Abstract
The impact of pre transplant (HCT) cytarabine consolidation therapy on post HCT outcomes has yet to be evaluated after reduced intensity or non-myeloablative conditioning. We analyzed 604 adults with acute myeloid leukemia (AML) in first complete remission (CR1) reported to the CIBMTR who received a RIC or NMA HCT from an HLA-identical sibling, HLA-matched unrelated donor (URD), or umbilical cord blood (UCB) donor in 2000–2010. We compared transplant outcomes based on exposure to cytarabine post remission consolidation. Three year survival rates were 36% (29–43%, 95% CI) in the no consolidation arm and 42% (37–47%, 95% CI) in the cytarabine consolidation arm (p=0.16). Disease free survival was 34% (27–41%, 95% CI) and 41% (35–46%, 95% CI) (p=0.15), respectively. Three year cumulative incidences of relapse were 37% (30–44%, 95% CI) and 38% (33–43%, 95% CI), respectively (p=0.80). Multivariate regression confirmed no effect of consolidation on relapse, DFS and survival. Prior to RIC/NMA HCT, these data suggest pre-HCT consolidation cytarabine does not significantly alter outcomes and support prompt transition to transplant as soon as morphologic CR1 is attained. If HCT is delayed while identifying a donor, our data suggest that consolidation does not increase transplant TRM and is reasonable if required.
Keywords: AML, RIC, cytarabine consolidation
Introduction
Decision-making regarding type of consolidation therapy following first complete remission (CR1) for acute myeloid leukemia (AML) depends on many patient and disease related variables. Post remission consolidation cytarabine chemotherapy can potentially cure a subset of AML patients, especially those with core binding factor leukemias.1–3 However, a recent meta-analysis has suggested a survival benefit for a broader application of allografts for all intermediate and high risk AML patients in CR1, excluding only those with good risk cytogenetic or molecular features.4
When an allograft is planned in a patient with AML in CR1, an abbreviated course of cytarabine consolidation therapy is often offered prior to HCT while a donor is being identified. Despite this common practice, the impact of pre-transplant consolidation chemotherapy on post-HCT outcomes for AML CR1 patients has not been prospectively evaluated. This question has been retrospectively addressed by prior CIBMTR and EBMT (European Group for Blood and Marrow Transplanation) analyses with myeloablative (MA) conditioning. Pre-transplant consolidation therapy did not alter survival or relapse and did not increase transplant-related mortality (TRM).5,6 The influence of pre-transplant cytarabine consolidation chemotherapy in the setting of reduced intensity conditioning (RIC)/non-myeloablative (NMA) HCT for this patient population is uncertain. Prior retrospective analyses comparing outcomes following MA or RIC/NMA conditioning suggest a higher rate of relapse following RIC/NMA HCT but less TRM and thus similar survivals, even in the older population receiving RIC HCT.7–9 These data would theoretically lead to the hypothesis that pre-HCT chemotherapy might reduce relapse risk after RIC all-HCT. Most recent retrospective and prospective publications, however, have challenged this earlier supposition showing relatively similar relapse and TRM, regardless of conditioning intensity. 10–13
In the context of expanding use of RIC/NMA HCT, a setting where more stringent disease control may be desirable, the effectiveness of pre-HCT consolidation chemotherapy is largely unknown. A retrospective analysis from the University of Minnesota compared the outcomes of 60 AML patients in CR1 undergoing the same RIC HCT in 2001–2008 based on exposure to pre-HCT consolidation chemotherapy. The investigators reported similar relapse and survival in subjects who did or did not receive pre-HCT consolidation.14 In order to define the value of pre-RIC/NMA HCT consolidation chemotherapy for AML in CR1, we addressed this question in a large dataset from the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
Data source
The CIBMTR includes a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; patients are followed longitudinally and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patient selection
All adult patients reported to the CIBMTR who received a RIC or NMA conditioning HCT for AML in CR1 from either an HLA-identical sibling, unrelated donor (URD), or umbilical cord blood (UCB) donor in 2000–2010 were included in this analysis. Patients with French American British (FAB) subtype M3 were excluded. The very few patients with favorable risk cytogenetics (n=8) were also excluded.
