Abstract
Objective
The purpose of this study is to investigate the prognostic role of pretreatment anemia in patients with early cervical cancer who underwent radical hysterectomy.
Methods
In this study, we retrospectively enrolled patients with early cervical cancer (International Federation of Obstetrics and Gynecology stage IB to IIA) who were treated at Samsung Medical Center, Seoul, Korea, from 1996 to 2007.
Results
We retrospectively enrolled 805 patients. Median pretreatment hemoglobin (Hb) level was 12.8 g/dL (4.0-16.9) in all patients. Ninety-ninth out of 805 patients had pretreatment anemia (12.3%). Pretreatment anemia was significantly associated with large tumor size, advanced clinical stage, and parametrial invasion. In multivariate analysis, higher pretreatment Hb entailed better prognostic significance in disease free survival (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.078-0.99) but not in overall survival (HR, 0.94; 95% CI, 0.80-1.10).
Conclusion
In conclusion, we found that the negative association between pretreatment Hb level and tumor size and the impact of anemia before treatment on disease free survival adjusted for other factors including clinical stage and pathological findings in early stage cervical cancer.
Keywords: Anemia, Hemoglobins, Prognosis, Survival, Uterine cervical neoplasms
Introduction
Cervical cancer is still the one of the most common malignancies in female, which is the third common cancer in women worldwide, even though the incidence has been steadily decreased in recent years [1,2]. The most of deaths from cervical cancer come from the recurrence of the disease [3]. As a result, predicting recurrence after primary treatment is important not only for counseling the patients about the disease prognosis but also for applying additional treatment to prevent recurrence.
In predicting recurrence of the cervical cancer, clinical staging system has limited value because of inter-observer variability of physical examination and not considering of other important factors such as lymph node (LN) metastasis [4]. As a result many clinicopathological factors are used to stratify the risk for recurrence of the disease [5-7]. However there is still a lack of strong predictor for recurrence up to date and many investigation searching for risk factors for recurrence in cervical cancer is ongoing.
Anemia is a common condition in cancer patients [8]. Especially, cervical cancer is among the tumors characterized by higher prevalence of anemia at diagnosis [9]. And, interestingly, lower pretreatment hemoglobin (Hb) level or anemia before treatment has been reported as an independent prognostic factor for poor prognosis in locally advanced cervical cancer (LACC) [10,11]. For this reason Hb level before treatment was considered as one of the parameters used in prognostic model predicting recurrence in LACC [12]. However, the prognostic role of pretreatment anemia or lower Hb level in patients with early stage cervical cancer (ECC) is still unclear.
As a result, this study was designed to investigate the role of pretreatment anemia as a prognostic factor in ECC and association between pretreatment Hb level and clinicopathological factors in these patients.
Materials and methods
1. Patients
With institutional review board approval, patients with ECC (International Federation of Obstetrics and Gynecology stage IB to IIA) who were treated at Samsung Medical Center, Seoul, Korea from 1996 to 2007 were retrospectively enrolled in this study. The patients' clinical data and pathological findings after surgery as well as laboratory results were collected. We excluded patients with IA1 and IA2; atypical histological subtypes including clear cell, melanoma, metastatic carcinoma, etc.; patients who underwent fertility-saving surgery; patients with concurrent hematologic diseases; patients with para-aortic LN metastasis; patients who did not have the results of Hb level within two weeks before starting initial treatment; patients who received transfusion before blood sampling; and patients who had radiation therapy (RT) oriented therapy as a primary treatment. At our institution, anemia is diagnosed when Hb level is less than 11.2 g/dL for adult female. As a result we use the Hb level of 11.2 g/dL as a cut-off value for the analysis.
2. Treatment
We usually performed surgery as a primary treatment in patients with ECC (IB1 to IIA). However, the choice for primary treatment was dependent on the attending physician's preference. Since 2000, platinum based concurrent chemoradiation therapy (CCRT) has been recommended as adjuvant treatment in cases with more than one high-risk pathological factor for recurrence after surgery, which is described below.
As we prescribed previously [13], standard surgery consisted of type III radical hysterectomy with bilateral pelvic LN dissection. Additional procedures such as bilateral salpingo-oophorectomy and para-arotic LN sampling or dissections were not routinely performed. Adjuvant therapy after surgery was considered based on pathological risk factors. Patients who had more than one of the three high-risk factors (positive pelvic LN, microscopic parametrial invasion, and positive resection margins with tumor) received adjuvant platinum based CCRT. Patients with at least two of the three intermediate risk factors (stromal invasion of more than half of the cervix or stromal invasion more than 1 cm, lympho-vascular space invasion [LVSI], and the largest pathological diameter of 4 cm or greater) received adjuvant RT alone.
