Abstract
Objective
To investigate the effects of EZH2 gene on the growth and migration of hepatocellular carcinoma HepG2 cell in vitro and in vivo.
Methods
EZH2 shRNA plasmid vectors were constructed and transfected into HepG2 cells. A model of EZH2 gene-silencing HepG2 cell lines was constructed, and the experimental cells were classified into 3 groups: HepG2 blank control group, HepG2-V vector control group and HepG2-EZH2 (-) group. The mRNA and protein expressions of EZH2 in these three cells were detected by real-time fluorogenic quantitative PCR and Western blotting, respectively. Cell proliferation was analyzed by MTT assay. Cells were inoculated subcutaneously in nude mice, and the growth of tumor cells in vivo was observed. Transwell chamber assay was performed to observe any change in the migration ability of cells.
Results
The mRNA expression of HepG2 and HepG2-V was 100% and (95.27±10.87)%, respectively. Compared with the control group, the mRNA level of HepG2-EZH2 (-) were significantly decreased to (20.55±13.21)% (P<0.001). Similarly, the EZH2 protein expression were inhibited in HepG2-EZH2 (-) cells. The inhibition rate of tumor growth was 36.3% in vitro and 52.5% in vivo. The migration rate of the HepG2-EZH2 (-) group [(7.15±1.13)%] was significantly lower than those in the HepG2 group [(14.57±4.32)%] and the HepG2-V group [(15.21±5.22)%], with significant differences (both P<0.05).
Conclusions
EZH2 silencing can effectively inhibit the proliferation and growth of HepG2 cells in vitro and in vivo and inhibit cell migration. Therefore, the EZH2 gene may be a novel target for the treatment of liver cancer.
Key Words: EZH2 gene, liver cancer, cell migration, HepG2 cell
Primary liver cancer is the fifth most common malignancy in the world, or the third in China, with a globally increasing prevalence. The 5-year survival rate of liver cancer patients is less than 10%, making this type of tumor among the most malignant ones. China has the highest incidence of hepatocellular carcinoma (HCC) worldwide. With a low surgical resection rate, the overall prognosis of this condition is poor. EZH2 (enhancer of zeste homolog 2), a newly identified human gene, is the human homolog of the gene enhancer in Drosophila zeste, and one of the important members of the Polycomb group gene family, which is associated with cell cycle regulation. In the event of sustained activation, this gene can lead to abnormal proliferation and malignant transformation of cells, and is thus considered to be a new candidate cancer gene (1). Upregulated expression of EZH2 has been confirmed in prostate cancer (2), breast cancer (3), liver cancer (4), and many other malignancies, suggesting close correlation between the abnormal regulation of EZH2 and tumor development and metastasis. In this study, we constructed a model of EZH2 gene-silencing HepG2 cell lines to identify the effects of EZH2 on the growth of liver cancer cells both in vitro and in vivo, and on cell migration using the Transwell assay.
Materials and methods
Materials
The following products and services were used in the present study: T4 polynucleotide kinase, Trizol, T4 DNA ligase and liposomes LipofectAMINE2000, purchased from Invitrogen Corporation; pSilencer 2.1-U6 plasmids, purchased from Ambion, Inc.; HindIII and BamHI restriction endonucleases, purchased from TakaRa; reverse transcription kit and SYBR Green Rea l-time PCR Master Mix, purchased from Toyobo Co., Japan; plasmid DNA extraction kit, provided by Omega, with primer synthesis and nucleotide sequencing completed by Shanghai Sangon Biotechnology Company and Shanghai Boya Biotechnology Co., Ltd., respectively; rat anti-human EZH2 monoclonal antibodies and mouse anti-human β-actin monoclonal antibodies, purchased from Cell Signaling Technology, Inc.; horseradish peroxidase labeled secondary antibodies, purchased from Santa Cruz Biotechnology, USA; electrochemiluminescence color kit and BCA protein quantitation kit, purchased from Beyotime Institute of Biotechnology; and Transwell chamber, purchased from Corning Inc. Human hepatoma cell line HepG2 was preserved in our laboratory. Eighteen BALB/c nu/nu male nude mice, aged four to six weeks, weighing about 20 g, were used in the study.
