Abstract
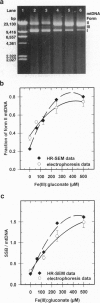
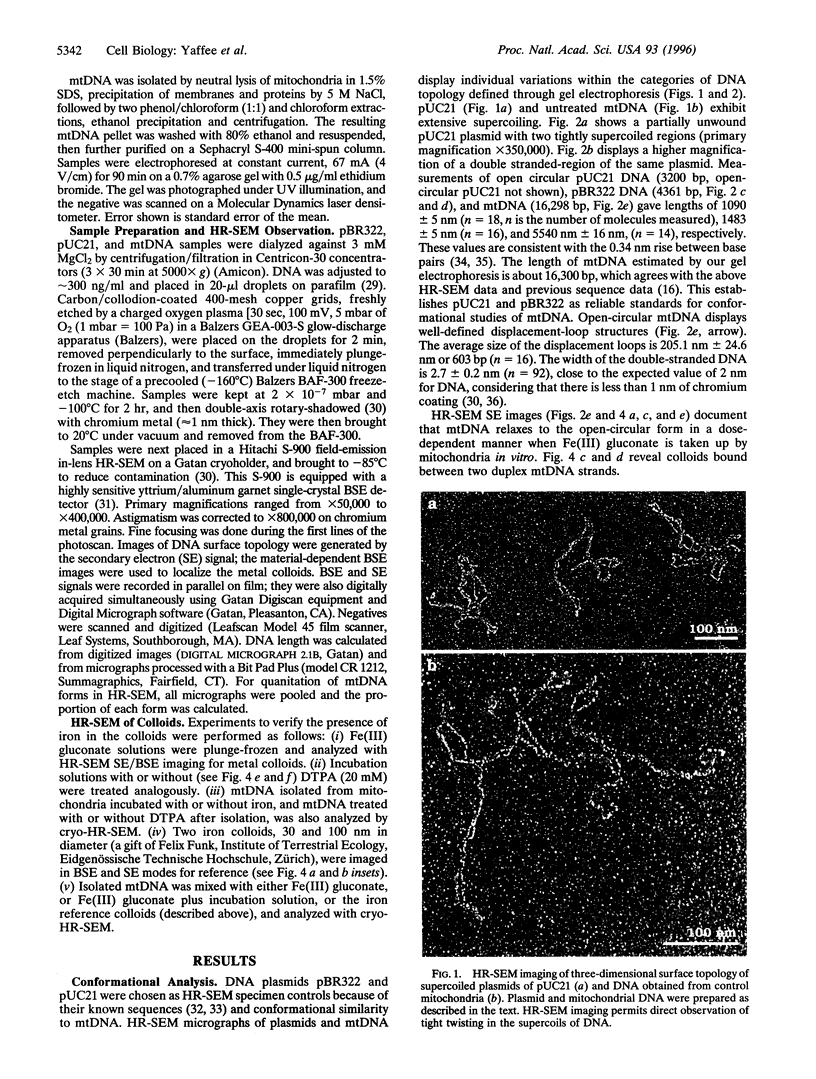
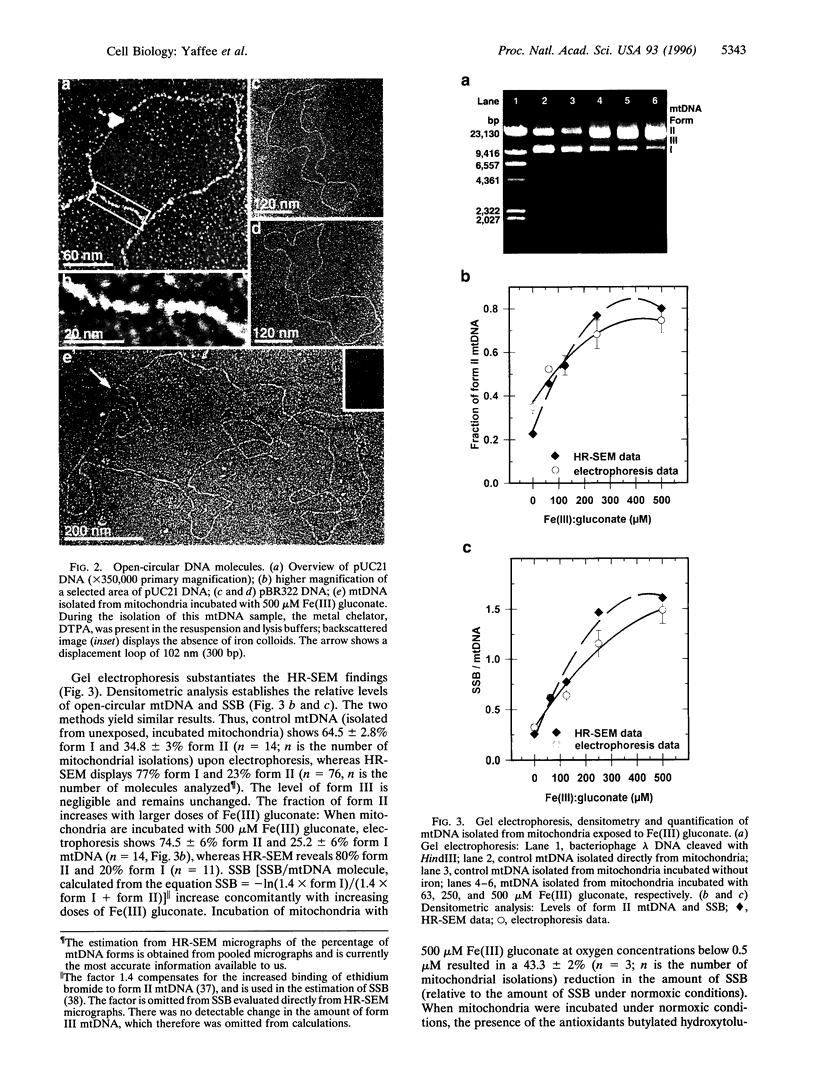
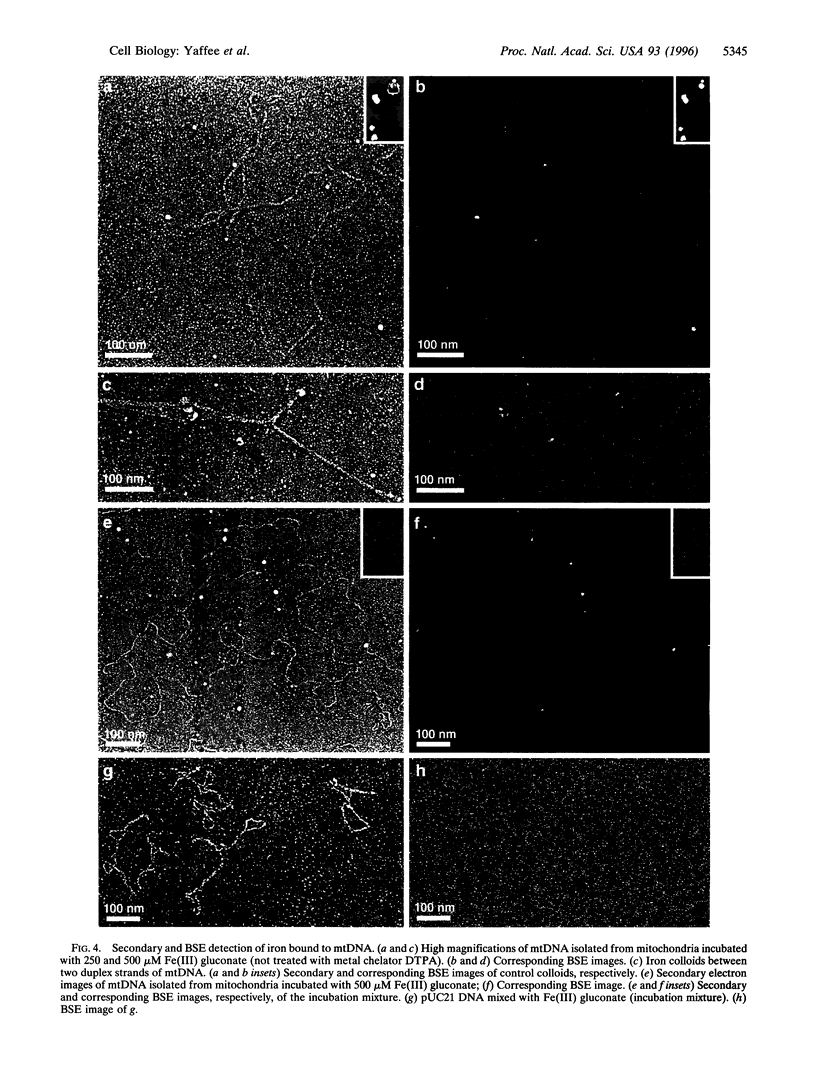
When respiring rat liver mitochondria are incubated in the presence of Fe(III) gluconate, their DNA (mtDNA) relaxes from the supercoiled to the open circular form dependent on the iron dose. Anaerobiosis or antioxidants fail to completely inhibit the unwinding. High-resolution field-emission in-lens scanning electron microscopy imaging, in concert with backscattered electron detection, pinpoints nanometer-range iron colloids bound to mtDNA isolated from iron-exposed mitochondria. High-resolution field-emission in-lens scanning electron microscopy with backscattered electron detection imaging permits simultaneous detailed visual analysis of DNA topology, iron dose-dependent mtDNA unwinding, and assessment of iron colloid formation on mtDNA strands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bednar J., Furrer P., Stasiak A., Dubochet J., Egelman E. H., Bates A. D. The twist, writhe and overall shape of supercoiled DNA change during counterion-induced transition from a loosely to a tightly interwound superhelix. Possible implications for DNA structure in vivo. J Mol Biol. 1994 Jan 21;235(3):825–847. doi: 10.1006/jmbi.1994.1042. [DOI] [PubMed] [Google Scholar]
- EISINGER J., SHULMAN R. G., BLUMBERG W. E. Relaxation enhancement by paramagnetic ion binding in deoxyribonucleic acid solutions. Nature. 1961 Dec 9;192:963–964. doi: 10.1038/192963a0. [DOI] [PubMed] [Google Scholar]
- Epe B., Mützel P., Adam W. DNA damage by oxygen radicals and excited state species: a comparative study using enzymatic probes in vitro. Chem Biol Interact. 1988;67(1-2):149–165. doi: 10.1016/0009-2797(88)90094-4. [DOI] [PubMed] [Google Scholar]
- Fairfield F. R., Bauer W. R., Simpson M. V. Mitochondria contain a distinct DNA topoisomerase. J Biol Chem. 1979 Oct 10;254(19):9352–9354. [PubMed] [Google Scholar]
