ABSTRACT
BACKGROUND:
A standard neoadjuvant regimen has not been defined for borderline resectable (BR) pancreatic cancer. This phase II trial was designed to determine the safety of accelerated fraction radiotherapy (AFRT) with capecitabine in patients with BR pancreatic cancer.
METHODS:
The patients had newly diagnosed BR adenocarcinoma of the pancreas and normal organ function. Intensity-modulated (n = 11) or 3D conformal (n = 2) radiotherapy was given to a dose of 50 Gy in 2.5-Gy fractions with capecitabine 825 mg/m2 twice on radiation days. The primary outcome was the frequency of severe treatment-related adverse events (AEs). The study was stopped before planned interim analysis because of 2 severe (grades 4 and 5) gastric ulcerations.
RESULTS:
Thirteen patients were enrolled with a median age of 66 years. All patients completed treatment. Seven (54%) experienced grade 3+ treatment-related AEs. Severe gastric ulceration occurred in 2 patients despite receipt of ≥43 Gy to only 1% (2–3 cm3) of the stomach. Lymphopenia (n = 7) was the only other severe AE that occurred in >1 patient. In 7 of the 13 patients, disease had progressed outside the pancreas at restaging. Five of the 13 underwent resection, and all had >10% viable tumor. Median progression-free survival (PFS) was 2.4 months (95% CI 1.9–5.9), and median survival was 9.1 months (95% CI 5.9–not reached). Among those who underwent resection, median PFS was 13.0 months (95% CI 4.4–not reached). Median survival was not reached.
CONCLUSIONS:
Given the limited efficacy signal and severe gastric ulcerations, we do not recommend this regimen for pancreatic cancer. We also do not recommend the use of high doses per fraction outside a clinical trial.
Surgical resection remains the only hope for a cure of pancreatic cancer. Patients who undergo a microscopically margin negative (R0) resection followed by adjuvant chemotherapy or chemoradiotherapy have the best prognosis, with a 5-year survival of approximately 20%.1–4 In contrast, patients with locally advanced, unresectable (but not metastatic) pancreatic cancer have a median survival of only 1 year.5
The emerging category of borderline resectable (BR) pancreatic cancer applies to patients who present with cancers that are neither overtly unresectable nor clearly resectable. This classification encompasses patients with cancer that may be technically amenable to surgical resection, but the involvement of vascular structures portends an excessively high risk of a microscopically involved margin.6 Some groups also include patients with indeterminate nodules on baseline imaging.5 Together, these definitions create a group of patients for whom neoadjuvant therapy may increase the chance of a curative R0 resection. Neoadjuvant therapy also allows patients with radiographically occult metastatic disease at presentation adequate time to manifest cancer progression and thereby avoid the morbidity of surgery. Resectability rates in recent series of BR pancreatic cancer range between 40% and 60%.7–14 When the patients with progression on treatment are selected, those with an initial diagnosis of BR cancer who go on to have surgical resection have survival similar to that of patients in whom the cancer was deemed resectable at diagnosis.5,9
Randomized trials have yet to define the optimal neoadjuvant approach for patients with BR pancreatic cancer. Investigators at the MD Anderson Cancer Center (MDACC) have led the way with neoadjuvant therapy for resectable and BR cancers, using gemcitabine and gemcitabine-based combinations with external beam radiotherapy (RT) to 30 Gy over 2 weeks.15,16 Even with these aggressive regimens, only 14% of resected tumors have a major pathologic response, as defined by <10% viable residual tumor, suggesting substantial chemoradiotherapy resistance.
Given the importance of achieving local control in patients who undergo resection, approaches that optimize chemoradiotherapy delivery have the potential to improve outcomes in patients with BR pancreatic cancer. Accelerated fraction radiotherapy (AFRT) provides a greater dose intensity per fraction and decreases the total interval of dose delivery, theoretically improving local control over standard fractionation.17 Our group has reported excellent outcomes with neoadjuvant AFRT in a retrospective series of patients with BR patients.8 We undertook this phase II trial to evaluate the safety and efficacy of an AFRT scheme as neoadjuvant therapy for BR pancreatic cancer and its suitability as a platform on which to test novel systemic targeted agents.
