Abstract
China Liver Transplant Registry (CLTR) is the official national liver transplant registry in Mainland China that has been authorized by the National Health and Family Planning Commission of the People’s Republic of China (NHFPC) and serves for both regulatory and scientific purposes. The CLTR 2011 annual scientific reports released national statistics describing current status of liver transplant (LT) in China. This article, as an accompanying document of CLTR 2011 annual scientific report, provides an overview of scientific results for LT in China. Up to December 2011, a total number of 20,877 LT performed during 1980-2011 in 81 certified transplant centers had been reported to CLTR. Of these donated livers, 92.63% were procured from deceased donors (N=19,338) and 7.37% were from living donors (N=1,539). In March 2010, the pilot project of the new deceased organ donation was initiated. From the initiation of the pilot program to the end of 2011, there were 115 LT (0.55% of all LT) using the liver grafts from Chinese categories donors. The recipient post-transplant survival had been significantly improved over years. The median post-transplant follow-up was 14.74 months, of which the longest follow-up time was 192.47 months. The 1-year, 3-year and 5-year cumulative survival rate for all recipients was 77.97%, 65.38% and 60.53%, respectively.
Key Words: Liver transplantation (LT), statistics, deceased donors, living donors, organ donation, China, scientific registry of transplant recipients, MELD, survival outcome
Introduction
China Liver Transplant Registry (CLTR) is the official national liver transplant (LT) registry in Mainland China that has been authorized by the National Health and Family Planning Commission of the People’s Republic of China (NHFPC) and serves for both regulatory and scientific purposes.
The CLTR 2011 annual scientific reports released national statistics showing current status of liver transplant (LT) in China. The analyses of the annual reports were performed by CLTR research center based on the data submitted by the transplant centers in mainland China, which is mandated by NHFPC. The annual reports had been reviewed by the scientific committee of CLTR (ScC) and approved by the NHFPC for formal release.
This article, as an accompanying document of CLTR 2011 annual scientific report, provides an overview of scientific results for LT in China.
The overview
