SUMMARY
Considerable advances have been made in the understanding of the pathogenesis and treatment of secondary hyperparathyroidism (SHPT) in chronic kidney disease (CKD). These include the discovery that the calcium-sensing receptor has an important role in the regulation of parathyroid gland function, the development of calcimimetics to target this receptor, the recognition that vitamin D receptor activation has important functions beyond the regulation of mineral metabolism, the identification of the phosphaturic factor fibroblast growth factor 23 and the contribution of this hormone to disordered phosphate and vitamin D metabolism in CKD. However, despite the availability of calcimimetics, phosphate binders, and vitamin D analogs, control of SHPT remains suboptimal in many patients with advanced kidney disease. In this Review, we explore several unresolved issues regarding the pathogenesis and treatment of SHPT. Specifically, we examine the significance of elevated circulating fibroblast growth factor 23 levels in CKD, question the proposition that calcitriol deficiency is truly a pathological state, explore the relative importance of the vitamin D receptor and the calcium-sensing receptor in parathyroid gland function and evaluate the evidence to support the treatment of SHPT with calcimimetics and vitamin D analogs. Finally, we propose a novel treatment framework in which calcimimetics are the primary therapy for suppressing parathyroid hormone production in patients with end-stage renal disease.
Keywords: calcimimetics, calcium-sensing receptor, chronic kidney disease, secondary hyperparathyroidism, vitamin D
INTRODUCTION
Over the past decade, considerable progress has been made in understanding the pathogenesis and treatment of secondary hyperparathyroidism (SHPT) in patients with chronic kidney disease (CKD). Basic science investigations have led to a better understanding of the biological functions of the calcium-sensing receptor (CaSR), the phosphaturic hormone fibroblast growth factor 23 (FGF23), the vitamin D receptor (VDR), and the regulation of 1,25-dihydroxy-vitamin D (1,25[OH]2D; calcitriol) production and metabolism by the CYP27B1 (25-hydroxy-vitamin D-1α-hydroxylase)/CYP24A1 (25-hydroxyvitamin D-24-hydroxylase) enzyme system. The development of cinacalcet, a calcimimetic that acts as an allosteric modulator of the CaSR, is a major clinical milestone that has introduced a new option for the therapeutic control of SHPT. New discoveries are also taking place with respect to the physiological functions of vitamin D beyond its traditional role in maintenance of mineral homeostasis. In spite of these advances, however, the clinician's ability to control circulating levels of calcium, phosphate, and parathyroid hormone (PTH) and to avoid parathyroidectomy in patients with CKD remains limited. Moreover, conceptual divides have emerged with regard to the relative importance of pathogenic factors and treatment strategies for SHPT.
In this Review, we explore the experimental basis for the controversies surrounding the pathogenesis and treatment of SHPT in end-stage renal disease (ESRD). In particular, we re-examine whether reduced circulating levels of 1,25(OH)2D in CKD are a pathological condition requiring treatment or simply an adaptive response, compare the safety and efficacy of therapeutic agents that target the VDR or CaSR to modulate parathyroid gland function and review randomized controlled trials of cinacalcet and vitamin D analogs in patients with ESRD who have SHPT. Finally, we propose a new treatment framework for SHPT in ESRD.
PARADIGM-SHIFTING CONSIDERATIONS IN THE PATHOGENESIS OF SHPT
Is calcitriol deficiency a pathological state or an adaptive response?
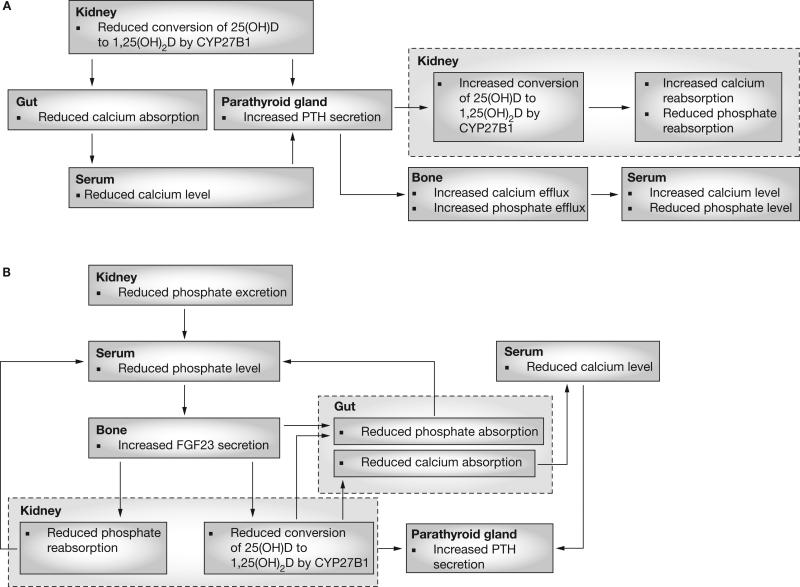
The traditional understanding of SHPT (Figure 1A) has emphasized the importance of decreased synthesis of 1,25(OH)2D by the kidney. In this conceptualization, SHPT in CKD effectively represents a state of functional vitamin D deficiency,1,2 principally caused by the compromised capacity of the poorly functioning kidney to convert 25-hydroxyvitamin D (25[OH]D; calcidiol) to 1,25(OH)2D by the action of the enzyme CYP27B1. Diminished availability of 25(OH)D, possibly due to superimposed nutritional factors or abnormalities of liver metabolism3,4—as well as diminished substrate delivery to the kidney as a result of decreased glomerular filtration rate1—might also contribute to decreased 1,25(OH)2D levels in CKD. The reduction in 1,25(OH)2D production impairs gastrointestinal calcium absorption and stimulates PTH secretion. PTH then increases calcium and phosphate efflux from bone and stimulates 1,25(OH)2D production by the kidney, all of which serve to increase renal calcium reabsorption and inhibit renal phosphate reabsorption. Elevated PTH levels maintain serum calcium and phosphate levels in the normal range until compensatory mechanisms become insufficient, and parathyroid gland hypertrophy and hyperplasia ensue. This vitamin-D-centric view of SHPT implies that primary treatment should consist of active vitamin D analogs to suppress PTH production, possibly in combination with nutritional vitamin D supplementation.
