Abstract
It has been shown recently that PrP (prion protein) and the calcium channel auxiliary α2δ subunits interact in neurons and expression systems [Senatore, Colleoni, Verderio, Restelli, Morini, Condliffe, Bertani, Mantovani, Canovi, Micotti, Forloni, Dolphin, Matteoli, Gobbi and Chiesa (2012) Neuron 74, 300–313]. In the present study we examined whether there was an effect of PrP on calcium currents. We have shown that when PrP is co-expressed with calcium channels formed from CaV2.1/β and α2δ-1 or α2δ-2, there is a consistent decrease in calcium current density. This reduction was absent when a PrP construct was used lacking its GPI (glycosylphosphatidylinositol) anchor. We have reported previously that α2δ subunits are able to form GPI-anchored proteins [Davies, Kadurin, Alvarez-Laviada, Douglas, Nieto-Rostro, Bauer, Pratt and Dolphin (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1654–1659] and show further evidence in the present paper. We have characterized recently a C-terminally truncated α2δ-1 construct, α2δ-1ΔC, and found that, despite loss of its membrane anchor, it still shows a partial ability to increase calcium currents [Kadurin, Alvarez-Laviada, Ng, Walker-Gray, D’Arco, Fadel, Pratt and Dolphin (2012) J. Biol. Chem. 1287, 33554–33566]. We now find that PrP does not inhibit CaV2.1/β currents formed with α2δ-1ΔC, rather than α2δ-1. It is possible that PrP and α2δ-1 compete for GPI-anchor intermediates or trafficking pathways, or that interaction between PrP and α2δ-1 requires association in cholesterol-rich membrane microdomains. Our additional finding that CaV2.1/β1b/α2δ-1 currents were inhibited by GPI–GFP, but not cytosolic GFP, indicates that competition for limited GPI-anchor intermediates or trafficking pathways may be involved in PrP suppression of α2δ subunit function.
Keywords: α2δ, auxiliary subunit, calcium channel, GPI anchor, prion protein
Abbreviations: CaV, voltage-gated Ca2+; DRM, detergent-resistant membrane; GPI, glycosylphosphatidylinositol; HA, haemagglutinin; KO, knockout; MBS, Mes-buffered saline; PI-PLC, phosphatidylinositol-specific phospholipase C; PNGase F, peptide N-glycosidase F; PrP, prion protein; PrPC, normal cellular PrP; PrPSc, PrP scrapie; WT, wild-type
Short abstract
PrP, but not GPI-anchorless PrP, suppresses CaV2.1/β/α2δ-1 or α2δ-2 calcium channel currents. However, PrP does not inhibit CaV2.1/β currents formed with anchorless α2δ-1, rather than full-length α2δ-1. The results of the present study suggest that α2δ subunits and PrP may compete for GPI-anchor pathways.
INTRODUCTION
CaV (voltage-gated Ca2+) channels contain a pore-forming α1 subunit, which determines the main properties, both biophysical and pharmacological, of the channels. For the CaV1 and CaV2 subfamilies, the α1 subunit is associated with a membrane-anchored predominantly extracellular α2δ subunit (for a review, see [1]) and a cytoplasmic β subunit (for reviews, see [2,3]). The β subunits have been shown to enhance calcium channel trafficking by binding to the I–II linker of the α1 subunits [4,5], and thus inhibiting proteasomal decay as has been shown for the CaV2.2 [6] and CaV1.2 [7] channels. In contrast, the mechanism of enhancement of the CaV channel functional expression by α2δ subunits has not been firmly established, although we have found that it involves their von Willebrand Factor A domain [8], and results in increased plasma membrane expression and active zone targeting of the channel complex [8,9].
The major mechanism whereby α2δ subunits increase the functional expression of calcium channels is due to an increase in the amount of channel protein at the plasma membrane ([8,10] and J.S. Cassidy and A.C. Dolphin, unpublished work). Furthermore, it has been found that the α2δ subunits do not increase single channel conductance or open probability [11,12], two other mechanisms whereby macroscopic current could be increased. Nevertheless, there are effects of α2δ subunits to increase voltage-dependent inactivation and to hyperpolarize the voltage-dependence of steady-state inactivation, providing evidence that these subunits do remain associated with the functional channel complex on the plasma membrane [8,13,14].
Mammalian genes encoding four α2δ subunits have been identified (for reviews, see [3,15]). The topology of the α2δ protein was first determined for α2δ-1, and is thought to generalize to all four α2δ subunits (for reviews, see [1,16]). They all have predicted N-terminal signal sequences, indicating that the N-terminus is extracellular. The α2 and δ subunits are the product of a single gene, encoding the α2δ pre-protein, which is post-translationally cleaved into α2 and δ [17]. The extracellular α2 subunit then remains disulfide-bonded to the membrane-bound δ subunit. Although α2δ subunits were originally described as transmembrane proteins, we have shown previously that they can form GPI (glycosylphosphatidylinositol)-anchored proteins [13].
The PrPC [normal cellular PrP (prion protein)] is a GPI-anchored sialoglycoprotein [18] exposed on the outer leaflet of the plasma membrane, that is widely distributed throughout the nervous system [19,20]. Its normal physiological function is still being elucidated [20] and genetic ablation of PrP KO (knockout) only produces subtle phenotypes in mice [21,22]. However, it appears to play a role in signal transduction [23], copper binding [24], synaptic function [25] and myelin maintenance [26]. Furthermore, misfolding of PrPC results in the formation of the partially protease-resistant isoform PrPSc (PrP scrapie), which accumulates in the brain and results in neurodegeneration, giving rise to Creutzfeldt–Jakob disease [19,27]. PrPSc is both neurotoxic and infectious. In the absence of PrPC, PrPSc cannot be generated and PrP-null mice do not propagate infectivity or develop pathology on infection with PrPSc [28–30]. Thus it has been postulated that the presence of PrPC in neurons is essential for the initiation of a cascade of signalling events leading to prion disease pathology.
Initial evidence that the α2δ subunits are closely associated with PrP came from the finding that the α2δ-1, α2δ-2 and α2δ-3 subunits were co-immunopurified with PrP from the brains of transgenic mice expressing Myc-tagged PrP [31]. The α2δ subunits are expressed strongly in cholesterol-rich detergent-resistant membranes [13,32], as is PrP [33,34], which may represent the basis for their association. It has been shown recently that α2δ-1 and PrP can co-immunoprecipitate from both brain tissue and cell lines [35], and that the interaction between pathogenic mutant PrP and α2δ-1 results in the intracellular retention of both species [35].
We were interested to determine whether PrP co-expression has any direct effect on calcium channel currents via a direct or indirect effect on α2δ subunit processing or function. We therefore utilized full-length and C-terminally truncated PrP and α2δ-1 to examine the effect of these species on calcium channel functional expression.
MATERIALS AND METHODS
Heterologous expression of cDNAs
The calcium channel cDNAs used were rat CaV2.1 (GenBank® accession number M64373), mouse α2δ-2 (GenBank® accession number AF247139), rat α2δ-1 (GenBank® accession number M86621), α2δ-1 mid HA (haemagglutinin) [36], α2δ-1ΔC–HA [36], rat β4 (GenBank® accession number NM001105733) and rat β1b [37]. The PrP constructs used (in pcDNA3) were WT (wild-type) PrP (mouse) and ΔGPI–PrP [38]. The GFP constructs used were mut3 GFP [39] and GPI–GFP in pcDNA3 [40]. The cDNAs were in the pMT2 expression vector, unless stated above. Mammalian tsA-201 cells were transfected with the following cDNA combinations (Cav2.1/β1b/α2δ-1/PrP at 3:2:2:0.5 or Cav2.1/β4/α2δ-2/PrP at 3:2:2:1), with empty vector replacing constructs not used unless otherwise stated, and transfection was performed as described previously [41]. The cDNA for GFP was also included to identify transfected cells from which electrophysiological recordings and imaging were performed, unless GPI–GFP was used.
