Abstract
Multiple Myeloma (MM) consists of several distinct cytogenetic subtypes, and we hypothesized that each subtype may have a unique mode of initial presentation and end-organ damage. We studied 484 patients with newly diagnosed MM were to determine the relationship between specific myeloma-defining event (MDE) and the cytogenetic subtype. Patients were divided into 4 non-overlapping groups based on the MDE at diagnosis: isolated renal failure, isolated anemia, isolated lytic bone disease, or a combination (mixed). MM with translocations without trisomies accounted for 30% of all patients, but accounted for 50% of patients with renal failure. Specifically, the t(14;16) translocation accounted for only 5% of all MM patients, but was present in 13.5% of patients with renal failure as MDE. Among patients with t(14;16) 25% presented with renal failure only as MDE. Patients with isolated renal failure as MDE had significantly poorer survival compared with all other groups, while patients with bone disease as MDE had the best outcome (p < 0.001). Our findings support the hypothesis that in addition to prognostic differences, there is significant heterogeneity in clinical presentation associated with the cytogenetic subtype, suggesting that MM encompasses a group of cytogenetically and phenotypically distinct disorders rather than a single entity.
Keywords: Mutliple myeloma, cytogenetic abnormalities, myeloma defining event, renal failure, translocations, bone lesions
INTRODUCTION
Multiple myeloma (MM) is the third most common hematologic malignancy, accounting for approximately 12,000 deaths per year in the United States alone.1 MM is defined by monoclonal proliferation of plasma cells in the bone marrow. Unlike other malignancies, the diagnosis of MM is based on the presence of one or more markers of end-organ damage attributable to the plasma cell proliferative disorder: hypercalcemia, renal failure, anemia, and lytic bone disease (also known as CRAB symptoms).2 There is marked variation in prognosis across patients, which in part can be explained by the heterogeneity in clinical presentation of disease, stage, disease biology, and response to therapy.3
In current practice, fluorescence in situ hybridization (FISH) and cytogenetics are routinely performed on samples taken from those diagnosed with MM. There are several cytogenetically distinct subtypes of MM: trisomies (involving the odd-numbered chromosomes), immunoglobulin heavy chain (IgH) translocations involving chromosome 14q32, and a small subset in which there is evidence of both trisomies and IgH translocations. The IgH translocated subgroup is heterogeneous, and consists of several molecular subtypes based on the specific partner chromosome involved in the translocation. The most common reciprocal translocations in the IgH translocated subgroup of MM are t(11;14), t(4;14), t(14;16), t(14;20), and t(6;14).4
It is well known that the prognosis of MM varies according to the underlying cytogenetic subtype. Presence of certain cytogenetic abnormalities such as t(4;14), t(14;16), t(14;20), and del17p are markers of high risk myeloma.56 In many studies, patients with these subtypes have a poorer overall survival rate compared to patients with trisomies, t(11;14), and t(6;14) subtypes of MM. 7–14,15 More recent studies indicate that the underlying cytogenetic subtype also influences risk of progression from a benign clonal state to MM.16
Although the impact of the underlying cytogenetic subtype on prognosis has been well studied, the relationship between the molecular subtype of MM and spectrum of end-organ damage has not been well studied. This is of particular importance in a disease like myeloma where the disease definition is primarily clinical and based on several disparate types of end organ damage. For example, plasma cell proliferation leading to light chain cast nephropathy is termed myeloma even in the absence of lytic bone disease, and is likely quite a different disease from osteolytic bone lesions due to plasma cell proliferation, which is also termed myeloma. Indeed, if clinical presentation and end-organ damage varied significantly across cytogenetic subtypes, when coupled with prior studies on prognosis, we could begin to consider MM as a collection of genetically and clinically diverse group of disorders rather than as a single entity. In this paper, we examine the relationship between the primary cytogenetic subtypes of MM and initial clinical presentation.