RT protocols were also as previously described [13]. In brief, each patient received external beam RT therapy using 10 to 15-MV photons to the whole pelvis for a total dose of 50.4 Gy. The daily fraction size was 1.8 Gy, administered five times per week. Patients were irradiated with a four-field box technique (anterior, posterior, and bilaterals) to spare some of the small bowel anterior to the iliac nodes.
We follow-up the patients with examinations approximately every three months for the first two years, every six months for the next three years, and every year thereafter. During the routine follow-up, computed tomography or magnetic resonance imaging, and chest X-ray was performed annually. We defined disease free survival as the time from the initial treatment to relapse or to the final follow-up visit, and overall survival was defined as the time from the initial treatment to death or to the final follow-up visit.
3. Statistical analysis
The Wilcoxon rank sum test or two-sample t-test was used to compare the median or mean values, respectively, after checking whether the data had non-normal or normal distributions according to the Shapiro-Wilks test. Comparisons of means or medians among three groups were performed using the one way ANOVA as a parametric test or Kruskal-Wallis test as a non-parametric test. Spearman correlation analysis was used to investigate the association between tumor diameter and pretreatment Hb level. Frequency distributions between categorical variables were compared using the χ2 test. The Fisher's exact test was used if the expected frequency was <5. The overall and progression-free survival curves were calculated according to the Kaplan-Meier method with the logrank test. The Cox proportional-hazards model was used for the multivariate analyses. Statistical analyses were performed using PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered statistically significant and all P-values were two-sided.
Results