A total number of 20,877 LT performed during 1980-2011 in 81 certified transplant centers has been captured by CLTR, which grants CLTR the status of the third largest liver transplant database worldwide next to UNOS [113,432 cases during 1988-2011 (1)] and Eurotransplant [100,542 cases during 1968-2010 (2)];
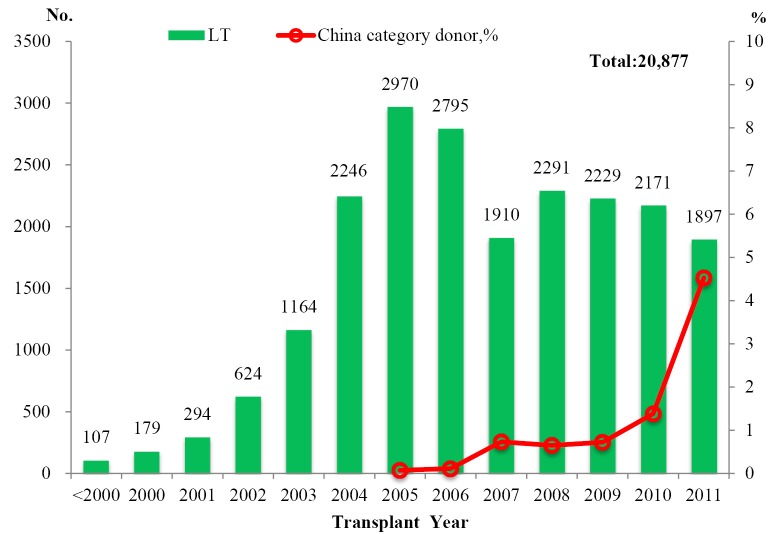
The number of LT per year steadily increased since 1993 with a peak of 2,970 cases in 2005 (Figure 1). Deceased donor liver transplantation (DDLT) accounted for the majority of LT (19,338, 92.63%) while living donor liver transplantation (LDLT) accounted for 7.37% (N=1,539) of LT;
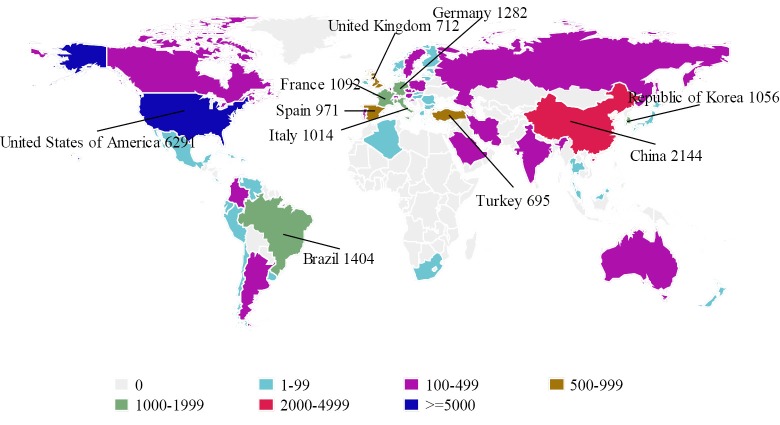
Results from the 2010 Global Observatory on Donation and Transplantation (GODT) report, produced by the WHO-ONT collaboration, indicated that among 58 data reporting countries, China was ranked at second place next to the United States (6,291 cases) in terms of the number of LT in 2010 (Figure 2). Meanwhile, in all the 99 member countries/regions investigated, China was among the 90 countries/regions which have had organ transplantation legislation in place (3);
The promulgation of the Regulation on Human Organ Transplantation in 2007 followed by a series of stringent polices led to a decline in the number of LT program. The number of current certified LT hospitals reduced to 81 by the year of 2009 and 70 (86.4%) of them located in the provincial capital. With the implementation of LDLT specific regulation policy of NHFPC in 2009, the number of LDLT decreased considerably, and the number of transplant centers practicing living donor organ transplantation dropped from 37 in 2009 to 17 in 2011;
-
In March 2010, the pilot project of the new deceased organ donation was initiated. Meanwhile, the classification of decease organ donation in China, namely “China Category” was developed by the national organ transplantation committee (OTC) of NHFPC. The China Category is summarized as the following:
China Category I (C-I): organ donation after brain death;
China Category II (C-II): organ donation after circulatory death;
China Category III (C-III): organ donation after brain death followed by circulatory death.
Figure 1.
Number of liver transplantation by year. *From March 2010 to the end of 2011, there were 115 LT using grafts from China Categories donors
Figure 2.
Liver transplantation by country in 2010 (3)
The classification was designed to be consistent with international classification standard for decease organ donation and respect the current cultural and societal value of Chinese people. From the initiation of the pilot program to the end of 2011, there were 115 LT (0.55% of all LT) performed using the liver grafts from China Categories donors. So far, 33 transplant centers from 19 provinces have started the LT in terms of China Categories;