A total of 604 patients were identified from 165 centers. Patients were initially divided into 3 cohorts for analysis: 1) no post-remission therapy before transplant, or 2) standard-dose cytarabine consolidation therapy (defined as ≤ 1 g/m2/day on earlier CIBMTR data submission forms (pre 2008), or ≤ 2 g/m2/day on current forms), or 3) high-dose cytarabine consolidation therapy (defined as >1 g/m2/day on earlier forms, or > 2 g/m2/day on current forms. However, as no difference was seen between lower and higher dose consolidation cohorts, the final analysis compared no cytarabine consolidation versus any dose of cytarabine consolidation. Patients included in the study cohort received a maximum of 2 cycles of induction therapy to obtain CR1 status. CIBMTR classifications of URD matching were used to define well-matched, partially matched, or mismatched categories.15 Preparative regimens were classified as either RIC or NMA by established CIBMTR functional definitions. RIC included any regimen with either: 1) 500cGy or less of total body irradiation (TBI) as a single fraction or 800 cGy or less if fractionated; 2) <9 mg/kg busulfan oral (or intravenous equivalent); 3) <140 mg/m2 melphalan; 4) <10mg/kg thiotepa; or 5) BEAM regimen (carmustine, etoposide, cytarabine, and melphalan).16,17 All other regimens were classified as NMA conditioning according to Champlin et al. where prompt hematopoietic recovery could reasonably be expected without a transplant, and would produce mixed chimerism after engraftment post-transplant. 18 Based on these classifications, the most common RIC regimens included: 1) fludarabine + busulfan; 2) fludarabine + melphalan; 3) fludarabine + cyclophosphamide; and 4) other. NMA regimens included: 1) fludarabine + low dose TBI (≤200 cGy); and 2) fludarabine + anti-thymocyte globulin (ATG).
Study endpoints
The primary endpoint was overall survival (OS) in those with or without pre-HCT consolidation chemotherapy. Secondary endpoints included hematopoietic recovery, occurrence of acute graft versus host disease (aGVHD) and chronic (cGVHD), treatment-related mortality (TRM), incidence of relapse, and disease-free survival (DFS). Overall survival was defined as time to death from any cause with surviving patients censored at time of last contact. Hematopoietic recovery was defined as time to absolute neutrophil count ≥ 500 neutrophils/mcL sustained for 3 consecutive days. Criteria for aGVHD and cGVHD were based on consensus criteria as previously defined.19,20 TRM was defined as any death in the first 28 days post-transplant or any death after day 28 without recurrent leukemia. Relapse was defined as hematologic evidence of disease recurrence with those surviving without relapse censored at the date of last contact and using death in remission as the competing hazard. DFS was defined as survival without death or relapse with those who survived without recurrence or persistent disease censored at the date of last contact.
Statistical analysis
Patient, disease, treatment history, and transplant-related factors were compared between groups using the Chi-square test for categorical variables and the Wilcoxon two sample test for continuous variables. The product-limit estimator proposed by Kaplan-Meier 21 was used to estimate the median and range of follow-up time.
Univariate probabilities of disease-free and overall survival were calculated using the Kaplan-Meier estimator with the variance estimated by Greenwood’s formula.21 Probabilities of acute and chronic GVHD, TRM and relapse were calculated using cumulative incidence curves to accommodate competing risks. Ninety-five percent confidence intervals for all probabilities and p-values of pairwise comparisons were derived from pointwise estimates and calculated using an arcsine square root transformation.
The final consolidation therapy groups used for analysis were no consolidation versus any dose cytarabine consolidation after initial analyses showing no cytarabine dose effect. The proportional hazards assumption for all the variables was examined and its violations were addressed by using a stratified Cox model when needed. A backward elimination method was used to build the regression model for the outcomes of TRM, relapse, DFS and overall survival. As exposure to cytarabine based consolidation was the main interest of the study, the risk factor of cytarabine consolidation was included in all steps of model building. Patient-related variables including age (< 45, 45–60, > 60), gender, Karnofsky Performance Status (KPS) (< 90% versus ≥ 90%) were considered in the analysis. Disease-related variables included FAB or World Health Organization (WHO) subtype (FAB M0/M1/M2 versus M4/M5/M6/M7 versus AML not otherwise specified (NOS) versus all remaining categories versus missing), antecedent hematologic disorder (yes or no), cytogenetics at diagnosis by Southwest Oncology Group (SWOG) criteria2 (intermediate versus unfavorable versus unknown significance), and number of cycles of induction chemotherapy (1 versus 2). Transplant-related variables considered included year of transplant (2000–2005 versus 2006–2008 versus 2008–2010), donor source (matched sibling versus matched URD versus UCB versus other URD), recipient CMV serostatus (negative versus positive versus missing), conditioning regimen (fludarabine + busulfan versus fludarabine + melphalan versus fludarabine +cyclophosphamide and “other” versus NMA), GVHD prophylaxis (tacrolimus-based versus cyclosporin- based), and ATG or alemtuzumab exposure (yes or no). The risk factors with significance level p < 0.05 were included in the model. The potential interaction between main effect of pre-transplant consolidation therapy exposure and all significant covariates were examined. Adjusted probability of DFS and overall survival were computed based on the final Cox regression model, stratified by age, and weighted by the pooled sample proportion value for all significant risk factors. SAS software (SAS Institute, Vary, NC) was used to perform all statistical analyses.
