Abstract
The role of nanoparticle surface hydrophobicity on its hemolytic property is established in the absence and the presence of plasma proteins. Significantly, the formation of plasma protein corona on NP surface protects red blood cells from both hydrophilic and hydrophobic NP-mediated hemolysis.
Intravascular rupture of red blood cells (RBCs) upon systemic administration of therapeutic NPs can elevate plasma level of cell-free hemoglobin leading to endothelial cell dysfunction and vascular thrombosis.1 This acute hemolysis can further cause hemolytic anemia that result in multiple organ failure2 and death.3 Several reports have recently demonstrated the hemolytic activities of therapeutic NPs both in vitro4 and in vivo,5 indicating potential adverse side-effects of NPs that can limit applications in nanomedicine. However, a lack of parametric study in vitro on NP hemolytic behavior makes their interaction profile difficult to predict in vivo.
Surface functionality of NPs is central to their effective use in therapeutic applications, imparting functional properties 6 and dictating their circulation profile in the blood stream.7 However, in contrast to small molecule therapeutics the formation of a protein corona on the NP surface during blood circulation8 alters surface chemical behavior of NPs and defines its physiological responses.9 This interplay of NP surface functionality and the protein corona formed in situ would be expected to dictate the overall interaction of NPs with RBCs, regulating the hemolytic behavior of NPs. To date, however, there is no clear understanding how changes on NP surfaces coupled with the formation of protein corona control the hemolytic properties of NPs.10
Herein, we report the role of NP surface functionality on hemolytic activity in the presence and absence of plasma proteins. To this end, we have synthesized a class of cationic gold NPs of same core size (~2 nm), differing in their surface hydrophobicity (Figure 1). These NPs showed a direct increase in hemolytic activity with the increase of surface hydrophobicity in the absence of plasma proteins. Significantly, the presence of protein corona dramatically altered the hemolytic activity of these NPs; hemolysis was only observed in the most hydrophobic of surface coverage. These studies demonstrate the importance of serum proteins in moderating hemolytic activity, expanding the range of particle surfaces that can be employed without hemolytic consequences.
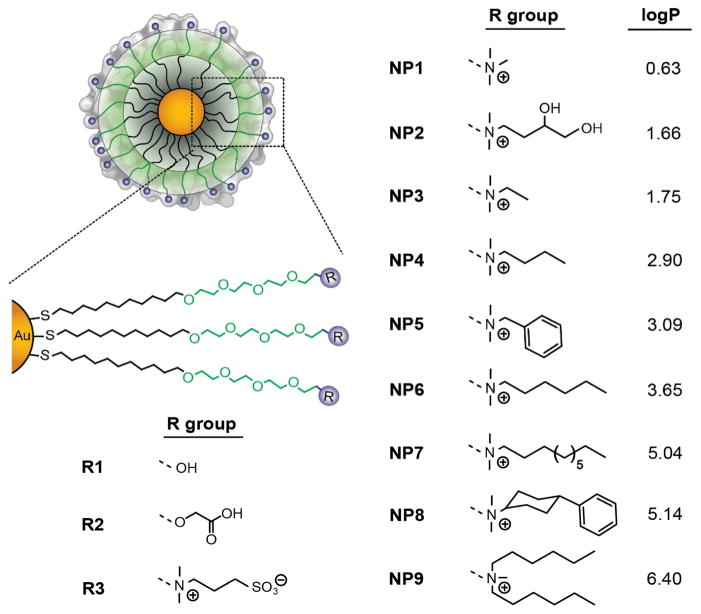
Figure 1.
The structure of the NPs used in the current study. logP denotes the n-octanol/water partition coefficients of R groups.
The NP surface hydrophobicity has a critical role in the cellular uptake, 11 toxicity, 12 and immune responses 13 of nanomaterials. To understand the role of NP surface hydrophobicity on the blood compatibility, a family of nine cationic NPs was synthesized systematically changing the degree of surface hydrophobicity by the use of specific chemical groups (Figure 1).14 This chemical approach allows us to control the nature of the monolayer (nanoparticle surface), while maintaining the other physicochemical properties (e.g. size and surface charge) constant. Using this system, the NP properties can be translated into numerical descriptors, as demonstrated previously by interfacial tension experiments.15 As such, the logP of the headgroups (R groups, Figure 1) was employed to describe the relative hydrophobic nature of the NP surface.
