Abstract
Background
Green chemistry is a rapidly developing new field that provides us with a proactive avenue for the sustainable development of future science and technologies. Green chemistry uses highly efficient and environmentally benign synthetic protocols to deliver lifesaving medicines, accelerating lead optimization processes in drug discovery, with reduced unnecessary environmental impact. From this view point, it is desirable to use water instead of organic solvents as a reaction medium, since water is safe, abundant and an environmentally benign solvent.
Results
A convenient one-pot method for the efficient synthesis of the novel Zwitterion derivatives 4a-pvia a three-component condensation reaction of barbituric acid derivatives 1a,b, dimedone 2, and various aldehydes 3 in the presence of aqueous diethylamine media is described. This new approach is environmentally benign, with clean synthetic procedure, short reaction times and easy work-up procedure which proceeded smoothly to provide excellent yield (88-98%). The synthesized products were characterized by elemental analysis, IR, MS, NMR and CHN analysis. The structure of 4a was further confirmed by single crystal X-ray diffraction. The compound crystallizes in the orthorhombic space group Pbca with α = 14.6669 (5) Å, b = 18.3084 (6) Å, c = 19.0294 (6) Å, α = 90°, β = 90°, = 90°, V = 5109.9 (3) Å3, and Z = 8. The molecules are packed in crystal structure by weak intermolecular C–H⋅ ⋅ ⋅O hydrogen bonding interactions.
Conclusions
An environmentally benign Aldol-Michael protocol for the synthesis of dimedone-barbituric derivatives using aqueous diethylamine medium is achieved.
Keywords: Tandem Aldol-Michael reactions, MCRs, Barbituric acid, Aqueous media, Green chemistry, Dimedone, Zwitterions
Background
Recently, the development of environmentally benign and clean synthetic procedures has become the goal of organic synthesis. Water plays an essential role in life processes and also as a medium for organic reactions [1,2]. The use of water as a reaction medium exhibits remarkable benefit because of its high polarity and therefore immiscibility with most organic compounds. Reactions in aqueous media are environmentally safe and have less carcinogenic effects with a simple work up procedure which are especially important in industry. Thus, there is a need for developing multicomponent reactions (MCR’s) in water, without the use of any harmful organic solvents.
On the other hand, due to the diverse biological properties of barbituric acid derivatives (1), there is a widespread interest in their synthesis [3-7]. Compounds alkylated in the fifth position have demonstrated anticancer, HIV-1 and HIV-2 protease inhibitors [8], sedative-hypnotic [9,10] and anticonvulsant [11] properties. Many of their representatives have clinical use as anti-inflammatory [12] and hypnotic drugs, such as veronal, phenobarbital, seconal, bucolone and sodium pentothal (Figure 1) [13-15]. A number of compounds having these systems have been synthesized with diverse pharmacological activities [16,17].
Figure 1.
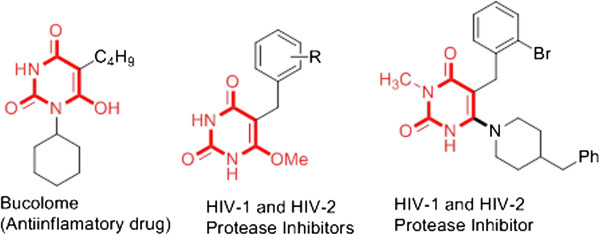
Bioactive compounds containing the barbituric acid framework.
Dimedone (5,5-dimethylcyclohexane-1,3-dione) 2 belongs to the cyclic 1,3-diketones – a very important class of organic compounds. A wide range of practical applications of dimedone include their uses as versatile precursors for synthesis of numerous hetero and spirocyclic compounds [18], xanthene derivatives with their industrial [19] and synthetic [20] applications, and also as reagent for various analytical determinations [21].
As a part of our work on one-pot multicomponent reactions (MCRs) for the synthesis of various heterocyclic compounds, we report here a highly efficient procedure for the preparation of dimedone-barbituric derivatives based on tandem Aldol-Michael reactions using aqueous diethylamine medium.
Results and discussion
In a typical experimental procedure, a mixture of barbituric acid 1a,b, dimedone 2 and aromatic aldehyde 3 in water was stirred in the presence of a stoichiometric amount of diethylamine (1.0 equiv.) to afford the ‘Zwitterion adduct salts’ of dimedone-barbituric acid derivative 4a in high yields (Scheme 1).
Scheme 1.
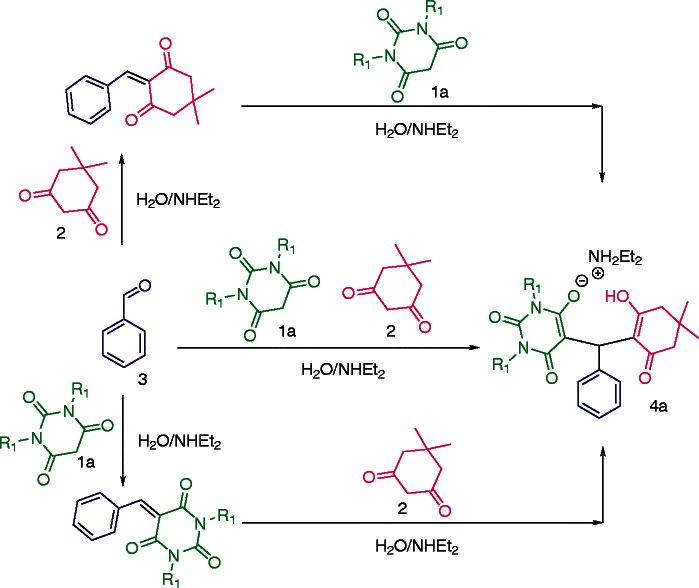
Synthesis of 4a.
A possible mechanism for the tandem Aldol- Michael reaction is shown in Figure 2. In the first step of the reaction, olefin is produced by a Aldol condensation between aryl aldehyde 3 and 1a,b promoted by DEA. Dimedone in the presence of DEA is then converted to its corresponding diethylammonium dimedonate that easily reacts with olefin to give product 4a-p[22-31].