Mice
Cerebella were obtained from C57BL/6J (WT) mice or from Tg(WT-E1) mice expressing WT mouse PrP with an epitope for the monoclonal antibody 3F4 at approximately 4-fold the endogenous PrP level, referred to throughout the text as PrP Tg(WT) [42], or from PrP-KO mice with a pure C57BL/6J background (European Mouse Mutant Archive, Monterotondo, Rome; EM:01723) [21].
Preparation of Triton X-100-insoluble membrane fractions [DRMs (detergent-resistant membrane)]
All steps were performed on ice. One cerebellum was used as the starting material from WT, PrP Tg(WT) and PrP-KO mice. The cerebella were homogenized using a Teflon homogenizer in MBS [Mes-buffered saline; 25 mM Mes (pH 6.5), 150 mM NaCl and Complete™ protease inhibitor cocktail (Roche)], containing 1% (v/v) Triton X-100 (Thermo Scientific), and left on ice for 1 h. An equal volume of 90% (w/v) sucrose in MBS was then added. The sample was transferred into a 13 ml ultracentrifuge tube and overlaid with 10 ml of discontinuous sucrose gradient, consisting of 35% (w/v) sucrose in MBS (5 ml) and 5% (w/v) sucrose in MBS (5 ml). The sucrose gradients were centrifuged at 138000 g for 18 h at 4°C (Beckman SW40 rotor). Fractions (1 ml) were subsequently harvested from the top to the bottom of the tube. When necessary, protein fractions from the gradient were washed free of sucrose by dilution in 25 volumes of ice-cold PBS and ultracentrifugation (150000 g for 1 h at 4°C) to pellet the cholesterol-enriched microdomain material. Triton X-100-insoluble protein was resuspended in deglycosylation buffer and treated with PNGase F (peptide N-glycosidase F; Roche), as described below.
Treatment of Triton X-100-insoluble protein fractions with PI-PLC (phosphatidylinositol-specific phospholipase C)
Triton X-100-insoluble fractions from brain tissue were collected, washed free of sucrose and centrifuged as described above. The resultant pellet of Triton X-100-insoluble material was resuspended in an appropriate volume of PI-PLC reaction buffer [10 mM Tris/HCl (pH 7.4) and 150 mM NaCl containing Complete™ protease inhibitor cocktail (Roche)], to a final protein concentration of ~2 mg/ml. The samples were sonicated and treated with 25 units of PI-PLC enzyme (Sigma) for 3 h at 37°C.
Phase separation of PI-PLC-treated proteins using Triton X-114
Membrane-associated proteins were separated from soluble proteins in two phases of Triton X-114 as described previously [43]. Briefly, the pellet of detergent-insoluble material was resuspended in an appropriate volume of reaction buffer (final concentration of ~2 mg/ml of protein) and incubated with PI-PLC as described above. Control experiments omitting the enzyme were also performed. After PI-PLC incubation the samples were supplemented with Triton X-114 (Thermo Scientific) to a final concentration of 1%. A cushion of 6% (w/v) sucrose, 10 mM Tris/HCl (pH 7.4), 150 mM NaCl and 0.06% Triton X-114 was placed at the bottom of a 1.5 ml Eppendorf tube. The protein sample was then overlaid on this sucrose cushion and the tube incubated for 3 min at 30°C and centrifuged at 300 g for 4 min at room temperature (20°C) in a swinging bucket rotor. Following centrifugation, the detergent phase was present as an oily droplet at the bottom of the tube. Fresh Triton X-114 was then added to the upper aqueous phase to 0.5% and the procedure was repeated using the same sucrose cushion. In the last step the aqueous phase was removed from the cushion, supplemented with fresh Triton X-114 to 2% and subjected to another centrifugation. The detergent phase of this last procedure was discarded. The aqueous and detergent phases from this procedure were adjusted to the equal volume with 10 mM Tris/HCl (pH 7.4) and 150 mM NaCl plus protease inhibitors.
Acetone precipitation and PNGase F deglycosylation
To remove the remaining Triton X-114 in the detergent and aqueous phases from the phase separation experiment the proteins were precipitated by the addition of 4 volumes of ice-cold acetone and subsequent incubation for 1 h at −20°C. The precipitated material was centrifuged at 16000 g for 10 min and the pellet was washed once with an acetone/water (4:1) mixture (−20°C). The pellets of the precipitated proteins were then resuspended in 45 μl of PNGase F buffer [10 mM Tris/HCl (pH 7.5) and 150 mM NaCl supplemented with 75 mM 2-mercaptoethanol, 0.5% Triton X-100, 0.1% SDS and protease inhibitors]. A total of 1 unit of PNGase F was added per 10 μl volume followed by incubation at 37°C for 5–12 h. The samples were then resuspended in an appropriate volume of SDS gel loading buffer and heated for 10 min at 56°C in order to terminate the reaction.
Immunoblotting
Western blotting was performed as described previously [41]. The samples were resolved on either 3–8% Tris/acetate or 4–12% Bis-Tris gels with the relevant buffer systems (Life Technologies). The samples were then blotted on to PVDF membranes (Bio-Rad Laboratories), blocked with 3% BSA in TBS provided with 0.5% Igepal and incubated with the following primary antibodies: anti-α2-1 (1:1000 dilution; mouse monoclonal; Sigma), anti-α2-2 (residues 102–117; rabbit polyclonal; [44]), anti-PrP (3F4 epitope), anti-PrP (residues 45–66; rabbit polyclonal; [45]) and anti-flotillin-1 (1:2000 dilution; mouse monoclonal; BD Biosciences). The secondary antibodies used were goat anti-mouse and goat anti-rabbit coupled to HRP (horseradish peroxidase; Bio-Rad Laboratories).
Electrophysiology
Calcium channel expression in tsA-201 cells was investigated by whole-cell patch-clamp recording essentially as described previously [46]. The internal (pipette) and external solutions and recording techniques were similar to those described previously [47]. The patch pipette solution contained: 140 mM caesium aspartate, 5 mM EGTA, 2 mM MgCl2, 0.1 mM CaCl2, 2 mM K2-ATP and 10 mM Hepes, pH 7.2 at 310 mM mOsm, with sucrose. The external solution for recording Ba2+ currents contained: 150 mM tetraethylammonium Br, 3 mM KCl, 1 mM NaHCO3, 1 mM MgCl2, 10 mM Hepes, 4 mM glucose and 1 mM BaCl2, pH 7.4 at 320 mOsm, with sucrose. Pipettes of resistance 2–4 MΩ were used. An Axopatch 1D amplifier (Axon Instruments) was used, and data were filtered at 1–2 kHz and digitized at 5–10 kHz. Current records were subjected to leak and residual capacitance current subtraction (P/8 protocol). Analysis was performed using Pclamp 9 (Molecular Devices) and Origin 7 (Microcal Origin). All comparisons between different groups of transfected cells were performed in parallel on the same experimental days.
When stated, current–voltage (I–V) plots were fit with a modified Boltzmann equation for determination of the voltage for 50% activation (V50, activation) [48], and steady-state inactivation curves were fit with a single Boltzmann function for determination of the voltage for 50% inactivation (V50, inactivation). Where data are given as means±S.E.M., statistical comparisons were performed using either Student's t test or ANOVA with the appropriate post-hoc test.