METHODS
This single-institution, prospective, phase II trial was approved by the Institutional Review Board at the University of Virginia and registered with clinicaltrials.gov (NCT01333332). All patients gave written informed consent. Eligible patients were 18 years of age or older and had a histologically or cytologically confirmed diagnosis of BR adenocarcinoma of the pancreas. Cancers were classified as BR by our multidisciplinary hepatobiliary tumor board, according to the MDACC classification: category A, abutment of <180° of the superior mesenteric artery and/or celiac axis, abutment or encasement of a short segment hepatic artery, and involvement of the portal vein or superior mesenteric vein amenable to vascular reconstruction; category B, concern for extrapancreatic metastatic disease based on either an indeterminate nodule on imaging or pathologically confirmed nodal disease; or category C, borderline performance status or medical comorbidities that raise concern for the patient's ability to tolerate pancreatic resection.5
Those with prior therapy for pancreatic cancer were excluded. Laboratory requirements included the following: absolute neutrophil count (ANC) ≥1.5 × 109 cells/L, platelets ≥100,000 × 109 cells/L, AST/ALT ≤5 times the upper limits of laboratory normal, creatinine clearance (as measured by Cockcroft-Gault) of >30 mL/min, and total bilirubin ≤3 times the laboratory upper limits of normal. Patients with total bilirubin 3–5 times the upper limit because of cancer-associated obstruction were eligible after an attempt to relieve biliary obstruction.
Treatment
Treatment consisted of AFRT with concomitant capecitabine, which was started within 14 days of study enrollment. External beam RT was given to a dose of 50 Gy in 20 fractions of 2.5 Gy per fraction delivered Monday through Friday. The gross tumor volume (GTV) consisted of the manually contoured tumor volume, including draining lymph nodes, to account for microscopic disease extension. The porta hepatis and para-aortic nodes were not included. Manual contouring was performed with a computed tomographic (CT) scan with either intravenous contrast or co-registration with diagnostic magnetic resonance imaging (MRI). The planning tumor volume (PTV) consisted of the GTV plus 0.5–1.5-cm radial and 1–2-cm craniocaudal extensions. Treatment was delivered to 95% of the PTV with intensity-modulated radiotherapy (IMRT) with daily soft tissue CT image guidance on Trilogy (n = 6) or Tomotherapy (n = 5) machines. In 2 cases where insurance coverage precluded the use of IMRT, 3-D conformal radiation was permitted, with tight margins and daily image guidance to the same volumes. The dose was constrained to ensure that <20% of the gastric volume received 45 Gy. In all cases, a 4-D CT was used to evaluate the need for gating; none met the motion criteria for gating, deep-inspiration breath hold, or active breathing control. Thus, all treatments were administered with BodyFix and vacuum cover sheet immobilization without other motion management. Because of the concurrent morning dose of capecitabine, which must be taken within 30 minutes of a meal, the degree of gastric distension was not controlled by making patients NPO before daily radiation treatments. Concurrent capecitabine was administered twice on radiation days. The first 5 patients received flat, asymmetric doses of capecitabine at 1000 mg in the morning and 2000 mg in the evening, based on prior institutional experience. Thereafter, a more conventional dose of 825 mg/m2 twice daily was used. Patients with a creatinine clearance of 30–50 mL/min received a 25% reduction in capecitabine dose rounded to the nearest 150-mg tablet size. All patients were instructed to take a proton pump inhibitor, but data on adherence were not collected.
Restaging with CT or MRI to determine resectability was performed 2–4 weeks after completion of treatment. The tumor was deemed resectable if the disease had been stable or improved. Surgery occurred 4–8 weeks after completion of treatment. Grading of treatment response in resected tumors was performed by a single expert pathologist as follows: grade 1, >90% viable; grade IIa, 50–89% viable; grade IIb, 10–49% viable; grade 3, <10% viable; and grade 4, no viable tumor.18
Monitoring for adverse events (AEs) continued for 30 days after the last protocol therapy (from the last day of radiation for patients with inoperable tumors and from the day of surgery for those with resected tumors). The patients were followed up for recurrence and survival via telephone or routine physicians' visits every 6 months until the time of death or for 5 years in the surviving patients.
Toxicity was measured according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.0). The response was measured according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1).
Statistical Considerations
The primary objective was to determine the safety of AFRT to 50 Gy with concomitant capecitabine in patients with BR pancreatic cancer. Safety was measured by the rate of unacceptable treatment-related grade 3+ AEs. The following AEs were considered unacceptable: any grade 3–5 nonhematologic toxicity requiring dose reduction in capecitabine and/or delay in radiation, grade 4 neutropenia or grade 3–5 neutropenia with fever or infection, and grade 4 thrombocytopenia or grade 3 thrombocytopenia with major bleeding. A target sample size of 40 patients was chosen with an interim analysis planned at 20 patients, to assess safety. Specifically, we planned to test for AE rates of 50% (unacceptable) vs. 30% (deemed safe).
The study was stopped before the planned interim analysis because of 2 severe (1 grade 4 and 1 grade 5) gastric ulcerations. Although gastric ulceration is a known complication of RT, the efficacy signal at the time of the second event was not promising, and the decision was made by the investigative team to close the study to further accrual.