In prior studies, we have demonstrated that cationic NPs showed significantly higher hemolysis compared to anionic counterpart.16 To this end, NPs with different surface charge e.g. cationic, anionic, neutral, and zwitterionic (500 nM each) were incubated with RBCs for 30 min and the absorbance of released hemoglobin from the hemolyzed RBCs were measured at 570 nm.17 We observed a significantly higher hemolytic activity of cationic NPs compared to NPs with other surface charges (anionic, neutral, and zwitterionic) (see SI), corroborating previous reports.18
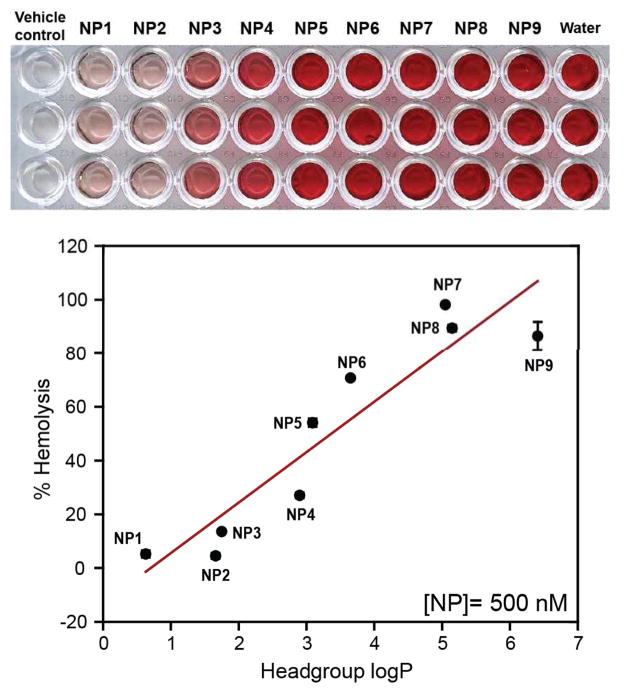
The role of NP surface hydrophobicity on hemolytic behavior was established incubating NP1-NP9 (500 nM each) with RBCs as mentioned above. A direct increase in hemolytic activity was observed for NP1-NP9 with the increase of hydrophobicity (Figure 2), demonstrating the role of NP surface hydrophobicity on the hemolysis. Significantly, NPs with similar headgroup logP but different chemical functionalities e.g. NP2 and NP3 demonstrated different degree of hemolysis (~4.5% and 13.5% respectively), evidencing the role of specific functional groups on the observed hemolytic behavior.
Figure 2.
Hemolytic activities of NP1-NP9 (500 nM each) in the absence of plasma proteins. RBCs were incubated with NPs for 30 min in PBS at 37 °C and the mixture was centrifuged to detect the cell-free hemoglobin in the supernatant. % Hemolysis was calculated using water as the positive control. Error bars represent standard deviation (n=3).