Results
Patient disease, treatment, and transplant-related factors are shown in Table 1. All patients received induction chemotherapy with either 3+7 based (anthracycline + cytarabine) (85%) or other multi-drug induction regimens including mitoxantrone and etoposide (4%), cytarabine regimens without anthracycline (9%), and others (2%) (clofarabine, gemtuzumab, topotecan, amsacrine, enocitabine, or anthracycline alone). Median age, performance status, presence of extramedullary disease, median WBC count at diagnosis, graft source, type of GVHD prophylaxis, and use of ATG or alemtuzumab was similar between the two groups. Between the consolidation groups, those receiving no consolidation had a slightly higher percentage of AML NOS (not otherwise specified) (25% compared to 18%), had a slightly higher percentage of “other” multi-agent induction chemotherapy (22% versus 11%), were more likely to have previously undergone 2 cycles of induction prior to CR1 documentation (33% versus 19%), and were more likely to have an antecedent hematologic disorder (myelodysplastic(MDS)/myeloproliferative neoplasm MPN) (34% versus 19%). Those receiving no consolidation also had a slightly higher percentage of fludarabine + melphalan conditioning (25% versus 13%) compared to the cytarabine consolidation group. Those receiving no cytarabine consolidation were slightly less likely to be classified as an FAB subtype of M4/M5/M6//M7(20% versus 32%) and have intermediate risk cytogenetics (38% versus 48%). The median follow up between the groups was similar (36 months for no consolidation versus 35 months for those receiving consolidation).
Table 1.
Characteristics of adult patients (≥18 years) receiving RIC/NMA HCT for AML in CR1 between 2000 and 2010
| Characteristics | No Cytarabine Consolidation | Cytarabine Consolidation | P Value |
|---|---|---|---|
| Number of Patients | 202 | 402 | |
| Number of Centers | 90 | 75 | |
| Patient Related Characteristics: | |||
| Age at Transplant (Median, range) | 60(18–75) | 59(19–76) | 0.18 |
| Age | 0.29 | ||
| Less than 45 years | 21(10%) | 41(10%) | |
| 45–60 | 76(38%) | 177(44%) | |
| Greater than 60 | 105(52%) | 184(46%) | |
| Gender | 0.16 | ||
| Male | 125(52%) | 225(55%) | |
| Female | 77(38%) | 177(45%) | |
| Karnofsky Performance Status (KPS) | 0.84 | ||
| 90–100% | 133(66%) | 268(67%) | |
| Less than 90% | 60(30%) | 120(30%) | |
| Missing | 9(4%) | 14(3%) | |
| Disease Related Characteristics: | |||
| FAB Subtype: | <0.01 | ||
| M0,M1/M2 | 64(32%) | 139(35%) | |
| M4/M5/M6/M7 | 40(20%) | 127(32%) | |
| AML NOS | 51(25%) | 74(18%) | |
| Miscellaneous: | 21(10%) | 31(7.5%) | |
| Other/Missing | 26(13%) | 31(7.5%) | |
| WBC at Diagnosis | 0.16 | ||
| ≥ to 5×107/Liter | 110(54%) | 195(49%) | |
| < 5×107/Liter | 92(46%) | 207(51%) | |
| Extramedullary Disease at Diagnosis | 0.67 | ||
| Absent | 193(96%) | 382(95%) | |
| Present | 8(4%) | 44(5%) | |
| Cytogenetics (SWOG Classification) | 0.03 | ||
| Intermediate | 77(38%) | 193(48%) | |