Construction and identification of the EZH2 eukaryotic expression vector (5)
The sequence of the double-stranded oligonucleotide that had a complementary sequence and encoded short hairpin RNA (shRNA) was: 5'-gatccGAGGTTCAGACGAGCTGATTTCAAGAGAATCAGCTCGTCTGAACCTCttttttggaaa-3' and 5'-agcttttccaaaaaaGAGGTTCAGACGAGCTGATTCTCTTGAAATCAGCTCGTCTGAACCTCg-3'. The two chain primer sequences were supplemented with BamHI and HindIII enzyme sites (underlined), respectively, and the target of the siRNA formed by their transcription products was human EZH2 mRNA 256-274 nucleotides (Gene Bank no. NM-004456). According to the Blast database, the fragment was a specific sequence. The sequence as a target of the siRNA generated by the transcription products of irrelevant double-stranded oligonucleotides was 5'-GAGGTTCAGACGAGTCGAT-3', which was not homologous with any of the human genome sequences. Mixed in equal volumes, the two complementary sequences were incubated at 95 °C for 5 minutes, and cooled to ambient temperature. After annealing overnight, the mixture was phosphorylated with T4 polynucleotide kinase at 37 °C. The pSilencer2.1-U6 was linearized with BamHI and HindIII enzymes, and ligated with T4 DNA ligase at 16 °C overnight. Following transformation into chemically competent E. coli, positive clones were picked and grown overnight with shaking. Plasmids were then extracted for DNA sequencing.
Transfection and screening of the recombinant vector
HepG2 cells were cultured to logarithmic phase in DMEM medium supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 incubator. The cells were trypsinized 24 h before transfection, and inoculated into 6-well plates, 5×105 cells per well. The pSilencer2.1-U6 and pSilencer2.1-U6-EZH2 (-) were transfected into HepG2 cells following experimental method specified in the LipofectAMINE 2,000 package insert. G418 pressure screening was conducted at the concentration of 500 mg/L 24 h after transfection. Positive clone cell lines were picked three weeks later for expanding culture. HepG2 cells transfected with plasmids were named after HepG2-V cells (empty vector) and HepG2-EZH2 (-) cells (silenced EZH2).
Quantitative detection of EZH2 mRNA expression in HepG2 cells using PCR method
Total cellular RNA was extracted from each group using the Trizol method, and the mRNA was reverse transcribed into cDNA following the kit instructions. PCR was performed on the ABI 7300 quantitative PCR system, with EZH2 upstream primer 5'-TTGTTGGCGGAAGCGTGTAAAATC-3', reverse primer 5'-TCCCTAGTCCCGCGCAATGAGC-3'. GAPDH was used as internal reference, with upstream primer being 5'-TGAACGGGAAGCTCACTGG-3', and reverse primer 5'-TCCACCACCCTGTTGCTGTA-3'. Reaction conditions: denaturation at 95 °C for 60 s, 95 °C for 15 s, 56 °C for 15 s, and 72 °C for 45 s, repeated for 45 cycles in total. The EZH2/GAPDH ratio was calculated using the 2-ΔΔCt method. A template-free negative control was established in the PCR process for the melting curve analysis after the end of the experiment, for confirming that only a single product was generated. The whole procedure was repeated three times.
Determination of EZH2 protein expression levels in HepG2 cells by Western blot assay
Cells collected from each group were subject to RIPA lysis to extract total protein, the concentration of which was determined by BCA assay. Fifty grams of total protein from each group was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. After fixation, mouse anti-human EZH2 monoclonal antibodies and mouse anti-human β-actin monoclonal antibodies (1:1,000 dilution) were added, and the reaction was incubated at 4 °C overnight. The secondary antibodies (1:5,000 dilution) were added to react at room temperature for 1 h, and then exposed to electrochemiluminescence. The above procedure was repeated three times using β-actin as the equal loading control.
Determination of the effects of the recombinant vector on proliferation of HepG2 cells using methyl thiazolyl tetrazolium (MTT)
After being digested, the exponentially growing HepG2 HepG2-V and HepG2-EZH2 (-) cells were respectively added into 96-well cell plates as 5×103/100 µL cell suspension per well for culture. Twenty-four hours later, 20 µL of MTT was added into each well and incubated at 37 °C for 4 hours. After removal of the supernatant, DMSO was added (150 µL/well), and the system was shaken for 10 minutes at room temperature. The absorbance at 490 nm was read using an absorbance reader. Four duplicate wells were established in each group and observed for four consecutive days to develop the cell growth curve.