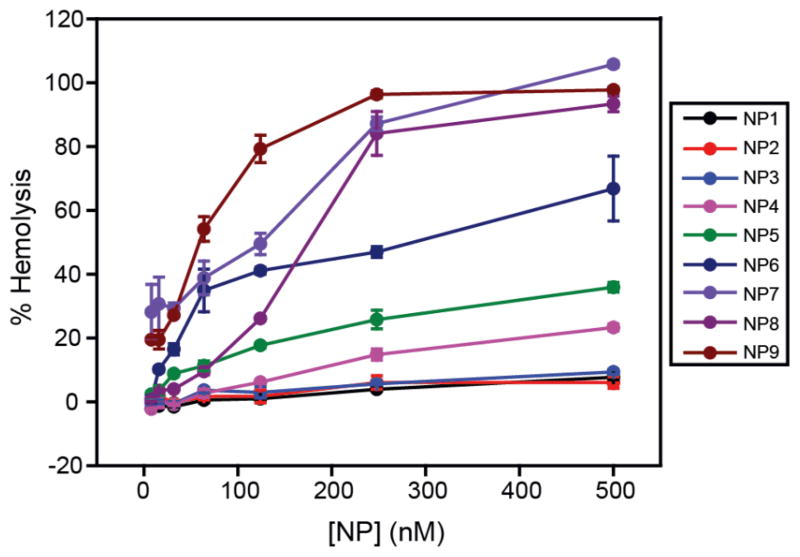
The dose-dependent hemolytic behavior of these NPs was investigated exposing RBCs to a range of concentrations of NPs from 8 nM to 500 nM. As shown in Figure 3, a dose-dependent increase of hemolysis was observed for all NPs. NP1-NP3 did not show significant hemolysis at the highest concentration tested (500 nM), demonstrating biocompatibility of these particles towards RBCs. Significantly, a sigmoidal dose-response curve was observed for more hydrophobic NPs (see SI), demonstrating the co-operative nature of hemolytic process for these NPs.19 This result demonstrated that the subtle changes on NP surfaces can modulate the interaction profile with RBCs, leading to different hemolytic profile.
Figure 3.
Dose-dependent hemolytic activity of NP1-NP9 in the absence of plasma proteins. % Hemolysis was calculated using water as the positive control. Error bars represent standard deviation (n=3).
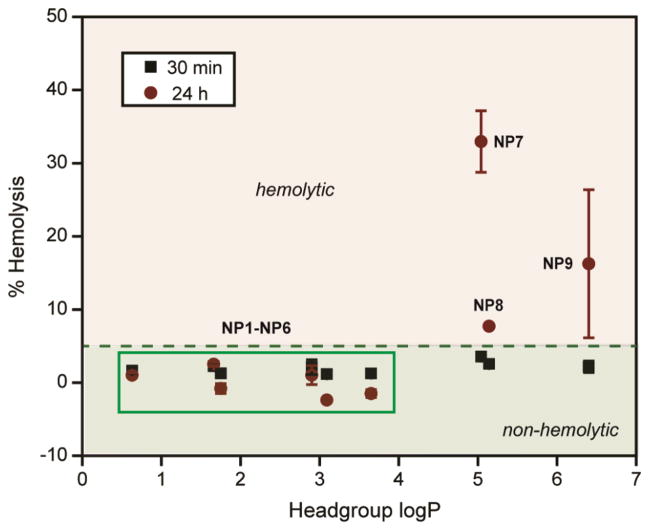
Binding of plasma proteins on NP can mask the chemical nature of the NP surface, altering their activity in vivo.20 The hemolytic activity of NP1-NP9 was further studied in the presence of 55% of plasma protein, a condition NPs will meet while administered in the blood stream. NPs were pre-incubated with 55% plasma in PBS for 30 min and then added to the RBCs. The pre-incubation time was chosen to ensure protein absorption, although protein coronas form within minutes after NP exposure.21 Following incubation with RBCs, the absorbance of the supernatant was measured at 570 nm to monitor the extent of hemolysis (vide supra). In presence of plasma, little to no hemolysis was observed for NP1-NP6 (Figure 4). Significantly, NP6 that showed ~70% hemolysis in the absence of plasma protein, demonstrated no hemolytic activity in the presence of plasma even after 24 hr, a striking example of the effect of protein corona formation on NP surface. Likewise, NP7-NP9 demonstrated significant decrease in hemolytic activities (more than 20-fold) in presence of plasma within 30 minutes. However, NP7-NP9 still showed ~5% hemolysis within 30 min, demonstrating hemolytic potential of these NPs even in the presence of plasma proteins. This behavior was more pronounced after 24 hr where NP7 and NP9 showed severe hemolysis (Figure 4). Significantly, NP7 and NP8 showed different hemolytic activities after 24 hr in the presence of serum protein, despite their similar headgroup hydrophobicity, suggesting surface topology plays a role in hemolytic propensity. Nonetheless, this study signifies the interplay of the chemical functionalities on NP surface and the protein corona formed in situ, making NPs ‘silent’ towards hemolytic consequences. However, NPs with high degree of surface hydrophobicity (headgroup logP > 4) demonstrated severe hemolysis, providing a limit of the NP surface hydrophobicities tested in this study.