Figure 1.
Alternative paradigms for the pathogenesis of SHPT in chronic kidney disease. (A) According to the traditional paradigm, a primary decrease in 1,25(OH)2D production occurs because of the loss of functional kidney mass, which results in decreased activity of CYP27B1 (25[OH]D-1α-hydroxylase); the decrease in 1,25(OH)2D leads to SHPT both indirectly, by reducing gastrointestinal calcium absorption, and directly, by loss of suppressive effect on the parathyroid glands. Increased circulating PTH levels act on the kidney (to increase CYP27B1 activity, increase calcium reabsorption, and decrease phosphate reabsorption) and on the bone (to increase calcium and phosphate efflux); the net effect is that serum calcium levels are preserved while serum phosphate levels are reduced. (B) In the alternative paradigm, a primary decrease in renal phosphate excretion due to the loss of functioning kidney mass leads to increased FGF23 secretion from bone; increased FGF23 levels act on the kidney to inhibit phosphate reabsorption and reduce 1,25(OH)2D synthesis. Phosphate homeostasis is restored by the effects of both decreased 1,25(OH)2D levels, which diminish gastrointestinal calcium absorption, and increased FGF23 levels, which boost renal phosphate excretion. Reductions in 1,25(OH)2D lead to increased PTH production, as in the traditional paradigm, but this event occurs later; FGF23 suppresses PTH during the early stages of chronic kidney disease. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; SHPT, secondary hyperparathyroidism.
Several observations, however, give cause to reconsider the traditional view that SHPT in CKD is the same as true vitamin D deficiency. First, phosphate retention is the principal abnormality that initiates SHPT in CKD, as evidenced by the decreased capacity of the failing kidney to excrete phosphate and the observation that a reduction in dietary phosphate alone in proportion to the declining glomerular filtration rate can prevent the development of SHPT in models of CKD.5 Second, nutritional vitamin D deficiency is typically associated with hypophosphatemia,6 rather than with the hyperphosphatemia that is usually observed in CKD. Third, the principal function of the PTH–vitamin D axis is to prevent hypocalcemia, not hyperphosphatemia. The effect of PTH on phosphate excretion is mainly designed to address the phosphate retention that accompanies the absorption of calcium via the gastrointestinal tract and the efflux of phosphate from the bone that accompanies calcium resorption. Thus, the elevated PTH levels seen in both vitamin D deficiency and in renal failure have more to do with maintenance of circulating calcium levels than with phosphate homeostasis.
The discovery of FGF23, which is involved in novel adaptive responses that have evolved to prevent hyperphosphatemia and vitamin D intoxication, and the elucidation of FGF23's function as a 1,25(OH)2D counter-regulatory hormone7,8 provides a new conceptual framework for understanding of the pathogenesis of SHPT. Although PTH and FGF23 both have phosphaturic actions, they have opposite effects on CYP27B1 and CYP24A1 enzyme activity; PTH stimulates the production of 1,25(OH)2D and inhibits its degradation, whereas FGF23 inhibits the production9–11 and increases the degradation10 of 1,25(OH)2D. The difficulty of increasing 1,25(OH)2D levels in CKD via nutritional vitamin D supplementation could illustrate the difficulty of overcoming the counter-regulatory forces of FGF23 and CYP24A1. In the setting of CKD, in which FGF23 levels are increased,12 FGF23 was a strong independent predictor of diminished 1,25(OH)2D levels even after adjustment for 25(OH)D levels;13 therefore, the FGF23–bone–kidney axis might be the effector of a ‘phosphate trade-off’ that compensates for the limited renal phosphate excretion caused by the reduced nephron mass. In this view (Figure 1B), reduced renal phosphate excretion is the primary stimulus for a cascade of events, in which FGF23-dependent suppression of renal 1,25(OH)2D production is a necessary adaptive response to limit the gastrointestinal absorption of phosphate, as opposed to a functionally deficient state requiring treatment. Along with preventing the stimulation of gastrointestinal phosphate absorption by 1,25(OH)2D, the phosphaturic action of FGF23 represents a mechanism to maintain neutral phosphate balance. Thus, incremental increases in FGF23 would be a very early event in CKD,12–14 and measurement of FGF23 levels early in CKD might someday be warranted to detect sub-clinical SHPT. Indeed, FGF23 levels have recently been shown to independently predict mortality in incident dialysis patients.15 However, other investigators have suggested alternative ideas to this ‘limited phosphate excretion’ hypothesis, based on the unexplained observation of post-prandial hypercalciuria and hypocalcemia in patients with CKD, leading to elevations in circulating PTH levels without changes in serum FGF23 levels.16
FGF23 also acts directly on the parathyroid gland to suppress PTH secretion,9 which abolishes PTH-mediated stimulation of CYP27B1, and further suppresses production of 1,25(OH)2D. As such, elevation of circulating PTH is almost certainly a later event following FGF23-mediated reductions in 1,25(OH)2D production and probably results from impaired gastrointestinal calcium absorption. The end-organ effects of FGF23 are mediated by Klotho, a cell-surface glucosidase that binds to the FGF receptor and the C-terminus of FGF23 to convert the canonical FGF receptor into a specific receptor for FGF23.17 Since expression of messenger RNA for Klotho in the kidneys is reduced in patients with CKD, end-organ resistance to FGF23 could also contribute to the increased circulating FGF23 levels in advancing renal failure,18 in a manner analogous to the role of end-organ resistance to PTH in hyperparathyroidism.19 Regardless, dietary phosphate restriction increases renal production of 1,25(OH)2D in CKD,20 possibly via reductions in FGF23 production.