Xenopus oocytes were prepared, injected and utilized for electrophysiology as described previously [49], with the following exceptions. Plasmid cDNAs for the calcium channel α1, α2δ-1 and β1b subunits and for PrP were mixed at 2:1:2:x ratios at 1 μg/μl, where x=2, 1 or 0.5 unless otherwise stated, and the empty vector was included to maintain the total cDNA mixture constant. A 9 nl volume was injected intranuclearly, after 2-fold dilution of the cDNA mixtures. Recordings in Xenopus oocytes were performed at 18°C as described previously [48], and all recordings were performed 48–60 h after injection for CaV2.2. The Ba2+ concentration was 10 mM.
RESULTS
α2δ subunits and PrP show similar biochemical properties
We have shown previously that endogenous α2δ-1 and α2δ-2 are strongly expressed in DRM fractions [13,32], as is PrP [18]. Therefore we prepared DRM fractions from the cerebella of WT, PrP Tg(WT) and PrP-KO mice. We found the typical localization of α2δ-1, α2δ-2 and also PrP in DRMs (Figure 1A).
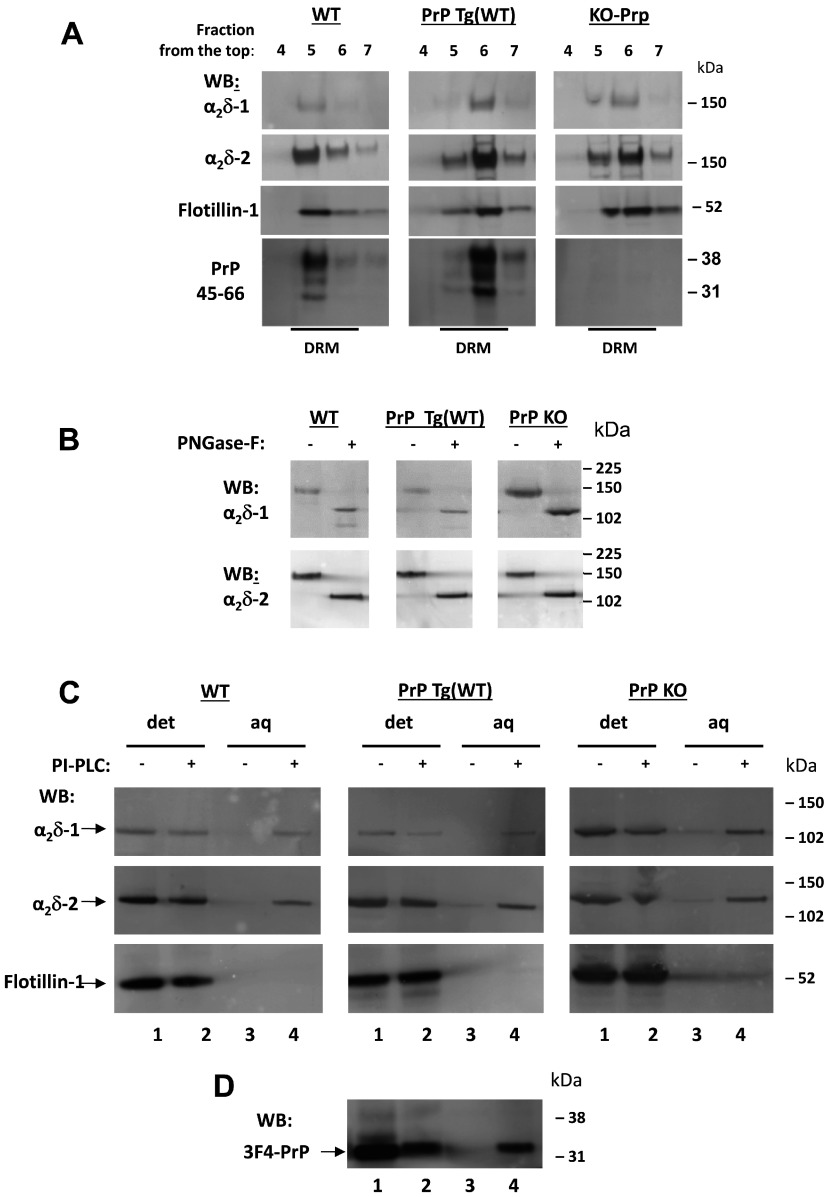
Figure 1. PI-PLC treatment and phase separation of α2δ-1, α2δ-2 and PrP in mouse cerebella.
(A) DRM fractions from WT (left-hand panel), PrP Tg(WT) (middle panel) and PrP KO (right-hand panel) mouse cerebella were prepared as described in the Materials and methods section. Aliquots were resolved on 3–8% Tris/acetate (to resolve α2δs) or 4–12% Bis-Tris gels (to resolve PrP in the same samples), and analysed by Western blotting (WB) with relevant antibodies as indicated. The full profile is not shown, but only the fractions of the sucrose gradient corresponding to DRMs identified by the presence of flotillin-1 (fractions 4–7 harvested from the top). The anti-α2δ-1 and anti-α2δ-2 antibodies recognize the α2-1 and α2-2 moieties. (B) Aliquots of concentrated DRM fractions from WT (left-hand panel), PrP Tg(WT) (middle panel) and PrP KO (right-hand panel) cerebella were treated with PNGase F and analysed by Western blotting with the indicated antibodies. (C) DRM fractions analysed in (A) were subjected to PI-PLC treatment and Triton X-114 phase separation (see the Materials and methods section), followed by PNGase F deglycosylation. The proteins remaining in the aqueous (aq) and detergent (det) phase were then resolved on 4–12% Bis-Tris gels and analysed with the indicated antibodies. Lanes 1 and 2 in each panel are from detergent phase fractions, whereas lanes 3 and 4 are from the respective aqueous phase, treated or not with PI-PLC as indicated. (D) The PrP Tg(WT) fractions from (C, middle panel) were also blotted for PrP using the 3F4 antibody. Molecular mass is shown on the right-hand side of the gels in kDa.
It is well-established that PrP is GPI-anchored [18], and our previous findings indicate that the α2δ subunits can form GPI-anchored proteins [13]. One important piece of evidence supporting this premise is that following PI-PLC treatment, both endogenous α2δ subunits from the rat brain and heterologously expressed α2δ proteins redistribute from the detergent phase into the aqueous phase upon Triton X-114 phase separation [13]. This demonstrates that PI-PLC treatment has converted the protein into a hydrophilic species, most probably by removing the bulky hydrophobic GPI anchor. To assess if the behaviour of α2δ proteins in this phase-separation assay was affected by the presence or absence of PrP, we concentrated the DRM fractions enriched in both PrP and α2δ and subjected them to PI-PLC treatment as described previously [13]. An aliquot of the initial DRM material from the three mouse genotypes, before PI-PLC treatment, was deglycosylated with PNGase F to demonstrate the presence of α2δ-1 and α2δ-2 (Figure 1B).
PI-PLC treatment caused α2δ-1 and α2δ-2 to redistribute into the aqueous phase upon Triton X-114 phase separation in all three of the mouse genotypes tested (Figure 1C). As a control, the PrP Tg(WT) samples were also blotted for PrP, and redistributed similarly upon PI-PLC treatment (Figure 1D).