Figure 4.
Hemolytic activities of NP1-NP9 (500 nM each) in the presence of plasma proteins. NPs were pre-incubated with 55% of plasma in PBS following incubation with RBCs for 30 min and 24 hr at 37 °C. The mixture was centrifuged to detect the cell-free hemoglobin in the supernatant. % Hemolysis was calculated using water as positive control. Error bars represent standard deviation (n=3).
Conclusion
In summary, we have demonstrated the critical synergy between NP surface chemical functionalities and the protein corona, leading to dramatically attenuated hemolytic behaviour of NPs. In the absence of plasma proteins, a linear hemolytic profile was observed with increasing NP surface hydrophobicity. Significantly, the presence of plasma protein passivated both hydrophilic and hydrophobic NP surfaces leading to no hemolysis within 30 min. NPs with the highest degrees of hydrophobicity (headgroup logP>4), however, maintained their hemolytic properties (24 hr) potentially due to aggregation in the presence of plasma proteins. This study signifies the protective role of protein corona for improved blood compatibility and provides extended design parameters for therapeutic nanomaterials without toxic consequences.
Supplementary Material
Acknowledgments
This research was supported in part by a grant from the National Institutes of Health (grants EB014277). NSF support is acknowledged for NSEC Center for Hierarchical Manufacturing (CMMI-1025020, BY) and MRSEC facilities (DMR-0820506).
Footnotes
Electronic Supplementary Information (ESI) available: NP synthesis, hydrodynamic size and zeta potential, pictures of NP hemolysis. See DOI: 10.1039/b000000x
Notes and references
- 1.Rother RP, Bell L, Hillmen P, Gladwin MT. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 2.(a) Stroncek D, Procter JL, Johnson J. Am J Hematol. 2000;64:67–70. doi: 10.1002/(sici)1096-8652(200005)64:1<67::aid-ajh12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (b) Saitoh D, Kadota T, Senoh A, Takahara T, Okada Y, Mimura K, Yamashita H, Ohno H, Inoue M. Am J Emerg Med. 1993;11:355–359. doi: 10.1016/0735-6757(93)90167-a. [DOI] [PubMed] [Google Scholar]; (c) Scharte M, Fink MP. Crit Care Med. 2003;31:S651–S657. doi: 10.1097/01.CCM.0000098036.90796.ED. [DOI] [PubMed] [Google Scholar]
- 3.(a) Perkins J. Asian J Tranfus Sci. 2008;2:20–23. [Google Scholar]; (b) Yuerek S, Riess H, Kreher S, Doerken B, Salama A. Transfusion Med. 2010;20:265–268. doi: 10.1111/j.1365-3148.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Love SA, Thompson JW, Haynes CL. Nanomedicine. 2012;7:1355–1364. doi: 10.2217/nnm.12.17. [DOI] [PubMed] [Google Scholar]; (b) Choi J, Reipa V, Hitchins VM, Goering PL, Malinauskas RA. Toxicol Sci. 2011;123:133–143. doi: 10.1093/toxsci/kfr149. [DOI] [PubMed] [Google Scholar]; (c) Zhao YN, Sun XX, Zhang GN, Trewyn BG, Slowing II, Lin VSY. ACS Nano. 2011;5:1366–1375. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lu SL, Duffin R, Poland C, Daly P, Murphy F, Drost E, MacNee W, Stone V, Donaldson K. Environ Health Perspect. 2009;117:241–247. doi: 10.1289/ehp.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li YT, Liu J, Zhong YJ, Zhang J, Wang ZY, Wang L, An YL, Lin M, Gao ZQ, Zhang DS. Int J Nanomedicine. 2011;6:2805–2819. doi: 10.2147/IJN.S24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Saha K, Bajaj A, Duncan B, Rotello VM. Small. 