Therapeutic approaches to SHPT would, by necessity, differ depending on which of the above views of the pathogenesis of SHPT is correct. If CKD is a state of functional vitamin D deficiency, then administration of vitamin D analogs to suppress PTH is rational. However, if phosphate retention leads to increases in circulating FGF23 levels, suppression of CYP27B1 activity, and stimulation of CYP24A1 activity leading to a fall in circulating 1,25(OH)2D levels, then the primary treatment of SHPT should be phosphate restriction rather than use of vitamin D analogs, since the latter would serve to increase calcium and phosphate absorption, leading to hyperphosphatemia and further stimulation of FGF23 production.
If phosphate retention secondary to limited phosphate excretion is the main cause of SHPT, why does treatment with vitamin D analogs in stage 4 and 5 CKD suppress circulating PTH levels without raising serum phosphate levels?21 The answer might be that vitamin D further stimulates FGF23 production, increasing the stimulus for phosphaturia in the setting of residual renal function. By contrast, in ESRD, treatment with active vitamin D analogs worsens hyperphosphatemia, probably reflecting the unopposed effect of increased gastrointestinal phosphate absorption,22 whereas treatment with cinacalcet results in a slight decrease in serum phosphate levels,23,24 possibly via a decrement in PTH-mediated phosphate efflux from bone and/or a vitamin D-analog-sparing effect.
Relative importance of CaSR and VDR in regulating parathyroid gland function
The CaSR regulates PTH secretion, PTH gene transcription25,26 and parathyroid cell proliferation.27 In humans with severe congenital neonatal hyperparathyroidism28 and in the analogous homozygous Casr knockout mice,29 increases in serum PTH and calcium levels and hyperplasia of the parathyroid gland are evident in spite of marked elevations in circulating 1,25(OH)2D levels. Ablation of the VDR in mice also results in SHPT and parathyroid gland hyperplasia, but normalization of serum calcium levels is sufficient to fully correct abnormal parathyroid gland function in this model, unlike in the Casr knockout mice or in cases of severe neonatal hyperparathyroidism.30 The fact that 1,25(OH)2D is ineffective in suppressing PTH production in the absence of CaSR, but calcium is sufficient to normalize parathyroid gland function in the absence of VDR, indicates that CaSR is the dominant regulator of parathyroid gland function.31–34 The principal direct function of the VDR in the parathyroid gland, then, is to suppress PTH gene transcription; in addition, the VDR has an indirect action on the parathyroid gland through stimulation of gastrointestinal calcium absorption and elevation of serum calcium levels, which serves to affect parathyroid gland function via the CaSR.
Animal and clinical studies that investigated calcimimetics, which are allosteric modulators of the CaSR, also support the dominant role of this receptor in SHPT. As reviewed by Drueke et al.,31 cinacalcet has consistently been shown to prevent the development of, mitigate the effects of, or even reverse, established parathyroid gland hyperplasia in rodents.35,36 The effects of cinacalcet on calcium-mediated PTH release have been examined by de Francisco et al.,37 who studied 10 individuals with severe SHPT (mean serum PTH level 1,116 ng/l). Patients were exposed to alternating low-calcium and high-calcium dialysate baths to maximally stimulate and suppress the parathyroid gland in turn. They then received cinacalcet (mean of 13 weeks), after which the patients were again exposed to the alternating dialysate concentrations to determine if cinacalcet had any effect on the PTH release set point (the serum calcium level associated with 50% maximal PTH stimulation). Cinacalcet significantly reduced both the set point and the maximal level of PTH release, an important finding because the set point might be a marker of the severity of SHPT and of parathyroid gland mass. Lomonte et al.38 provide more direct, but still rather preliminary, evidence of a potential association between cinacalcet and parathyroid gland histology. These investigators examined parathyroid glands from patients with SHPT and found that nodular hyperplasia was more common in individuals treated with vitamin D sterols and cinacalcet than in individuals treated with oral phosphate binders alone. However, no patients were exposed to cinacalcet monotherapy; therefore, Lomonte and colleagues relied on linear regression to isolate the independent effects of cinacalcet, and they found that the drug was associated with a significant increase in oxyphil cell:chief cell ratio in the parathyroid gland. Oxyphil cells have been reported to proliferate at a lower rate than chief cells,39 perhaps indicating that cinacalcet could slow the histologic progression of SHPT.