We could enroll 805 patients with ECC (IB1 to IIA) who were treated with surgery with or without adjuvant therapies. The characteristics of patients are described in Table 1. The median age of the patients was 47 years (range, 2-83 years). Median pretreatment Hb level was 12.8 g/dL (range, 4.0-16.9 g/dL) in this study population. Stage IB1 was most common (602, 74.8%) and about a half of patients (409/805, 50.8%) underwent surgery alone, 199 (24.7%) patient underwent surgery with RT, 153 (19.0%) patients underwent surgery with CCRT and the rest of the patients (44/805, 5.5%) had neoadjuvant chemotherapy before surgery. Histologically, squamous cell was the most frequent histologic subtype (611/805, 75.9).
Table 1.
Patients' characteristics
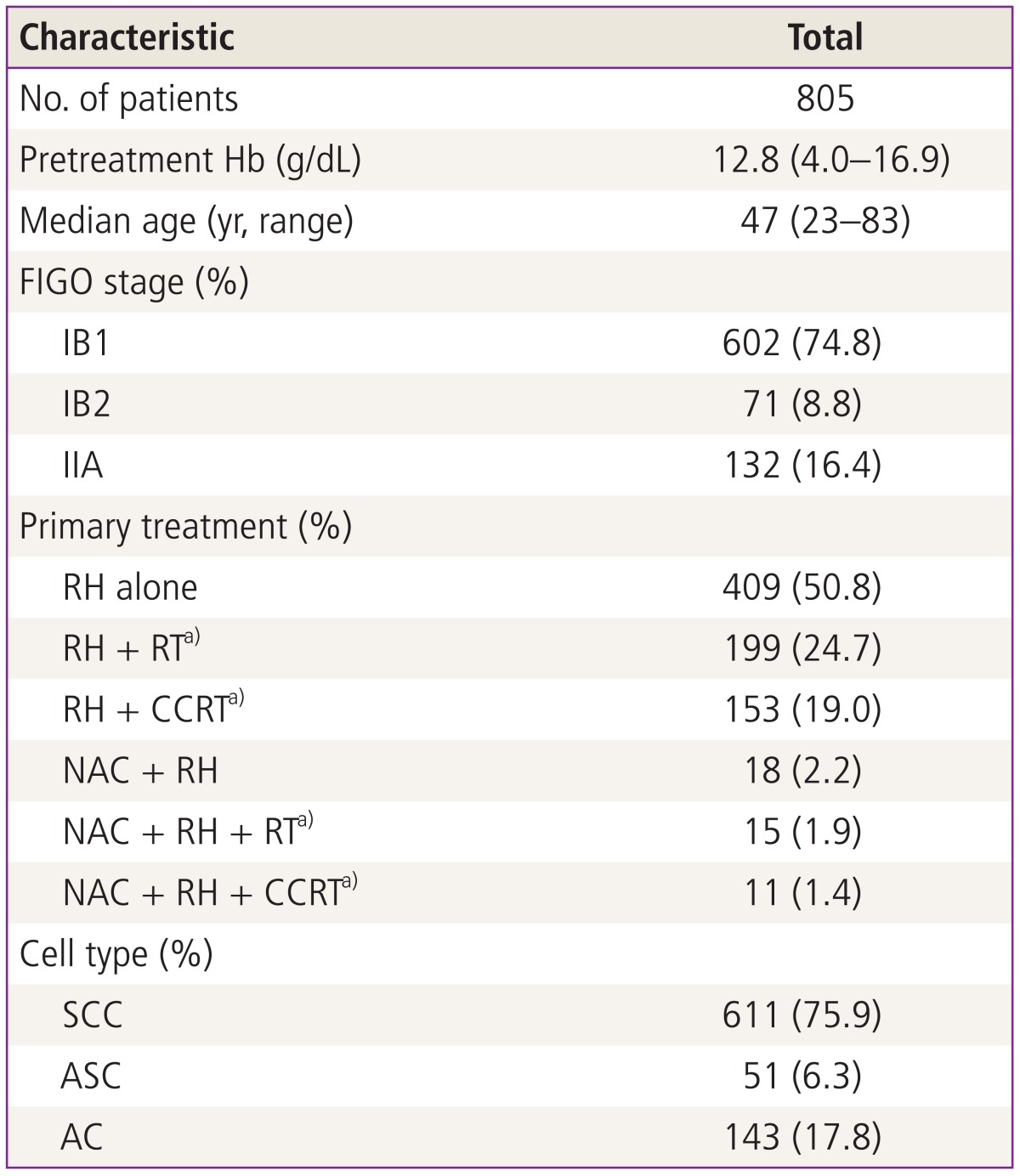
Hb, hemoglobin; FIGO, International Federation of Obstetrics and Gynecology; RH, type III radical hysterectomy; RT, radiation therapy; CCRT, concurrent chemoradiation therapy; NAC, neoadjuvant chemotherapy; SCC, squamous cell carcinoma; ASC, adenosquamous cell carcinoma; AC, adenocarcinoma.
a)Adjuvant setting.
The median follow-up for the patient group was 58.3 months with a range of 2 to 181 months and the five-year survival rate was 92.4%. There were 96 (11.9%) recurrences and 56 (7.0%) deaths during the study period.
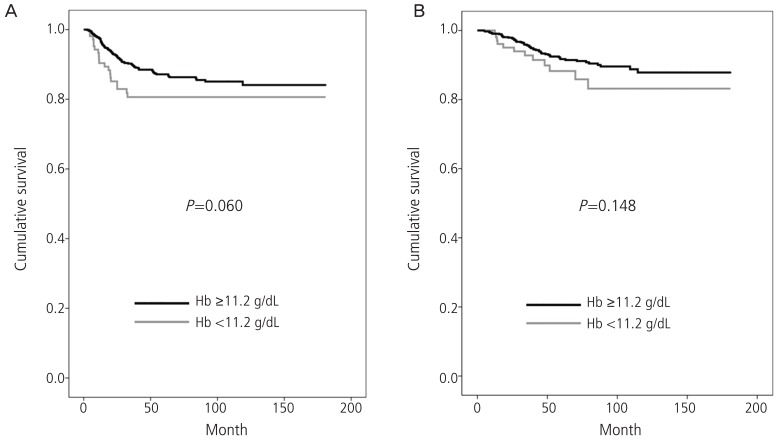
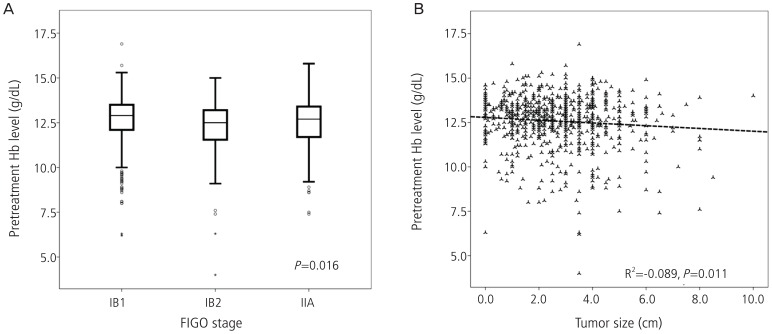
When we divided the patients into two groups according to whether the patients had pretreatment anemia or not, there was no statistical significant difference between groups but trends were shown that the patients with pretreatment anemia may have worse survival outcomes when compared with the patients without anemia (Fig. 1). Then we compared the association between pretreatment anemia and clinico-pathological findings which have been known as risk factors for recurrence. First, patients with IB2 had lower pretreatment Hb level compared with the patients with IB1, which was statistically significant (P=0.016), and negative correlation was observed between tumor size and pretreatment Hb level with statistical significance (R2=-0.089, P=0.011) as shown in Fig. 2. Second, among the high risk factors for recurrence, microscopic parametrial invasion was significantly associated with pretreatment anemia (P=0.002) (Table 2). However, pretreatment Hb level was not associated with lymphatic invasion of tumor cells including lympho-vascular space invasion or LN metastasis as well as histologic subtypes and vaginal resection margin with tumor.