Figure 2.
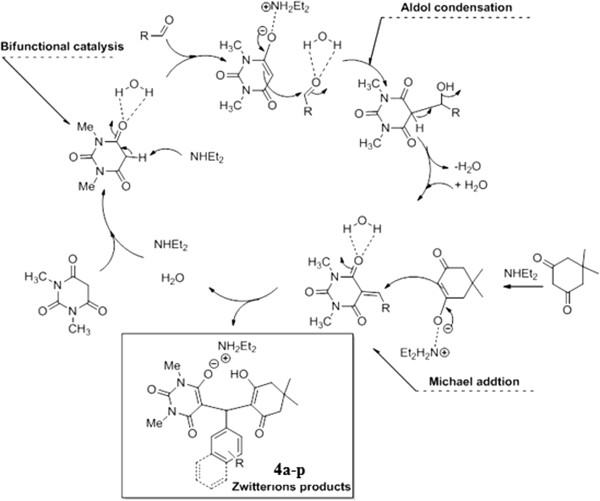
A possible mechanistic pathway.
In the absence of DEA, the reaction does not proceed efficiently and only a poor yield of products was obtained after 10 h. The structures of products were confirmed by physical and spectroscopic (IR, MS, NMR) data, and by elemental analysis. The workup procedure is very simple and the products do not require further purification.
The X-ray zwitterion structure of 4a (Figure 3) was obtained using X-ray structure determination from a single crystal grown from CHCl3/Et2O as solvents. The structure shows interesting characteristics (Table 1). We were unable to determine the location of the C6 and C14 hydrogens by 1HNMR analysis. This is because the hydrogen from C6 dimedone, rather than hydrogen from C14 of the barbituric acid moiety, is removed by the basicity of diethylamine. This was confirmed by the X-ray structure because one hydrogen is on the diethylamine and the other is involved in hydrogen bonding interactions between both barbituric acid and dimedone moiety. The hydrogen-bonding interactions are listed in Table 2. Figure 4 depicts the packing of the molecules in the crystal structure. The crystal structure is stabilized by C–H⋅ ⋅ ⋅O hydrogen bonds into a three-dimensional framework structure. It is noteworthy to mention that 1HNMR have also shown a singlet signal at δ 15.28 ppm which can be assigned to the OH group which makes a hydrogen bond.
Figure 3.
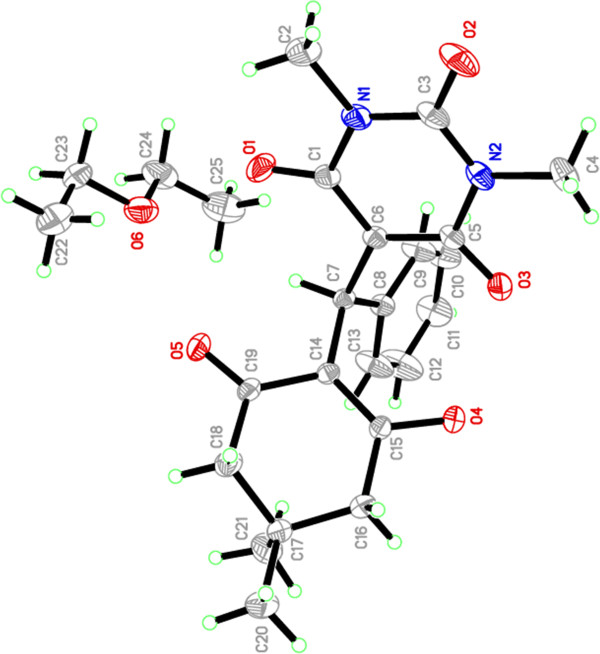
ORTEP representation of the structure of 4a.
Table 1.
Crystallographic data and refinement information of 4a
| Empirical formula | C 25 H 32 N 2 O 6 |
|---|---|
| Formula weight |
456.53 |
| Temperature (K) |
293 |
| Crystal system |
Orthorhombic |
| Space group |
Pbca |
| Cu Kα radiation, λ |
1.54178 Å |
|
a = |
14.6669 (5) Å |
|
b = |
18.3084 (6) Å |
|
c = |
19.0294 (6) Å |
| α = |
90º |
| β = |
90º |
| γ = |
90º |
|
V = |
5109.9 (3) Å3 |
|
Z = |
8 |
| Theta range for data collection |
3.0–69.2° |
| μ = |
0.70 mm−1 |
| Density clac. (g/cm3) |
1.187 |
| Crystal shape and colour |
Plate, colourless |
| Crystal size |
0.89 × 0.78 × 0.22 mm |
| h/k/l |
−17,17/-22,22/-22,23 |
| Measured reflections |
32924 |
| Independent reflections |
4796 (Rint = 0.088) |
| Reflections with I > 2σ(I) |
3997 |
| Goodness-of-fit on F
2
|
1.04 |
|
R[F2 > 2σ(F2)] = |
0.067 |
|
wR(F2) = |
0.195 |
| Δρmax = |
0.47 e Å−3 |
| Δρmin = | −0.40 e Å−3 |
Table 2.
Hydrogen-bond geometry (Å, °)
| D —H · · · A | D —H | H · · · A | D · · · A | D —H · · · A |
|---|---|---|---|---|
| C2—H2B · · · O1 |
0.9600 |
2.2600 |
2.655(3) |
104.00 |
| C4—H4B · · · O3 |
0.9600 |
2.2300 |
2.682(3) |
108.00 |
| C7—H7A · · · O1 |
0.9800 |
2.3700 |
2.894(2) |
113.00 |
| C7—H7A · · · O5 |
0.9800 |
2.2800 |
2.821(2) |
114.00 |
| C22—H22A · · · O3i | 0.9600 | 2.5400 | 3.376(3) | 146.00 |
Symmetry code: (i) x, −y + 3/2, z + 1/2.
Figure 4.