PATIENTS AND METHODS
Patients
From among consecutive patients seen at Mayo Clinic with multiple myeloma from January 1, 2004 and December 31, 2009, we identified 500 patients who were seen at the Mayo Clinic within 90 days of their diagnosis and had bone marrow FISH studies performed within one year preceding their diagnosis or within 6 months following the diagnosis. Among this group, 16 patients did not have sufficient plasma cells observed during the FISH analysis and were excluded from the analysis. The study was approved by the Mayo Clinic Institutional Review Board and was done in accordance with the Declaration of Helsinki.
FISH Studies
Aspirate samples were enriched for mononuclear cells using the Ficoll method and cytospin slides were prepared. FISH analysis was performed as previously described using the following probes 3cen (D3Z1), 7cen (D7Z1), 9cen (D9Z1), 15cen (D15Z4), 11q13 (CCND1-XT), 14q32 (IGH-XT), 13q14 (RB1), 13q34 (LAMP1), 14q32 (5′IGH,3′IGH), 17p13.1 (p53), 17cen (D17Z1).2 Patients were considered to have high risk disease if FISH studies demonstrated one of the following abnormalities: t(4;14), t(14;16), t(14;20), or loss of p53 gene locus (del 17p or monosomy 17), in the absence of any trisomy (www.msmart.org).8, 17 Patients with any of the other abnormalities or a normal FISH were considered to have standard risk multiple myeloma.
Myeloma Defining Event (MDE) subgroups
All patient records were reviewed by A.J.G. and P.P.S and subsequently assigned to MDE groupings. MDE was defined as the specific end-organ damage (CRAB event) attributable to the plasma cell disorder that met the disease-definition for MM. Based on the principal MDE at initial diagnosis, patients were classified into 4 non-overlapping groups: lytic bone disease only without renal failure or anemia (Bone), renal failure (creatinine clearance < 30 ml/min) in the absence of any evidence of bone disease (Renal), anemia (hemoglobin < 12.5) in the absence of bone disease or renal failure (Anemia), or a combination of symptoms (Mixed). Of the CRAB events, hypercalcemia alone was not included in the MDE classification as patients with hypercalcemia from MM almost always have lytic bone disease and will be in the lytic bone disease group. The presence of lytic bone lesions was considered as a separate category regardless of the presence of concurrent renal failure or anemia since it represents the dominant clinical presentation of most patients with MM. However, patients with renal failure in absence of lytic bone disease, and patients with anemia alone without any lytic bone disease or renal failure were felt to merit a separate MDE subgroup class. As expected, a certain proportion of patients had two or more MDEs at presentation and they were considered as “Mixed”.
Statistical analysis
We first examined the relationship between the primary cytogenetic subtype of MM and the four non-overlapping MDE groups. We and then examined the relationship between the underlying cytogenetic subtype and each of the individual CRAB symptoms, with overlap permitted. The effect of MDE on survival was also studied. Chi-squared tests were used to test differences in between MDE groups; where numbers were insufficient, Fisher’s exact test and chi-squared tests with simulated p-values (10,000 iterations) were conducted. Overall survival (OS) was defined as the time from diagnosis to death, with patients alive at the time of last follow-up censored at that date. Survival curves were constructed according to the Kaplan-Meier method and the survival curves were compared using the log-rank test. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The distributions of primary cytogenetic subtypes of MM in this cohort have been previously published.15 Of the 484 patients in this cohort, 201 patients had trisomies without IgH translocations (42%), 146 had IgH abnormalities and no trisomies (30%), 74 had both trisomies and IgH translocations (15%), and 63 had no detectable abnormalities (13%). The distribution of patients across the groups based on MDE included Bone (n=168), Renal (n=37), Anemia (n=71), and Mixed MM (n=208).
Myeloma variant by myeloma defining event and FISH abnormalities
The distribution of the cytogenetic types according to MDE-based groups is shown in Table 1. There was a significant association between the subtype of MM with IgH translocation without trisomies and renal failure as the MDE (p=0.012). Thus, MM with immunoglobulin heavy chain translocations without trisomies was present in only 30% of all patients in the study cohort, but accounted for 50% of patients with renal failure as the MDE. Specifically, the t(14;16) translocation accounted for only 5% of all MM patients, but was present in 13.5% of patients with renal failure as the MDE. Then we examined the relationship between the presence of individual abnormalities and the MDE grouping and results are shown in Tables 2 and 3. Twenty five percent of patients with the t(14;16) translocation presented with renal failure only as the initial MDE. Interestingly, we found that patients with t(11;14) and t(6;14) tended to present more often in the bone disease group compared with patients who had t(4;14) or t(14;16).