Fig. 1.
(A) Disease free survival and (B) overall survival according to the pretreatment hemoglobin (Hb, with anemia vs. without anemia) in patients with early cervical cancer.
Fig. 2.
The association between pretreatment hemoglobin (Hb) level and International Federation of Obstetrics and Gynecology (FIGO) clinical stage (A) and tumor size (B).
Table 2.
Clinico-pathological findings based on the pretreatment Hb level
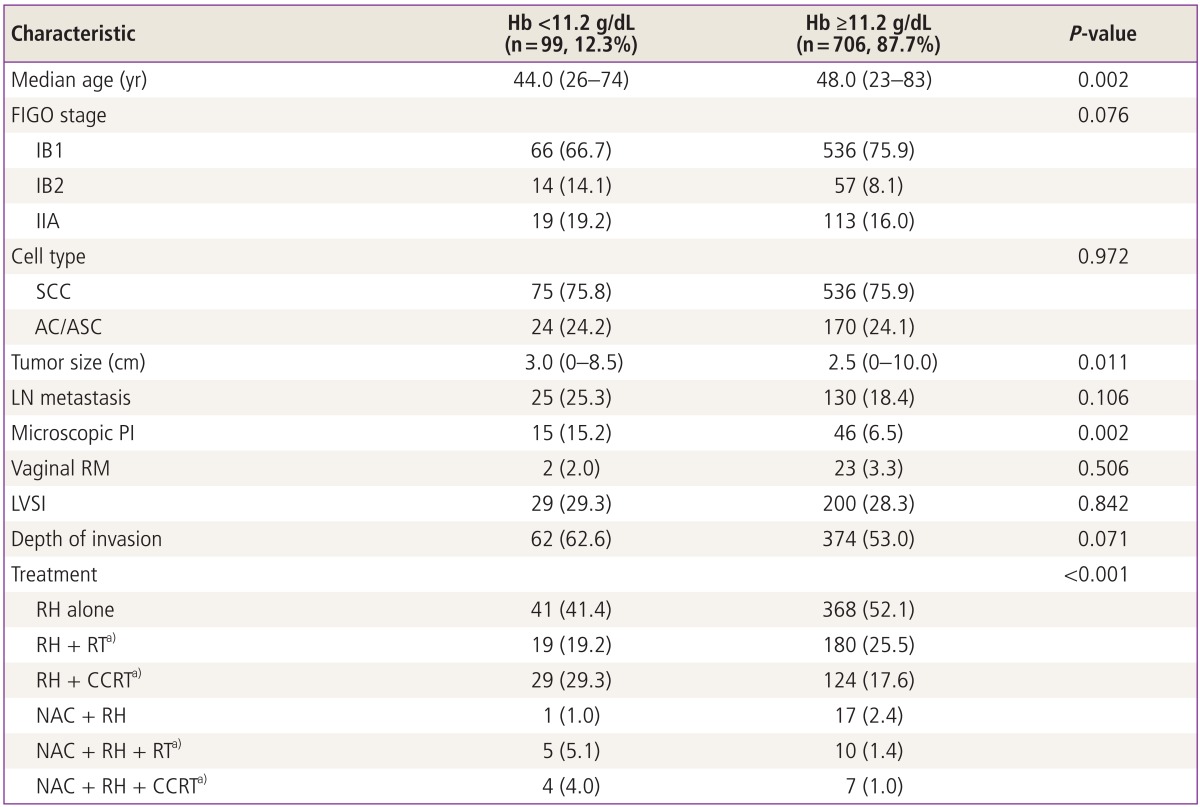
Values are presented as number (range) or (%).
Hb, hemoglobin; FIGO, International Federation of Obstetrics and Gynecology; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; LN, lymph node; PI, parametrial invasion; RM, resection margin with tumor; LVSI, lympho-vascular space invasion; RH, type III radical hysterectomy; RT, radiation therapy; CCRT, concurrent chemoradiation therapy; NAC, neoadjuvant chemotherapy.
a)Adjuvant setting.
In all patients, the univariate analysis revealed that pretreatment anemia and other clinicopathological findings such as stage, type of treatment, histological subtype, and intermediate/high risk factors for recurrence had prognostic significance for both disease free and overall survival (Tables 3, 4). In multivariate analyses, lower pretreatment Hb remained as a significant independent poor prognostic factor in disease free survival (P=0.039) even after adjusting for other factors including tumor size but this finding was not observed in overall survival (P=0.404) (Tables 3, 4).