An upward trend was observed in the number of LT from China Category donors since the initiation of the pilot program. There was a dramatic increase in number of China category donors by 186.67% in 2011 when comparing to that of the previous year. In addition, China Category donors represented 4.53% (86/1,897) of donor pool in 2011, which was close to the fraction of living donors (4.90%, 93/1,897) (Figure 1);
Central and south parts of China have the highest density in both the number of LT and the number of transplant centers. Among them, Shanghai (21.61%, 4,511/20,877), Beijing (19.84%, 4,142/20,877), Tianjin (17.85%, 3,727/20,877), Guangdong (11.05%, 2,306/20,877) and Zhejiang (5%, 1,044/20,877) were the top five regions in term of the cumulative number of LT;
In 2011, the LT rate was 1.41 per million populations in China. It was 20.92 PMP in Shanghai, 15.56 PMP in Beijing, and 15.42 PMP in Tianjin, all of which presented a much higher rate than those in the rest of the country;
The cumulative number of pediatric LT was 540, accounting for 2.59% of total LT. There was a jump in the number of pediatric LTs from 39 in 2010 to 75 in 2011, an increase of 92.3%, probably due to the increase of the decease pediatric organ donors;
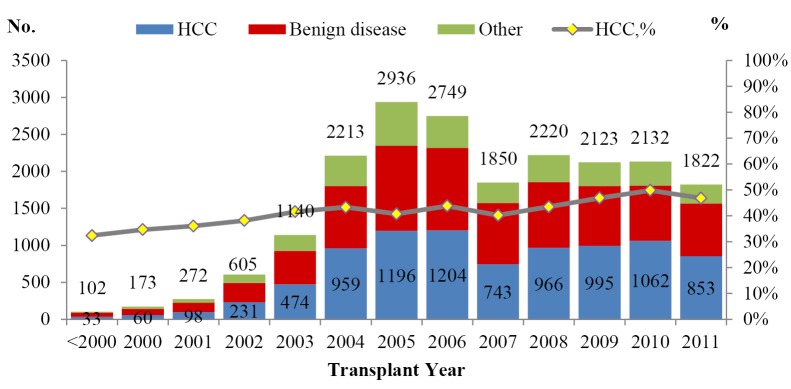
Among adult recipients, the proportion of LT for HCC recipients was 43.63% (N=8,874). The proportion roughly remained unchanged over years (Figure 3). 64.9% HCC patients were beyond Milan criteria, probably due to a lack of consensus on HCC patient selection and national organ allocation system;
There were 289 liver-related multi-organ transplants performed nationwide, 82.70% of which (239/289) were liver-kidney transplantations. During the same period, 767 cases of re-transplantation were documented in the registry. Meanwhile, 19 cases of dual-donor LT had been reported by 15 transplant centers since 2002;
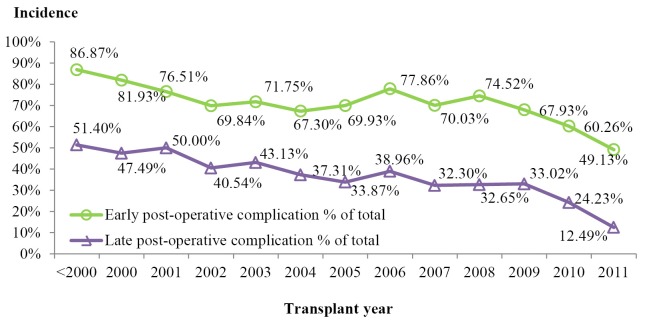
The recipient post-transplant survival had been significantly improved over years (Figure 4). Comparing to 2010, post-transplant complication rate in 2011 (49.13%) dropped more than 10% in both early and late post-transplant period. Hospital mortality rate decreased to 6.08% in 2011 from 36.79% in the years before 2000. There were improvements in graft survival rates over years. The unadjusted 1-year survival rate was 47.97% before 2000 and it reached at 84.51% by 2010-2011.
Figure 3.
Adult LT by diagnosis
Figure 4.