2011;7:1903–1918. doi: 10.1002/smll.201100478. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mout R, Moyano DF, Rana S, Rotello VM. Chem Soc Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo JW, Chambers E, Mitragotri S. Curr Pharm Des. 2010;16:2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 8.(a) Walkey CD, Chan WCW. Chem Soc Rev. 2012;41:2780–2799. doi: 10.1039/c1cs15233e. [DOI] [PubMed] [Google Scholar]; (b) Monopoli MP, Aberg C, Salvati A, Dawson KA. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]; (c) Arvizo RR, Giri K, Moyano D, Miranda OR, Madden B, McCormick DJ, Bhattacharya R, Rotello VM, Kocher JP, Mukherjee P. Plos One. 2012:7. doi: 10.1371/journal.pone.0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green MLH, Sim RB. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]; (b) Owens DE, Peppas NA. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.(a) Joglekar M, Roggers RA, Zhao YN, Trewyn BG. Rsc Advances. 2013;3:2454–2461. [Google Scholar]; (b) Kwon T, Woo HJ, Kim YH, Lee HJ, Park KH, Park S, Youn B. J Nanosci Nanotechnol. 2012;12:6168–6175. doi: 10.1166/jnn.2012.6433. [DOI] [PubMed] [Google Scholar]
- 11.Zhu ZJ, Posati T, Moyano DF, Tang R, Yan B, Vachet RW, Rotello VM. Small. 2012;8:2659–2663. doi: 10.1002/smll.201200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chompoosor A, Saha K, Ghosh PS, Macarthy DJ, Miranda OR, Zhu ZJ, Arcaro KF, Rotello VM. Small. 2010;6:2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. J Am Chem Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyano DF, Rotello VM. Langmuir. 2011;27:10376–10385. doi: 10.1021/la2004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rana S, Yu X, Patra D, Moyano DF, Miranda OR, Hussain I, Rotello VM. Langmuir. 2012;28:2023–2027. doi: 10.1021/la204017z. [DOI] [PubMed] [Google Scholar]
- 16.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Bioconjugate Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 17.(a) Lin YS, Haynes CL. Chem Mater. 2009;21:3979–3986. [Google Scholar]; (b) Lin YS, Haynes CL. J Am Chem Soc. 2010;132:4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]; (c) Yu T, Malugin A, Ghandehari H. ACS Nano. 2011;5:5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Nano Lett. 2010;10:2543–2548. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schaeublin NM, Braydich-Stolle LK, Schrand AM, Miller JM, Hutchison J, Schlager JJ, Hussain SM. Nanoscale. 2011;3:410–420. doi: 10.1039/c0nr00478b. [DOI] [PubMed] [Google Scholar]; (c) Leroueil PR, Berry SA, Duthie K, Han G, Rotello VM, McNerny DQ, Baker JR, Orr BG, Holl MMB. Nano Lett. 2008;8:420–424. doi: 10.1021/nl0722929. [DOI] [PubMed] [Google Scholar]
- 19.Andreeva ZI, Nesterenko VF, Fomkina MG, Ternovsky VI, Suzina NE, Bakulina AY, Solonin AS, Sineva EV. Biochim Biophys Acta. 2007;1768:253–263. doi: 10.1016/j.bbamem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 20.(a) Lynch I, Dawson KA. Nano Today. 2008;3:40–47. [Google Scholar]; (b) Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 21.(a) Dell’Orco D, Lundqvist M, Oslakovic C, Cedervall T, Linse S. Plos One. 2010;5:e10949. doi: 10.1371/journal.pone.0010949. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barran-Berdon AL, Pozzi D, Caracciolo G, Capriotti AL, Caruso G, Cavaliere C, Riccioli A, Palchetti S, Lagana A. Langmuir. 29:6485–6494. doi: 10.1021/la401192x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.