Table 3.
Univariate and multivariate analysis for disease free survival
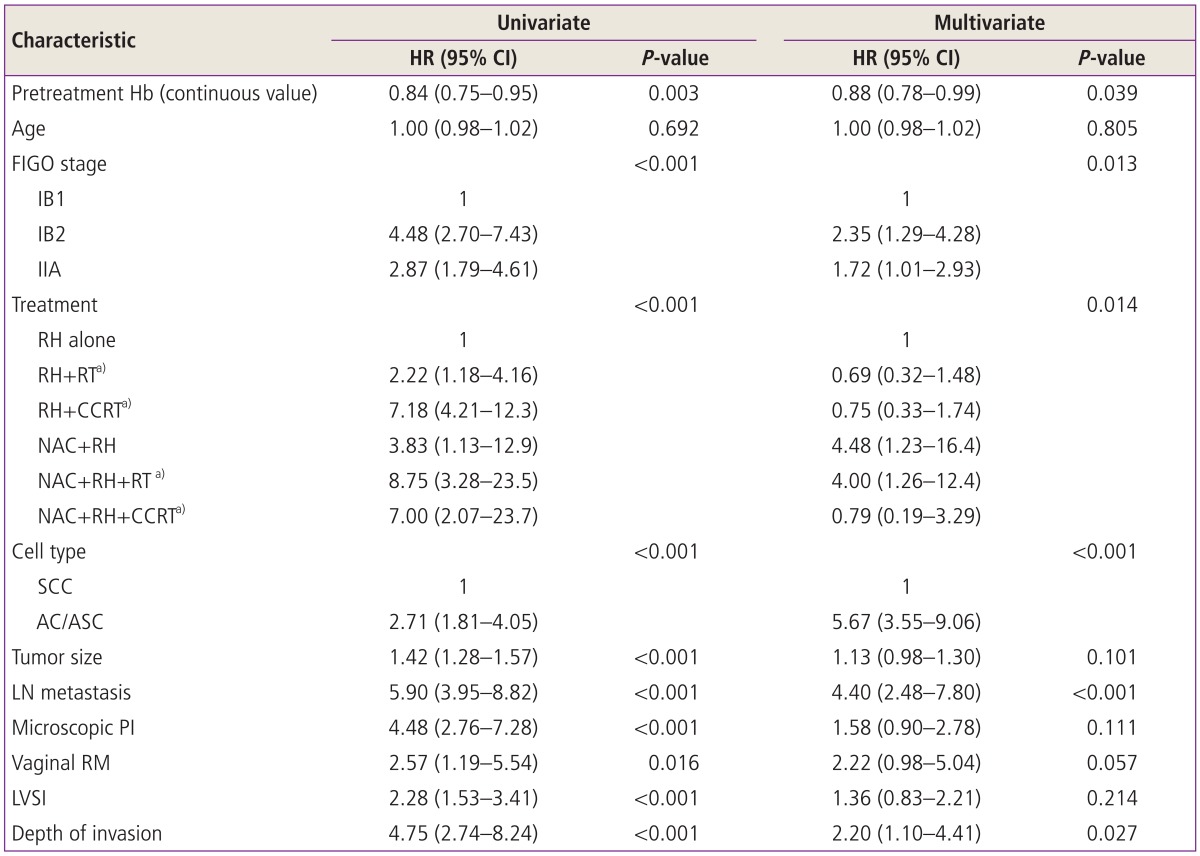
Pretreatment Hb levels were analyzed as a continous variable.
HR, hazard ratio; CI, confidence interval; Hb, hemoglobin; FIGO, International Federation of Obstetrics and Gynecology; RH, type III radical hysterectomy; RT, radiation therapy; CCRT, concurrent chemoradiation therapy; NAC, neoadjuvant chemotherapy; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; LN, lymph node; PI, parametrial invasion; RM, resection margin with tumor; LVSI, lympho-vascular space invasion.
a)Adjuvant setting.
Table 4.