AE rates, pathologic response, and resection rates are presented descriptively. The Kaplan-Meier method was used to calculate progression free and overall survivals from the time of first radiation dose until progression or death. Patients lost to follow-up were censored at the date of their last contact with study personnel.
RESULTS
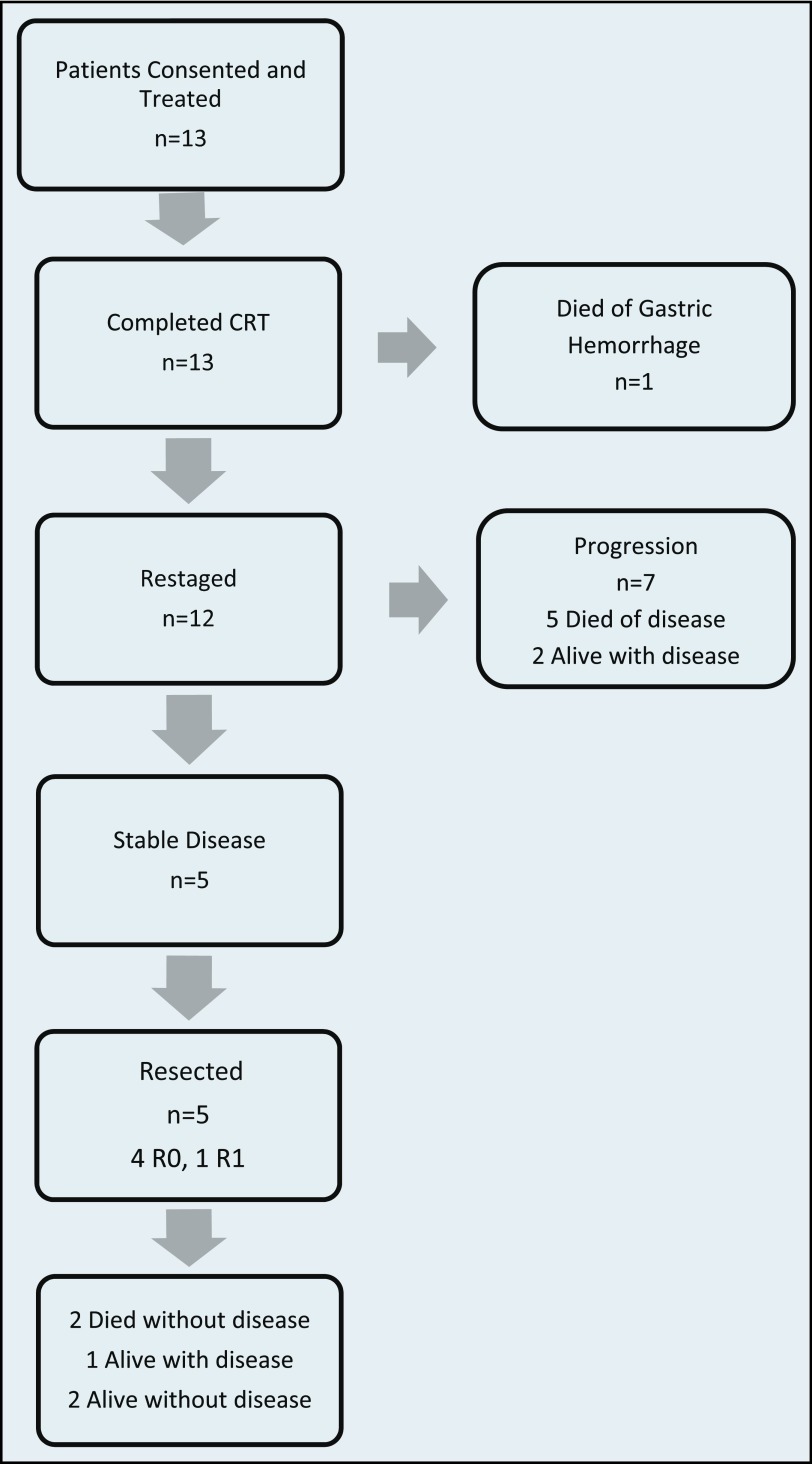
Thirteen patients were consented and enrolled between September 2010 and February 2012 (Figure 1). The median age of the cohort was 66 (range, 54–82) years (Table 1). The majority (85%) of patients were white, and there was a predominance of women (8/13). Two patients were classified as having BR cancer on the basis of indeterminate nodules that were possible signs of extrapancreatic disease; the remainder had vascular involvement.
Figure 1.