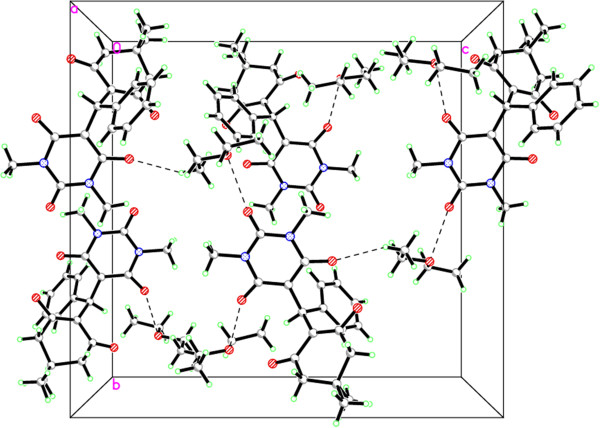
Crystal packing showing intermolecular C–H⋅O hydrogen bonds as dashed lines. 4a.
With the optimal reaction conditions established, the generality of the Aldol-Michael reactions was next investigated by using a series of aryl aldehyde 3 (Table 3). Various aldehydes derivatives with either electron-withdrawing or electron-donating groups at the para-, meta-, or even sterically hindered ortho-position on the aromatic ring were tolerated and gave the corresponding condensed products 4a-p in excellent chemical yield up to 98% (Scheme 2).
Table 3.
Tandem Aldol-Michael reactions of barbituric acid 1a,b and dimedone 2 with aldehydes 3 in aqueous diethylamine medium a
| # | 3 | R 1 | R 2 | yield (%) b |
|---|---|---|---|---|
| 1 |
4a |
CH3 |
Ph |
98 |
| 2 |
4b |
CH3 |
p-CH3Ph |
97 |
| 3 |
4c |
CH3 |
p-ClPh |
97 |
| 4 |
4d |
CH3 |
p-BrPh |
95 |
| 5 |
4e |
CH3 |
m-BrPh |
93 |
| 6 |
4f |
CH3 |
p-CH3OPh |
92 |
| 7 |
4 g |
CH3 |
o-NO2Ph |
93 |
| 8 |
4 h |
CH3 |
2,4-Cl2Ph |
90 |
| 9 |
4i |
CH3 |
2,6-Cl2Ph |
89 |
| 10 |
4j |
CH3 |
2-Naphthaldehyde |
94 |
| 11 |
4 k |
CH3 |
p-HO-Ph |
91 |
| 12 |
4 l |
H |
Ph |
93 |
| 13 |
4 m |
H |
p-CH3Ph |
91 |
| 14 |
4n |
H |
p-ClPh |
90 |
| 15 |
4o |
H |
p-BrPh |
89 |
| 16 | 4p | H | 2-Naphthaldehyde | 90 |
aAll reactions were carried out with barbituric acid derivatives 1a,b (1.5 mmol), dimedone 2 (1.5 mmol) aldehydes 3 (1.5 mmol) and diethylamine (1.5 mmol) in water (1.5 mL) for the specified time. bYield of isolated product 4a-p.
Scheme 2.
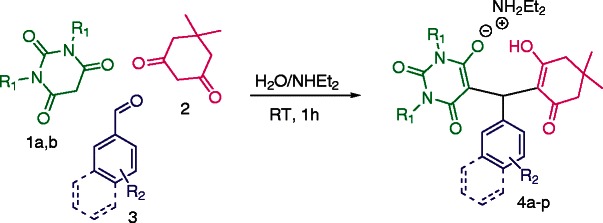
Synthesis of 4a-p.
Conclusions
In summary, a mild, efficient, and expeditious method has been developed for the synthesis of zwitterion-condensed products 4a-pvia a three component; one-pot cyclocondensation reaction of aromatic aldehyde, barbituric acid, and dimedone using aqueous diethylamine medium. The main advantage of the present methodology is a simple work-up procedure with milder reaction conditions. This method provides excellent yields of the products with high selectivity. Further studies on expanding the application of this method and the biological evaluation of these dimedone-barbituric derivatives are in progress.
Experimental section
General
All chemicals were purchased from Aldrich, Sigma-Aldrich, Fluka etc., and were used without further purification, unless otherwise stated. All melting points were measured on a Gallenkamp melting point apparatus in open glass capillaries and are uncorrected. IR Spectra were measured as KBr pellets on a Nicolet 6700 FT-IR spectrophotometer. The NMR spectra were recorded on a Jeol-400 NMR spectrometer. 1H NMR (400 MHz), and 13C NMR (100 MHz) were run in either deuterated dimethylsulphoxide (DMSO-d6) or deuterated chloroform (CDCl3). Chemical shifts (δ) are referred in terms of ppm and J -coupling constants are given in Hz. Mass spectra were recorded on a Jeol of JMS-600H. Elemental analysis was carried out on an Elmer 2400 Elemental Analyzer; CHN mode.
General procedure for aldol condensation Michael addition for the synthesis of 4a-p (GP1)
A mixture of aldehyde 3 (1.5 mmol), dimedone 2 (1.5 mmol), barbituric acid derivatives 1a,b (1.5 mmol) and Et2NH (1.5 mmol, 155 μL) in 1.5 mL of degassed H2O was stirred at room temperature for 1–2 hours until TLC showed complete disappearance of the reactants. The product precipitated and the mixture was filtered and washed with ether (3 × 20 mL). The solid was recrystallized from a mixture of CH2Cl2/Et2O to afford pure product 4a-p.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4a)
4a was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and benzaldehyde according to the general procedure (GP1) yielding colorless crystalline material (671 mg, 1.47 mmol, 98%). m.p: 159°C; IR (KBr, cm –1 ): 3150, 2959, 1667, 1617, 1585, 1422, 1256, 1227; 1H NMR (400 MHz, CDCl3): δ 15.28 (s, 1H, OH), 7.17-7.04 (m, 5H, Ph), 5.85 (s, 1H, benzyl-H), 3.29 (s, 12H, 4CH3), 2.96 (q, 4H, J = 7.3 Hz, CH2CH3), 2.42 (d, 2H, J = 5.1 Hz, CH2), 2.29 (m, 2H, CH2), 1.24 (t, 6H, J = 7.3 Hz, CH2CH3), 1.14 (s, 3H, CH3), 1.05 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 192.5, 180.8, 152.5, 142.5, 128.0, 126.7, 125.1, 116.3, 90.9, 51.4, 45.9, 42.2, 33.0, 31.5, 29.6, 28.4, 27.6, 11.4; LC/MS (ESI): 457 [M]+; Anal. for C25H35N3O5; calcd: C, 65.62; H, 7.71; N, 9.18; Found: C, 65.61; H, 7.73; N, 9.20.