- Flatmark T., Romslo I. Energy-dependent accumulation of iron by isolated rat liver mitochondria. Requirement of reducing equivalents and evidence for a unidirectional flux of Fe(II) across the inner membrane. J Biol Chem. 1975 Aug 25;250(16):6433–6438. [PubMed] [Google Scholar]
- Frei B., Winterhalter K. H., Richter C. Mechanism of alloxan-induced calcium release from rat liver mitochondria. J Biol Chem. 1985 Jun 25;260(12):7394–7401. [PubMed] [Google Scholar]
- Funk F., Lecrenier C., Lesuisse E., Crichton R. R., Schneider W. A comparative study on iron sources for mitochondrial haem synthesis including ferritin and models of transit pool species. Eur J Biochem. 1986 Jun 2;157(2):303–309. doi: 10.1111/j.1432-1033.1986.tb09669.x. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., SWOBODA B. E., MASSEY V. KINETICS AND MECHANISM OF ACTION OF GLUCOSE OXIDASE. J Biol Chem. 1964 Nov;239:3927–3934. [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Grasso J. A., Myers T. J., Hines J. D., Sullivan A. L. Energy-dispersive X-ray analysis of the mitochondria of sideroblastic anaemia. Br J Haematol. 1980 Sep;46(1):57–72. doi: 10.1111/j.1365-2141.1980.tb05935.x. [DOI] [PubMed] [Google Scholar]
- Hermann R., Schwarz H., Müller M. High precision immunoscanning electron microscopy using Fab fragments coupled to ultra-small colloidal gold. J Struct Biol. 1991 Aug;107(1):38–47. doi: 10.1016/1047-8477(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Linn S. Purification of all forms of HeLa cell mitochondrial DNA and assessment of damage to it caused by hydrogen peroxide treatment of mitochondria or cells. J Biol Chem. 1995 Apr 7;270(14):7950–7956. doi: 10.1074/jbc.270.14.7950. [DOI] [PubMed] [Google Scholar]
- Hruszkewycz A. M. Evidence for mitochondrial DNA damage by lipid peroxidation. Biochem Biophys Res Commun. 1988 May 31;153(1):191–197. doi: 10.1016/s0006-291x(88)81207-5. [DOI] [PubMed] [Google Scholar]