Table 1.
Relationship between Non-overlapping primary molecular cytogenetic abnormalities and myeloma defining event at diagnosis.
| FISH abnormality | Overall (n=484) No. of patients (%) |
Bone disease variant (n=168) No. of patients (%)* |
Renal Failure variant (n=37) No. of patients (%)* |
Anemia variant (n=71) No. of patients (%)* |
Mixed variant (n=208) No. of patients (%)* |
|---|---|---|---|---|---|
| Trisomy (ies) without IgH abnormality | 201 (42%) | 70 (41.7%) | 7 (18.9%) | 32 (45.1%) | 92 (44.2%) |
| IgH abnormality without trisomy (ies) | 146 (30%) | 42 (25.0%) | 19 (51.4%) | 20 (28.2%) | 65 (31.3%) |
| t(11;14) | 74 (15%) | 26 (15.5%) | 6 (16.2%) | 9 (12.7%) | 33 (15.9%) |
| t(4;14) | 28 (6%) | 6 (3.6%) | 2 (5.4%) | 5 (7.0%) | 15 (7.2%) |
| t(14;16) | 19 (4%) | 2 (1.2%) | 5 (13.5%) | 3 (4.2%) | 9 (4.3%) |
| t(14;20) | 1 (<1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) |
| Unknown partner/deletion of IgH region | 24 (5%) | 8 (4.8%) | 6 (16.2) | 3 (4.2%) | 7 (3.4%) |
| IgH abnormality with Trisomy (ies) | 74 (15%) | 26 (15.5%) | 4 (10.8) | 13 (18.3%) | 31 (14.9%) |
| t(11;14) | 12 (3%) | 4 (2.4%) | 0 (0.0%) | 3 (4.2%) | 5 (2.4%) |
| t(4;14) | 19 (4%) | 6 (3.6%) | 1 (2.7%) | 6 (8.5%) | 6 (2.9%) |
| t(14;16) | 5 (1%) | 1 (0.6%) | 1 (2.7%) | 1 (1.4%) | 2 (1.0%) |
| t(6;14) | 3 (<1%) | 1 (0.6%) | 0 (0.0%) | 1 (1.4%) | 1 (.5%) |
| Unknown partner/deletion of IgH region | 35 (7%) | 14 (8.3%) | 2 (5.4%) | 2 (2.8%) | 17 (8.2%) |
| Monosomy 14 in absence of IgH translocations or trisomy (ies) | 22 (4.5%) | 12 (7.1%) | 3 (8.1%) | 2 (2.8%) | 5 (2.4%) |
| Other cytogenetic abnormalities in absence of IgH translocations or trisomy (ies) or monosomy 14* | 26 (5.5%) | 9 (5.4%) | 1 (2.7%) | 3 (4.2%) | 13 (6.3%) |
| Normal | 15 (3%) | 9 (5.4%) | 3 (8.1%) | 1 (1.4%) | 2 (1.0%) |
Number and proportion represents the breakdown of patients presenting with each MDE according to cytogenetic subtype
Table 2.
Relationship between Non-overlapping primary molecular cytogenetic abnormalities and myeloma defining event at diagnosis in patients with immunoglobulin heavy chain (IgH) translocations.
| FISH Abnormality | No. Of patiets | Bone Disease variant | Renal Failure variant | Anemia Variant | Mixed Variant |
|---|---|---|---|---|---|
| t(11;14), No. of patients (%) | 86 | 30 (34.9%) | 6 (7.0%) | 12 (14.0%) | 38 (44.2%) |
| t(4;14), No. of patients (%) | 47 | 12 (25.5%) | 3 (6.4%) | 11 (23.4%) | 21 (44.7%) |
| t(14;16), No. of patients (%) | 24 | 3 (12.5%) | 6 (25.0%) | 4 (4.2%) | 11 (45.8%) |
| t(14;20), No. of patients (%) | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.0%) |
| t(6;14), No. of patients (%) | 3 | 1 (33.3%) | 0 (0%) | 1 (33.3%) | 1 (33.3%) |
| Unknown Partner/Deletion of IgH Region, No. of patients (%) | 59 | 22 (37.3%) | 8 (13.6%) | 5 (8.5%) | 24 (40.7%) |
Number and proportion represents the breakdown of patients with each cytogenetic subtype according to MDE
Table 3.