The structure of 4a was confirmed by X-ray crystal structure analysis. CCDC- 933624 contains the supplementary crystallographic data for this compound. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre viahttp://www.ccdc.cam.ac.uk/data_request/cif. A colorless crystal suitable for X-ray analysis was obtained from recrystallization of the compound from CHCl3/Et2O at room temperature after 2 days.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(p-tolyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4b)
4b was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and p-tolualdehyde according to the general procedure (GP1) yielding an oily material (685 mg, 1.45 mmol, 97%). IR (KBr, cm –1 ): 3150, 2954, 2867, 1675, 1580, 1508, 1447, 1380, 1256, 1145; 1H NMR (400 MHz, CDCl3): δ 15.25 (s, 1H, OH), 7.00-6.93 (m, 4H, Ph), 5.84 (s, 1H, benzyl-H), 3.28 (s, 12H, 4CH3), 2.90 (q, 4H, J = 7.3 Hz, CH2CH3), 2.30 (d, 4H, J = 5.1 Hz, CH2), 2.22 (s, 3H, CH3), 1.20 (t, 6H, J = 7.3 Hz, CH2CH3), 1.16 (s, 3H, CH3), 1.04 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 196.5, 180.1, 152.8, 140.5, 134.2, 129.8, 128.7, 126.8, 126.7, 115.6, 91.0, 51.4, 45.9, 42.5, 32.6, 31.5, 29.6, 28.4, 27.6, 20.9, 11.9; LC/MS (ESI): 471 [M]+; Anal. for C26H37N3O5; calcd: C, 66.22; H, 7.91; N, 8.91; Found: C, 66.24; H, 7.92; N, 8.87.
5-((4-Chlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4c)
4c was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and p-chlorobenzaldehyde 3 according to the general procedure (GP1) yielding an oily material (715 mg, 1.45 mmol, 97%). IR (KBr, cm –1 ): 3151, 2955, 2868, 2497, 1675, 1580, 1481, 1444, 1379, 1258, 1206; 1H NMR (400 MHz, CDCl3): δ 15.02 (s, 1H, OH), 7.12-6.95(m, 4H, Ph), 5.87 (s, 1H, benzyl-H), 3.30 (s, 12H, 4CH3), 2.90 (q, 4H, J = 7.3 Hz, CH2CH3), 2.38 (s, 4H, CH2), 1.20 (t, 6H, J = 7.3 Hz, CH2CH3), 1.16 (s, 3H, CH3), 1.04(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198.1, 181.0, 152.5, 141.5, 130.6, 128.3, 128.2, 128.0, 127.9, 115.2, 90.7, 65.9, 49.8, 42.3, 32.4, 31.5, 31.2, 29.6, 28.4, 27.6, 15.3, 11.4; LC/MS (ESI): 492 [M]+; Anal. for C25H34ClN3O5; calcd: C, 61.03; H, 6.97; Cl, 7.21; N, 8.54; Found: C, 61.06; H, 7.00; Cl, 7.18; N, 8.57.
5-((4-Bromophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4d)
4d was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and p-bromobenzaldehyde 3 according to the general procedure (GP1) yielding an oily material (761 mg, 1.42 mmol, 95%). IR (KBr, cm –1 ): 3155, 2955, 2867, 2500, 1674, 1579, 1430, 1376, 1204;1H NMR (400 MHz, CDCl3): δ 15.20 (s, 1H, OH), 7.34 (d, 2H, J = 8.0 Hz, Ph), 6.98 (d, 2H, J = 8.0 Hz, Ph), 5.79 (s, 1H, benzyl-H), 3.27 (s, 12H, 4CH3), 2.99 (q, 4H, J = 7.3 Hz, CH2CH3), 2.40 (d, 2H, J = 5.1 Hz, CH2), 2.28 (m, 2H, CH2), 1.29 (t, 6H, J = 7.3 Hz, CH2CH3), 1.18 (s, 3H, CH3), 1.04 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.1, 191.2, 164.8, 152.4, 142.8, 132.5, 131.0, 129.9, 128.7, 128.6, 118.9, 115.9, 90.6, 51.2, 45.8, 42.3, 32.7, 31.5, 29.5, 28.5, 28.3, 27.6, 11.4; LC/MS (ESI): 536 [M]+; Anal. for C25H34BrN3O5; calcd: C, 55.97; H, 6.39; Br, 14.89; N, 7.83; Found: C, 56.00; H, 6.40; Br, 14.86; N, 7.82.