| Unfavorable | 63(31%) | 119(30%) | |
| Unknown Significance | 62(31%) | 90(22%) | |
| Pre-Existing MDS/MPN | <0.01 | ||
| No | 134(66%) | 327(81%) | |
| Yes | 68(34%) | 75(19%) | |
| Time From Diagnosis to CR1 median(range), months | 1.6 (<1–89) | 1.3(<1–160) | <0.0001 |
| 0–2 | 120 (59%) | 306 (76%) | |
| 2–6 | 60(30%) | 88(22%) | |
| 6+ | 22(11%) | 8(2%) | |
| Treatment Characteristics: | |||
| Induction Regimen | <0.01 | ||
| 3+7 or “similar” | 157(78%) | 358(89%) | |
| Other Multiagent Induction | 45(22%) | 44(11%) | |
| Cycles Induction Chemotherapy | <0.01 | ||
| 1 Cycle | 136(67%) | 326(81%) | |
| 2 Cycles | 66(33%) | 76(19%) | |
| Cycles of Cytarabine Consolidation | |||
| 1 | N/A | 168(42%) | |
| 2 or more | N/A | 134(33%) | |
| Unknown Number | N/A | 100(25%) | |
| Year of Transplant | <0.01 | ||
| 2000–2005 | 53(26%) | 106(26%) | |
| 2006–2008 | 84(42%) | 148(37%) | |
| 2008–2010 | 65(32%) | 148(37%) | |
| Graft Source | 0.51 | ||
| Matched Sibling Donor | 106(52%) | 185(46%) | |
| Matched URD | 52(26%) | 117(29%) | |
| Partially Matched URD | 16(8%) | 38(9%) | |
| UCB | 28(14%) | 62(15%) | |
| Recipient CMV Serostatus | 0.29 | ||
| Negative | 61(30%) | 128(32%) | |
| Positive | 141(70%) | 269(67%) | |
| Missing | 0(0%) | 5(1%) | |
| Conditioning Regimens | |||
| RIC | 0.045 | ||
| Flu/Bu | 89(44%) | 173(43%) | |
| Flu/Mel | 51(25%) | 54(13%) | |
| Flu/Cy +Other | 36(18%) | 97(24%) | |
| NMA | |||
| Flu/TBI (200–500 cGy) | 23(11%) | 71(18%) | |
| Flu/ATG | 3 (2%) | 7(2%) | |
| GVHD Prophylaxis | 0.32 | ||
| Tacrolimus-based | 115(57%) | 212(53%) | |
| Cyclosporine-based | 87(43%) | 190(47%) | |
| ATG/Alemtuzumab Use | 0.06 | ||
| No | 133(66%) | 235(58%) | |
| Yes | 69(34%) | 167(42%) | |
| Time From CR1 to Transplant: Median (interquartile range, 25–75%), (months) | 2(1–4) | 4(3–5) | <0.0001 |
| Follow Up of Survivors: Median, (range) (months) | 36(3.9–115) | 35(3.2–132) | 0.25 |
Miscellaneous AML Subtypes Includes: AML with Abnormal Eosinophils + AML with 11q23 + AML with Multilineage Dysplasia,
Induction Chemotherapy: 3+7 = anthracycline (idarubicin or daunorubicin) + cytarabine
Other Multi-agent Induction (n=89) included: Mitoxantrone +etoposide (4%), cytarabine without anthracycline (9%), and others (2%) (clofarabine, gemtuzumab, topotecan, amsacrine, enocitabine, anthracycline alone)
AML NOS (Not Otherwise Specified); WBC=White blood cell counts; SWOG = Southwest Oncology Group; MDS=myelodysplastic syndrome; MPN=myeloproliferative neoplasm; CMV= cytomegalovirus; RIC=reduced intensity conditioning; NMA= non-myeloablative; Flu=fludarabine; Bu= busulfan; Mel=melphalan; Cy=cyclophosphamide; TBI=total body irradiation; ATG= anti-thymocyte globulin; GVHD=graft versus host disease; CR1 = complete remission 1; URD=unrelated donor; UCB=umbilical cord blood.
Outcomes
After a median follow up time of 36 months (3–132), 239 patients were alive at last contact. Two hundred and seventeen patients had relapsed and died and 20 patients had relapsed, but were alive at last contact.