The dominant role of the CaSR does not mean, however, that the VDR is unimportant. In fact, there are important, albeit complex, inter-relationships between the CaSR and the VDR in the regulation of mineral homeostasis, and the VDR has nonclassical actions that might affect the function of multiple organs. Both the CaSR40–43 and the VDR44–48 can become down-regulated in humans and animals with CKD and severe hyperparathyroidism, making the parathyroid gland progressively more resistant to treatment with vitamin D analogs. Data also indicate that extracellular calcium level regulates the expression of the VDR49 and that the VDR upregulates expression of the CaSR.19,50,51
RATIONALE FOR A NEW TREATMENT PARADIGM FOR SHPT
Results of clinical trials
Calcimimetics
A surprisingly large number of randomized controlled trials have been undertaken to investigate the effects of calcimimetics on the control of SHPT in patients with ESRD. The results of at least eight studies have been published, and the findings of the ACHIEVE (Optimizing the Treatment of Secondary Hyperparathyroidism: A Comparison of Sensipar and Low Dose Vitamin D vs Escalating Doses of Vitamin D Alone) trial have been presented in abstract form.52 Although a detailed review of each trial is beyond the scope of this Review, some broad conclusions can be drawn. Several studies compared cinacalcet plus existing therapies (including continued, but restricted, vitamin D analog use) with standard-of-care (consisting of unrestricted use of phosphate binders and vitamin D analogs).24,53,54 In other words, no attempt was made to minimize the use of vitamin D analogs in the control groups. A meta-analysis by Strippoli et al.55 examined most of these studies. The authors of the meta-analysis concluded that the addition of cinacalcet to standard-of-care significantly improved control of calcium–phosphate product and serum PTH, calcium and phosphate levels, and resulted in a greater percentage of patients achieving the Kidney Disease Outcomes Quality Initiative (KDOQI) targets for these parameters compared with the standard-of-care. When stratified by sex, race, age, diabetic status, duration of dialysis, and mineral metabolism parameters, cinacalcet plus standard therapy proved consistently superior to standard therapy alone in attainment of PTH reduction.
Subsequent studies have produced further concordant results. In a trial that was not included in the meta-analysis, Fukugawa et al.56 randomly allocated 144 Japanese patients to receive lower doses of cinacalcet than in US studies (maximum dose 100 mg/day vs 180 mg/day) or placebo and found that cinacalcet was associated with significant improvements in control of calcium–phosphate product and of serum PTH, calcium and phosphate levels. The response to cinacalcet seemed to be independent of concomitant vitamin D use.
In the above trials, vitamin D analog doses remained relatively equal in the two arms, enabling the investigators to isolate the effects of cinacalcet. However, other recent clinical trials have directly investigated the role of cinacalcet with concomitant, low-dose vitamin D analog therapy in the treatment of SHPT in ESRD. In a study by Block et al.,57 375 patients with a serum PTH level of greater than 300 ng/l initiated cinacalcet treatment, and pre-existing doses of vitamin D analogs were then reduced (for example to 2 μg paricalcitol per dialysis session). Participants experienced consistent improvements in serum PTH levels and calcium–phosphate product after exposure to cinacalcet, and the mean reduction in vitamin D dose was analogous to halving of weekly paricalcitol dose from 21.0 μg to 10.2 μg. Chertow et al.58 employed a similar protocol of reduced vitamin D analog dosage in cinacalcet-treated patients, but these investigators enrolled 53 patients who had serum PTH levels of 150–300 ng/l. Both studies showed that cinacalcet plus low-dose vitamin D analogs had greater efficacy at reducing circulating PTH levels without increasing calcium–phosphate product than did vitamin D analogs alone.
Messa et al.23 recently published the results of the OPTIMA (Open-Label, Randomized Study Using Cinacalcet to Improve Achievement of KDOQI Targets in Patients with ESRD) study, which compared cinacalcet with standard-of-care by use of a study protocol that rigorously adhered to the KDOQI mineral metabolism guidelines. A total of 552 patients were enrolled. The authors found that cinacalcet-based treatment was superior to vitamin-D-only therapy at achieving KDOQI targets for calcium–phosphate product and for serum PTH, calcium and phosphate levels individually, and at achieving both calcium–phosphate product and serum PTH level goals. Although there was only a modest overall difference in the change in dose of vitamin D analogs between the groups (a 6% decrease in the cinacalcet group vs a 14% increase in the vitamin-D-only group), in the subgroup of patients who were receiving vitamin D analogs at baseline (68% of the total study sample), a 22% decrease in vitamin D analog dose was seen in the cinacalcet arm compared with a 3% increase in the control arm. Finally, the ACHIEVE study—which included a washout period from vitamin D analog therapy—randomly allocated 173 patients to receive either cinacalcet plus low-dose vitamin D analogs, or flexible doses of vitamin D analogs. A significantly higher number of patients in the cinacalcet plus low-dose vitamin D analog group attained a greater than 30% reduction in serum PTH levels than in the vitamin-D-only group.52
The above clinical data provide strong evidence that cinacalcet is an extremely effective agent for the suppression of PTH in SHPT. Moreover, the effects of cinacalcet are independent of vitamin D analogs, and doses of vitamin D analogs can typically be reduced in the presence of cinacalcet. To our knowledge, no published studies have investigated the use of cinacalcet as true monotherapy (i.e. in the complete absence of nutritional vitamin D supplementation and active vitamin D analog treatment).