Consort diagram of patient outcomes.
Table 1.
Patient characteristics
| Median age (range), y | 66 (51–82) |
| Sex, n (%) | |
| Male | 5 (38) |
| Female | 8 (62) |
| Race, n (%) | |
| Caucasian | 11 (85) |
| African American | 2 (15) |
| Borderline resectable category (%) | |
| A: vascular involvement | 11 (85) |
| B: indeterminate metastatic | 2 (15) |
Adverse Events
Only 1 patient required a delay in radiation therapy because of sepsis from cholangitis. The same patient also had multiple other dose delays because of nonadherence to radiation appointments. No patient required capecitabine dose reduction. All patients completed the protocol.
Seven patients experienced grade 3 or higher AEs (Table 2). Lymphopenia was the most common severe AE, with 7 patients experiencing grade 3–4 lymphopenia and 4 patients with grade 1–2 lymphopenia. Other severe treatment-related events were elevated INR (n = 1) in a patient on warfarin, hyponatremia (n = 1), and anemia (n = 1). The most common grade 1–2 AEs during treatment were nausea and vomiting (n = 5 and 2, respectively), fatigue (n = 6), and thrombocytopenia (n = 5). After surgery, 1 patient developed sepsis from an intra-abdominal abscess that ultimately led to his death. One patient experienced atrial fibrillation followed by cardiac arrest in the recovery room, but was resuscitated.
Table 2.
Treatment-related AEs
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total affected n (%) |
|---|---|---|---|---|---|---|
| Worst grade per patient: severe events, n | ||||||
| Lymphopenia | 2 | 2 | 5 | 2 | 0 | 11 (85) |
| Anemia | 2 | 0 | 1 | 0 | 0 | 3 (23) |
| Increased INR | 0 | 1 | 1 | 0 | 0 | 2 (15) |
| Acute coronary syndrome | 0 | 0 | 1 | 0 | 0 | 1 (8) |
| Gastric hemorrhage | 0 | 0 | 0 | 0 | 1 | 1 (8) |
| Gastric ulcer | 0 | 0 | 1 | 0 | 0 | 1 (8) |
| Hyponatremia | 0 | 0 | 1 | 0 | 0 | 1 (8) |
| Worst grade per patient: mild and moderate events, n | ||||||
| Nausea or vomiting | 6 | 1 | 0 | 0 | 0 | 7 (54) |
| Fatigue | 4 | 2 | 0 | 0 | 0 | 6 (46) |
| Thrombocytopenia | 4 | 1 | 0 | 0 | 0 | 5 (38) |
| Anorexia | 2 | 0 | 0 | 0 | 0 | 2 (15) |
| Chills | 2 | 0 | 0 | 0 | 0 | 2 (15) |
| Leukopenia | 1 | 1 | 0 | 0 | 0 | 2 (15) |
| Oral mucositis | 1 | 1 | 0 | 0 | 0 | 2 (15) |
| Diarrhea | 0 | 1 | 0 | 0 | 0 | 1 (8) |
| Dry skin | 1 | 0 | 0 | 0 | 0 | 1 (8) |
| Hand-foot syndrome | 0 | 1 | 0 | 0 | 0 | 1 (8) |
| Hypoalbuminemia | 1 | 0 | 0 | 0 | 0 | 1 (8) |
| Hypocalcemia | 1 | 0 | 0 | 0 | 0 | 1 (8) |
| Hypomagnesemia | 1 | 0 | 0 | 0 | 0 | 1 (8) |
| Skin ulceration | 1 | 0 | 0 | 0 | 0 | 1 (8) |
| Transaminase elevation | 1 | 0 | 0 | 0 | 0 | 1 (8) |
All events considered to be at least possibly related to treatment are shown.
In the 30-day posttreatment follow-up period, 2 patients had severe gastric complications. One presented to the emergency department with unstable angina from anemia caused by a gastric ulceration. She recovered to undergo restaging, at which time the ulceration was incidentally found to have a contained perforation. Liver metastases also developed, and she chose to be discharged home with hospice. A second patient died of hemorrhagic shock from a gastric bleed approximately 1 month after completion of therapy. Endoscopy showed diffuse gastritis with ulceration. Biopsy confirmed radiation gastritis and concurrent active cytomegalovirus infection. The mean gastric radiation dose for these patients was 7.6 Gy and 15.7 Gy, respectively (Table 3). In both patients, only 1% (2–3 cm3) of the stomach received a dose of 43 Gy or higher.
Table 3.