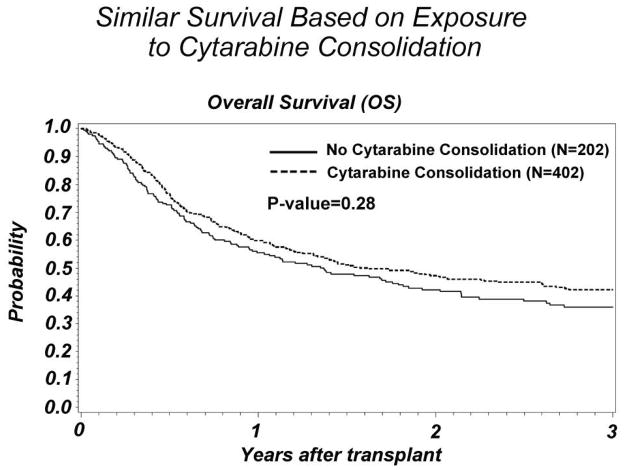
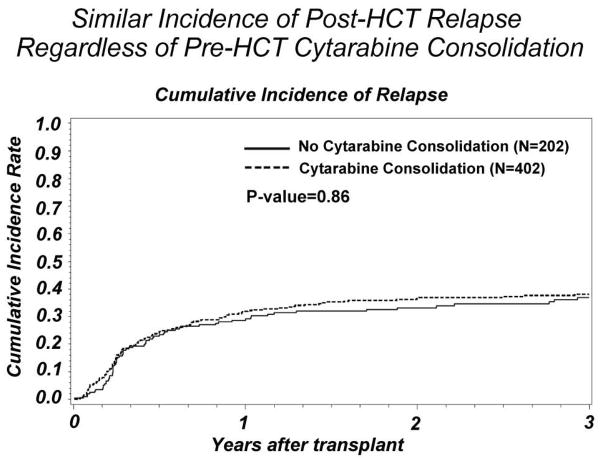
Three year OS was similar between the groups: 36% (29–43%, 95% CI) for those receiving no consolidation compared to 42% (37–47%, 95% CI) in the consolidation group (p=0.15) (Figure 1). There was no significant difference in the incidence of relapse. The no consolidation group had a 3 year cumulative incidence of relapse of 37% (33–43%, 95% CI) versus consolidation 38% (33–43%, 95% CI)(p=0.80) (Figure 2).
Figure 1.
Comparison of survival post-transplant between AML CR1 subjects who did or did not receive pre-transplant cytarabine consolidation
Figure 2.
Comparison of post-transplant relapse incidence between AML CR1 subjects who did or did not receive pre-transplant cytarabine consolidation
Neutrophil engraftment at Day +28 was similar between groups at no consolidation 86% (77–92%, 95% CI) and consolidation 82% (75–87%, 95% CI). The cumulative incidence of grade III-IV acute GVHD was similar at 16% (12–22%, 95% CI) and 13% (10–16%, 95% CI), respectively (p=0.26). The incidence of chronic GVHD at three years was identical between the two groups at 41% (p=0.96).
TRM at Day +100 was slightly higher in the no consolidation group at 12% (8–17%, 95% CI) compared to 5% (4–8%, 95% CI) in the consolidation group (p=0.01) and this difference was maintained at 1 year with a TRM of 23% (17–29%, 95% CI) in the no consolidation group versus 16% (12–20%, 95% CI) in the consolidation group (p=0.04).
Three year DFS for the no consolidation group was 34% (27–41%, 95% CI) compared with 41% (35–46%, 95% CI) for the group receiving consolidation (p=0.15) (Table 2). Additional supplemental univariate analyses investigating the impact of cytogenetic risk group, type of induction chemotherapy, conditioning regimen, and donor source on post-HCT outcomes revealed no unique factors of significance (data not shown). These analyses found that unfavorable cytogenetics, UCB donor source, and fludarabine plus melphalan conditioning were the only factors of significance influencing relapse or TRM mirroring the findings of our primary analysis focusing on cytarabine consolidation exposure.
Table 2.