Vitamin D analogs
The clinical trial data for vitamin D analogs are less extensive than those for cinacalcet, which is probably because the demonstrable capacity of vitamin D analogs to lower serum PTH levels caused these agents to become standard-of-care without evidence from multiple randomized controlled trials. Few randomized controlled trials have compared the efficacy of vitamin D sterols and placebo in the treatment of SHPT.59,60 Most trials have been designed to test one analog against another over timeframes ranging from a few days61,62 to 32 weeks,63 although longer-term studies of cohorts exposed to single agents have been conducted over a year or more;64–66 as such, control of mineral metabolism parameters, and not mortality, was the principal end point in these studies.
However, several large observational studies have shown a consistent positive association between use of vitamin D analogs and survival in patients with ESRD. By use of the Fresenius Medical Care database, Teng et al.67 examined over 67,000 patients on hemodialysis to compare the effects of paricalcitol, a selective vitamin D sterol, with the nonselective agent calcitriol. They reported an adjusted hazard ratio for mortality of 0.84 (95% CI 0.79–0.90) for paricalcitol relative to calcitriol. In a subsequent study of over 50,000 dialysis patients from the Fresenius Medical Care database,68 these investigators observed that vitamin D analogs were associated with a 20% improvement in 2-year survival rate (hazard ratio 0.80, 95% CI 0.76–0.83). Kalantar-Zadeh et al.69 examined over 58,000 incident and prevalent hemodialysis patients and also found a significant survival advantage associated with vitamin D analog use. This study, however, identified a dose-dependent effect of vitamin D analogs, such that individuals who received high doses (e.g. ≥15 μg paricalcitol per week) had worse survival, indicating a possible toxic effect at this level. The other large study to examine this issue was conducted by Tentori et al.,22 who studied over 7,700 prevalent hemodialysis patients from the Dialysis Clinic Inc. database. Although median follow-up was relatively short (37 weeks), these investigators also found a 20% improvement in survival associated with use of any vitamin D analog.
Caution should be used in extrapolating these results, for several reasons. First, treatment with vitamin D analogs can increase serum phosphate levels,66,70 and high phosphate levels are associated with mortality.71 Second, the influence of confounding factors, such as bias by indication (i.e. nonrandom treatment allocation), cannot be excluded. Given the disappointing history of the discordance between observational data and the results of randomized controlled trials in the dialysis population, a randomized controlled trial is needed to establish unequivocally whether high-dose vitamin D analogs offer a survival advantage. The potential of cinacalcet to control SHPT with reduced fixed doses of vitamin D analogs, or possibly as ‘monotherapy’ (i.e. with only calcium-based phosphate binders and nutritional vitamin D supplementation) now provides an opportunity to compare alternative protocols with the traditional standard-of-care, which relies on titrating the dose of vitamin D analogs to high levels to lower serum PTH levels.
Testing the new paradigm
The biological plausibility that vitamin D has actions in multiple organs and especially the role of this vitamin in innate immunity,72–74 along with the survival advantage associated with vitamin D analog therapy in retrospective database analyses, has led to an increasing belief that vitamin D analogs may be essential ‘life-saving’ therapies for patients with ESRD.75 Although intriguing, this theory has yet to be borne out by randomized controlled trials in patients with CKD or ESRD. Since there is clinical equipoise on the critical issue of cinacalcet versus high-dose vitamin D analogs for SHPT control and mortality, a randomized controlled trial would be ethical. Such a trial would enable not only determination of whether one agent is more effective than the other for the control of SHPT and, more importantly, for the prevention of mortality, but also whether one therapy (i.e. cinacalcet) could replace the other (i.e. vitamin D analogs) as the primary treatment for SHPT.