Relationship between Non-overlapping primary molecular cytogenetic abnormalities and myeloma defining event at diagnosis in patients with Trisomies.
| FISH Abnormality | Overall | Bone Disease variant No. of patients (%) |
Renal Failure variant No. of patients (%) |
Anemia variant No. of patients (%) |
Mixed variant No. of patients (%) |
|---|---|---|---|---|---|
| All trisomies | 275 | 96 (34.9%) | 11 (4.0%) | 45 (16.4%) | 123 (44.7%) |
| Trisomy alone without other abnormality | 236 | 84 (35.6%) | 9 (3.8%) | 34 (14.4%) | 106 (44.9%) |
| No trisomies | 209 | 72 (34.4%) | 26 (12.4%) | 26 (12.4%) | 85 (40.7%) |
Number and proportion represents the breakdown of patients with each cytogenetic subtype according to MDE
Relationship between CRAB feature and FISH abnormalities
We then examined the relationship between the underlying cytogenetic subtype of MM and specific CRAB abnormalities present at diagnosis, without consideration of the overlap between the different CRAB features at initial presentation (Table 4). There was a significant association between the cytogenetic subtype of MM in patients with renal failure (n=97) compared to patients without renal failure (n=371), P<0.001. Of the 97 patients with any renal failure, 45.4% had an IgH translocation without trisomy.
Table 4.
Relationship between primary molecular cytogenetic abnormalities and specific CRAB (hypercalcemia, renal failure, anemia, bone disease) feature at diagnosis*
| FISH abnormality | Overall (n=484) | Hypercalcemia (n=59) | Renal Insufficiency (n=98) | Anemia (n=242) | Bone Disease (n=336) |
|---|---|---|---|---|---|
| Trisomy (ies) without IgH abnormality | 201 (42%) | 26 (44.1%) | 33 (33.7%) | 100 (41.3%) | 146 (43.5%) |
| IgH abnormality without trisomy (ies) | 146 (30%) | 21 (35.6%) | 44 (44.9%) | 84 (34.7%) | 95 (28.3%) |
| t(11;14) | 74 (15%) | 7 (11.9%) | 16 (16.3%) | 38 (15.7%) | 52 (15.5%) |
| t(4;14) | 28 (6%) | 7 (11.9%) | 10 (10.2%) | 18 (7.4%) | 18 (5.4%) |
| t(14;16) | 19 (4%) | 3 (5.1%) | 9 (9.2%) | 15 (6.2%) | 10 (3.0%) |
| t(14;20) | 1 (<1%) | 0 (0.0%) | 1 (1.0%) | 1 (0.4%) | 1 (0.3%) |
| Unknown partner/deletion of IgH region | 24 (5%) | 4 (6.8%) | 9 (9.2%) | 13 (5.4%) | 15 (4.5%) |
| IgH abnormality with Trisomy (ies) | 74 (15%) | 6 (10.2%) | 7 (7.1%) | 33 (13.6%) | 47 (14.0%) |
| t(11;14) | 12 (3%) | 1 (1.7%) | 1 (1.0%) | 5 (2.1%) | 7 (2.1%) |
| t(4;14) | 19 (4%) | 2 (3.4%) | 2 (2.0%) | 11 (4.5%) | 10 (3.0%) |
| t(14;16) | 5 (1%) | 0 (0.0%) | 1 (1.0%) | 4 (1.7%) | 3 (0.9%) |
| t(6;14) | 3 (<1%) | 0 (0.0%) | 1 (1.0%) | 1 (0.4%) | 2 (0.6%) |
| Unknown partner/deletion of IgH region | 35 (7%) | 3 (5.1%) | 2 (2.0%) | 12 (5.0%) | 25 (7.4%) |
| Monosomy 14 in absence of IgH translocations or trisomy (ies) | 22 (4.5%) | 2 (3.4%) | 4 (4.1%) | 9 (3.7%) | 17 (5.1%) |
| Other cytogenetic abnormalities in absence of IgH translocations or trisomy (ies) or monosomy 14* | 26 (5.5%) | 3 (5.1%) | 5 (5.1%) | 10 (4.1%) | 18 (5.4%) |
| Normal | 15 (3%) | 1 (1.7%) | 4 (4.1%) | 5 (2.1%) | 12 (3.6%) |
Patients may appear in multiple columns.