Effects of highly expressed EZH2 on tumor growth determined using the model of subcutaneously inoculated nude mice
Eighteen male mice were randomly divided into three groups, with six in each. Expotentially growing cells in the HepG2 group, the HepG2-V group and the HepG2-EZH2 (-) group were collected, and 0.1 mL of each 1×108/mL cell suspension was inoculated to the right forelimb armpit of nude mice. The time to tumor development was observed, and the tumor diameter was then measured once every week to calculate the tumor volume. Tumor volume = tumor long diameter × short diameter2/2. The mice were sacrificed 28 days after inoculation, with the tumors stripped and weighed. Tumor inhibition rate = (tumor weight of control group - tumor weight of experimental group)/tumor weight of control group ×100%.
Determination of EZH2 effects on migration of HepG2 cells using cell migration assay
The cells were divided into three groups: HepG2 group, HepG2-V group and HepG2-EZH2 (-) group. Three duplicate wells were set in each group for the assay in a transwell chamber with an 8 µm pore size. Cells at the logarithmic phase were digested with 0.25% trypsin and washed twice with serum-free DMED. A total of 1×105 cells/100 µL were counted and inoculated in the upper chamber. In the lower chamber, 600 µL of DMEM medium containing 10% fetal bovine serum was added for incubation at 37 °C in a 5% CO2 incubator for 12 and 24 h, respectively. After removal of the upper chamber, the lower chamber was washed twice with PBS to digest the cells, which were then counted to calculate the number of cells that had migrated to the lower chamber. Cell migration rate = number of cells in the lower chamber/inoculated cells ×100%.
Statistical analysis
Statistical analysis was completed in SPSS12.0 with general data expressed as mean ± standard deviation, and mean values between the two groups were compared using Student’s t-test. A two-sided P of <0.05 was considered statistically significant.
Results
EZH2 mRNA expression levels in HepG2 cells by group
With the EZH2 mRNA level in untransfected HepG2 cells as 100%, the mRNA expression declined to (20.55±13.21)% in HepG2-EZH2 (-) cells, with significant difference (P<0.001). The EZH2 mRNA expression level in HepG2-V cells was (95.27±10.87)%, with no significant difference compared with HepG2 cells.
EZH2 protein expression levels in HepG2 cells by group
The EZH2 protein expression level was significantly higher in the HepG2 group and the HepG2-V group, compared with HepG2-EZH2 (-) cells, as shown in Figure 1.
Figure 1.
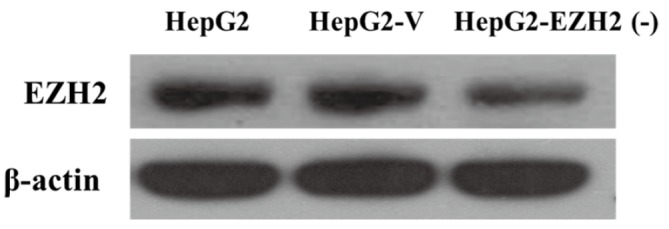
EZH2 protein expression in different groups
Proliferation of HepG2 cells in different groups
The cell growth curves of the HepG2, HepG2-V and HepG2-EZH2 (-) groups are shown in Figure 2. The HepG2 group and the HepG2-V group had very similar growth curves, without statistical difference in the cell growth conditions at any given time point. The HepG2-EZH2 (-) group, however, was associated with significantly lower cell proliferation at 24 h. As the incubation time prolonged, significant suppression of cell proliferation was observed, with the inhibition rate being 36.3% at 96 h (P<0.05).
Figure 2.