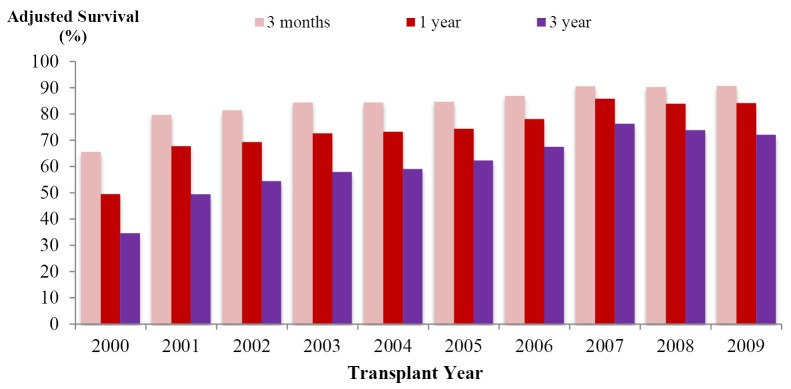
Adjusted graft survival. *Adjusted by population patterns of patient’s age, sex and diagnosis (malignant & benign disease) in 2009
Donors’ characteristics
95.74% of liver donors were male. However, the sex distribution showed different patterns across donor type (% of male donors: 98.32% among uncontrolled cardiac death donors, 82.58% among China Categories donors and 66.98% among living donors). In addition, there were changes in the sex-ratio in the group of living donors since 2008. By 2011, the proportion of female living donors exceeded that of male living donors for the first time (51.65%/48.35%, female/male);
The median age of donors was 29.84 years. It was 30 for China Categories donors, 30.33 for living donors and 29 for uncontrolled cardiac death donors;
30.35% of donated livers were from blood type O donors while 30.98% were from blood type A donors, and 28.60% and 10.06% were from blood type B and AB donors. There were 370 (1.91%) cases of donor-recipient ABO blood type incompatible LT. Among them, A-O (donor-recipients) incompatible LT represented the largest fraction (29.19%, 108/370);
The majority of China Categories donors were C-II (DCD, 42.98%) and C-III (DBCD, 38.84%). Among the C-II group, 74.29% of them were Maastricht III donors. 55.67% of causes of donor death were trauma and 18.29% were cerebral vascular accident.
Recipients’ characteristics
83.65% of LT recipients were male and the median age for all LT recipients was 48.5 years. The median age of LDLT recipients dropped gradually in the past four years, from 44.50 in 2008 to 23.90 in 2011;
The pre-transplant diabetes mellitus was documented in 10.76% of recipients while pre-transplant hepatitis B infection was recorded in 76.64% of recipients;
Cirrhosis (72.27%) was the leading pathological diagnosis for adult LT recipients (Table 1). HCC were found in 43.63% of adult LT recipients and the majority of whom (64.90%) were with tumor status beyond Milan criteria;
Biliary atresia (37.13%) and Wilson’s disease (23.23%) were two leading pathological and etiological diagnosis of pediatric recipients (Table 2);
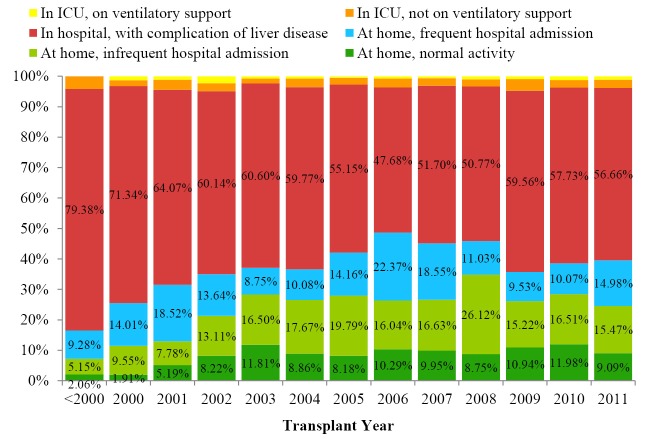
0.86% of the recipients required ICU stay and respiratory support prior to transplant. 55.57% of the LT recipients had been admitted to the general ward due to liver disease related complications and 40.88% of them stayed in their home while waiting for LT (Figure 5);
The median MELD score at the time of transplant dropped gradually in the recent years (Figure 6). But the median PELD score at the time of transplant increased steadily in the recent years (Figure 7). The national liver and kidney allocation policy of People’s Republic of China promulgated by the NHFPC in Dec. 2010 adopted MELD/PELD-based ranking system for allocation of deceased donor livers. Prior to this, no consensus has been reached with respect to the allocation rules at the national level so that the extent to which the MELD/PELD was applied as the ranking criteria for LT varied among centers.