CONCLUSIONS
Any treatment paradigm for SHPT must take into consideration the pathogenesis of the disorder, the mechanism of action and potential toxicity of drugs, and the results and ramifications of clinical studies. Although vitamin D is the principal therapy for SHPT caused by nutritional vitamin D deficiency in patients with normal renal function, we suggest that treatment protocols for SHPT in ESRD should comprise a combination of phosphate restriction and/or phosphate binders (to lower serum phosphate levels), cinacalcet (as first-line therapy to lower serum PTH levels), and a more judicious use of vitamin D analogs (principally for their potential non-mineral-metabolism-related effects). This approach (Box 1) is most likely to lead to optimal outcomes for several reasons. First, decreased levels of 1,25(OH)2D seem to be an adaptive response in advanced renal disease to limit the toxic effects of hyperphosphatemia, and the use of vitamin D analogs at doses necessary to suppress serum PTH levels consistently leads to hyperphosphatemia or increases the need for phosphate binders. Second, molecular genetic studies in mice and humans demonstrate the greater importance of the CaSR than the VDR in the regulation of parathyroid gland function. Third, the vitamin-D-sparing effects of cinacalcet could result in less calcium loading than therapy with vitamin D analogs. Calcium loading by use of calcium-based phosphate binders, which are generally administered in the setting of concurrent vitamin D therapy, is associated with increased vascular calcifications.76 Cinacalcet lowers serum calcium levels; therefore, the combination of cinacalcet and calcium-based phosphate binders could gain greater acceptance. Fourth, and most importantly, clinical trials have demonstrated the superior suppression of PTH production and control of calcium–phosphate product, compared with traditional therapy, in patients with ESRD who use calcimimetics, both as adjunctive therapy to vitamin D analogs and as primary therapy with reduced doses of vitamin D analogs.
Although the survival benefit of vitamin D analogs has not yet been demonstrated in a randomized controlled trial, such a benefit is biologically plausible, and physiological ‘replacement’ doses of vitamin D analogs (i.e. those that are likely to be nonhypercalcemic and nonhyperphosphatemic, such as 2 μg paricalcitol, 1 μg doxercalciferol, or 0.5 μg calcitriol thrice weekly with dialysis57,58), possibly in combination with nutritional vitamin D replacement, seem warranted in the majority of patients who lack obvious contraindications. As our collective understanding moves forward, low, fixed doses of vitamin D analogs might become the standard of care as an adjunctive therapy to cinacalcet. Such ‘tonic’ doses could be titrated upward if maximal doses of calcimimetics have failed to control SHPT, if calcimimetics have intolerable adverse effects at effective doses, or if calcimimetic use results in hypocalcemia—an effect that can frequently be remedied by vitamin D analogs.
The prevention and treatment of uremic SHPT is complex and requires multiple interventions in addition to vitamin D analogs and calcimimetics, including optimization of nutritional intake, control of gastrointestinal phosphate absorption, and prevention of metabolic acidosis, as well as provision of adequate dialysis. Establishing the optimal combination of calcium supplements, phosphate binders, vitamin D supplements (in terms of dose, type and route of administration) and cinacalcet requires additional data. The nephrology community should now focus its efforts on conducting randomized controlled trials to determine whether calcimimetics provide mortality benefits relative to vitamin D analogs. Although a study in which cinacalcet is tested directly against a vitamin D analog would theoretically be the most rigorous option, the widespread use of vitamin D analogs, as well as their capacity to protect against cinacalcet-induced hypocalcemia, makes such a study unfeasible. A trial in which use of vitamin D analogs is minimized in the cinacalcet arm would reflect the reality of clinical practice and increase the likelihood that individuals who are randomly allocated to cinacalcet would continue to receive the drug over a lengthy treatment period, which is critical to show unambiguous differences in cumulative event rates. In the meantime, calcimimetics should be strongly considered as primary therapy for SHPT in most patients with ESRD.
REVIEW CRITERIA.
We searched PubMed using the terms “vitamin D”, “vitamin D receptor”, “calcium-sensing receptor”, “calcimimetics”, “cinacalcet”, “FGF23”, or “fibroblast growth factor”, in combination with the terms “secondary hyperparathyroidism”, “end-stage renal disease”, “chronic kidney disease”, “chronic kidney failure”, or “chronic renal failure”. Original English-language reports and reviews from 1988 to the present were considered; references to original reports were sought from reviews. Bibliographies of articles retrieved were examined for relevant articles not captured by the initial search strategy. This approach was augmented by manual searches for seminal publications in the authors’ personal libraries.
Box 1 Suggested principles for the treatment of secondary hyperparathyroidism in end-stage renal disease.
■ Control serum phosphorus levels by use of dietary phosphorus restriction and oral phosphate binders
■ Consider giving low or ‘physiologic’ doses of vitamin D analogs to patients without high serum calcium or phosphorus levels (for the potential survival benefit)
■ Use calcimimetics as first-line therapy to titrate serum parathyroid hormone (PTH) levels to the target recommended by the Kidney Disease Outcomes Quality Initiative in patients without hypocalcemia and in those in whom phosphate binders and ‘low-dose’ vitamin D alone are insufficient to control PTH
■ Increase the dose of vitamin D analogs to reduce serum PTH levels only in patients who have insufficient control of secondary hyperparathyroidism on high-dose calcimimetic therapy, in those who cannot tolerate adequate doses of calcimimetics, or in those who have symptomatic and/or severe hypocalcemia while on calcimimetics
■ The role of nutritional vitamin D supplementation (e.g. with ergocalciferol or cholecalciferol) in the treatment of secondary hyperparathyroidism due to chronic kidney disease is controversial and unproven; evidence-based recommendations cannot yet be made
KEY POINTS.
