Abstract
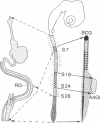
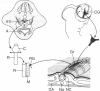
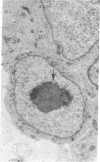
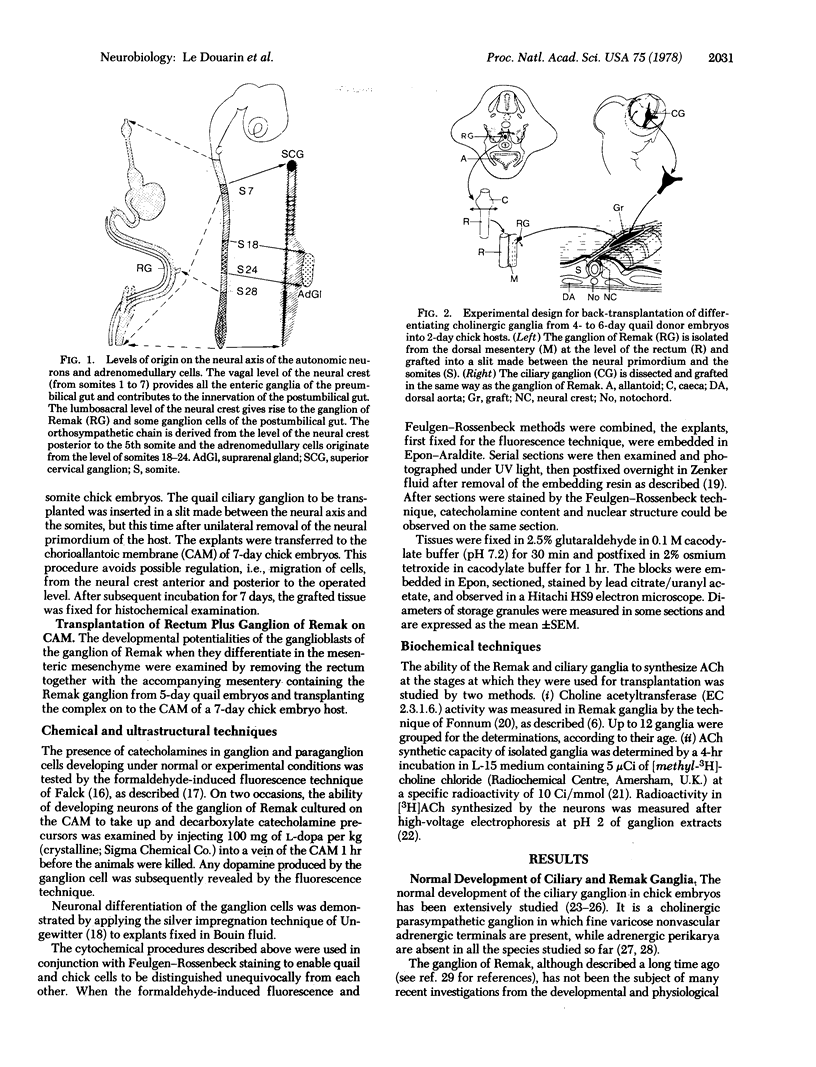
We have previously shown that the neural crest is regionalized early into "adrenergic" and "cholinergic" areas from which arise, respectively, the sympathetic and parasympathetic ganglioblasts of the autonomic nervous system. This regionalization does not correspond, however, to an irreversible determination of the neural crest cells since, under certain experimental conditions, cholinergic cells can arise from the adrenergic region of the crest and vice versa. The phenotypic expression of the presumptive ganglion cells appears to be responsive to the environmental conditions they encounter during and/or after their migration. In the present study we show that the developmental behavior of parasympathetic ganglion cells which have stopped migrating and at least some of which have started to differentiate into cholinergic neurons can be profoundly modified if they are transplanted into a younger embryo at the trunk neural crest level. The crest level. The grafted ganglion cells start migrating and stop in the same sites as the host neural crest cells. Their further differentiation depends on their localization. When situated in the adrenergic ganglia and in the suprarenal gland they synthesize catehcolamines, whereas they differentiate into nonfluorescent, silver-staining ganglion cells if they migrate in the gut wall. Thus, the differentiation of autonomic neurons is dependent on tissue interactions even after the neural crest cells have grouped to form ganglionic structures in which biochemical differentiation is already in progress.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Malmfors T. The adrenergic nervous system of the domestic fowl (Gallus domesticus [L.]). Z Zellforsch Mikrosk Anat. 1970;106(1):22–50. doi: 10.1007/BF01027715. [DOI] [PubMed] [Google Scholar]
- Burton H., Bunge R. P. A comparison of the uptake and release of [3H]norepinephrine in rat autonomic and sensory ganglia in tissue culture. Brain Res. 1975 Oct 24;97(1):157–162. doi: 10.1016/0006-8993(75)90924-5. [DOI] [PubMed] [Google Scholar]
- Cantino D., Mugnaini E. Adrenergic innervation of the parasympathetic ciliary ganglion in the chick. Science. 1974 Jul 19;185(4147):279–281. doi: 10.1126/science.185.4147.279. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V., Giacobini E., Pilar G., Uchimura H. Induction of cholinergic enzymes in chick ciliary ganglion and iris muscle cells during synapse formation. J Physiol. 1976 Jun;257(3):749–766. doi: 10.1113/jphysiol.1976.sp011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. M. Factors directing the expression of sympathetic nerve traits in cells of neural crest origin. J Exp Zool. 1972 Feb;179(2):167–182. doi: 10.1002/jez.1401790204. [DOI] [PubMed] [Google Scholar]