Outcomes after RIC/NMA HCT based on cytarabine consolidation exposure: univariate analysis
| No Cytarabine consolidation | Cytarabine consolidation | P-value | |||
|---|---|---|---|---|---|
| N eval. | Incidence (95% CI) | N eval. | Incidence (95% CI) | ||
| OS | |||||
| @ 2 year | 202 | 42 (35–49) | 402 | 47 (42–52) | 0.25 |
| @ 3 year | 202 | 36 (29–43) | 402 | 42 (37–47) | 0.16 |
| Relapse | |||||
| @ 2 year | 197 | 33 (27–40) | 393 | 37 (32–41) | 0.42 |
| @ 3 year | 197 | 37 (30–44) | 393 | 38 (33–43) | 0.80 |
| aGVHD III–IV | 202 | 402 | |||
| @ Day +100 | 16(12–22) | 13(10–16) | 0.26 | ||
| cGVHD | 195 | 395 | |||
| @ 3 year | 41(34–49) | 41(36–47) | 0.96 | ||
| TRM | |||||
| @ 100 days | 197 | 12 (8–17) | 393 | 5 (4–8) | 0.01 |
| @ 1 year | 197 | 23 (17–29) | 393 | 16 (12–20) | 0.04 |
| DFS | |||||
| @ 2 year | 197 | 39 (32–46) | 393 | 44 (39–49) | 0.20 |
| @ 3 year | 197 | 34 (27–41) | 393 | 41(35–46) | 0.15 |
A forward stepwise method was used to build the regression models and compare risks for TRM, relapse, DFS, and OS in multivariate analyses adjusting for the effects of other significant covariates (Table 3). Similar outcomes regardless of consolidation exposure were confirmed for OS, DFS, and relapse. The modest univariate difference in TRM between the two groups was not confirmed in multivariate analysis. Unfavorable cytogenetics was the only significant factor influencing relapse. TRM was worse with UCB donor source, fludarabine + melphalan conditioning, and age > 60 and was better in females. Overall survival was worse with UCB donor source, unfavorable cytogenetics, age > 60, and male gender.
Table 3.
Outcome after RIC/NMA HCT: multivariate analysis
| Outcome | Variable | RR | 95% CI | P Value |
|---|---|---|---|---|
| Overall Survival | Consolidation | |||
| No | 1 | |||
| Yes | 0.886 | 0.71 – 1.10 | 0.28 | |
| Donor Source: | ||||
| Matched Sibling | 1 | |||
| Matched URD | 0.88 | 0.68 – 1.14 | 0.34 | |
| Partially matched URD | 1.03 | 0.71 – 1.51 | 0.86 | |
| UCB | 1.60 | 1.18 – 2.16 | 0.002 | |
| Cytogenetics | ||||
| Intermediate | 1 | |||
| Unfavorable | 1.74 | 1.36–2.22 | <0.0001 | |
| Other/Missing | 1.20 | 0.92 – 1.56 | 0.18 | |
| Age | ||||
| <45 | 1 | |||
| 45–60 | 1.22 | 0.83 – 1.79 | 0.31 | |
| >60 | 1.51 | 1.03 – 2.2 | 0.03 | |
| Gender | ||||
| Male | 1 | |||
| Female | 0.78 | 0.63 – 0.97 | 0.02 | |
| TRM | Consolidation | |||
| No | 1 | |||
| Yes | 0.74 | 0.53 – 1.04 | 0.08 | |
| Donor Source | ||||
| Matched Sibling | 1 | |||
| Matched URD | 0.998 | 0.65 – 1.52 | 0.99 | |
| Partially matched URD | 1.371 | 0.79 – 2.39 | 0.26 | |
| UCB | 3.83 | 2.25 – 6.54 | <0.0001 | |
| Conditioning | ||||
| Flu/Bu | 1 | |||
| Flu/Mel | 1.6 | 1.05 – 2.43 | 0.03 | |
| Flu/Other | 0.65 | 0.38 – 1.14 | 0.13 | |
| TBI-based | 0.94 | 0.56 – 1.6 | 0.80 | |
| Age | ||||
| <45 | 1 | |||
| 45–60 | 1.2 | 0.63–2.28 | 0.58 | |
| >60 | 1.96 | 1.04–3.67 | 0.04 | |
| Gender | ||||
| Male | 1 | |||
| Female | 0.65 | 0.46–0.91 | 0.013 | |
| Relapse | Consolidation | |||
| No | 1 | |||
| Yes | 1.03 | 0.77–1.36 | 0.86 | |
| Cytogenetics | ||||
| Intermediate | 1 | |||
| Unfavorable | 1.87 | 1.38–2.5 | <0.0001 | |
| Other/Missing | 1.17 | 0.83 – 1.66 | 0.37 | |
| WBC | ||||
| < 5.0 | 1 | |||
| >= 5.0 | 0.77 | 0.59 – 0.99 | 0.05 | |
| DFS | Consolidation | |||
| No | 1 | |||
| Yes | 0.87 | 0.7–1.07 | 0.19 | |
| Cytogenetics | ||||
| Intermediate | 1 | |||
| Unfavorable | 1.65 | 1.29–2.1 | <0.0001 | |
| Other/Missing | 1.19 | 0.92 – 1.55 | 0.19 | |
| Gender | ||||
| Male | 1 | |||
| Female | 0.75 | 0.60–0.92 | 0.0071 | |
| Donor Source | ||||
| Matched Sibling Donor | 1 | |||
| Matched URD | 0.84 | 0.64 – 1.09 | 0.20 | |
| Partially matched URD | 0.94 | 0.64 – 1.36 | 0.72 | |
| UCB | 1.37 | 1.01 – 1.85 | 0.04 |
Note: Relapse and DFS models were stratified on ATG/Alemtuzumab use
Discussion