■ Calcitriol (1,25[OH]2D) deficiency in advanced kidney disease might be an adaptive response mediated by an increase in fibroblast growth factor 23 (FGF23) production
■ Increases in circulating FGF23 levels seem to occur earlier than increases in serum PTH levels in patients with kidney disease, and FGF23 exerts the opposite effect to PTH on the CYP27B1 and CYP24A1 enzyme systems that are responsible for the synthesis and metabolism of calcitriol
■ Both the calcium-sensing receptor and the vitamin D receptor are important in the development of secondary hyperparathyroidism (SHPT), but the former seems more important and, as such, a more promising target for therapy
■ Calcimimetics have consistently been shown to provide superior control of mineral metabolic parameters when added to vitamin D analog therapy and, increasingly, when tested against high-dose vitamin D therapy; these agents should, therefore, be considered first-line therapy (after phosphorus control) in most patients with SHPT
■ Randomized controlled trials of calcimimetics plus low-dose vitamin D analogs versus escalating doses of vitamin D analogs should be undertaken to determine the relative survival benefits of each regimen
Footnotes
Competing interests
The authors have declared associations with the following company: Amgen. See the article online for full details of the relationships.
References
- 1.Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clin J Am Soc Nephrol. 2008;3:1555–1560. doi: 10.2215/CJN.01150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AJ, Slatopolsky E. Drug Insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab. 2007;3:134–144. doi: 10.1038/ncpendmet0394. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zehnder D, et al. Cross-sectional analysis of abnormalities of mineral homeostasis, vitamin D and parathyroid hormone in a cohort of pre-dialysis patients. The chronic renal impairment in Birmingham (CRIB) study. Nephron Clin Pract. 2007;107:c109–c116. doi: 10.1159/000108652. [DOI] [PubMed] [Google Scholar]
- 5.Slatopolsky E, et al. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis. 2001;37:S54–S57. doi: 10.1053/ajkd.2001.20740. [DOI] [PubMed] [Google Scholar]
- 6.Rowe PS. The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15:264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito H, et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 9.Krajisnik T, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 10.Shimada T, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 11.Perwad F, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 12.Larsson T, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez O, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 14.Shigematsu T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez OM, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakova T, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–623. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 18.Koh N, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 19.Tokumoto M, et al. Parathyroid cell growth in patients with advanced secondary hyperparathyroidism: vitamin D receptor, calcium sensing receptor, and cell cycle regulating factors. Ther Apher Dial. 2005;9(suppl 1):S27–S34. doi: 10.1111/j.1744-9987.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 20.Portale AA, et al. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest. 1987;80:1147–1154. doi: 10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coburn JW, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43:877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Tentori F, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 23.Messa P, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block GA, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 25.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 26.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 27.Silver J, Levi R. Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int Suppl. 2005;95:S8–S12. doi: 10.1111/j.1523-1755.2005.09501.x. [DOI] [PubMed] [Google Scholar]
- 28.Pollak MR, et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 29.Ho C, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 30.Li YC, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 31.Drueke T, et al. Can calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studies. Nephrol Dial Transplant. 2007;22:1828–1839. doi: 10.1093/ndt/gfm177. [DOI] [PubMed] [Google Scholar]
- 32.Goodman WG. Calcimimetics: a remedy for all problems of excess parathyroid hormone activity in chronic kidney disease? Curr Opin Nephrol Hypertens. 2005;14:355–360. doi: 10.1097/01.mnh.0000172722.52499.71. [DOI] [PubMed] [Google Scholar]
- 33.Kos CH, et al. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111:1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu Q, et al. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–1037. doi: 10.1172/JCI17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colloton M, et al. Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int. 2005;67:467–476. doi: 10.1111/j.1523-1755.2005.67103.x. [DOI] [PubMed] [Google Scholar]
- 36.Chin J, et al. Activation of the calcium receptor by a calcimimetic compound halts the progression of secondary hyperparathyroidism in uremic rats. J Am Soc Nephrol. 2000;11:903–911. doi: 10.1681/ASN.V115903. [DOI] [PubMed] [Google Scholar]
- 37.de Francisco AL, et al. Calcium-mediated parathyroid hormone release changes in patients treated with the calcimimetic agent cinacalcet. Nephrol Dial Transplant. 2008;23:2895–2901. doi: 10.1093/ndt/gfn191. [DOI] [PubMed] [Google Scholar]