Table 1. Diagnosis [pathology].
| Diagnosis: pathology | Adult, No. [%] | Pediatric, No. [%] |
|---|---|---|
| Cirrhosis | 14,685 [72.27] | 168 [31.34] |
| Tumor [not associated with cirrhosis] | 2,152 [10.59] | 21 [3.92] |
| Graft failure | 596 [2.93] | 11 [2.05] |
| CAH | 555 [2.73] | 1 [0.19] |
| Fulminant hepatic failure | 550 [2.71] | 13 [2.43] |
| Chronic liver disease [CAH or cirrhosis] | 497 [2.45] | 0 [0] |
| Primary biliary cirrhosis | 360 [1.77] | 24 [4.48] |
| CAH acute flare | 270 [1.33] | 1 [0.19] |
| Cirrhosis with acute deterioration or complications | 148 [0.73] | 2 [0.37] |
| Polycystic disease | 76 [0.37] | 1 [0.19] |
| Secondary biliary cirrhosis | 63 [0.31] | 3 [0.56] |
| Primary sclerosing cholangitis | 51 [0.25] | 2 [0.37] |
| Budd-Chiari syndrome | 45 [0.22] | 3 [0.56] |
| Other metabolic disease | 43 [0.21] | 38 [7.09] |
| Biliary atresia | 14 [0.07] | 199 [37.31] |
| Familial amyloidotic polyneuropathy | 5 [0.02] | 1 [0.19] |
| Unspecified | 210 [1.03] | 48 [8.96] |
| Total | 20,320* [100] | 536* [100] |
*Excluded cases (Adult: missing N=17; Pediatric: missing N=4) with missing pathological diagnosis
Table 2. Diagnosis [etiology].
| Diagnosis: etiology | Adult, No. [%] | Pediatric, No. [%] |
|---|---|---|
| HBV | 15,834 [78.32] | 30 [5.91] |
| HCV | 1,313 [6.49] | 0 [0] |
| Idiopathic/cryptogenic | 718 [3.55] | 50 [9.84] |
| ETOH | 561 [2.77] | 0 [0] |
| Auto-immune | 475 [2.35] | 12 [2.36] |
| Non-A to C | 267 [1.32] | 13 [2.56] |
| Wilson’s Disease | 140 [0.69] | 118 [23.23] |
| Drug induced | 77 [0.38] | 6 [1.18] |
| Technical | 74 [0.37] | 0 [0] |
| Congenital | 72 [0.36] | 227 [44.69] |
| HAV | 22 [0.11] | 0 [0] |
| Unspecified | 664 [3.28] | 52 [10.24] |
| Total | 20,217* [100] | 508* [100] |
*Excluded cases (Adult: missing N=120; Pediatric: missing N=32) with missing etiological diagnosis
Figure 5.
Recipients’ status prior to transplant
Figure 6.
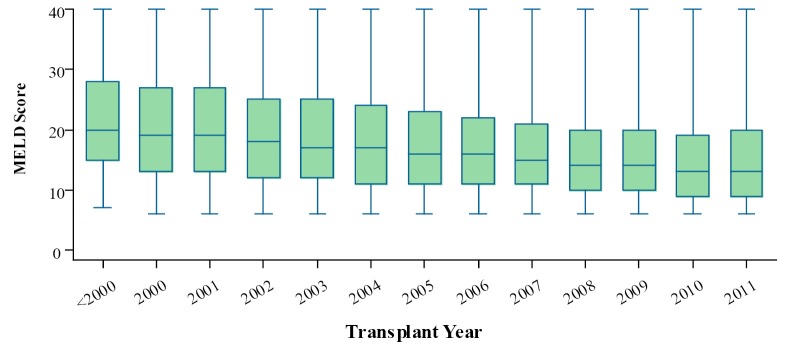
Median MELD score at the time of transplant
Figure 7.