Number and proportion represents the breakdown of patients presenting with each MDE according to cytogenetic subtype
We compared patients with bone disease at diagnosis (n=335) versus patients who did not initially present with any lytic bone lesions or fractures (n=145) (Table 4). No significant difference in distribution of cytogenetic subtypes was found (p=0.56). However, as with MDE, patients with t(4;14) represented 10% of the study cohort, but accounted for only 5% of the cohort with bone disease. No differences in distribution of MM patients who presented with anemia (n=241) at diagnosis were compared with individuals who did not present with that symptom (n=240).
Myeloma Defining Events and survival
Patients presenting with renal failure as the MDE had significantly shorter median overall survival compared to patients who presented with lytic bone disease (41.9 months versus 59.3 months; p < 0.001) (Figure 1).
Figure 1.
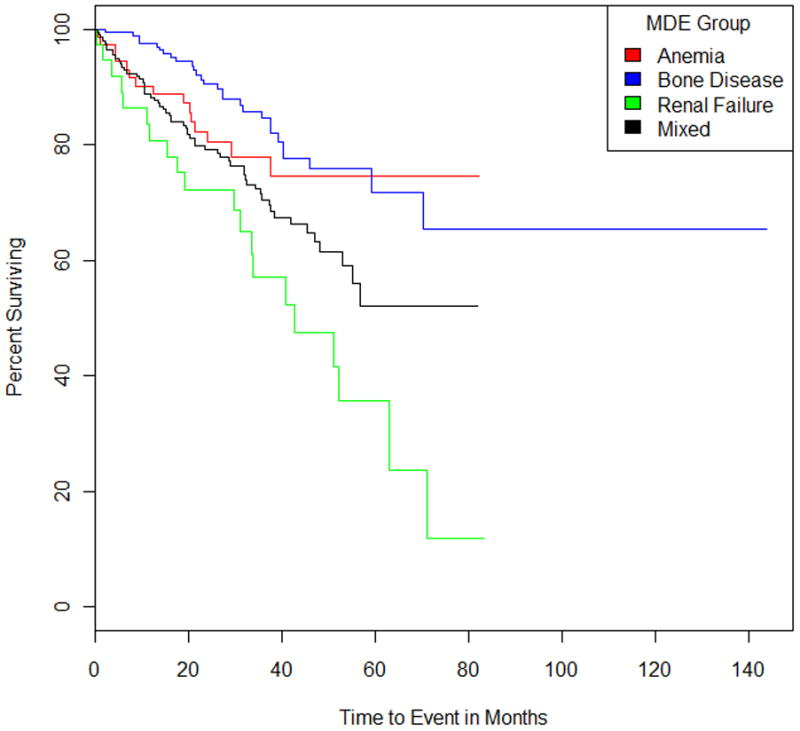
Kaplan-Meier curves for overall survival from diagnosis for myeloma patients grouped by Myeloma Defining Event (Anemia, Bone Disease, Renal Failure, or Mixed); p=0.0008.
Laboratory features and cytogenetic classification
We then compared the other clinical and laboratory parameters with the underlying cytogenetic classification to identify any associations (Table 5).
Table 5.