- 38.Lomonte C, et al. Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol. 2008;3:794–799. doi: 10.2215/CJN.04150907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushita H, et al. Proliferation of parathyroid cells negatively correlates with expression of parathyroid hormone-related protein in secondary parathyroid hyperplasia. Kidney Int. 1999;55:130–138. doi: 10.1046/j.1523-1755.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 40.Gogusev J, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 41.Kifor O, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 42.Brown AJ, et al. Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int. 1999;55:1284–1292. doi: 10.1046/j.1523-1755.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 43.Ritter CS, et al. Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int. 2001;60:1737–1744. doi: 10.1046/j.1523-1755.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 44.Martin LN, et al. Parathyroid glands in uraemic patients with refractory hyperparathyroidism: histopathology and p53 protein expression analysis. Histopathology. 1998;33:46–51. [PubMed] [Google Scholar]
- 45.Fukuda N, et al. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92:1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, et al. Vitamin D receptor and PCNA expression in severe parathyroid hyperplasia of uremic patients. Chin Med J (Engl) 2001;114:410–414. [PubMed] [Google Scholar]
- 47.Tokumoto M, et al. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62:1196–1207. doi: 10.1111/j.1523-1755.2002.kid585.x. [DOI] [PubMed] [Google Scholar]
- 48.Yano S, et al. Decrease in vitamin D receptor and calcium-sensing receptor in highly proliferative parathyroid adenomas. Eur J Endocrinol. 2003;148:403–411. doi: 10.1530/eje.0.1480403. [DOI] [PubMed] [Google Scholar]
- 49.Maiti A, Beckman MJ. Extracellular calcium is a direct effecter of VDR levels in proximal tubule epithelial cells that counter-balances effects of PTH on renal Vitamin D metabolism. J Steroid Biochem Mol Biol. 2007;103:504–508. doi: 10.1016/j.jsbmb.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Brown AJ, et al. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol. 1996;270:F454–F460. doi: 10.1152/ajprenal.1996.270.3.F454. [DOI] [PubMed] [Google Scholar]
- 51.Canaff L, Hendy GN. Human calcium-sensing receptor gene: vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 52.Fishbane S, et al. Cinacalcet HCl with low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients versus vitamin D alone—the ACHIEVE study. Clin J Am Soc Nephrol. doi: 10.2215/CJN.01040308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quarles LD, et al. The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol. 2003;14:575–583. doi: 10.1097/01.asn.0000050224.03126.ad. [DOI] [PubMed] [Google Scholar]
- 54.Lindberg JS, et al. The calcimimetic AMG 073 reduces parathyroid hormone and calcium x phosphorus in secondary hyperparathyroidism. Kidney Int. 2003;63:248–254. doi: 10.1046/j.1523-1755.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- 55.Strippoli GF, et al. Meta-analysis of biochemical and patient-level effects of calcimimetic therapy. Am J Kidney Dis. 2006;47:715–726. doi: 10.1053/j.ajkd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Fukagawa M, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23:328–335. doi: 10.1093/ndt/gfm534. [DOI] [PubMed] [Google Scholar]
- 57.Block GA, et al. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2008;23:2311–2318. doi: 10.1093/ndt/gfn026. [DOI] [PubMed] [Google Scholar]
- 58.Chertow GM, et al. Cinacalcet hydrochloride (Sensipar) in hemodialysis patients on active vitamin D derivatives with controlled PTH and elevated calcium x phosphate. Clin J Am Soc Nephrol. 2006;1:305–312. doi: 10.2215/CJN.00870805. [DOI] [PubMed] [Google Scholar]
- 59.Martin KJ, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9:1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 60.Delmez JA, et al. A controlled trial of the early treatment of secondary hyperparathyroidism with calcitriol in hemodialysis patients. Clin Nephrol. 2000;54:301–308. [PubMed] [Google Scholar]
- 61.Coyne DW, et al. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis. 2002;40:1283–1288. doi: 10.1053/ajkd.2002.36899. [DOI] [PubMed] [Google Scholar]
- 62.Joist HE, et al. Differential effects of very high doses of doxercalciferol and paricalcitol on serum phosphorus in hemodialysis patients. Clin Nephrol. 2006;65:335–341. doi: 10.5414/cnp65335. [DOI] [PubMed] [Google Scholar]
- 63.Sprague SM, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 64.Brandi L, et al. Long-term suppression of secondary hyperparathyroidism by intravenous 1 alpha-hydroxyvitamin D3 in patients on chronic hemodialysis. Am J Nephrol. 1992;12:311–318. doi: 10.1159/000168465. [DOI] [PubMed] [Google Scholar]
- 65.Lindberg J, et al. A long-term, multicenter study of the efficacy and safety of paricalcitol in end-stage renal disease. Clin Nephrol. 2001;56:315–323. [PubMed] [Google Scholar]
- 66.Akizawa T, et al. Long-term effect of 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in haemodialysis patients: one-year administration study. Nephrol Dial Transplant. 2002;17(suppl 10):S28–S36. doi: 10.1093/ndt/17.suppl_10.28. [DOI] [PubMed] [Google Scholar]
- 67.Teng M, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 68.Teng M, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 69.Kalantar-Zadeh K, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 70.Sprague SM, et al. Suppression of parathyroid hormone secretion in hemodialysis patients: comparison of paricalcitol with calcitriol. Am J Kidney Dis. 2001;38:S51–S56. doi: 10.1053/ajkd.2001.28110. [DOI] [PubMed] [Google Scholar]
- 71.Block GA. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 72.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 73.Bikle DD. What is new in vitamin D: 2006–2007. Curr Opin Rheumatol. 2007;19:383–388. doi: 10.1097/BOR.0b013e32818e9d58. [DOI] [PubMed] [Google Scholar]
- 74.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 75.Andress D. Nonclassical aspects of differential vitamin D receptor activation: implications for survival in patients with chronic kidney disease. Drugs. 2007;67:1999–2012. doi: 10.2165/00003495-200767140-00003. [DOI] [PubMed] [Google Scholar]
- 76.Chertow GM, et al. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]