5-((3-Bromophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4e)
4e was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and m-bromobenzaldehyde 3 according to the general procedure (GP1) yielding an oily material (745 mg, 1.39 mmol, 93%). IR (KBr, cm –1 ): 3050, 2955, 2868, 2500, 1675, 1581, 1444, 1378, 1255, 1205; 1H NMR (400 MHz, CDCl3): δ 15.63 (s, 1H, OH), 7.22 (d, 1H, J = 7.3 Hz, Ph), 7.19 (s, 1H, Ph), 7.07 (d, 1H, J = 7.3 Hz, Ph), 7.05 (d, 1H, J = 7.3 Hz, Ph), 5.84 (s, 1H, benzyl-H), 3.34 (s, 6H, 2CH3), 3.32 (s, 6H, 2CH3), 2.98 (q, 4H, J = 7.3Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 1.24 (t, 6H, J = 7.3 Hz, CH2CH3), 1.12 (s, 3H, CH3), 1.03 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 190.8, 186.4, 165.2, 164.4, 151.7, 144.7, 129.7,129.6, 128.7, 125.3, 91.5, 42.1, 34.4, 28.9, 28.7, 11.5; LC/MS (ESI): 536 [M]+; Anal. for C25H34BrN3O5; calcd: C, 55.97; H, 6.39; Br, 14.89; N, 7.83; Found: C, 56.01; H, 6.41; Br, 14.86; N, 7.84.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4f)
4f was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and anisaldehyde 3 according to the general procedure (GP1) yielding an oily material (672 mg, 1.38 mmol, 92%). IR (KBr, cm –1 ): 3047, 2953, 2866, 2499, 1679, 1577, 1510, 1427, 1373, 1255, 1214;1H NMR (400 MHz, CDCl3): δ 15.26 (s, 1H, OH), 6.98 (d, 2H, J = 8.0 Hz, Ph), 6.72 (d, 2H, J = 8.0 Hz, Ph), 5.69 (s, 1H, benzyl-H), 3.71 (s, 3H, CH3), 3.29 (s, 12H, 4CH3), 2.87 (q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 1.19 (t, 6H, J = 7.3 Hz, CH2CH3), 1.12 (s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 195.1, 187.2, 157.1, 134.5, 133.9, 127.8, 127.6, 115.6, 113.4, 55.2, 42.6, 31.5, 31.1, 27.9, 12.2; LC/MS (ESI): 487 [M]+; Anal. for C26H37N3O6; calcd: C, 64.05; H, 7.65; N, 8.62;Found: C, 64.11; H, 7.64; N, 8.59.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-nitrophenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4 g)
4b was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and p-nitrobenzaldehyde 3 according to the general procedure (GP1) yielding a beige material (700 mg, 1.39 mmol, 93%). m.p: 148°C; IR (KBr, cm –1 ): 3050, 2950, 2865, 2500, 1669, 1580, 1510, 1427, 1373, 1255, 1214;1H NMR (400 MHz, CDCl3): δ 15.26 (s, 1H, OH), 6.99 (d, 2H, J = 8.0 Hz, Ph), 6.72 (d, 2H, J = 8.8 Hz, Ph), 5.69 (s, 1H, benzyl-H), 3.71 (s, 12H, 4CH3), 2.85 (q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d,4H, J = 14.7 Hz, CH2), 1.19 (t, 6H, J = 7.3 Hz, CH2CH3), 1.12 (s, 3H, CH3), 1.03 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 161.6, 153.2, 145.5, 141.6, 129.1, 128.2, 127.8, 125.8, 88.5, 49.1, 41.9, 27.5, 11.5; LC/MS (ESI): 502 [M]+; Anal. for C25H34N4O7; calcd: C, 59.75; H, 6.82; N, 11.15; Found: C, 59.73; H, 6.81; N, 11.17.
5-((2,4-Dichlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4 h)
4 h was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and 2,4-dichlorobenzaldehyde 3 according to the general procedure (GP1) yielding a beige solid material (710 mg, 1.35 mmol, 90%). m.p: 164°C; IR (KBr, cm –1 ): 3059, 2995, 2867, 2114, 1741, 1658, 1591, 1463, 1429, 1370, 1341, 1256, 12011H-NMR (400 MHz, CDCl3): δ 14.80 (s, 1H, OH), 7.29 (d, 1H, J = 8.0 Hz, Ph), 7.19 (s, 1H, Ph), 7.12 (d, 2H, J = 8.0 Hz, Ph), 5.76 (s, 1H, benzyl-H), 3.28 (s, 12H, 4CH3), 3.07 (q, 4H, J = 7.3 Hz, CH2CH3), 2.37 (s, 2H, CH2), 2.27 (d, 2H, J = 5.1 Hz, CH2), 1.34 (t, 6H, J = 7.3 Hz, CH2CH3), 1.04 (s, 3H, CH3), 1.01 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.1, 165.4, 164.4, 152.5, 139.8, 133.6, 131.7, 131.2, 129.3, 126.4, 115.7, 89.8, 51.2, 45.7, 41.9, 32.4, 31.2, 28.3, 28.2, 11.3; LC/MS (ESI): 526 [M]+; Anal. for C25H33Cl2N3O5; calcd: C, 57.04; H, 6.32; Cl, 13.47; N, 7.98; Found: C, 57.09; H, 6.31; Cl, 13.44; N, 8.01.