- Itoh H., Shioda T., Matsura T., Koyama S., Nakanishi T., Kajiyama G., Kawasaki T. Iron ion induces mitochondrial DNA damage in HTC rat hepatoma cell culture--role of antioxidants in mitochondrial DNA protection from oxidative stresses. Arch Biochem Biophys. 1994 Aug 15;313(1):120–125. doi: 10.1006/abbi.1994.1367. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG M., SLENCZKA W. [Pyridine nucleotide in liver mitochondria. An analysis of their redox relationships]. Biochem Z. 1959;331:486–517. [PubMed] [Google Scholar]
- Kosovsky M. J., Soslau G. Immunological identification of human platelet mitochondrial DNA topoisomerase I. Biochim Biophys Acta. 1993 Jun 24;1164(1):101–107. doi: 10.1016/0167-4838(93)90117-a. [DOI] [PubMed] [Google Scholar]
- LORING H. S., WARITZ R. S. Occurrence of iron, copper, calcium, and magnesium in tobacco mosaic virus. Science. 1957 Apr 5;125(3249):646–648. doi: 10.1126/science.125.3249.646-a. [DOI] [PubMed] [Google Scholar]
- Lim L. O., Neims A. H. Mitochondrial DNA damage by bleomycin. Biochem Pharmacol. 1987 Sep 1;36(17):2769–2774. doi: 10.1016/0006-2952(87)90263-2. [DOI] [PubMed] [Google Scholar]
- Lloyd R. S., Haidle C. W., Robberson D. L. Bleomycin-specific fragmentation of double-stranded DNA. Biochemistry. 1978 May 16;17(10):1890–1896. doi: 10.1021/bi00603a014. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Luo Y., Han Z., Chin S. M., Linn S. Three chemically distinct types of oxidants formed by iron-mediated Fenton reactions in the presence of DNA. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12438–12442. doi: 10.1073/pnas.91.26.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini A., Trenti T., Ceccarelli-Stanzani D., Ventura E. The effect of ferric iron complex on isolated rat liver mitochondria. I. Respiratory and electrochemical responses. Biochim Biophys Acta. 1985 Oct 29;810(1):20–26. doi: 10.1016/0005-2728(85)90202-6. [DOI] [PubMed] [Google Scholar]
- Richardson C. L., Verna J., Schulman G. E., Shipp K., Grant A. D. Metal mutagens and carcinogens effectively displace acridine orange from DNA as measured by fluorescence polarization. Environ Mutagen. 1981;3(5):545–553. doi: 10.1002/em.2860030506. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romslo I., Flatmark T. Energy-dependent accumulation of iron by isolated rat liver mitochondria. I. General features. Biochim Biophys Acta. 1973 Apr 27;305(1):29–40. doi: 10.1016/0005-2728(73)90228-4. [DOI] [PubMed] [Google Scholar]