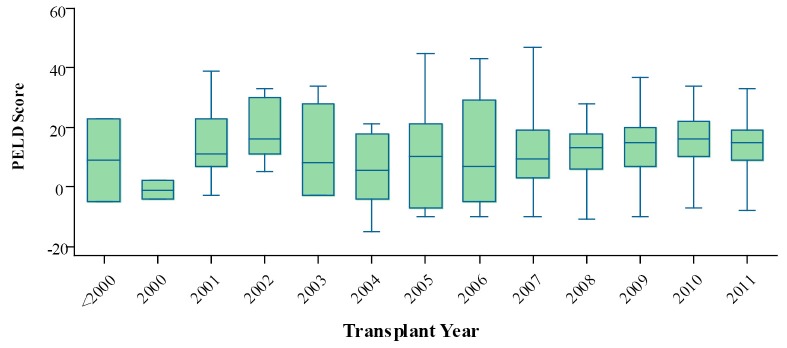
Median PELD score at the time of transplant
Recipients’ intra-operative data
The median anhepatic phase time ranged between 55 to 60 minutes in the past 9 years. LT from China Categories donors achieved the shortest anhepatic phase (51.50 minutes in median) among three groups of donors (57 minutes for LT from uncontrolled cardiac death donors and 70 minutes for LT from living donors);
The median cold ischemia time for all LT was 8 hours. It was 6.19 hours for LT from China Categories donors, which was shorter than those from uncontrolled cardiac death donors (8.17 hours);
Biliary stent was used in 38.56% of total LT. There was a decreased trend on the usage of biliary stent over years (25.36% in 2011);
The median volume of blood loss in LT was 2,000 mL, 66.10% of which were less than 3,000 mL;
The median operation time of all LT was 8 hours. It was 7.5 hours in 2011, the first time less than 8 hours. For China Categories donors, it was 7 hours in 2010 and 6.5 hours in 2011, both of which were less than that from uncontrolled cardiac donors during the same period;
The overall rate of intra-operative complications was 12.22%, among which intra-operative blood loss (8.57%) was ranked on the top. The overall intra-operative complications rate had decreased from 17.73% in 2010 to 11.70% in 2011;
The immunosuppressant regimen of the combination of Tacrolimus and mycophenolate mofetil were used in 70.85% of LT recipients. Steroids were used in 87.85% of the recipients in conjunction with this regimen.
Post-transplant outcome
The early (<30 days) post-operative complications were observed in 68.51% of the patients receiving LT. Among which, pleural effusion (47.05%), diabetes mellitus (32.06%), peritoneal effusion/abscess (32.02%), and post-transplant infections (30.35%) were the leading complications. The rate of early post-transplant complications was 49.13% in 2011, the lowest level over the years (Figure 8);
The late postoperative complication occurred in 32.76% of the patients receiving LT during the reported time period. Diabetes mellitus (16.55%), biliary complications (8.74%), and hypertension (8.41%) were the leading types (Figure 8);
The hospital mortality rate significantly decreased over the decade. This was confirmed by a downward trend from 26.26% in 2000 to 6.08% in 2011. Nevertheless, it was reported that 58.69% of the patients suffering from post-transplant renal failure died before discharge. The hospital mortality rate was lower in adult transplant (7.82%) than in pediatric transplants (9.07%), but with no statistical significance. Differences in hospital mortality rate were also observed across donor types. In 2011, patients receiving LT from China category donors were observed highest hospital mortality rate (7.06%) compared to the other two donor types. (6.45% for LDLT and 6.01% for LT from uncontrolled cardiac death donors);
The 1-year, 3-year, and 5-year cumulative rate of biliary complications were 11.41%, 15.41%, and 17.73%, respectively. There was significant difference (P<0.001) across donor types. The cumulative rates of biliary complications was the highest in cadaver split LTs, which was 19.37%, 30.81%, 30.81% at 1, 3 and 5 years, respectively;
The 1-year, 3-year and 5-year cumulative rate of post-transplant heptocellular carcinoma recurrence was 19.28%, 29.53%, and 33.69%, respectively;
The 1-year, 3-year and 5-year cumulative post-transplant rate of recurrence for hepatitis B was 1.67%, 3.18%, 4.23%, respectively.
Figure 8.