5-((2,6-Dichlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4i)
4i was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and 2,6-dichlorobenzaldehyde 3 according to the general procedure (GP1) yielding an oily material (702 mg, 1.33 mmol, 89%). IR (KBr, cm –1 ): 3048, 2955, 2869, 2728, 2494, 1676, 1575, 1428, 1372, 1238, 1196;1H NMR (400 MHz, CDCl3): δ 14.80 (s, 1H, OH), 7.36 (d, 2H, J = 8.0 Hz, Ph), 7.29 (t, 1H, J = 8.0 Hz, Ph), 7.12 (d, 2H, J = 8.0 Hz, Ph), 5.98 (s, 1H, benzyl-H), 3.26 (s, 12H, 4CH3), 2.92(q, 4H, J = 7.3 Hz, CH2CH3), 2.37 (s, 2H, CH2), 2.27 (d, 2H, J = 5.1 Hz, CH2), 1.24(t, 6H, J = 7.3 Hz, CH2CH3), 1.094(s, 3H, CH3), 1.04 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 192.8, 188.9, 165.3, 164.3, 152.5, 149.7, 137.4, 131.5, 129.8, 126.5, 124.2, 115.5, 114.7, 89.9, 53.5, 41.4, 31.9, 28.7, 28.2, 11.4 ; LC/MS (ESI): 526 [M]+; Anal. for C25H33Cl2N3O5; calcd: C, 57.04; H, 6.32; Cl, 13.47; N, 7.98; Found: C, 57.08; H, 6.30; Cl, 13.45; N, 8.00.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(naphthalen-2-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4j)
4j was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and 2-naphthaldehyde 3 according to the general procedure (GP1) yielding a white solid material (715 mg, 1.41 mmol, 94%). m.p: 170 °C; IR (KBr, cm –1 ): 2994, 2948, 2866, 2506, 1742, 1651, 1603, 1570, 1526, 1473, 1431, 1362, 1245;1H NMR (400 MHz, CDCl3): δ 14.26 (s, 1H, OH), 7.46-7.22 (m, 7H, naphthyl), 6.20 (s, 1H, benzyl-H), 3.26 (s, 6H, 2CH3), 3.23 (s, 6H, 2CH3), 3.14 (q, 4H, J = 7.3 Hz, CH2CH3), 2.41 (q, 4H, J = 5.1 Hz, CH2), 2.23 (s, 2H, CH2), 1.37 (t, 6H, J = 7.3 Hz, CH2CH3), 1.07 (s, 3H, CH3), 1.01 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.0, 180.5, 165.3, 164.3, 152.5, 149.7, 136.8, 131.5, 129.9, 126.5, 124.2, 115.5, 114.7, 89.9, 50.9, 45.5, 41.7, 31.3, 30.7, 28.2, 11.1; LC/MS (ESI): 507 [M]+; Anal. for C29H37N3O5; calcd: C, 68.62; H, 7.35; N, 8.28; Found: C, 68.65; H, 7.34; N, 8.30.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-hydroxyphenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4 k)
4 k was prepared from 1,3-dimethylbarbituric acid 1a, dimedone 2 and p-hydroxybenzaldehyde 3 according to the general procedure (GP1) yielding a white solid material (645 mg, 1.36 mmol, 91%). m.p: 162°C; IR (KBr, cm –1 ): 23097, 2939, 2884, 2828, 2498, 1747, 1574, 1530, 1506, 1466, 1384, 1241;1H NMR (400 MHz, DMSO-d6): δ 14.52 (s, 1H, OH), 8.50 (brs, 1H, OH), 6.76 (d, 2H, J = 8.0 Hz, Ph), 6.50 (d, 2H, J = 8.0 Hz, Ph), 6.04 (s, 1H, benzyl-H), 3.07 (s, 12H, 2CH3), 3.14 (q, 4H, J = 7.3 Hz, CH2CH3), 2.92 (q, 4H, J = 13.9 Hz, CH2), 206 (s, 4H, CH2), 1.12 (t, 6H, J = 7.3 Hz, CH2CH3), 0.98 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ = 198.0, 188.5, 154.1, 136.6, 128.3, 115.3, 114.3, 90.1, 50.9, 45.5, 42.1, 31.6, 30.7, 29.7, 11.7; LC/MS (ESI): 473 [M]+; Anal. for C25H35N3O6; calcd: C, 63.41; H, 7.45; N, 8.87; Found: C, 63.40; H, 7.43; N, 8.85.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4 l)
4 m was prepared from barbituric acid 1b, dimedone 2 and benzaldehyde 3 according to the general procedure (GP1) yielding a white solid material (598 mg, 1.39 mmol, 93%). m.p: 215°C; IR (KBr, cm –1 ): 3027, 2948, 2867, 2156, 1683, 1593, 1451, 1374, 1291, 1257, 11411H-NMR (400 MHz, CDCl3): δ 12.26 (s, 1H, OH), 9.31 (brs, 2H, NH), 7.12 (m, 5H, Ph), 5.52 (s, 1H, benzyl-H), 2.99 (q, 4H, J = 7.3 Hz, CH2CH3), 2.45 (d, 4H, J = 5.1 Hz, CH2), 1.24(t, 6H, J = 7.3 Hz, CH2CH3), 1.09 (s, 3H, CH3), 1.03 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198.5, 180.8, 152.5, 142.5, 128.0, 126.7, 125.1, 116.3, 90.9, 51.4, 45.9, 42.2, 33.0, 28.4, 27.6, 11.3; LC/MS (ESI): 429 [M]+; Anal. for C23H31N3O5; calcd: C, 64.32; H, 7.27; N, 9.78; Found: C, 64.29; H, 7.29; N, 9.80.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(p-tolyl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4 m)
4n was prepared from barbituric acid 1a, dimedone 2 and tolualdehyde 3 according to the general procedure (GP1) yielding a white solid material (604 mg, 1.36 mmol, 91%). m.p: 213°C; IR (KBr, cm –1 ): 3150, 2955, 2867, 1690, 1592, 1508, 1375, 1256, 1232, 1167; 1H NMR (400 MHz, CDCl3): δ 13.31 (s, 1H, OH), 8.83 (brs, 2H, NH), 7.27 (d, 2H, J = 8.0 Hz, Ph), 7.00 (d, 2H, J = 8.0Hz, Ph), 5.88 (s, 1H, benzyl-H), 2.83 (q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 2.23 (s, 3H, CH3), 1.19 (t, 6H, J = 7.3 Hz, CH2CH3), 1.04 (s, 3H, CH3), 1.02 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 196.5, 180.1, 152.8, 140.5, 131.4, 130.7, 128.7, 128.6, 118.5, 115.6, 91.0, 50.9, 42.8, 31.6, 31.5, 29.2, 28.3, 27.8, 20.9, 11.3; LC/MS (ESI): 443 [M]+; Anal. for C24H33N3O5; calcd: C, 64.99; H, 7.50; N, 9.47; Found: C, 64.95; H, 7.49; N, 9.50.
5-((4-Chlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4n)
4o was prepared from barbituric acid 1a, dimedone 2 and p-chlorobenzaldehyde 3 according to the general procedure (GP1) yielding an oily product (625 mg, 1.35 mmol, 90%). IR (KBr, cm –1 ): 3049, 2954, 2865, 2499, 1738, 1699, 1590, 1483, 1375, 1292, 1258, 1225, 1205; 1H NMR (400 MHz, CDCl3δ 13.32 (s, 1H, OH), 8.83 (brs, 2H, NH), 7.27 (d, 2H, J = 8.0 Hz, Ph), 7.00 (d, 2H, J = 8.0 Hz, Ph), 5.89 (s, 1H, benzyl-H), 2.88 (q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 1.19 (t, 6H, J = 7.3 Hz, CH2CH3), 1.09(s, 3H, CH3), 1.03 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 190.9, 141.0, 134.8, 131.0, 129.5, 128.3, 115.3, 91.1, 47.1, 42.7, 31.6, 31.5, 29.1, 28.2, 27.8, 11.3; LC/MS (ESI): 463 [M]+; Anal. for C23H30ClN3O5; calcd: C, 59.54; H, 6.52; Cl, 7.64; N, 9.06; Found: C, 59.57; H, 6.51; Cl, 7.60; N, 9.02.