Importantly, the response of α2δ-1 and α2δ-2 to PI-PLC treatment was not affected by the absence of PrP, in the PrP KO cerebellar material (Figure 1C). It was also not affected by the overexpression of PrP in the PrP Tg(WT) cerebellar material (Figure 1C). These data indicate that although PrP has been reported to interact with α2δ subunits [35], interaction with PrP was not responsible for the presence of α2δ subunits in DRM fractions or their redistribution into the aqueous phase following PI-PLC cleavage of GPI-anchor sites.
Co-expression of PrP decreased calcium channel currents containing CaV2.1/β4/α2δ-2
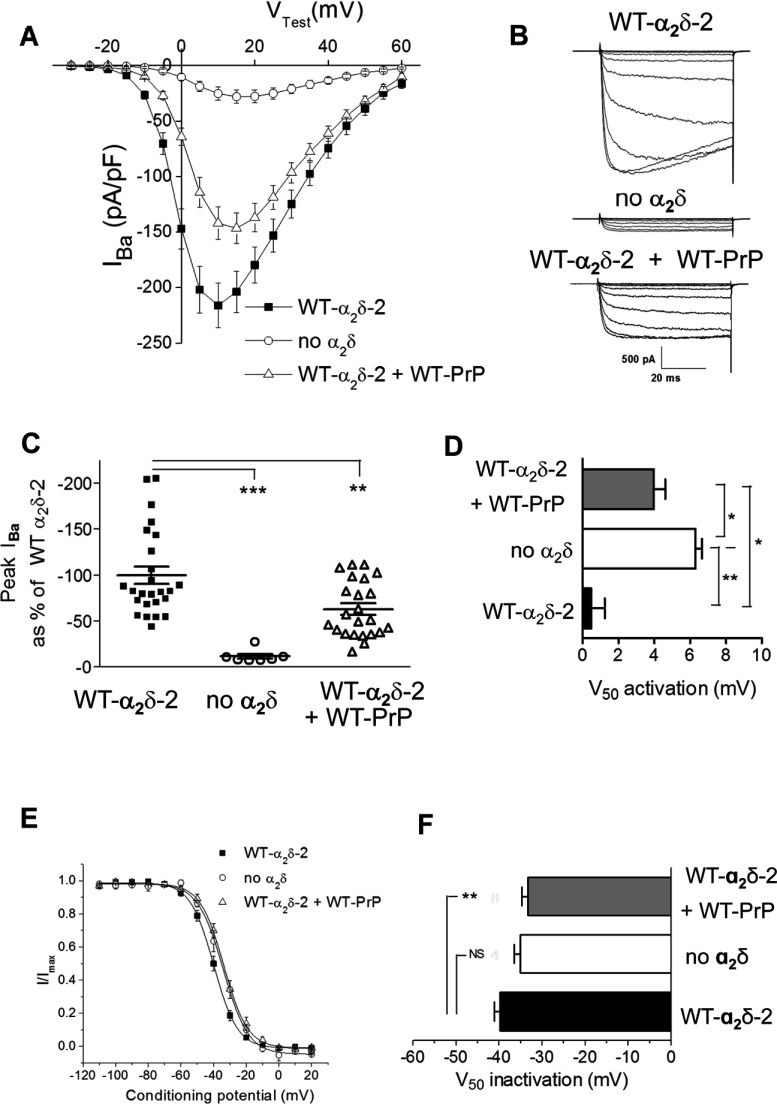
We then examined whether PrP would influence the ability of α2δ to increase calcium channel currents. We expressed CaV2.1/β4 and α2δ-2 to mimic the calcium channel combination present in cerebellar Purkinje cells, either with or without PrP. We found that PrP co-expression produced a moderate, but consistent, reduction in peak calcium channel currents at +10 mV of approximately 34%, although the currents in the presence of PrP and α2δ remained significantly larger than those in the absence of α2δ (Figures 2A–2C). The voltage-dependence of activation of the CaV2.1/β4/α2δ-2 currents in the presence of PrP was also significantly depolarized, compared with in its absence, although not to the same extent as in the absence of α2δ (Figure 2D). Similarly, the voltage-dependence of steady-state inactivation of CaV2.2/β4/α2δ-2 was depolarized significantly in the additional presence of PrP (Figures 2E and 2F). All these effects are indicative of a reduced enhancement by α2δ-2 of CaV2.1/β4 calcium channel currents when PrP was co-expressed.
Figure 2. Effect of PrP on CaV2.1/β4/α2δ-2 calcium channel currents.
(A) Current–voltage (I–V) relationships for IBa recorded from tsA-201 cells expressing CaV2.1/β4/α2δ-2 (■; n=25), CaV2.1/β4 alone (○; n=7) and CaV2.1/β4/α2δ-2/WT PrP (△; n=23). The ratio of cDNAs used for transfection for CaV2.1/β4/α2δ-2/WT-PrP was 3:2:2:1, with empty vector used where α2δ or PrP was absent. (B) Examples of families of IBa current traces resulting from step potentials from −100 mV to between −30 and +15 mV in 5 mV increments for CaV2.1/β4/α2δ-2 (top panel), CaV2.1/β4 alone (middle panel) and CaV2.1/β4/α2δ-2/WT PrP (bottom panel). (C) Individual peak IBa currents at +10 mV, expressed as the mean±S.E.M. percentage of the control condition with WT α2δ-2 and CaV2.1/β4/α2δ-2 (■; n=25), CaV2.1/β4 alone (○; n=7) and CaV2.1/β4/α2δ-2/WT PrP (△; n=23). **P<0.01 and ***P<0.001. (D) Voltage-dependence of activation (V50 activation) determined by fitting a modified Boltzmann function to the individual I–V relationships shown in (A) for CaV2.1/β4/α2δ-2 (black bar), CaV2.1/β4 alone (white bar) and CaV2.1/β4/α2δ-2/WT PrP (grey bar). *P<0.05 and **P<0.01. (E) Steady-state inactivation curves for IBa recorded from cells expressing CaV2.1/β4/α2δ-2 (■; n=12), CaV2.1/β4 alone (○; n=4) and CaV2.1/β4/α2δ-2/WT PrP (△; n=8). (F) Voltage-dependence of steady-state inactivation (V50 inactivation) determined by fitting a Boltzmann function to the individual steady-state inactivation relationships for the data shown in (E); CaV2.1/β4/α2δ-2 (black bar), CaV2.1/β4 alone (white bar) and CaV2.1/β4/α2δ-2/WT PrP (grey bar). **P<0.01; NS, not significant. All statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test.