- Ryan T. P., Aust S. D. The role of iron in oxygen-mediated toxicities. Crit Rev Toxicol. 1992;22(2):119–141. doi: 10.3109/10408449209146308. [DOI] [PubMed] [Google Scholar]
- Sakurai K., Haga K., Ogiso T. A role of iron in lambda DNA strand breaks in the reaction system of alloxan with reduced glutathione: iron(III) binding to the DNA. Biol Pharm Bull. 1994 Feb;17(2):227–231. doi: 10.1248/bpb.17.227. [DOI] [PubMed] [Google Scholar]
- Shepherd R. E., Isaacson Y., Chensny L., Zhang S., Kortes R., John K. Lactobionic and gluconic acid complexes of FeII and FeIII; control of oxidation pathways by an organ transplantation preservant. J Inorg Biochem. 1993 Jan;49(1):23–48. doi: 10.1016/0162-0134(93)80046-c. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires T. K. Iron-induced DNA damage and synthesis in isolated rat liver nuclei. Biochem J. 1982 Aug 1;205(2):321–329. doi: 10.1042/bj2050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Hauswirth W. W., Ross W. E., Neims A. H. A method for assessing damage to mitochondrial DNA caused by radiation and epichlorohydrin. Mol Pharmacol. 1985 Jan;27(1):167–170. [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Jiji R. M., Mergner W. J., Bulger R. E. Energy dispersive x-ray microanalysis of mitochondrial deposits in sideroblastic anemia. Lab Invest. 1978 Oct;39(4):375–380. [PubMed] [Google Scholar]
- Vieira J., Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991 Apr;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- WACKER W. E., GORDON M. P., HUFF J. W. METAL CONTENT OF TOBACCO MOSAIC VIRUS AND TOBACCO MOSAIC VIRUS RNA. Biochemistry. 1963 Jul-Aug;2:716–719. doi: 10.1021/bi00904a016. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Watson N. A new revision of the sequence of plasmid pBR322. Gene. 1988 Oct 30;70(2):399–403. doi: 10.1016/0378-1119(88)90212-0. [DOI] [PubMed] [Google Scholar]