Gastric radiation doses
| Technique | Min dose (Gy) | Mean dose (Gy) | Max dose (Gy) | Receiving ≥43 Gy (%) | Volume receiving ≥43 Gy (cm3) |
|---|---|---|---|---|---|
| 3D | 0.42 | 7.28 | 47.08 | 21 | 24 |
| IMRT | 2.23 | 26.73 | 52.23 | 2 | 5 |
| IMRT | 1.68 | 22.83 | 54.24 | 15 | 41 |
| 3D | 1.11 | 14.17 | 50.88 | 1 | 3 |
| IMRT | 0.78 | 3.09 | 54.41 | 1 | 3 |
| IMRT* | 8.50 | 15.74 | 51.55 | 1 | 3 |
| IMRT | 4.20 | 8.23 | 51.33 | 1 | 8 |
| IMRT | 8.81 | 12.73 | 54.14 | 3 | 13 |
| IMRT | 1.16 | 17.09 | 52.65 | 6 | 17 |
| IMRT | 0.80 | 13.23 | 51.82 | 7 | 19 |
| IMRT | 0.60 | 1.80 | 13.10 | 0 | 0 |
| IMRT* | 1.07 | 7.66 | 52.31 | 1 | 2 |
| IMRT | 3.73 | 11.52 | 49.56 | 1 | 2 |
Patients experiencing gastric ulceration during treatment follow-up.
Outcomes
Twelve patients were subsequently restaged after completing therapy, 7 of whom had evidence of extrapancreatic progression (Figure 1). No patient had a radiographically detected response to treatment. All 5 patients without progression underwent resection: 4 had R0 resection, and 1 had R1. Posttreatment stages were ypT1N0 (n = 1), ypT2N0 (n = 1), and ypT3N0 (n = 3). Two patients had a grade IIa (50–89% viable tumor) and 3 had grade IIb (10–49% viable tumor) treatment responses. Two of the 5 patients with resected tumors received adjuvant gemcitabine. The remaining 3 did not receive any adjuvant therapy: 2 declined treatment and 1 died of postoperative complications.
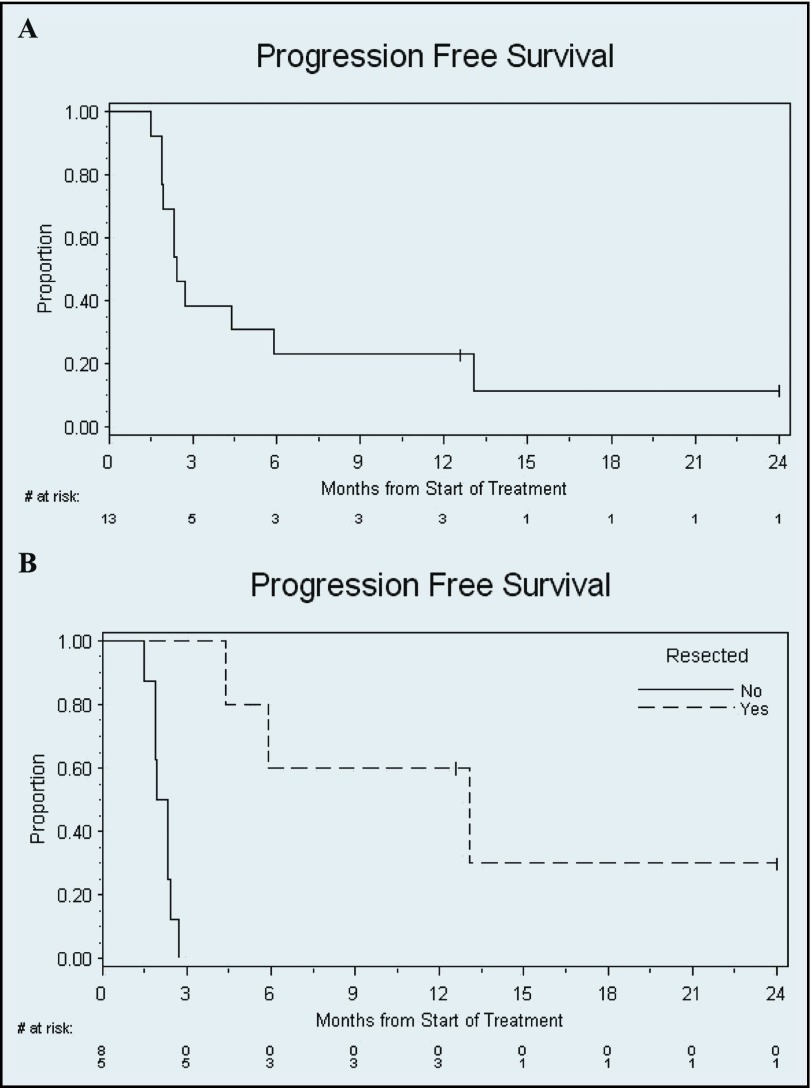
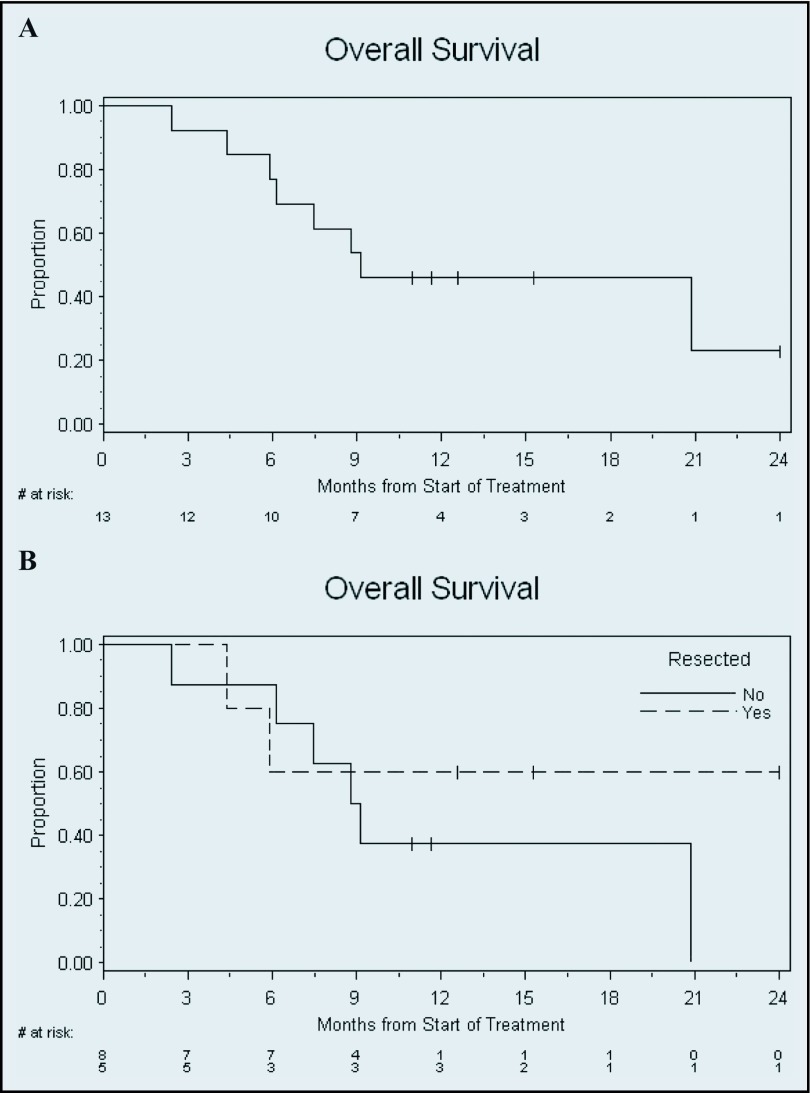