Univariate and multivariate analysis for overall survival
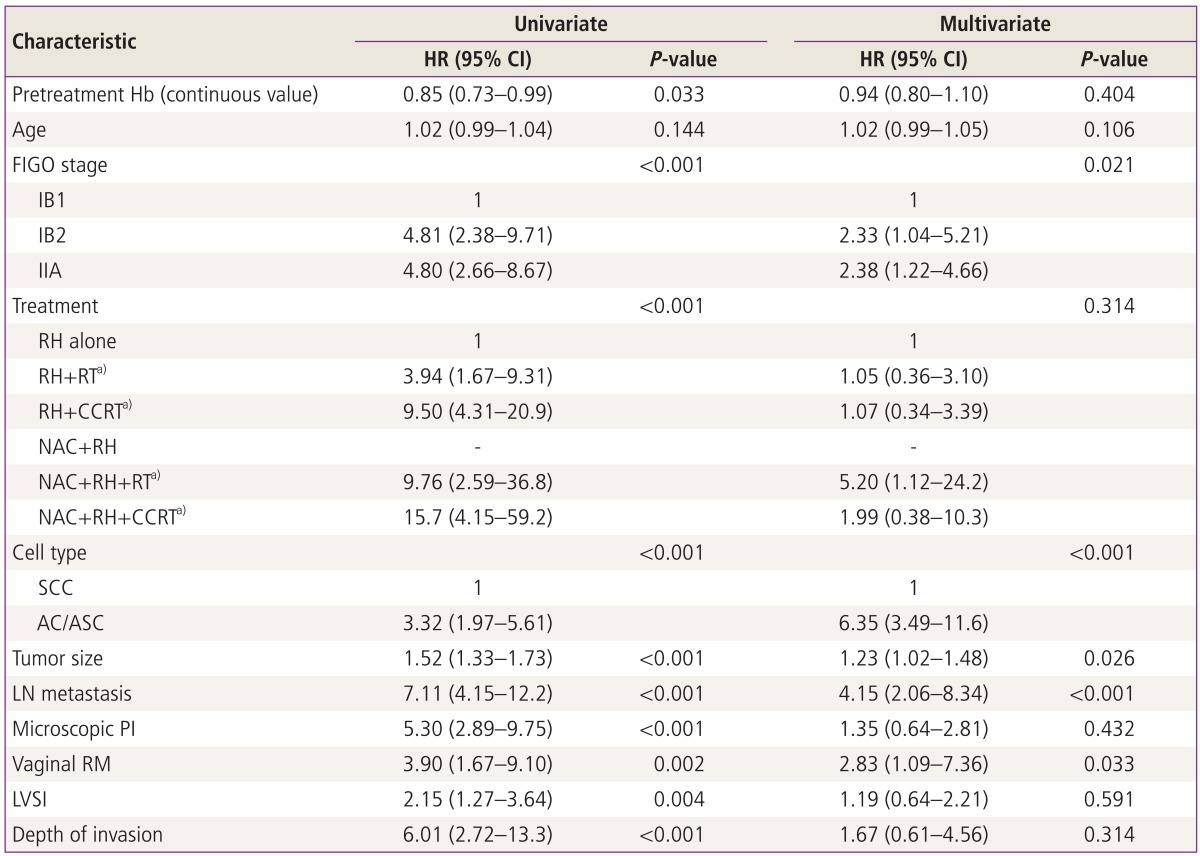
Pretreatment Hb levels were analyzed as a continous variable.
HR, hazard ratio; CI, confidence interval; Hb, hemoglobin; FIGO, International Federation of Obstetrics and Gynecology; RH, type III radical hysterectomy; RT, radiation therapy; CCRT, concurrent chemoradiation therapy; NAC, neoadjuvant chemotherapy; SCC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; LN, lymph node; PI, parametrial invasion; RM, resection margin with tumor; LVSI, lympho-vascular space invasion.
a)Adjuvant setting.
Discussion
In this study, we found that the negative association between pretreatment Hb level and tumor size and the impact of lower Hb before treatment on disease free survival adjusted for other factors including clinical stage and pathological findings in ECC.
Anemia is known to be a common finding in cancer patients and up to 30% of cancer patients suffer from anemia [14]. Decreased Hb from the normal range is reported to be associated with shorter survival times for patients with solid malignant tumors including lung, head and neck, prostate, and hematologic malignancies such as lymphoma and multiple myeloma and the overall estimate increase in risk of death was 65% (range, 54%-77%) in a meta-analysis [8]. Among gynecologic malignancies, anemia was also found to have an independent relationship to poor survival in epithelial ovarian cancer [15], endometrial cancer [16] and LACC [11,17-21]. However, there is no evidence of association between pretreatment Hb and prognosis in ECC and we found its significant independent prognostic role in this study.
In our study, the proportion of patients with pretreatment anemia was 12.3% which is relatively lower incidence from that of LACC reported in previous studies, which was about 25% [11,22]. It suggest that bulky tumors might have more anemic condition compared with small volume tumors and the finding of negative correlation between tumor size and pretreatment Hb level in this study is supporting this idea. A review article addressing the anemia and cervical cancer also reported that Hb levels prior to and during radiotherapy are strongly correlated with tumor size (R2=-0.46, P<0.001) measured based on imaging studies in LACC [9], which is corresponding well with the result shown in our ECC patients. Overall, pretreatment Hb may be a surrogate marker for disease burden itself and may partially explain the prognostic impact on survival in patients with ECC in this respect.