Clinical characteristics of primary molecular cytogenetic abnormalities at diagnosis.
| Cytogenetic Abnormalities | Median Age (Years) | Percent with Bone Disease | Median Ratio Of Involved and Uninvolved Serum Free Light Chains (Range) | Percent with Serum Free Light Chain Ratio Of 100 Or Greater | Median Involved Serum Free Light Chain Level (Range) | Percent with Involved Serum Free Light Chain Level of 100 Or Greater | Median Bone Marrow Plasma Cell Percentage (Range) |
|---|---|---|---|---|---|---|---|
| Trisomy (ies) without IgH abnormality | 66 | 72.6% | 53.5 (1.7–6135.7) | 39.2% | 28.1 (0.4–3520) | 26.8% | 35.8 (1.6–90) |
| IgH abnormality without trisomy (ies) | 64 | 65.1% | 173.9 (4.2–7312.5) | 60.0% | 78.3 (1.2–1690) | 43.0% | 39 (1–93) |
| t(11;14) | 64 | 70.3% | 111.0 (5.5–7312.5) | 54.7% | 44.3 (2.4–1540) | 37.7% | 40 (1–93) |
| t(4;14) | 64.5 | 64.3% | 128.0 (4.2–2764.7) | 50.0% | 69.5 (1.2–905) | 35.0% | 49.8 (8–98) |
| t(14;16) | 66 | 52.6% | 458.8 (49.0–3038.1) | 80.0% | 129.5 (50.1–1690) | 60.0% | 30.5 (20–75) |
| t(14;20) | 55 | 100.0% | 232 (n=1) | 100.0% | 39.9 | 0.0% | 18 (n=1) |
| Unknown partner/deletion of IgH region | 58 | 62.5% | 649.3 (11.2–5777.8) | 75.0% | 174 (4.1–1560) | 62.5% | 30 (4–84) |
| IgH Abnormality with trisomy (ies) | 64 | 63.5% | 147.9 (2.6–16788.5) | 52.5% | 68.2 (4.6–2200) | 44.4% | 33 (0.8–85.6) |
| t(11;14) | 62.5 | 58.3% | 229.7 (6.6–1913.0) | 55.6% | 88.2 (4.9–2200) | 44.4% | 41 (9.5–80) |
| t(4;14) | 62 | 52.6% | 118.2 (7.5–16788.5) | 38.9% | 38.1 (4.6–1760) | 38.5% | 34 (3.3–85.6) |
| t(14;16) | 69 | 60.0% | 38.75 (9.3–13057.1) | 40.0% | 11.1 (21.7–914) | 20.0% | 14 (11–37) |
| t(6;14) | 68 | 66.7% | 263.9 (198.3–329.6) | 100.0% | 155.9 (75.8–236) | 50.0% | 33 (n=1) |
| Unknown partner/deletion of IgH region | 65 | 71.4% | 175.3 (2.6–1881.8) | 60.0% | 115 (1.3–1160) | 52.0% | 29.5 (0.8–74) |
| Monosomy 14 in absence of IgH translocations or trisomy (ies) | 65.5 | 77.3% | 112.1 (13.6–1614.9) | 50.0% | 113.5 (4.5–2600) | 57.1% | 22.5 (4–68) |
| Other cytogenetic abnormalities in absence of IgH translocations or trisomy (ies) or monosomy 14 | 64.5 | 69.2% | 102.0 (1.8–9759.4) | 50.0% | 68.9 (2.6–700) | 35.7% | 30.5 (0.2–89) |
| Normal | 63 | 80.0% | 68.0 (1.7–631.7) | 58.3% | 8.14 (1.3–360) | 8.3% | 54.2 (38–70.2) |
In particular, we found the highest FLC levels and ratio in the t(14;16) and other IgH translocation groups, similar to what we have described in the past.15 Given the relationship between renal MDE and t(14;16), and the known relationship between FLC levels and renal insufficiency, we focused further on this finding.
Specifically, we examined if the poor outcome known to be associated with t(14;16) was in part explained by a higher incidence of renal insufficiency. The median OS for t(14;16) without and with renal insufficiency was 44.2 and 9.3 months, respectively (p < 0.0001).