The median progression-free survival (PFS) of the entire cohort was 2.4 months (95% CI 1.9–5.9), and median overall survival was 9.1 months (95% CI 5.9–not reached) (Figures 2 and 3). The median PFS of the 5 patients with resection was 13.0 months (95% CI 4.4–not reached). Median survival with resection was not reached, whereas median survival in patients who did not undergo resection was 9.0 months (95% CI 3.4–20.9).
Figure 2.
Kaplan-Meier PFS. Time from start of protocol treatment until progression or death is shown for all patients. Censored patients are represented by ticks. (A) Entire cohort. Median PFS, 2.4 months (95% CI 1.9–5.9). (B) Stratified by resection. Median PFS in patients with resection, 13.0 months (95% CI 4.4–not reached). Median PFS in patients without resection, 2.1 months (95% CI 1.5–2.4).
Figure 3.
Kaplan-Meier overall survival. Time from start of protocol treatment until death is shown for all patients. Censored patients are represented by ticks. (A) Entire cohort. Median survival, 9.1 months (95% CI 5.9–not reached). (B) Stratified by resection. Median survival in patients with resection, not reached. Median survival in patients without resection, 9.0 months (95% CI 3.4–20.9).
DISCUSSION
A neoadjuvant treatment approach for patients with BR pancreatic cancer appears to increase the proportion able to undergo a potentially curative surgery. Accelerated fractionation of RT shortens the duration of RT by using a higher dose per fraction, rather than reducing the total dose, providing theoretical anticancer benefits to enhance locoregional control and improve resectability. It was our hope that this would be a well-tolerated platform on which to build novel targeted systemic therapy approaches in the neoadjuvant setting.