5-((4-Bromophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4o)
4n was prepared from barbituric acid 1a, dimedone 2 and p-bromobenzaldehyde 3 according to the general procedure (GP1) yielding a white solid material (678 mg, 1.33 mmol, 89%). m.p: 208°C; IR (KBr, cm –1 ): 3093, 2939, 2885, 2829, 2551, 1746, 1686, 1576, 1506, 1466, 1416, 1268, 1241; 1H NMR (400 MHz, CDCl3): δ 13.31 (s, 1H, OH), 8.67 (brs, 2H, NH), 7.05 (m, 4H, Ph), 5.79 (s, 1H, benzyl-H), 2.79(q, 4H, J = 7.3 Hz, CH2CH3), 2.35 (d, 4H, J = 5.1 Hz, CH2), 1.21(t, 6H, J = 7.3 Hz, CH2CH3), 1.11 (s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198.5, 180.1, 152.8, 140.5, 131.4, 130.7, 128.7, 128.6, 118.5, 115.6, 91.0, 50.9, 42.8, 31.6, 31.5, 29.2, 28.3, 27.8, 11.3; LC/MS (ESI): 508 [M]+; Anal. for C23H30BrN3O5; calcd: C, 54.34; H, 5.95; Br, 15.72; N, 8.27; Found: C, 54.35; H, 5.96; Br, 15.69; N, 8.30.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(naphthalen-2-yl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4p)
4q was prepared from barbituric acid 1a, dimedone 2 and 2-naphthaldehyde 3 according to the general procedure (GP1) yielding an oily product (646 mg, 1.35 mmol, 90%). IR (KBr, cm –1 ): 3049, 2948, 2863, 2725, 1685, 1594, 1508, 1371, 1252, 1216; 1H NMR (400 MHz, CDCl3): δ 14.25 (s, 1H, OH), 7.46-7.22 (m, 7H, naphthyl), 6.21 (s, 1H, benzyl-H), 3.27 (s, 6H, 2CH3), 3.25 (s, 6H, 2CH3), 3.14(q, 4H, J = 7.3 Hz, CH2CH3), 2.41 (q, 4H, J = 5.1 Hz, CH2), 2.23 (s, 2H, CH2), 1.37 (t, 6H, J = 7.3 Hz, CH2CH3), 1.07(s, 3H, CH3), 1.01 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.1, 180.5, 165.5, 164.2, 152.5, 149.7, 136.8, 131.5, 129.9, 126.5, 124.2, 115.5, 114.7, 89.9, 50.9, 45.5, 41.7, 31.3, 30.7, 28.2, 11.3; LC/MS (ESI): 479 [M]+; Anal. for C27H33N3O5; calcd: C, 67.62; H, 6.94; N, 8.76; Found: C, 67.65; H, 6.96; N, 8.80.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AB proposed the subject, designed the study. AMA carried out the synthesis of all the products. YNM and AMA helped in the results and discussion. MRHS carried out NMR spectroscopy and elemental analysis. HG and HKF carried out the X-ray crystallography part. AB prepared draft the manuscript. All the authors read and approved the final manuscript.
Contributor Information
Assem Barakat, Email: ambarakat@ksu.edu.sa.
Abdullah Mohammed Al-Majid, Email: amajid@ksu.edu.sa.
Abdulaziz Moshabab Al-Ghamdi, Email: hero-1206@hotmail.com.
Yahia Nasser Mabkhot, Email: yahia@ksu.edu.sa.
Mohammed Rafiq Hussain Siddiqui, Email: rafiqs@ksu.edu.sa.
Hazem A Ghabbour, Email: ghabbourh@yahoo.com.
Hoong-Kun Fun, Email: hfun.c@ksu.edu.sa.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project Number RGP- VPP- 257.