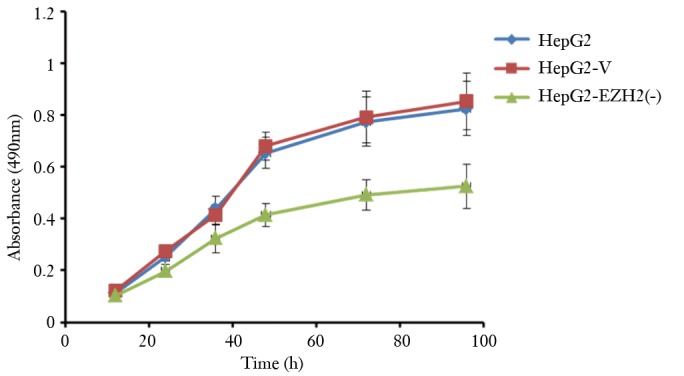
The cell growth curves of the HepG2, HepG2-V and HepG2-EZH2 (-) groups
Tumor growth in nude mice of different groups
Palpable masses were found from day 4 after inoculation in the nude mice of the HepG2 and HepG2-V groups, and tumors 4-7 mm in diameter were visible from day 7. On the other hand, palpable masses were found from day 7 after inoculation in the HepG2-EZH2 (-) group, and tumors 2-3 mm and 3-4 mm in diameter were visible from day 10 and day 14, respectively. Compared with the other two groups, nude mice in the HepG2-EZH2 (-) group presented significantly weakened tumor development and smaller tumor volume (P<0.05), while there was no difference in the tumor volume between the HepG2 group and the HepG2-V group (P>0.05, Table 1). The tumor weights in the HepG2, HepG2-V and HepG2-EZH2 (-) groups were 0.413±0.132, 0.397±0.162 and 0.196±0.119 g, respectively, 28 days after inoculation. Compared with the HepG2 group, silencing of EZH2 was associated with tumor shrinkage by up to 52.5% in the HepG2-EZH2 (-) group (P<0.05), whereas no difference was shown between the other two groups (P>0.05).
Table 1. Tumor volume at different time points in tumor-bearing nude mice (mm3, x̄±s).
| Group | n | Time point (days) |
|||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | ||
| HepG2 | 6 | 5.74±2.02 | 40.86±10.23 | 153.45±37.39 | 409.77±160.19 |
| HepG2-V | 6 | 4.92±1.91 | 32.19±5.66 | 139.28±30.86 | 323.81±90.28 |
| HepG2-EZH2(-) | 6 | 2.55±0.76* | 21.33±5.02* | 91.62±23.74* | 220.65±90.26* |
* P<0.05, compared with HepaG2 group and HepG2-V group
HepG2 cell migration in different groups
At 12 h, the cell migration rate was (8.13±2.25)% in the HepG2 group and (8.52±3.76)% in the HepG2-V group, without significant difference. Compared with the control group, however, the migration rate was significantly lower in the HepG2-EZH2 (-) group at (4.1±0.98)% (P<0.05). At 24 h, the migration rate of the HepG2-EZH2 (-) group [(7.15±1.13)%] was significantly lower than those in the HepG2 group [(14.57±4.32)%] and the HepG2-V group [(15.21±5.22)%], with significant differences (both P<0.05).
Discussion
EZH2, a newly identified human gene, is the human homolog of the gene enhancer in Drosophila zeste, and one of the important members of the Polycomb group gene family. The polycomb group (PcG) and the trithorax group (TrxG) constitute the widely conserved component in the cell memory system that is essential for preventing the change of cell identity. This system is established from the initial stage of embryonic development, exists throughout the development process, and continues to play a certain role until adulthood by generating PcG and TRX G protein complexes that contain different components to regulate the activity state of chromatins, thus maintaining transcription or inhibition of cell identity-associated genes (6). As an important PcG protein, EZH2 is involved in the formation of the chromatin structure, regulation of gene expression and growth control, and thus has pluripotency as it serves an important role in the formation process of PcG and Trx G protein complexes, differentiation and development of hematopoietic cells, X chromosome inactivation, and even cancer formation (7-11).
In 2005, Sudo et al. (12) reported significantly higher transcriptional and translational levels of EZH2 in hepatocellular carcinoma, compared to the corresponding adjacent tissues. The positive rates of portal vein tumor thrombus were significantly different in the high EZH2 expression and low EZH2 expression groups (P<0.001), suggesting a correlation between EZH2 and the malignant phenotype of hepatocellular carcinoma. In subsequent studies, many of them have confirmed that EZH2 is closely linked with the occurrence and development of liver cancer (13-15).
