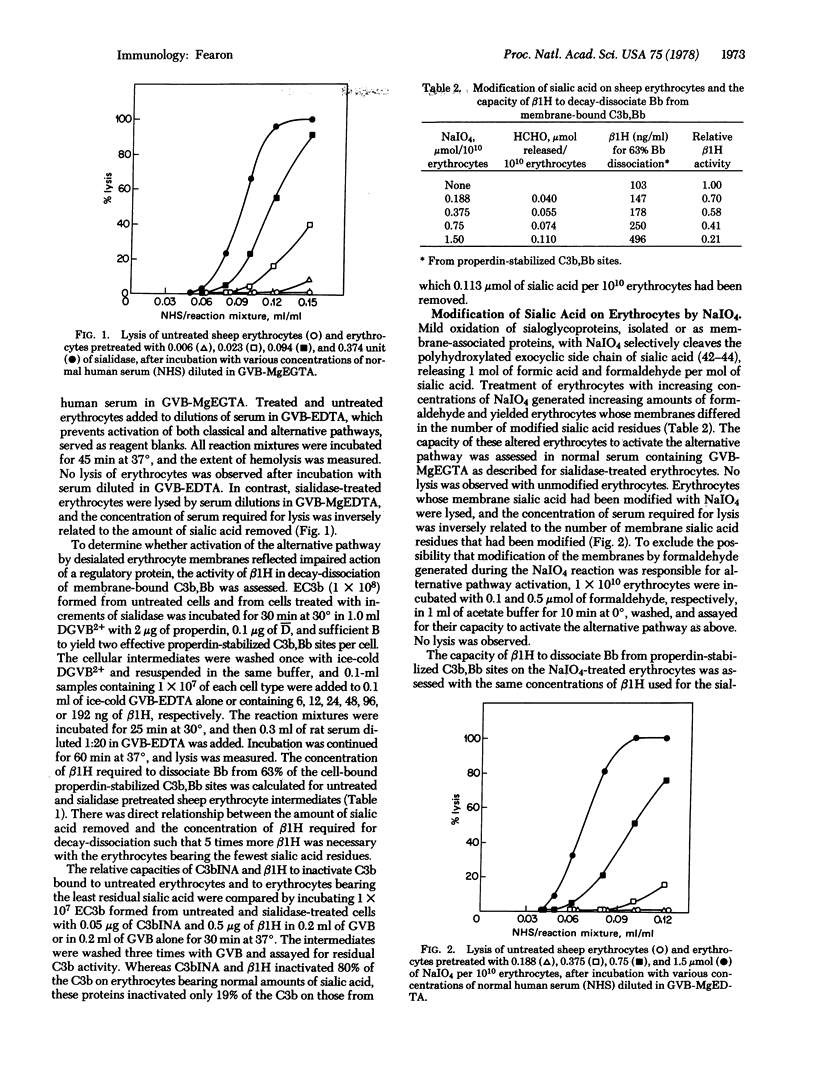
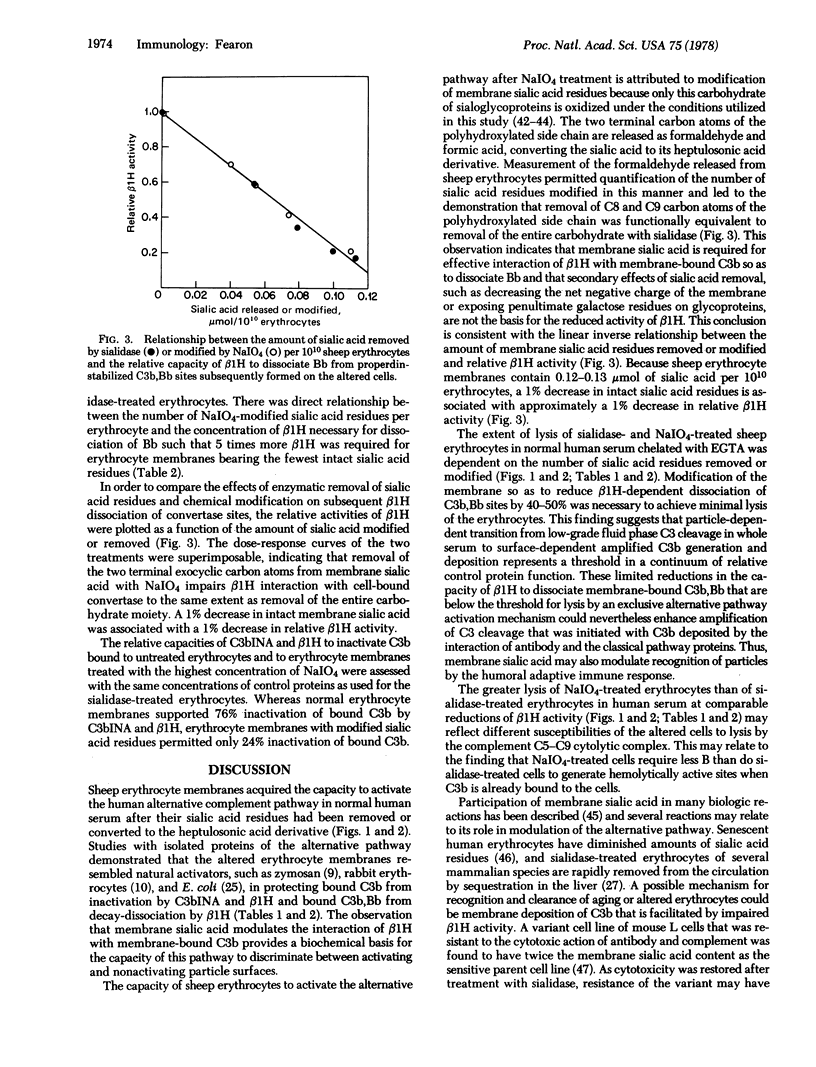
Sheep erythrocytes in their native state did not activate the alternative complement pathway, as measured by lysis in dilutions of normal human serum containing [ethylenebis(oxyethylenenitrilo)] tetraacetic acid but acquired this capacity after membrane sialic acid residues had been removed (by sialidase) or modified (by NaIO4). Activation of the alternative pathway by sheep erythrocytes required removal or modification of at least 40% of the membrane sialic acid to reach threshold, and it increased proportionately when larger amounts of sialic acid had been affected. Studies with isolated proteins of the alternative pathway demonstrated that the altered erythrocyte membranes resembled natural activators in protecting bound C3b from inactivation by C3b inactivator and β1H and protecting bound amplification C3 convertase (C3b,Bb) from decay-dissociation by β1H. A 1% decrease in intact sialic acid was associated with a 1% decrease in β1H activity in decay-dissociation of membrane bound C3b,Bb. Because removal of the C8 and C9 carbon atoms from the polyhydroxylated side chain of sialic acid by oxidation with NaIO4 was functionally equivalent to removal of the entire sialic acid moiety, secondary effects of the latter reaction, such as diminution of the negative charge of the membrane or exposure of penultimate galactose residues, were not considered to be responsible for the altered activity of β1H. These studies suggest that facilitation, by membrane sialic acid residues, of the interaction between bound C3b and β1H is essential to prevent the particle from effectively activating the alternative pathway.
Keywords: sialidase, NaIO4, sheep erythrocyte, C3b inactivator
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARQUILLA E. R., HAMASHIGE S., HAMLIN J., MILLER A. INTERFERENCE WITH IMMUNE HEMOLYSIS BY GLYCOPROTEIN ANTIGENS. J Exp Med. 1964 Nov 1;120:841–856. doi: 10.1084/jem.120.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff D., Bell W. C., Fulton I., Ibgebrigtsen N. Effect of sialidase on the viability of erythrocytes in circulation. Am J Hematol. 1976;1(4):419–432. doi: 10.1002/ajh.2830010407. [DOI] [PubMed] [Google Scholar]
- Baxter A., Beeley J. G. Changes in surface carbohydrate of human erythrocytes aged in vivo. Biochem Soc Trans. 1975;3(1):134–136. doi: 10.1042/bst0030134. [DOI] [PubMed] [Google Scholar]