Co-expression of PrP decreased calcium channel currents containing CaV2.1/β1b/α2δ-1
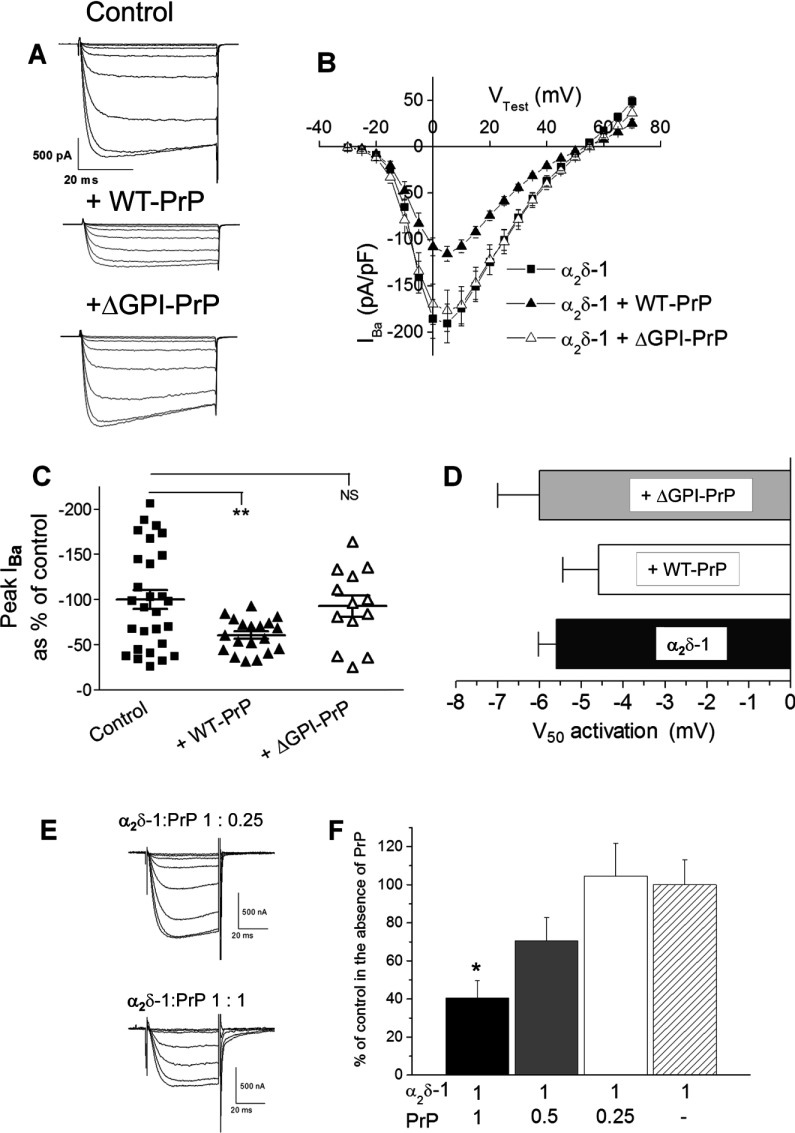
We then examined whether PrP would also inhibit calcium channel combinations containing α2δ-1, and tested whether any effect could be due to the demonstrated interaction at the level of the two polypeptides [35] or a result of lipid anchoring and co-localization in the same cholesterol-rich microdomains. We therefore expressed CaV2.1/β1b/α2δ-1 with either WT PrP or anchorless PrP, truncated at the GPI-anchor site (ΔGPI–PrP) [38]. We observed that co-expression with WT PrP reduced the peak calcium channel currents to a similar extent (39%) to that shown for the CaV2.1/β4/α2δ-2 combination (Figures 3A–3C), whereas ΔGPI–PrP had no effect on the CaV2.1/β1b/α2δ-1 calcium channel currents (Figures 3A–3C). In these experiments WT PrP did not produce a significant depolarization of the voltage-dependence of activation of the currents (Figure 3D).
Figure 3. Effect of PrP constructs on CaV2.1/β1b/α2δ-1 calcium channel currents.
(A) Examples of families of IBa current traces resulting from step potentials from −90 mV to between −30 and +10 mV in 5 mV steps for CaV2.1/β1b/α2δ-1 alone (top panel) with WT PrP (middle panel) or with ΔGPI–PrP (bottom panel). The ratio of cDNAs used for the transfection of CaV2.1/β1b/α2δ-1/PrP was 3:2:2:1, with empty vector used where α2δ or PrP was absent. (B) I–V relationships for IBa recorded from tsA-201 cells expressing CaV2.1/β1b/α2δ-1 alone (■; n=28) with WT PrP (▲; n=19) or with ΔGPI–PrP (△; n=13). (C) Individual mean±S.E.M. peak IBa currents at +5 mV for CaV2.1/β1b/α2δ-1 alone (■; n=28) with WT PrP (▲; n=19) or ΔGPI–PrP (△; n=13). **P<0.01. (D) V50 activation for CaV2.1/β1b/α2δ-1 alone (black bar) with WT PrP (white bar) or with ΔGPI–PrP (grey bar). All statistical differences were determined by one-way ANOVA and Dunnett's multiple comparison test. (E and F) CaV2.2/β1b/α2δ-1 was expressed in Xenopus oocytes either alone or together with PrP at the ratios shown. (E) Representative current traces elicited by steps to test potentials between −25 and +10 mV in 5 mV steps from a holding potential of −100 mV for CaV2.2/β1b/α2δ-1/PrP (1:0.25; upper panel) or CaV2.2/β1b/α2δ-1/PrP (1:1; lower panel). The residual voltage clamp transients have been truncated. (F) Peak currents measured at +10 mV for three α2δ-1/PrP ratios plotted as the mean±S.E.M. percentage of the mean control IBa recorded in the absence of PrP in the same experiment. α2δ-1/PrP 1:1 (black bar; n=6), α2δ-1/PrP ratio 1:0.5 (grey bar; n=18), α2δ-1/PrP ratio 1:0.25 (white bar; n=20) and mean normalized control for α2δ-1 in the absence of PrP (hatched bar; n=21). *P=0.016 between the individual conditions and their respective control condition performed in the same experiment as determined by Student's t test.
In another expression system, Xenopus oocytes with microinjected cDNAs and where the ratio of the different constructs can be controlled accurately, the effect of PrP to reduce CaV2.2/β1b/α2δ-1 calcium channel currents was observed at an α2δ-1/PrP ratio of 1:1, but not at 2:1 or 4:1 (Figures 3E and 3F), indicating that it was dependent on the amount of PrP expressed relative to α2δ. Taken together, these results suggest that the effect of PrP to reduce calcium channel currents is concentration-dependent, and the presence of a GPI anchor on PrP may be essential for the observed reduction in the functional expression of calcium currents.