In this study, we have successfully established the eukaryotic expression vector of EZH2, pSilencer2.1-U6-EZH2 (-), and the results showed that it could significantly lower the EZH2 mRNA and protein levels in HepG2 cells. MTT assay revealed that reduced EZH2 expression levels were associated with significantly slower cell growth in HepG2-EZH2 (-) cells, with a growth inhibition rate of up to 36.3%, compared with the other two groups. To further identify the effect of EZH2 on promoting tumor growth and the extent of this effect at the overall level, nude mice were subcutaneously inoculated with HepG2, HepG2-V and HepG2-EZH2 (-) cells, and the impact of EZH2 on tumorigenicity activity and tumor growth of HepG2 cells in vivo was then monitored. As shown in the results, the HepG2-EZH2 (-) group presented lower tumorigenicity and growth rate, as well as significantly reduced tumor volume, compared with the other two groups (P<0.05). The tumor growth inhibition rate was up to 52.5%. EZH2 has been associated with the invasion and metastasis of many tumors in previous reports (16-19). In one of these studies, Wang and colleagues have noted that high EZH2 expression in patients with oral tongue squamous cell carcinoma is often associated with tumor metastasis. In their cellular study, inhibition of EZH2 leads to reduced cell motility, while its high expression will enhance cell motility (19). To determine the impact of EZH2 on the invasion and metastasis of liver cancer cells, Transwell chamber assay was performed in the present study to observe any change in the migration ability of HepG2-EZH2 (-) cells. The results showed significantly reduced cell migration along with lower EZH2 expression (P<0.05), suggesting that EZH2 could be one of the genes that promoted liver cancer invasion and metastasis.
In summary, lower EZH2 expression can inhibit the growth of human hepatoma HepG2 cells, reduce tumorigenic activity, inhibit malignant proliferation of tumor cells, inhibit tumor growth, and lower the ability of tumor cells to migrate. In view of its involvement in tumor occurrence, development, invasion and metastasis, EZH2 can be used as a potential target in the gene treatment for liver cancer. However, further studies are still needed to uncover the underlying mechanisms.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res 2008;647:21-9 [DOI] [PubMed] [Google Scholar]
- 2.Shin YJ, Kim JH. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS One 2012;7:e30393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A 2003;100:11606-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonemitsu Y, Imazeki F, Chiba T, et al. Distinct expression of polycomb group proteins EZH2 and BMI1 in hepatocellular carcinoma. Hum Pathol 2009;40:1304-11 [DOI] [PubMed] [Google Scholar]
- 5.Gong ZB, Li YM, Yu ZH. Expression of EZH2 in hepatocellular liver carcinoma and its role in HepG2 proliferation. Journal of Practical Oncology 2006;21:523-6 [Google Scholar]
- 6.Orlando V.Polycomb, epigenomes, and control of cell identity. Cell 2003;112:599-606 [DOI] [PubMed] [Google Scholar]
- 7.Tie F, Prasad-Sinha J, Birve A, et al. A 1-megadalton ESC/E(Z) complex from Drosophila that contains polycomblike and RPD3. Mol Cell Biol 2003;23:3352-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lessard J, Sauvageau G.Polycomb group genes as epigenetic regulators of normal and leukemic hemopoiesis. Exp Hematol 2003;31:567-85 [DOI] [PubMed] [Google Scholar]
- 9.Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta 2002;1602:151-61 [DOI] [PubMed] [Google Scholar]
- 10.Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002;298:1039-43 [DOI] [PubMed] [Google Scholar]
- 11.Plath K, Fang J, Mlynarczyk-Evans SK, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science 2003;300:131-5 [DOI] [PubMed] [Google Scholar]
- 12.Sudo T, Utsunomiya T, Mimori K, et al. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer 2005;92:1754-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Lin MC, Wang H, et al. Proteomic analysis of EZH2 downstream target proteins in hepatocellular carcinoma. Proteomics 2007;7:3097-104 [DOI] [PubMed] [Google Scholar]
- 14.Sasaki M, Ikeda H, Itatsu K, et al. The overexpression of polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest 2008;88:873-82 [DOI] [PubMed] [Google Scholar]
- 15.Cheng AS, Lau SS, Chen Y, et al. EZH2-mediated concordant repression of Wnt antagonists promotes β-catenin-dependent hepatocarcinogenesis. Cancer Res 2011;71:4028-39 [DOI] [PubMed] [Google Scholar]
- 16.Au SL, Wong CC, Lee JM, et al. Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis. Hepatology 2012;56:622-31 [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Zhang M, Li Q.Enhancer of zeste homolog 2 expression is associated with tumor cell proliferation and invasion in cervical cancer. Am J Med Sci 2011;342:198-204 [DOI] [PubMed] [Google Scholar]
- 18.Liu DC, Yang ZL. Overexpression of EZH2 and loss of expression of PTEN is associated with invasion, metastasis, and poor progression of gallbladder adenocarcinoma. Pathol Res Pract 2011;207:472-8 [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Liu X, Chen Z, et al. Polycomb group protein EZH2-mediated E-cadherin repression promotes metastasis of oral tongue squamous cell carcinoma. Mol Carcinog. 2011 doi: 10.1002/mc.21848. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