DISCUSSION
MM consists of several cytogenetically distinct subtypes.184, 19, 20. These cytogenetic subtypes are typically non-overlapping, but approximately 10% of patients have both trisomies and IgH translocations.15 Almost all studies so far have focused on the prognostic implications of these cytogenetic subtypes. Myeloma as we define it today is likely a collection of cytogenetically distinct diseases, with not just varied prognosis, but also clinical presentation. In studies so far, we clearly know that some of the cytogenetic abnormalities in myeloma are recognized as “primary” events that are required for the initiation of the clone; this includes IgH translocations and trisomies. Thus although phenotypically myeloma is considered as one disease, from a genetic standpoint, it may represent distinct disorders. The current study was performed to examine if clinical presentation across the cytogenetic subtypes vary, just like the prognosis does. This remains a major unanswered question, and this represent the first systematic study looking at all the primary molecular types, and the specific myeloma defining event associated with each subtype.
The first major finding of this study was that the type of MDE was significantly influenced by the underlying cytogenetic subtype. MM with IgH translocations without trisomies accounted for 30% of all patients, but accounted for 50% of patients with renal failure as the MDE. Specifically, the t(14;16) translocation accounted for only 5% of all MM patients, but was present in 13.5% of patients with renal failure as the MDE. Twenty five percent of patients with the t(14;16) translocation presented with renal failure only as the initial MDE. Previous reports have indicated that patients with t(14;16) have an adverse prognosis.7–14 Our findings suggest that the mechanism by which t(14;16) is associated with poor survival may be at least in part due to the higher prevalence of renal failure as the MDE at diagnosis rather than true aggressive disease biology. In fact, after adjusting for renal failure, the outcome of t(14;16) in our study was comparable to other subtypes.
A second major finding of this study was that renal failure as initial MDE was associated with an adverse prognosis even in the absence of other concurrent CRAB features such as lytic bone disease. It is well known that renal failure in MM is associated with inferior outcome, but this is the first study to show that isolated renal failure as MDE has an adverse impact.21, 22 In fact, a priori we anticipated that patients presenting with renal failure as the sole MDE may actually have more of a paraprotein related disease state rather than true malignancy. But this study shows that there outcome is significantly worse than MM patients who present with lytic bone disease as the MDE. In fact, since lytic bone disease probably results in an early diagnosis of MM (lead-time bias since the term MM is applied more readily when lytic bone lesions are present), the outcome of these patients is superior to all other MDE groups. (Figure 1).
We also made several other interesting observations. For example, we found that patients with t(11;14) and t(6;14) tended to present more often with bone disease group as the initial MDE compared with patients who had t(4;14) or t(14;16). However, when all patients with bone disease were considered, no significant difference in the distribution of cytogenetic abnormalities was found in patients with and without lytic bone lesions. Similarly, there was no significant difference when the cytogenetic abnormalities were collapsed into prognostic risk groups commonly used in the clinic. There were no significant differences in the distribution of cytogenetic subtypes among patients with anemia as the MDE compared with the distribution seen when all MM patients are considered.
While this study provides insight into associations between clinical presentation, cytogenetic abnormalities, and survival in myeloma, there are limitations to these analyses. Although one of the strengths of the current study is the large number of patients, some subgroups were limited by small numbers. Second, the sample we used was a single-center referral population; future investigation using a multicenter approach would provide both numbers and diversity, subsequently solving these two limitations.
In summary, we show that there is significant heterogeneity in MDE associated with the cytogenetic subtype MM. The t(14;16) translocation was more frequently associated with renal failure as the MDE, and this association may explain in part the high risk phenotype associated with this abnormality. Patients classified as having renal failure alone as the initial MDE have significantly poorer survival compared with all other groups, while patients with bone disease as the MDE had the best outcome. Further validation of these findings in other patient samples is necessary, as is investigation of MDEs in patients with relapsed disease.
Acknowledgments
Funding: This work is supported in part by: Mayo Clinic Hematological Malignancies Program, Paul Calabresi K12 Award (CA96028). Also supported in part by Supported in part by grants CA 107476, CA 62242, CA100707, and CA 83724 from the National Cancer Institute, Rockville, MD, USA. Also supported in part by the Jabbs Foundation, Birmingham, United Kingdom and the Henry J. Predolin Foundation, USA.