In persons with AML in CR1 receiving a RIC/NMA allo-HCT we found no difference in outcomes between those who did or did not receive pre-HCT cytarabine consolidation. Our data highlight similar OS, DFS, and relapse post RIC/NMA HCT with no increased TRM following cytarabine consolidation. The precise evaluation of potential benefit or harm of giving cytarabine consolidation requires a randomized trial; however, no such study is reported or likely to be completed. These results are similar to the findings previously reported by others in the myeloablative setting.5,6
As the use of RIC regimens for HCT continues to expand, efforts to optimize both efficacy and safety of therapy continue. Several retrospective studies comparing MA to RIC conditioning have suggested an increased risk of relapse with RIC accompanied in some series by improved TRM 7–9 suggesting that RIC conditioning is potentially less effective in control of residual leukemia during CR. However, more recent studies11–13 have revealed similar relapse, TRM, OS regardless of conditioning intensity for AML patients transplanted in CR. The finding that post-HCT outcomes are not improved by cytarabine consolidation chemotherapy prior to RIC/NMA HCT supports the contention that RIC can effectively control disease recurrence in CR1 AML, and may challenge many clinicians’ pre-conceived theories.
Only limited data has been available to help guide consolidation chemotherapy decision-making before a RIC/NMA HCT. McCormack et al described the University of Minnesota experience with similar survival and relapse rates regardless of cytarabine consolidation using a uniform RIC regimen for AML.14 The current larger study, with diverse conditioning regimens, reveals similar findings and has substantially more power to identify any potentially differing outcomes regardless of cytarabine consolidation exposure—and none were found.
Pre-transplant cytarabine consolidation did not increase the risk of TRM. Physicians may, however, choose patients with a better performance status to receive consolidation chemotherapy prior to HCT and proceed directly to HCT for those patients who tolerated induction chemotherapy poorly or alternatively choose consolidation in those with higher relapse risks. We do not have data to directly examine the treating physician rationale for giving consolidation chemotherapy prior to RIC HCT. Our multivariate analysis did not demonstrate any difference in TRM based on consolidation exposure, but did show higher TRM following UCB HCT, in males, in those receiving fludarabine plus melphalan based conditioning, and in those older than 60.
Our analysis was designed to investigate cytarabine consolidation, but also any potential cytarabine dose effect. In the past, the advantage of high dose cytarabine consolidation without HCT was noted primarily for persons with good risk cytogenetics 1 or younger patients (< 60 years of age) with normal cytogenetics. 22 Thus, it is not surprising that we found similar outcomes regardless of cytarabine dose in this dataset which excluded patients with good risk cytogenetics and included a large subset of patients over the age of 60.1,22,23 These data support the theory that in AML patients in CR1, the graft versus leukemia effect provided by the allograft can eliminate residual disease in long-term survivors, even following RIC/NMA HCT. We analyzed only patients in morphologic CR as reported by the participating centers. Emerging literature suggest that minimal residual disease (MRD) defined by either cytogenetic, molecular, or multi-parameter flow cytometry may influence post HCT outcomes and may identify patients with higher risk of relapse.24,25 Future analysis should consider depth of remission defined by cytogenetic, molecular, and flow cytometric data to investigate the value of added pre-HCT cytarabine or other consolidation to reduce tumor burden prior to RIC allogeneic HCT, but this data suggests lesser importance of this minimal disease burden during CR1.