Membrane anchoring of both PrP and α2δ-1 is required for calcium channel current inhibition by PrP
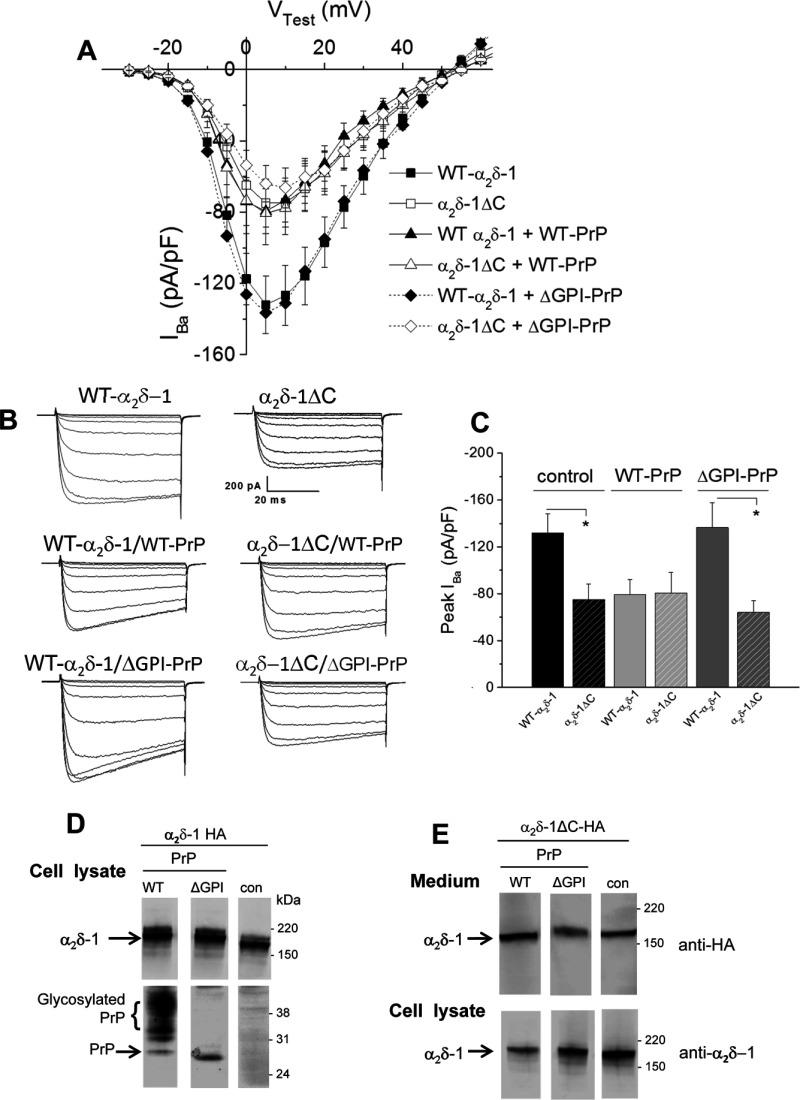
In order to test the importance of membrane anchoring of both α2δ-1 and PrP on the inhibition by PrP we expressed CaV2.1/β1b with either WT α2δ-1 or anchorless α2δ-1 (α2δ-1ΔC–HA), together with either WT PrP or anchorless PrP truncated at the GPI-anchor site (ΔGPI–PrP). We have shown previously that an anchorless form of α2δ-1, although being mainly secreted, is still able to promote an increase in calcium channel currents compared with no α2δ, although to a smaller extent than WT α2δ-1 [36]. In the present study we obtained the surprising result, shown in Figures 4(A)–4(C), that, whereas WT PrP inhibited calcium channel currents in the presence of WT α2δ-1, it did not produce any reduction in the presence of anchorless α2δ-1ΔC–HA (Figure 4C). In agreement with the data shown in Figure 3, ΔGPI–PrP did not produce any inhibition of calcium channel currents formed with WT α2δ-1 (Figure 4C). Similarly ΔGPI–PrP did not produce any reduction in the currents formed with anchorless α2δ-1ΔC–HA (Figure 4C). We found from Western blotting that the WT and truncated α2δ and PrP species used are all well-expressed in tsA-201 cells (Figures 4D and 4E), and lack of expression therefore could not account for the lack of effect of the truncated constructs when expressed together.
Figure 4. Comparison of the effect of PrP constructs on calcium channel currents containing α2δ-1 or anchorless α2δ-1.
(A) I--V relationships for IBa recorded from tsA-201 cells expressing CaV2.1/β1b/α2δ-1 alone (■; n=16) with WT PrP (▲; n=10) or ΔGPI–PrP (◆; n=14) or cells expressing CaV2.1/β1b/α2δ-1ΔC alone (□; n=11) with WT PrP (∆; n=13) or ΔGPI–PrP (◇; n=15). The ratio of cDNAs was the same as in Figure 3(A). (B) Examples of families of IBa current traces resulting from step potentials from −90 mV to between −30 and +10 mV in 5 mV steps for CaV2.1/β1b/α2δ-1 alone (top left-hand panel) or α2δ-1ΔC alone (top right-hand panel) with WT PrP (middle panel) or ΔGPI–PrP (bottom panels). (C) Peak IBa currents (means±S.E.M.) for the data shown in (A) for CaV2.1/β1b with α2δ-1 (solid bars) or with α2δ-1ΔC (hatched bars) either alone (black bars) or with WT PrP (light grey bars), or ΔGPI-PrP (dark grey bars). *P<0.05 as determined by one-way ANOVA and Bonferroni's post-hoc test. (D) Western blot of α2δ-1 (upper panels; 4–12% Bis-Tris gel) co-expressed (1:1) with WT PrP (left-hand lane), ∆GPI–PrP (middle lane) or empty vector (right-hand lane; con). PrP expression is shown in the lower panel (4–12% Bis-Tris gel). The lower expression of ∆GPI–PrP in the cell lysate is because much of it is secreted [53]. (E) Western blot of α2δ-1∆C in medium (upper panels; 3–8% Tris/acetate gel) and cell lysate (lower panels; 4–12% Bis-Tris gel) co-expressed (1:1) with PrP (left-hand panels), ∆GPI–PrP (middle panel) or empty vector (right-hand panels; con). In both (D) and (E), all three lanes are from the same blots from which irrelevant lanes have been excised and the molecular mass is given on the right-hand side in kDa.
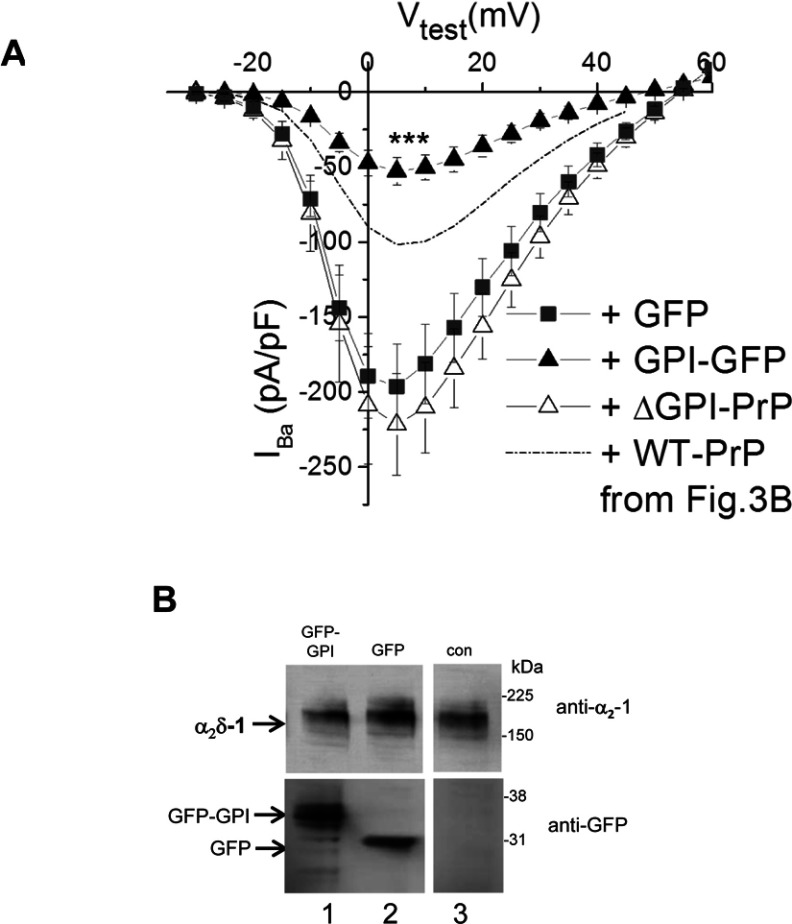
This result suggests either that association of the α2δ and PrP proteins in the plasma membrane, and potentially in the same cholesterol-rich membrane microdomains, facilitated by their GPI anchoring, is essential for the inhibition of calcium currents observed or that there is competition between α2δ-1 and PrP for the GPI-anchor intermediates, resulting in less maturation of α2δ subunit in the presence of PrP. We therefore examined whether the effect of PrP might be related to the GPI anchor and tested the effect of an unrelated protein engineered to contain a GPI anchor (GFP–GPI). We found that co-expression of GFP–GPI markedly reduced calcium channel currents formed by CaV2.1/β1b/α2δ-1 compared with the co-expression of cytosolic GFP, and in this experiment ∆GPI–PrP again had no effect (Figure 5A). This result is in agreement with the hypothesis that overexpression of a GPI-anchored protein can produce this inhibition. We then compared the level of α2δ-1 expression in the presence of co-expressed GFP–GPI and cytosolic GFP (Figure 5B). In all cases substantial α2δ-1 was co-expressed.