Apart from the association between pretreatment anemia and tumor size, we could also find that pretreatment anemia have independent prognostic power despite the impact of tumor size on anemia prior to treatment. Kapp et al. [23] reported that not only tumor size but also Hb was one of the significant prognostic factors in a multivariate analysis for survival which is similar with the result of our study. And some authors reported that lower Hb level is related with infiltrative phenotypes of tumors such as corpus invasion and nodal metastases in LACC [24]. Another possible explanation about poor survival and anemia in LACC is that anemia might be a surrogate marker of tumor hypoxia which is known to mediate resistance to radiotherapy [9,25]. So it is difficult to consider anemia as a direct or indirect marker for poor survival, however, it is clear that patients with anemia have lower survival.
Generally anemic conditions can be reversed by transfusion or administration of erythropoiesis stimulating agents (ESA). And some authors have suggested that the maintenance of Hb at normal levels would improve not only for quality of life [26,27] but also survival [21,22,28]. However, it is still unknown if transfusion ameliorate hypoxia of tumors and improve the outcome of anemic patients. A report showed that only 50% of patients with cervical cancer demonstrated an increase in tumor oxygenation following transfusion [29] and did not improved survival [9]. Furthermore, although transfusion may increase tumor oxygenation, does not necessarily improve treatment results as anemia is associated with tumor sizes which has an adverse prognosis independent of oxygenation. And in the treatment with ESA, there are still concerns about its safety on cancer prognosis and for example ESA are not recommended in early breast and non-small cell lung cancer patients with anemia [30]. Overall, further studies investigating the prognostic impact of manipulating anemic condition before or during treatment and the safety of ESA in patients with ECC should be needed in the future.
In this study, there are several limitations. First, we did not count the effects of transfusions before surgery due to preoperation anemia because the guidelines for transfusion such as Hb level to start transfusion and the amount of transfusion before surgery were based on the attending physicians' preferences which were heterogenic. So some effects of transfusion might have influenced the results of this study. Second, we could not see the prognostic effect of pretreatment anemia on overall survival. However, most of the patients with ECC show preferable overall survival that there may not be enough numbers of patients to see the survival difference based on the pretreatment anemia and anemic condition at the time of recurrence would be also important factor to overall survival. Third, anemia before treatment should be frequently associated with symptoms such as tumor related vaginal bleeding and menstrual cycles when the blood test was performed. With the retrospective study design, we could not consider these confounding factors and bias may be introduced in our results.
In conclusion, lower pretreatment Hb is independently associated with worse disease free survival and larger tumor size in patients with ECC. Measuring Hb before treatment may be an easy and cost-effective way to predict recurrence and incorporated into one of the contributing factors in prognostic models for recurrence of ECC in the future.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay J International Agency for Research on Cancer. Cancer incidence, mortality, and prevalence worldwide: IARC CancerBase GLOBOCAN 2008. Lyon: IARD Press; 2008. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Berek JS. Berek & Novak's gynecology. 14th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 4.Kupets R, Covens A. Is the International Federation of Gynecology and Obstetrics staging system for cervical carcinoma able to predict survival in patients with cervical carcinoma?: an assessment of clinimetric properties. Cancer. 2001;92:796–804. doi: 10.1002/1097-0142(20010815)92:4<796::aid-cncr1385>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Soisson AP, Soper JT, Clarke-Pearson DL, Berchuck A, Montana G, Creasman WT. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990;37:390–395. doi: 10.1016/0090-8258(90)90374-t. [DOI] [PubMed] [Google Scholar]