We acknowledge the inherent limitations of an analysis using an observational database. We cannot account for those who relapsed prior to HCT and were excluded, yet the median time to HCT we studied was only 2 and 4 months for the two cohorts capturing most of the higher risk period. In addition, some differences between the two groups merit discussion. Those requiring two rounds of induction prior to CR1 more often had no consolidation. However, it is uncertain if two rounds of induction chemotherapy were given following the absence of a remission after the first induction cycle. More patients in the no consolidation group had preceding MDS/MPN, another factor which might favor the consolidation arm. Lastly, in this data set we did not have the important data regarding FLT-3 ITD and NPM1 mutational status. Given this limitation, application of these data to those with known molecular signatures implying increased relapse risk, such as FLT-3 ITD, would not be recommended. In the absence of those molecular signatures; however, the results clearly show no difference in outcome based on pre-HCT consolidation exposure and support a recommendation to proceed promptly to transplant as soon as CR1 is attained. If HCT is delayed by the time required to identify a suitable donor, our data suggest that consolidation does not increase HCT TRM and its use is acceptable in that setting.
Acknowledgments
We would also like to acknowledge contributions to protocol development from the following individuals: Alison W. Loren, Ann E. Woolfrey, Ayman Saad, Camille Abboud, Dipnarine Maharaj, Edward A. Copelan, Franklin O. Smith, Jeffrey Szer, Jacob M. Rowe, James M. Foran, James L. Gajewski, Joseph H. Antin, Joseph Pidala, H. Kent Holland, Martin S. Tallman, Maxim Norkin, Michael R. Bishop, Mitchell S. Cairo, Muthalagu Ramanathan, Rodrigo Martino, Peter H. Wiernik, Robert K. Stuart, Stella Santarone, and William R. Drobyski.
Funding
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Financial Disclosure Statement: J Koreth has compensated consultant/advisory relationships with Spectrum Pharmaceuticals and Eleven Biotherapeutics; honoraria from Optum Health; and research funding from Millennium Pharmaceuticals, Otsuka Pharmaceuticals, and Prometheus Cabs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and leukemia group B. N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 2.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A southwest oncology group/eastern cooperative oncology group study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 3.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the united kingdom medical research council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 4.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallman MS, Rowlings PA, Milone G, et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood. 2000;96(4):1254–1258. [PubMed] [Google Scholar]
- 6.Cahn J, Labopin M, Sierra J, et al. No impact of high-dose cytarabine on the outcome of patients transplanted for acute myeloblastic leukaemia in first remission. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110(2):308–14. doi: 10.1046/j.1365-2141.2000.02178.x. [DOI] [PubMed] [Google Scholar]
- 7.Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 8.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12(10):1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108(3):836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 10.Martino R, Valcarcel D, Brunet S, Sureda A, Sierra J. Comparable non-relapse mortality and survival after HLA-identical sibling blood stem cell transplantation with reduced or conventional-intensity preparative regimens for high-risk myelodysplasia or acute myeloid leukemia in first remission. Bone Marrow Transplant. 2008;41(1):33–38. doi: 10.1038/sj.bmt.1705879. [DOI] [PubMed] [Google Scholar]
- 11.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: A prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Erkers T, Aschan J, et al. A prospective randomized toxicity study to compare reduced-intensity and myeloablative conditioning in patients with myeloid leukemia undergoing allogeneic haematopoietic stem cell transplantation. J Intern Med. 2013 doi: 10.1111/joim.12056. [DOI] [PubMed] [Google Scholar]
- 14.McCormack SE, Cao Q, Oran B, Weisdorf DJ, Warlick ED. Pre-transplant consolidation chemotherapy may not improve outcomes after reduced intensity conditioning hematopoietic stem cell transplantation for acute myeloid leukemia in first complete remission. Leuk Res. 2011;35(6):757–761. doi: 10.1016/j.leukres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum. report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: Working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champlin R, Khouri I, Shimoni A, et al. Harnessing graft-versus-malignancy: Non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111(1):18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 20.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH consensus criteria. Blood. 2011;118(15):4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Farag S, Ruppart A, Mrozek K, et al. Outcome of induction and postremission therapy in younger adults with acute myeloid leukemia with normal karyotype: a cancer and leukemia group B study. J Clin Oncol. 2005;23(3):482–93. doi: 10.1200/JCO.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 23.Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood. 2013;121(1):26–28. doi: 10.1182/blood-2012-07-444851. [DOI] [PubMed] [Google Scholar]
- 24.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2012;48(5):630–641. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 25.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]