Accumulative incidence of post-operative complications
Survival analysis
The median post-transplant follow-up was 14.74 months, of which the longest was 192.47 months. 5.01% of recipients were lost to follow up;
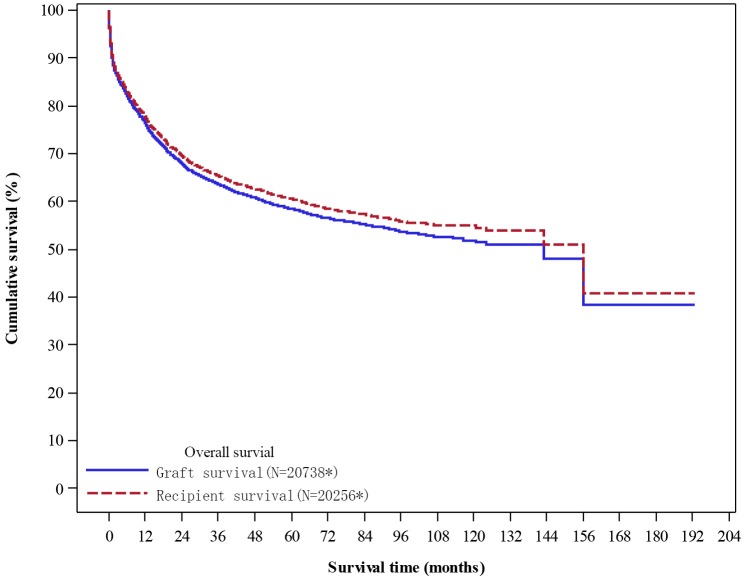
The 1-year, 3-year and 5-year cumulative survival rate for all recipients was 77.97%, 65.38% and 60.53%, respectively (Figure 9);
The 1-year, 3-year, and 5-year survival rate was 76.49%, 63.6%, and 58.42%, for adult recipients and 78.13%, 65.81%, and 59.25% for pediatric recipients;
Both LT from China Categories donors (1-year: 82.77%; 3-year: 69.67%; 5-years: 58.06%) and from living donors (1-year: 82.53%; 3-year: 72.05%; 5-year: 68.55%) yield higher unadjusted graft survival than LT from uncontrolled cardiac death donors (1-year: 76.01%; 3-year: 62.95%; 5-year: 57.65%) (P<0.001);
Up to 2011, there were 958 adult LT for acute liver failure/fulminate hepatic failure patients, with 1-year, 3-year, and 5-year graft survival rate of 67.79%, 60.54%, and 59.40%, respectively;
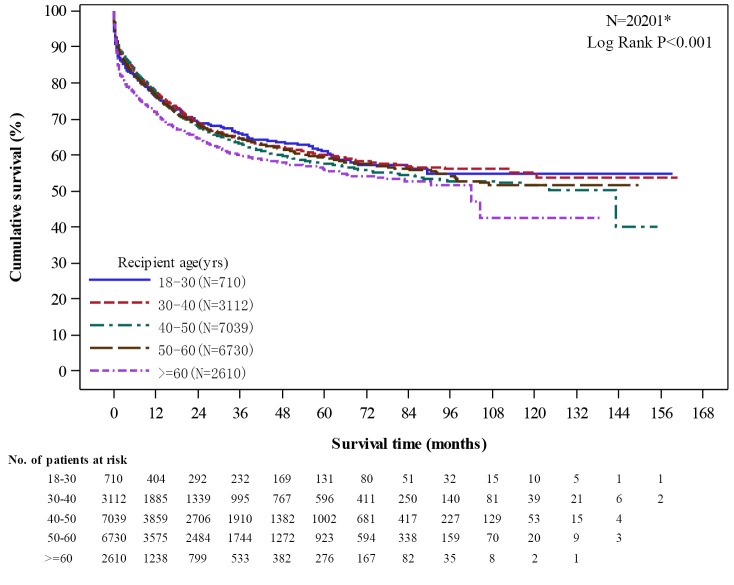
There were significant difference in graft survival rates of different age groups (P<0.001). Those of aged 60 years and older (2,610 recipients) had the lowest graft survival at 1-year (71.93%), 3-year (60.18%), and 5-year (55.94%) post-transplant among all adult recipients (Figure 10).