Figure 5. Effect of GPI–GFP on CaV2.1/β1b/α2δ-1 calcium channel currents.
(A) I–V relationships for IBa recorded from tsA-201 cells expressing CaV2.1/β1b/α2δ-1 with GFP (■; n=9), GPI–GFP (▲; n=9), or GFP and ΔGPI–PrP (△; n=6). The broken line indicates the level of IBa observed for CaV2.1/β1b/α2δ-1 plus WT PrP from Figure 3(B). ***P<0.001 between the peak IBa when GPI–GFP was co-expressed compared with when GFP was co-expressed (Student's t test). (B) Western blot of α2δ-1 (upper panels) co-expressed (1:1) with GFP–GPI (lane 1), GFP (lane 2) or empty vector (lane 3; con). GFP expression is shown in the lower panel. All three lanes are from the same blots from which irrelevant lanes have been excised and the molecular mass is given on the right-hand side in kDa.
DISCUSSION
In the present study we found that when PrP is co-expressed with CaV2.1 calcium channels together with a β-subunit and either α2δ-1 or α2δ-2, it produces a modest reduction in the expressed calcium channel currents. This reduction in calcium channel currents involves the membrane anchoring of PrP, as it is absent when PrP is truncated prior to its GPI-anchor site. Thus ΔGPI–PrP did not inhibit currents formed by the expression of CaV2.1/β1b/α2δ-1. This result suggests that the process might involve competition for pathways associated with the GPI anchoring of PrP, which might relate to our finding that α2δ subunits form GPI-anchored proteins [13]. The results of the present study reinforce that view, since native cerebellar α2δ-1, α2δ-2 and PrP all behave similarly following PI-PLC treatment and phase separation, being concentrated in the aqueous phase (Figure 1), which is supportive of evidence for GPI anchoring.
We therefore utilized an α2δ-1 subunit truncated at the predicted membrane attachment site (α2δ-1ΔC), which, as we have shown recently, is not an integral membrane protein [36]. We have characterized α2δ-1ΔC and found that, despite being mainly secreted, it still shows a partial ability to enhance calcium channel currents and to be associated extrinsically with the plasma membrane [36]. It is therefore possible that α2δ-1 enhances calcium currents by two mechanisms, one involving a trafficking process that is still maintained by anchorless α2δ-1∆C, and another mechanism that requires its membrane anchoring, for example a process that stabilizes channel complexes in the plasma membrane.
Importantly, we also found in the present study that WT PrP does not inhibit calcium channel currents when α2δ-1ΔC was substituted for WT α2δ-1. This suggested to us two possibilities. First, PrP and α2δ-1 may compete for GPI-anchor intermediates or subsequent trafficking processes specific for GPI-anchored proteins when both are overexpressed. Secondly, the interaction between PrP and α2δ-1 with other protein and lipid components in cholesterol-rich membrane microdomains [50,51] might be involved in the suppression of currents. Thus when either PrP or α2δ-1 is devoid of the lipid anchor no suppression occurs.
It is possible that WT α2δ-1 and PrP interact indirectly via an intermediary trafficking protein and compete for binding to that protein, whereas this does not occur when one of them is not membrane-anchored. Nevertheless, the additional finding that CaV2.1/β1b/α2δ-1 currents were also inhibited by GPI–GFP (when compared with the control GFP) suggests that the presence of a GPI anchor might be an important common denominator in this process. Only approximately 150 proteins are known to be GPI-anchored, and the GPI synthetic pathway, involving multiple enzymatic steps in the ER (endoplasmic reticulum) and Golgi, is likely to have limited capacity in mammalian cells [52]. Overexpression of multiple GPI-anchored proteins in the same cell might lead to saturation of limited resources. Further studies will be necessary to determine whether overexpression of other GPI-anchored proteins disrupts the function of α2δ within membrane microdomains, or possibly competes with it for GPI transamidase enzymes, GPI-anchoring intermediates or trafficking proteins subsequent to GPI anchoring. Taken together, the results of the present study provide supporting evidence that α2δ-1 can be GPI-anchored. More generally the results point to a competitive interaction between GPI-anchored proteins for resources that determine their level of post-translational processing or expression on the plasma membrane.
ACKNOWLEDGEMENTS
We thank Wendy S. Pratt and Kanchan Chaggar for technical assistance.
AUTHOR CONTRIBUTION
Annette Dolphin and Roberto Chiesa conceived the study. Anita Alvarez Laviada performed the patch-clamp electrophysiology. Annette Dolphin performed electrophysiology in Xenopus oocytes. Ivan Kadurin obtained all data shown in Figure 1. Assunta Senatore performed the Western blotting shown in Figures 4 and 5. Roberto Chiesa supplied constructs, mouse tissue and antibodies. Annette Dolphin, Ivan Kadurin and Roberto Chiesa wrote the paper.
FUNDING
This work was supported by the Medical Research Council [grant numbers G0801756 and G0901758 (to A.C.D.)], the Wellcome Trust [Programme grant number 098360/Z/12/Z] and the Fondazione Telethon [grant numbers TDRC00508TU and GGP12115 (to R.C.)]. A.A.-L. was supported by a Biotechnology and Biological Sciences Research Council PhD studentship. A.S. was supported by a fellowship from the Banca del Monte di Lombardia Foundation. R.C. is an Associate Telethon Scientist (Dulbecco Telethon Institute, Fondazione Telethon).