Of 13 patients enrolled in our study, only 5 (38%) went on to have resection. Despite an excellent preliminary experience,8 treatment effect in the resected tumors was modest. Only 2 patients are alive and cancer free after just 15 and 24 months. The majority of patients enrolled in our study exhibited disease progression at the time of restaging, approximately 2–3 months after diagnosis. This study began before the advent of FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin), and we chose not to include gemcitabine-based neoadjuvant chemotherapy as part of this regimen, given the high rate of primary progressive disease. However, the high rate of progressive disease highlights the critical need for better systemic therapies. Tumor specimens resected from patients enrolled in this trial have been incorporated into our ongoing orthotopic xenograft model program to study novel systemic and radiosensitizing treatment approaches.19 The high rate of early progression also suggests that with the aggressive biology of pancreatic cancer, a prolonged course of neoadjuvant therapy may not be needed to select patients destined to progress.
Although on-treatment tolerance of the accelerated fractionation was good, 2 patients developed severe gastritis and ulceration with hemorrhage in the month following treatment. One patient had a non-ST elevation myocardial infarction as a result, and the other died of gastric hemorrhage. Gastritis and ulceration are known complications of upper abdominal RT, the incidence and severity of which increase as the total dose and as the dose per fraction increase.20–23 At the doses received by our patients—a mean gastric dose range from 3 to 27 Gy and a volume of never more than 25 cm3 exposed to >43 Gy—the expected rate of gastric ulceration should have been very low. The estimated risk of gastric ulceration, according to Emami et al,22 is 5% at 5 years in patients receiving either 60 Gy over 6 weeks to one-third of the stomach or 50 Gy over 6 weeks to the entire stomach.22 The Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) update of the initial Emami estimates similarly reported that, at doses of approximately 50 Gy, the risk of gastric ulceration is <7%.24,25 However, the maximum dose point may be a better predictor of toxicity, and normal tissue tolerance is dependent not just on dose, but also on fraction size.25 Rates of late gastric toxicity at higher doses per fraction in the context of modern treatment planning and how these may be influenced by concurrent administration of a fluoropyrimidine are not well defined.
Recently, several single-institution series and phase II trials of accelerated RT have reported excellent tolerance of this approach. Investigators at MDACC have built their neoadjuvant program around a radiation course of 30 Gy in 3-Gy fractions over 2 weeks, and it has been extremely well tolerated.26,27 Adjuvant radiation to 55 Gy in 2.2-Gy fractions with concurrent capecitabine has also been reported without gastric toxicity, but only 6 patients were treated at this dose.28 A phase I/II study in locally advanced pancreatic cancer in which fixed-dose-rate gemcitabine was combined with escalating doses of IMRT from 50 to 60 Gy in 25 fractions found a dose of 55 Gy to be tolerable, with 6 of 20 patients experiencing dose-limiting toxicity at this dose level.29 Of the 50 patients treated, 3 experienced duodenal bleeding and 1 had duodenal perforation. This combination had promising efficacy. In our previously reported experience, the same accelerated fractionation scheme with capecitabine was administered to approximately 20 patients with BR or unresectable pancreatic cancer. The retrospective evaluation of these patients identified only rare grade 3–4 toxicity or hospitalization.8
In contrast to these reports of excellent tolerance of higher doses per fraction of abdominal radiation, there is also ample suggestion that such an approach results in more severe gastrointestinal mucosal toxicity. In a meta-analysis of individual patient data from recent prospective trials of modified radiation fractionation in lung cancer, accelerated fractionation schemes were associated with the highest rates of severe esophagitis, with an odds ratio of 3.21, (95% CI 2.41–4.28) compared with standard fractionation.30 At the extreme of accelerated fractionation is stereotactic body radiation (SBRT), in which a relatively high dose is delivered to a very narrow field in just a few fractions. With the use of SBRT in a variety of sites in the upper abdomen, including the pancreas, rates of severe gastroduodenal toxicity (ulceration, gastritis, and late stenosis) have been reported in the range of 15–43%.31–33
Whether the modest increase in dose per fraction in our study is truly associated with excessive AEs cannot be determined from this small, uncontrolled study. It is quite possible that our patients would have had severe gastric complications with standard fractionation as well. In addition, the fatal occurrence of gastric hemorrhage occurred in the setting of active cytomegalovirus infection, which probably exacerbated the radiation toxicity. However, given the lack of a definite efficacy signal and the toxicity observed, we do not recommend this accelerated fractionation regimen as a neoadjuvant approach to pancreatic cancer.