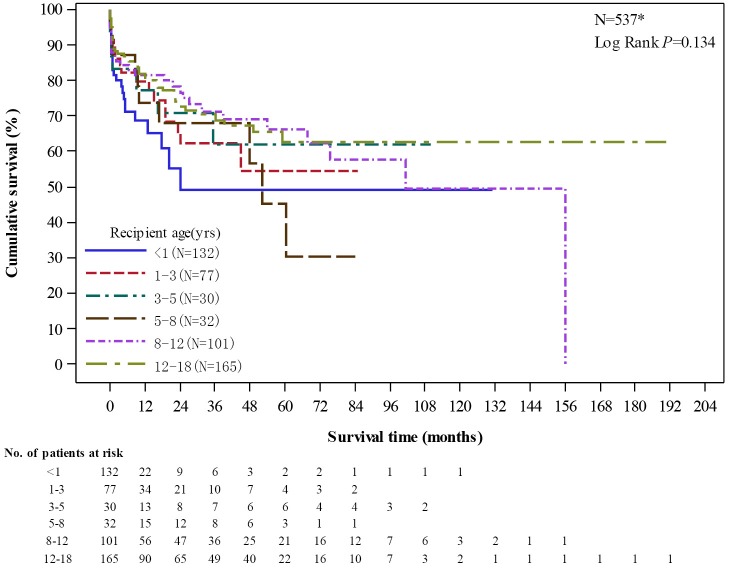
The graft survival rates of pediatric recipients had no significant difference among different age groups (P=0.134). There were total 132 recipients aged less than 1 year, of which 1-year, 3-year, and 5-year survival rate was 68.55%, 49.11%, and 49.11%, respectively (Figure 11).
Figure 9.
Unadjusted overall survival. *Excluded cases (graft survival: missing N=139; recipient survival: missing N=135) without the complete follow up or survival status report
Figure 10.
Unadjusted graft survival by age group among adult recipients. *Excluded cases (missing N=136) without the complete follow up or survival status report
Figure 11.
Unadjusted graft survival by age group among pediatric recipients. *Excluded cases (missing N=3) without the complete follow up or survival status report
Independent risk factors for survival were analyzed via univariate survival analysis and were summarized in the Appendix III of the CLTR 2011 annual scientific report. The analysis is to be updated every three years and will be reported in a separate publication.
Conclusions
The CLTR 2011 annual scientific report releases national statistics and scientific findings regarding LT in Mainland China during 1980-2011. This article, written as an accompanying document to the annual report, provides a brief summary of the scientific results along with updated information of the current LT activities in China. It is designed to meet the needs of readers from a wide range of fields, including health authorities, experts and scholars in transplant communities, officer of community service, as well as transplant patients and their families. Issues covered in the previous annual reports mainly focus on transplantation trend, transplant centers statistics, and recipients’ demographical pattern and post-transplant outcome. The 2011 report also briefed the readers on the current changes to the decease liver allocation and introduced the principles applied in the national deceased liver and kidney allocation policy of 2010. With the implementation of national liver allocation policy and national organ allocation computing system, we expect that the waitlist and the allocation statistics will be available to the public in the coming years. In addition to construction of the national allocation system, the data from China category donors is another new topic brought to this year’s report. The increase in the number of LT from the organ over years as well as the rapid expansion of the new national deceased organ donation program confirmed its values and feasibility in China. Conducted under the Human Organ Transplantation Regulation and governed by the health authorities, the new deceased organ donation program is expected to become the main organ source in Mainland China in the coming years.
Acknowledgements
Team work among the organ transplant committee, data analysis team, data management team and the medical writing team of CLTR involves throughout from the preparation to the publication of the annual report. Mandated by the NHFPC, CLTR is the national registry for LT serving regulatory and scientific purpose. However, interpretation of the results herein was made by the authors but not necessarily the NHFPC.
Disclosure: The authors declare no conflict of interest.
References
- 1.National data. Available online: http://optn.transplant.hrsa.gov/latestData/step2.asp
- 2.Evolution of LTs in Europe. Available online: http://www.eltr.org/spip.php?article152
- 3.Global Observatory on Donation and Transplantation. Available online: http://www.transplant-observatory.org