- Blumenfeld O. O., Gallop P. M., Liao T. H. Modification and introduction of a specific radioactive label into the erythrocyte membrane sialoglycoproteins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):242–251. doi: 10.1016/0006-291x(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Lysis of tumor cells by antibody and complement. VI. Enhanced killing of enzyme-pretreated tumor cells. J Immunol. 1976 Mar;116(3):661–668. [PubMed] [Google Scholar]
- Bryant R. E., Jenkins D. E., Jr Calcium requirements for complement dependent hemolytic reactions. J Immunol. 1968 Oct;101(4):664–668. [PubMed] [Google Scholar]
- Chapitis J., Lepow I. H. Multiple sedimenting species of properdin in human serum and interaction of purified properdin with the third component of complement. J Exp Med. 1976 Feb 1;143(2):241–257. doi: 10.1084/jem.143.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALMASSO A. P., MUELLER-EBERHARD H. J. INTERACTION OF AUTOLOGOUS COMPLEMENT WITH RED CELLS IN THE ABSENCE OF ANTIBODY. Proc Soc Exp Biol Med. 1964 Dec;117:643–650. doi: 10.3181/00379727-117-29658. [DOI] [PubMed] [Google Scholar]
- Ensky J., Hinz C. F., Jr, Todd E. W., Wedgwood R. J., Boyer J. T., Lepow I. H. Properties of highly purified human properdin. J Immunol. 1968 Jan;100(1):142–158. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Initiation of C3 cleavage in the alternative complement pathway. J Immunol. 1975 Nov;115(5):1357–1361. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: initiation of alternative complement pathway. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3220–3224. doi: 10.1073/pnas.72.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Formation of a hemolytically active cellular intermediate by the interaction between properdin factors B and D and the activated third component of complement. J Exp Med. 1973 Dec 1;138(6):1305–1313. doi: 10.1084/jem.138.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Properdin factor D: characterization of its active site and isolation of the precursor form. J Exp Med. 1974 Feb 1;139(2):355–366. doi: 10.1084/jem.139.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Purification of C3b inactivator and demonstration of its two polypeptide chain structure. J Immunol. 1977 Oct;119(4):1248–1252. [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Palmer P. D., Sanford B. H. Factors involved in the cytotoxicity of normal guinea pig serum for cells of murine tumor TA3 sublines treated with neuraminidase. J Immunol. 1973 Oct;111(4):1071–1080. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Lauf P. K. Immunological and physiological characteristics of the rapid immune hemolysis of neuraminidase-treated sheep red cells produced by fresh guinea pig serum. J Exp Med. 1975 Oct 1;142(4):974–988. doi: 10.1084/jem.142.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T. H., Gallop P. M., Blumenfeld O. O. Modification of sialyl residues of sialoglycoprotein(s) of the human erythrocyte surface. J Biol Chem. 1973 Dec 10;248(23):8247–8253. [PubMed] [Google Scholar]
- Lynen R., Vogt W., Schmidt G., Dieminger L. Purification of a human serum protein ("factor E") which enhances cobra venom factor-induced indirect lysis. Identification with the fifth component of complement. Z Immunitatsforsch Exp Klin Immunol. 1976 Apr;151(2):105–116. [PubMed] [Google Scholar]
- Minta J. O., Lepow I. H. Studies on the sub-unit structure of human properdin. Immunochemistry. 1974 Jul;11(7):361–368. doi: 10.1016/0019-2791(74)90189-x. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Porter R. R. Structure and activation of the early components of complement. Fed Proc. 1977 Aug;36(9):2191–2196. [PubMed] [Google Scholar]
- Schreiber R. D., Medicus R. G., Gïtze O., Müller-Eberhard H. J. Properdin- and nephritic factor-dependent C3 convertases: requirement of native C3 for enzyme formation and the function of bound C3b as properdin receptor. J Exp Med. 1975 Sep 1;142(3):760–772. doi: 10.1084/jem.142.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer W. T., Gottlieb C., Kornfeld S. Humoral immunostimulation. VII. Sialic acid masks antigenic sites on an antibody-selected bariant cell line. J Immunol. 1977 Aug;119(2):614–617. [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Perrin L. H. Lysis of human cultured lymphoblastoid cells by cell-induced activation of the properdin pathway. Science. 1977 Mar 4;195(4281):878–880. doi: 10.1126/science.402691. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Larsson I. Lactoperoxidase coupled to polyacrylamide for radio-iodination of proteins to high specific activity. Immunochemistry. 1974 Apr;11(4):203–206. doi: 10.1016/0019-2791(74)90329-2. [DOI] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of C3b hemolytic activity by a plasma protein distinct from C3b inactivator. Science. 1976 Sep 10;193(4257):1011–1013. doi: 10.1126/science.948757. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YACHNIN S., LAFORET M. T., GARDNER F. H. pH dependent hemolytic systems. I. Their relationship to paroxysmal nocturnal hemoglobinuria. Blood. 1961 Jan;17:83–96. [PubMed] [Google Scholar]