References
- 1.Davies A., Hendrich J., Van Minh A. T., Wratten J., Douglas L., Dolphin A. C. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Walker D., De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- 3.Dolphin A. C. β Subunits of voltage-gated calcium channels. J. Bioeng. Biomemb. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 4.Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T. P., Campbell K. P. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 5.Leroy J., Richards M. S., Butcher A. J., Nieto-Rostro M., Pratt W. S., Davies A., Dolphin A. C. Interaction via a key tryptophan in the I–II linker of N-type calcium channels is required for beta1 but not for palmitoylated β2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J. Neurosci. 2005;25:6984–6996. doi: 10.1523/JNEUROSCI.1137-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waithe D., Ferron L., Page K. M., Chaggar K., Dolphin A. C. β-Subunits promote the expression of Cav2.2 channels by reducing their proteasomal degradation. J. Biol. Chem. 2011;286:9598–9611. doi: 10.1074/jbc.M110.195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H. W., Hermosilla T., Zamponi G. W. The Cavβ subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat. Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- 8.Canti C., Nieto-Rostro M., Foucault I., Heblich F., Wratten J., Richards M. W., Hendrich J., Douglas L., Page K. M., Davies A., Dolphin A. C. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppa M., Lana B., Margas W., Dolphin A. C., Ryan T. A. α2δ couples calcium channels to neurotransmitter release sites to control release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassidy J., Dolphin AC. Using exofacially tagged functional Cav2.2 to investigate the modulation of pore subunit trafficking by auxiliary calcium channel subunits; 58th U.S. Biophysical Society meeting; San Francisco, CA, U.S.A.: 2014. 15–19 February 2014, Abstract 1672-Pos. [Google Scholar]
- 11.Brodbeck J., Davies A., Courtney J.-M., Meir A., Balaguero N., Canti C., Moss F. J., Page K. M., Pratt W. S., Hunt S. P., et al. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated a2d-2 protein with abnormal function. J. Biol. Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- 12.Wakamori M., Mikala G., Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J. Physiol. 1999;517:659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C. S., Pratt W. S., Dolphin A. C. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a post-translational modification essential for function. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurnett C. A., De Waard M., Campbell K. P. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 15.Catterall W. A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 16.Arikkath J., Campbell K. P. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 17.Jay S. D., Sharp A. H., Kahl S. D., Vedvick T. S., Harpold M. M., Campbell K. P. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J. Biol. Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 18.Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 19.Collinge J. Molecular neurology of prion disease. J. Neurol. Neurosurg. Psychiatry. 2005;76:906–919. doi: 10.1136/jnnp.2004.048660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biasini E., Turnbaugh J. A., Unterberger U., Harris D. A. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012;35:92–103. doi: 10.1016/j.tins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bueler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., Dearmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 22.Steele A. D., Lindquist S., Aguzzi A. The prion protein knockout mouse: a phenotype under challenge. Prion. 2007;1:83–93. doi: 10.4161/pri.1.2.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouillet-Richard S., Ermonval M., Chebassier C., Laplanche J. L., Lehmann S., Launay J. M., Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 24.Viles J. H., Cohen F. E., Prusiner S. B., Goodin D. B., Wright P. E., Dyson H. J. Copper binding to the prion protein: structural implications of four identical cooperative binding sites. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2042–2047. doi: 10.1073/pnas.96.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collinge J., Whittington M. A., Sidle K. C., Smith C. J., Palmer M. S., Clarke A. R., Jefferys J. G. Prion protein is necessary for normal synaptic function. Nature. 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- 26.Bremer J., Baumann F., Tiberi C., Wessig C., Fischer H., Schwarz P., Steele A. D., Toyka K. V., Nave K. A., Weis J., Aguzzi A. Axonal prion protein is required for peripheral myelin maintenance. Nat. Neurosci. 2010;13:310–318. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]
- 27.Prusiner S. B. Prions. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallucci G., Dickinson A., Linehan J., Klohn P. C., Brandner S., Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 29.Bueler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 30.Brandner S., Isenmann S., Raeber A., Fischer M., Sailer A., Kobayashi Y., Marino S., Weissmann C., Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 31.Rutishauser D., Mertz K. D., Moos R., Brunner E., Rulicke T., Calella A. M., Aguzzi A. The comprehensive native interactome of a fully functional tagged prion protein. PLoS ONE. 2009;4:e4446. doi: 10.1371/journal.pone.0004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies A., Douglas L., Hendrich J., Wratten J., Tran-Van-Minh A., Foucault I., Koch D., Pratt W. S., Saibil H., Dolphin A. C. The calcium channel α2δ-2 subunit partitions with CaV2.1 in lipid rafts in cerebellum: implications for localization and function. J. Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor D. R., Whitehouse I. J., Hooper N. M. Glypican-1 mediates both prion protein lipid raft association and disease isoform formation. PLoS Pathog. 2009;5:e1000666. doi: 10.1371/journal.ppat.1000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elfrink K., Nagel-Steger L., Riesner D. Interaction of the cellular prion protein with raft-like lipid membranes. Biol. Chem. 2007;388:79–89. doi: 10.1515/BC.2007.010. [DOI] [PubMed] [Google Scholar]
- 35.Senatore A., Colleoni S., Verderio C., Restelli E., Morini R., Condliffe S., Bertani I., Mantovani S., Canovi M., Micotti E., et al. Mutant prion protein suppresses glutamatergic neurotrasmission in cerebellar granule neurons by impairing membrane delivery of voltage-gated calcium channel α2δ-1 subunit. Neuron. 2012;74:300–313. doi: 10.1016/j.neuron.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadurin I., Alvarez-Laviada A., Ng S. F., Walker-Gray R., D’Arco M., Fadel M. G., Pratt W. S., Dolphin A. C. Calcium currents are enhanced by α2δ-1 lacking its membrane anchor. J. Biol. Chem. 2012;1287:33554–33566. doi: 10.1074/jbc.M112.378554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomlinson W. J., Stea A., Bourinet E., Charnet P., Nargeot J., Snutch T. P. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32:1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- 38.Stewart R. S., Harris D. A. Mutational analysis of topological determinants in prion protein (PrP) and measurement of transmembrane and cytosolic PrP during prion infection. J. Biol. Chem. 2003;278:45960–45968. doi: 10.1074/jbc.M307833200. [DOI] [PubMed] [Google Scholar]
- 39.Cormack B. P., Valdivia R. H., Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 40.Nichols B. J., Kenworthy A. K., Polishchuk R. S., Lodge R., Roberts T. H., Hirschberg K., Phair R. D., Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 2001;153:529–541. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page K. M., Heblich F., Davies A., Butcher A. J., Leroy J., Bertaso F., Pratt W. S., Dolphin A. C. Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J. Neurosci. 2004;24:5400–5409. doi: 10.1523/JNEUROSCI.0553-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiesa R., Piccardo P., Ghetti B., Harris D. A. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron. 1998;21:1339–1351. doi: 10.1016/s0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- 43.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 44.Barclay J., Balaguero N., Mione M., Ackerman S. L., Letts V. A., Brodbeck J., Canti C., Meir A., Page K. M., Kusumi K., et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehmann S., Harris D. A. A mutant prion protein displays an aberrant membrane association when expressed in cultured cells. J. Biol. Chem. 1995;270:24589–24597. doi: 10.1074/jbc.270.41.24589. [DOI] [PubMed] [Google Scholar]
- 46.Berrow N. S., Brice N. L., Tedder I., Page K., Dolphin A. C. Properties of cloned rat a1A calcium channels transiently expressed in the COS-7 cell line. Eur. J. Neurosci. 1997;9:739–748. doi: 10.1111/j.1460-9568.1997.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 47.Campbell V., Berrow N. S., Fitzgerald E. M., Brickley K., Dolphin A. C. Inhibition of the interaction of G protein Go with calcium channels by the calcium channel β-subunit in rat neurones. J. Physiol. 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canti C., Davies A., Berrow N. S., Butcher A. J., Page K. M., Dolphin A. C. Evidence for two concentration-dependent processes for β subunit effects on a1B calcium channels. Biophys. J. 2001;81:1439–1451. doi: 10.1016/S0006-3495(01)75799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canti C., Page K. M., Stephens G. J., Dolphin A. C. Identification of residues in the N-terminus of a1B critical for inhibition of the voltage-dependent calcium channel by Gbg. J. Neurosci. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuermer C. A. Reggie/flotillin and the targeted delivery of cargo. J. Neurochem. 2011;116:708–713. doi: 10.1111/j.1471-4159.2010.07007.x. [DOI] [PubMed] [Google Scholar]
- 51.Hooper N. M. Glypican-1 facilitates prion conversion in lipid rafts. J. Neurochem. 2011;116:721–725. doi: 10.1111/j.1471-4159.2010.06936.x. [DOI] [PubMed] [Google Scholar]
- 52.Fujita M., Kinoshita T. Structural remodeling of GPI anchors during biosynthesis and after attachment to proteins. FEBS Lett. 2010;584:1670–1677. doi: 10.1016/j.febslet.2009.10.079. [DOI] [PubMed] [Google Scholar]
- 53.Campana V., Caputo A., Sarnataro D., Paladino S., Tivodar S., Zurzolo C. Characterization of the properties and trafficking of an anchorless form of the prion protein. J. Biol. Chem. 2007;282:22747–22756. doi: 10.1074/jbc.M701468200. [DOI] [PubMed] [Google Scholar]