Footnotes
Authorship Contributions and Disclosure of Conflicts of Interest
S.V.R., S.K.K., and A.J.G. conceived of and designed the study; A.J.G., S.K.K., T.M.T, and P.P.S. collected and analyzed the data; A.J.G. and S.K.K. wrote the manuscript, with input from all authors.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60 (5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84(12):1095–110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle R, Rajkumar S. Criteria for diagnosis, staging, risk stratification, and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Guiterrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–21. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexaméthasone. Leukemia. 2010;24(3):623–8. doi: 10.1038/leu.2009.273. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar S. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8(8):479–91. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood. 2007;109(8):3489–95. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 8.Drach J, Ackermann J, Fritz E, Kromer E, Schuster R, Gisslinger H, et al. Presence of a p53 gene deletion in patients with multiple myeloma predicts for short survival after conventional-dose chemotherapy. Blood. 1998;92(3):802–9. [PubMed] [Google Scholar]
- 9.Facon T, Avet-Loiseau H, Guillerm G, Moreau P, Genevieve F, Zandecki M, et al. Chromosome 13 abnormalities identified by FISH analysis and serum beta2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy. Blood. 2001;97(6):1566–71. doi: 10.1182/blood.v97.6.1566. [DOI] [PubMed] [Google Scholar]
- 10.Gertz M, Lacy M, Dispenzieri A, Greipp P, Litzow M, Henderson K, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–40. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor P, Fonseca R, Rajkumar S, Sinha S, Gertz M, Stewart A, et al. Evidence for cytogenetic and fluorescence in situ hybridization risk stratification of newly diagnosed multiple myeloma in the era of novel therapie. Mayo Clin Proc. 2010;85(6):532–7. doi: 10.4065/mcp.2009.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroger N, Schilling G, Einsele H, Liebisch P, Shimoni A, Nagler A, et al. Deletion of chromosome band 13q14 as detected by fluorescence in situ hybridization is a prognostic factor in patients with multiple myeloma who are receiving allogeneic dose-reduced stem cell transplantation. Blood. 2004;103(11):4056–61. doi: 10.1182/blood-2003-12-4435. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Simon J, Garcia-0Sanz R, Tabernero M, Almeida J, Gonzalez M, Fernandez-Calco J, et al. Prognostic value of numerical chromosome aberrations in multiple myeloma: A FISH analysis of 15 different chromosomes. Blood. 1998;91(9):3366–71. [PubMed] [Google Scholar]
- 14.Shaughnessy JJ, Haessler J, Rhee Fv, Anaissie E, Pineda-Roman M, Cottler-Fox M, et al. Testing standard and genetic parameters in 220 patients with multiple myeloma with complete data sets: superiority of molecular genetics. Br J Haematol. 2007;173(5):530–6. doi: 10.1111/j.1365-2141.2007.06586.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Fonseca R, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA, et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood. 2012;119(9):2100–5. doi: 10.1182/blood-2011-11-390658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Gupta V, Fonseca R, Dispenzieri A, Gonsalves WI, Larson D, et al. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia. 2013 doi: 10.1038/leu.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chng W, Wier SV, Ahmann G, Winkler J, Jalal S, Bergsagel P, et al. A validated FISH trisomy index demonstrates the hyperdiploid and nonhyperdiploid dichotomy in MGUS. Blood. 2005;106(6):2156–61. doi: 10.1182/blood-2005-02-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, et al. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project. Leukemia. 2013;27(3):711–7. doi: 10.1038/leu.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87(1):78–88. doi: 10.1002/ajh.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Onc. 2011;8(8):497–1. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Talamo G, Faroog U, Zangari M, Liao J, Dolloff NG, Loughran TPJ, et al. Beyond the CRAB symptoms: a study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10(6):464–8. doi: 10.3816/CLML.2010.n.080. [DOI] [PubMed] [Google Scholar]