We believe that the observed toxicity should also serve as a caution. The extent to which accelerated fractionation schemes, SBRT, and other novel radiation techniques are used for pancreatic cancer in the community has not been reported. However, technological advances in cancer medicine, including altered fractionation schemes, recently made feasible because of modern radiation treatment planning and delivery devices, are often accompanied by rapid diffusion into routine practice.34 Ultimately, these RT advances are likely to improve outcomes for cancer patients. However, we believe our results highlight the paramount importance of prospectively evaluating these alternate RT dose schedules in clinical trials.
Acknowledgment
This work was presented in part as an abstract at the 2012 GI Cancers Symposium, San Francisco. The trial was made possible by the generous support of the Commonwealth Fund for Cancer Research of the University of Virginia Cancer Center and the American Cancer Society Institutional Research Grant awarded to the University of Virginia (IRG 81-001-26). Additional funding: National Cancer Institute (NCI K07160722-01 to HKS).
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Winter JM, Cameron JL, Campbell KA, et al. : 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10:1199–1210, 2006; discussion 1210-1211 [DOI] [PubMed] [Google Scholar]
- 2. Oettle H, Post S, Neuhaus P, et al. : Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Neoptolemos JP, Stocken DD, Bassi C, et al. : Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304:1073–81, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Kalser MH, Ellenberg SS: Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 120:899–903, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Katz MH, Pisters PW, Evans DB, et al. : Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 206:833–846, 2008; discussion 46-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abrams RA, Lowy AM, O'Reilly EM, et al. : Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 16:1751–1756, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Katz JN, Chang LC, Sangha O, et al. : Can comorbidity be measured by questionnaire rather than medical record review? Med Care 34:73–84, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Stokes JB, Nolan NJ, Stelow EB, et al. : Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 18:619–627, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Assifi MM, Lu X, Eibl G, et al. : Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery 150:466–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leone F, Gatti M, Massucco P, et al. : Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer 119:277–284, 2013 [DOI] [PubMed] [Google Scholar]
- 11. Lee JL, Kim SC, Kim JH, et al. : Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 152:851–862, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Hosein PJ, Macintyre J, Kawamura C, et al. : A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 12:851–199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel M, Hoffe S, Malafa M, et al. : Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 104:155–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landry J, Catalano PJ, Staley C, et al. : Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol 101:587–592, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varadhachary GR, Wolff RA, Crane CH, et al. : Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 26:3487–3495, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Evans DB, Varadhachary GR, Crane CH, et al. : Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 26:3496–3502, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Connell PP, Martel MK, Hellman S. Principles of radiation oncology, in DeVita V, Hellman S, Rosenberg S. (eds). Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 18. Evans DB, Rich TA, Byrd DR, et al. : Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 127:1335–1339, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Walters DM, Lindberg JM, Adair SJ, et al. : Inhibition of the growth of patient-derived pancreatic cancer xenografts with the MEK inhibitor trametinib is augmented by combined treatment with the epidermal growth factor receptor/HER2 inhibitor lapatinib. Neoplasia 15:143–155, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sell A, Jensen TS: Acute gastric ulcers induced by radiation. Acta Radiol 4:289–297, 1966 [DOI] [PubMed] [Google Scholar]
- 21. Coia LR, Myerson RJ, Tepper JE: Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys 31:1213–1236, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Emami B, Lyman J, Brown A, et al. : Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Cosset JM, Henry-Amar M, Burgers JM, et al. : Late radiation injuries of the gastrointestinal tract in the H2 and H5 EORTC Hodgkin's disease trials: emphasis on the role of exploratory laparotomy and fractionation. Radiat Oncol 13:61–68, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Kavanagh BD, Pan CC, Dawson LA, et al. : Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 76:S101–S107, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Marks LB, Yorke ED, Jackson A, et al. : Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 76:S10–S19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pisters PW, Abbruzzese JL, Janjan NA, et al. : Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol 16:3843–3850, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Pisters PW, Wolff RA, Janjan NA, et al. : Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol 20:2537–2544, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Morganti AG, Picardi V, Ippolito E, et al. : Capecitabine based postoperative accelerated chemoradiation of pancreatic carcinoma: a dose-escalation study. Acta Oncol 49:418–422, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Ben-Josef E, Schipper M, Francis IR, et al. : A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 84:1166–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mauguen A, Le Pechoux C, Saunders MI, et al. : Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 30:2788–2797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bae SH, Kim MS, Cho CK, et al. : Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys 84:e469–e474, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Hoyer M, Roed H, Sengelov L, et al. : Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 76:48–53, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Schellenberg D, Goodman KA, Lee F, et al. : Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 72:678–686, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Sheets NC, Goldin GH, Meyer AM, et al. : >Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307:1611–1620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]