References
- Grieco PA. Organic Synthesis in Water. London: Thomson Science; 1998. pp. 1–278. [Google Scholar]
- Li CJ. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: a decade update. Chem Rev. 2005;105:3095–3165. doi: 10.1021/cr030009u. [DOI] [PubMed] [Google Scholar]
- Bojarski JT, Mokrocz JL, Barton HJ, Paluchowska MH. Recent progress in barbituric acid chemistry. Adv Heterocycl Chem. 1985;38:229–297. [Google Scholar]
- Undheim K, Bennecke T, Katritzky AR, Rees CW, Scriven EFV, Boulton AJ. (Hrsg.): Comprehensive Heterocyclic Chemistry, Volume 6. Oxford: Elsevier Pergamon; 1996. Suppl. 93. [Google Scholar]
- Angerer S. Product class 12: pyrimidines. Sci Synth. 2004;16:379–572. [Google Scholar]
- Sans SRG, Chosaz MG. Historical aspects and applications of barbituric acid derivatives. Pharmazie. 1988;43:827–829. [PubMed] [Google Scholar]
- Taylor JB. Modern Medical Chemistry. New York: Prentice Hall; 1994. [Google Scholar]
- Guerin DJ, Mazeas D, Musale MS, Naguib FNM, Safarjalani ONA, Kouni MH, Panzica RP. Uridine phosphorylase inhibitors: chemical modification of benzyloxybenzyl barbituric acid and its effects on UrdPase inhibition. Bioorg Med Chem Lett. 1999;9:1477–1480. doi: 10.1016/S0960-894X(99)00238-3. [DOI] [PubMed] [Google Scholar]
- Andrews G. Medical pharmacology. Saint Louis, MO: The CV Mosby Co; 1976. pp. 243–250. [Google Scholar]
- Foye WO. Principles of medicinal chemistry. Pennsylvania, PA: Lea & Febiger; 1989. pp. 143–237. [Google Scholar]
- Goodman LS, Gilman A. The pharmacological basis of therapeutics. New Delhi: Mc Graw-Hill; 1991. pp. 358–360. [Google Scholar]
- Senda S, Izumi H, Fujimura H. Uracil derivatives and related compounds. VI. Derivatives of 5-alkyl-2,4,6-trioxoperhydropyrimidine as anti-inflammatory agents. Arznein Forsch. 1967;17:1519–1523. [PubMed] [Google Scholar]
- Fisher E, Moring JR. Ueber eine neue klasse von schlafmitteln. Ther Ggw. 1903;44:97–105. [Google Scholar]
- Doran WJ. Barbituric acid hypnotics. Med Chem. 1959;4:164–167. [Google Scholar]
- Bobranski B. Progress in chemistry of barbituric acid. Wiad Chem. 1977;31:231–278. [Google Scholar]
- Anderson GL, Shim JL, Brown AD. Pyrido[2,3-d]pyrimidines. IV. Synthetic studies leading to various oxopyrido[2,3-d]pyrimidines. J Org Chem. 1976;41:1095–1099. doi: 10.1021/jo00869a003. [DOI] [PubMed] [Google Scholar]
- Grivaky EM, Lee S, Siyal CW, Duch DS, Nichol CA. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J Med Chem. 1980;23:327–329. doi: 10.1021/jm00177a025. [DOI] [PubMed] [Google Scholar]
- El Ashry El SH, Awada LF, El Kilany Y, El Ibrahim S. Chapter 1 dimedone: a versatile precursor for annulated heterocycles. Adv Heterocycl Chem. 2009;98:1–141. [Google Scholar]
- Saitoh T, Taguchi K, Hiraide M. Derivatization of formaldehyde with dimedone and subsequent sorption on sodium dodecylsulfate/alumina admicelles for fluorometric analysi. Anal Sci. 2002;18:1267–1268. doi: 10.2116/analsci.18.1267. [DOI] [PubMed] [Google Scholar]
- Cremlyn RJ, Saunders D. Chlorosulfonation of 9-aryl 3,36,6-tetramethyloctahydroxanthen-1,8-diones. Phosphorus Sulfur Silicon. 1993;81:73–82. doi: 10.1080/10426509308034375. [DOI] [Google Scholar]
- Bodini ME, Pardo J, Arancibia V. Spectrophotometric determination of selenium(IV) with 5,5-dimethyl-1,3-cyclohexanedione. Talanta. 1990;37:439–442. doi: 10.1016/0039-9140(90)80237-A. [DOI] [PubMed] [Google Scholar]
- Gruttadauria M, Giacalone F, Marculesco AM, Meo PL, Riela S, Noto R. Hydrophobically directed aldol reactions: polystyrene-supported L-proline asa recyclable catalyst for direct asymmetric Aldol reactions in the presence of water. Eur J Org Chem. 2007;2007:4688. doi: 10.1002/ejoc.200700586. [DOI] [Google Scholar]
- Breslow R. Determining the geometries of transition states by use of antihydrophobic additives in water. Acc Chem Res. 2004;37:471–478. doi: 10.1021/ar040001m. [DOI] [PubMed] [Google Scholar]
- Blackmond DG, Armstrong A, Coombe V, Wells A. Water in organocatalytic processes: Debunking the Myths. Angew Chem Int Ed Engl. 2007;46:3798–3800. doi: 10.1002/anie.200604952. [DOI] [PubMed] [Google Scholar]
- Abaee MS, Cheraghi S, Navidipoor S, Mojtahedi MM, Forghani S. An efficient tandem aldol condensation-thia-Michael addition process. Tetrahedron Lett. 2012;53:4405–4408. doi: 10.1016/j.tetlet.2012.06.040. [DOI] [Google Scholar]
- Barakat A, Al-Majid AA. Shahidul Islam M, Al-Othman ZA: Highly enantioselective Friedel − Crafts alkylations of indoles with α, β-unsaturated ketones under Cu(II)-simple oxazoline-imidazoline catalysts. Tetrahedron. 2013;69:5185–5192. doi: 10.1016/j.tet.2013.04.063. [DOI] [Google Scholar]
- Jursic BS. Preparation of unsubstituted dipyridine-dibarbituric acid ylide through Dimerization of pyridin-2-ylmethylenepyrimidinetrione as a reactive intermediate. J Heterocyclic Chem. 2003;40:167–170. doi: 10.1002/jhet.5570400124. [DOI] [Google Scholar]
- Jursic BS, Neumann DM. Preparation of 5,5'-pyrilidene and 5,5'-quinolidene bis-barbituric acid derivatives. J Heterocyclic Chem. 2003;40:465–474. doi: 10.1002/jhet.5570400310. [DOI] [Google Scholar]
- Barakat A, Al-Majid AM, Al-Najjar HJ, Mabkhot YN, Ghabbour HA, Fun H-K. An efficient and green procedure for synthesis of rhodanine derivatives by Aldol-thia-Michael protocol using aqueous diethylamine. RSC Adv. 2014;4:4909–4916. doi: 10.1039/c3ra46551a. [DOI] [Google Scholar]
- Al-Najjar HJ, Barakat A, Al-Majid AM, Mabkhot YN, Weber M, Ghabbour HA, Fun H-K. A greener and efficient approach to Michael addition of barbituric acid to nitroalkene in aqueous diethylamine medium. Molecules. 2014;19:1150–1162. doi: 10.3390/molecules19011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majid AM, Barakat A, Al-Najjar HJ, Mabkhot YN, Ghabbour HA, Fun H-K. Tandem Aldol-Michael reactions in aqueous diethylamine medium: a greener and efficient approach to bis-pyrimidine derivatives. Int J Mol Sci. 2013;14:23762–23773. doi: 10.3390/ijms141223762. [DOI] [PMC free article] [PubMed] [Google Scholar]