- 6.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 7.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 9.Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000;57:13–19. doi: 10.1016/s0167-8140(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 10.Choi YS, Yi CM, Sin JI, Ye GW, Shin IH, Lee TS. Impact of hemoglobin on survival of cervical carcinoma patients treated with concurrent chemoradiotherapy is dependent on lymph node metastasis findings by magnetic resonance imaging. Int J Gynecol Cancer. 2006;16:1846–1854. doi: 10.1111/j.1525-1438.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 11.Obermair A, Cheuk R, Horwood K, Neudorfer M, Janda M, Giannis G, et al. Anemia before and during concurrent chemoradiotherapy in patients with cervical carcinoma: effect on progression-free survival. Int J Gynecol Cancer. 2003;13:633–639. doi: 10.1046/j.1525-1438.2003.13395.x. [DOI] [PubMed] [Google Scholar]
- 12.Seo Y, Yoo SY, Kim MS, Yang KM, Yoo HJ, Kim JH, et al. Nomogram prediction of overall survival after curative irradiation for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2011;79:782–787. doi: 10.1016/j.ijrobp.2009.11.054. [DOI] [PubMed] [Google Scholar]
- 13.Lee YY, Choi CH, Sung CO, Do IG, Hub SJ, Kim HJ, et al. Clinical significance of changes in peripheral lymphocyte count after surgery in early cervical cancer. Gynecol Oncol. 2012;127:107–113. doi: 10.1016/j.ygyno.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Tesarova P, Kvasnicka J. Treatment of anemia in patients with tumors. Cas Lek Cesk. 1995;134:647–650. [PubMed] [Google Scholar]
- 15.Obermair A, Handisurya A, Kaider A, Sevelda P, Kolbl H, Gitsch G. The relationship of pretreatment serum hemoglobin level to the survival of epithelial ovarian carcinoma patients: a prospective review. Cancer. 1998;83:726–731. [PubMed] [Google Scholar]
- 16.Wilairat W, Benjapibal M. Presence of anemia and poor prognostic factors in patients with endometrial carcinoma. Asian Pac J Cancer Prev. 2012;13:3187–3190. doi: 10.7314/apjcp.2012.13.7.3187. [DOI] [PubMed] [Google Scholar]
- 17.Schreiner P, Siracka E, Siracky J, Manka I. The effect of anemia on the radiotherapy results of the uterine cervix cancer. Neoplasma. 1975;22:655–660. [PubMed] [Google Scholar]
- 18.Thomson JM, Spratt JS., Jr Factors affecting survival in over 500 patients with stage II carcinoma of the cervix. Radiology. 1977;123:181–183. doi: 10.1148/123.1.181. [DOI] [PubMed] [Google Scholar]
- 19.Kapp DS, Fischer D, Gutierrez E, Kohorn EI, Schwartz PE. Pretreatment prognostic factors in carcinoma of the uterine cervix: a multivariable analysis of the effect of age, stage, histology and blood counts on survival. Int J Radiat Oncol Biol Phys. 1983;9:445–455. doi: 10.1016/0360-3016(83)90060-3. [DOI] [PubMed] [Google Scholar]
- 20.Girinski T, Pejovic-Lenfant MH, Bourhis J, Campana F, Cosset JM, Petit C, et al. Prognostic value of hemoglobin concentrations and blood transfusions in advanced carcinoma of the cervix treated by radiation therapy: results of a retrospective study of 386 patients. Int J Radiat Oncol Biol Phys. 1989;16:37–42. doi: 10.1016/0360-3016(89)90007-2. [DOI] [PubMed] [Google Scholar]
- 21.Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528–1536. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Kapp KS, Poschauko J, Geyer E, Berghold A, Oechs AC, Petru E, et al. Evaluation of the effect of routine packed red blood cell transfusion in anemic cervix cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:58–66. doi: 10.1016/s0360-3016(02)02896-1. [DOI] [PubMed] [Google Scholar]
- 23.Kapp KS, Stuecklschweiger GF, Kapp DS, Poschauko J, Pickel H, Lahousen M, et al. Prognostic factors in patients with carcinoma of the uterine cervix treated with external beam irradiation and IR-192 high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1998;42:531–540. doi: 10.1016/s0360-3016(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 24.Barkati M, Fortin I, Mileshkin L, Bernshaw D, Carrier JF, Narayan K. Hemoglobin level in cervical cancer: a surrogate for an infiltrative phenotype. Int J Gynecol Cancer. 2013;23:724–729. doi: 10.1097/IGC.0b013e31828a0623. [DOI] [PubMed] [Google Scholar]
- 25.Hockel M, Schlenger K, Hockel S, Vaupel P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res. 1999;59:4525–4528. [PubMed] [Google Scholar]
- 26.Ludwig H, Fritz E. Anemia of cancer patients: patient selection and patient stratification for epoetin treatment. Semin Oncol. 1998;25(3 Suppl 7):35–38. [PubMed] [Google Scholar]
- 27.Demetri GD, Kris M, Wade J, Degos L, Cella D Procrit Study Group. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. J Clin Oncol. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 28.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundfor K, Lyng H, Kongsgard UL, Trope C, Rofstad EK. Polarographic measurement of pO2 in cervix carcinoma. Gynecol Oncol. 1997;64:230–236. doi: 10.1006/gyno.1996.4571. [DOI] [PubMed] [Google Scholar]
- 30.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Erythropoietin or Darbepoetin for patients with cancer: meta-analysis based on individual patient data. Cochrane Database Syst Rev. 2009;(3):CD007303. doi: 10.1002/14651858.CD007303.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]