- Cohen A. M. Independent expression of the adrenergic phenotype by neural crest cells in vitro. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2899–2903. doi: 10.1073/pnas.74.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. La chaîne ganglionnée de Remak chez le poulet: les neurones adrénergiques. C R Assoc Anat. 1970 Dec;149:720–724. [PubMed] [Google Scholar]
- Cowell L. A., Weston J. A. An analysis of melanogenesis in cultured chick embryo spinal ganglia. Dev Biol. 1970 Aug;22(4):670–697. doi: 10.1016/0012-1606(70)90175-2. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Adrenergic nerves in the avian eye and ciliary ganglion. Z Zellforsch Mikrosk Anat. 1967;82(4):577–588. doi: 10.1007/BF00337123. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969 Nov;115(3):465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan E. J., MacLeish P. R., O'Lague P. H., Potter D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervonen H. Differentiation of sympathicoblasts in cultures of chick ganglia: light and electron microscopic, fluorescence and enzyme histochemical observations. Anat Embryol (Berl) 1975 May 16;146(3):225–243. doi: 10.1007/BF00302172. [DOI] [PubMed] [Google Scholar]
- Hildebrand J. G., Barker D. L., Herbert E., Kravitz E. A. Screening for neurotransmitters: a rapid radiochemical procedure. J Neurobiol. 1971;2(3):231–246. doi: 10.1002/neu.480020305. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Hendry I. A. Development of neurons synthesizing noradrenaline and acetylcholine in the superior cervical ganglion of the rat in vivo and in vitro. Neuroscience. 1977;2(5):741–749. doi: 10.1016/0306-4522(77)90027-6. [DOI] [PubMed] [Google Scholar]
- Landis S. C. Rat sympathetic neurons and cardiac myocytes developing in microcultures: correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4220–4224. doi: 10.1073/pnas.73.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synapse formation during embryogenesis on ganglion cells lacking a periphery. J Physiol. 1974 Sep;241(3):715–736. doi: 10.1113/jphysiol.1974.sp010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M. A Feulgen-positive nucleolus. Exp Cell Res. 1973 Mar 15;77(1):459–468. doi: 10.1016/0014-4827(73)90600-9. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Renaud D., Teillet M. A., Le Douarin G. H. Cholinergic differentiation of presumptive adrenergic neuroblasts in interspecific chimeras after heterotopic transplantations. Proc Natl Acad Sci U S A. 1975 Feb;72(2):728–732. doi: 10.1073/pnas.72.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974 Nov;41(1):162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973 Aug;30(1):31–48. [PubMed] [Google Scholar]
- Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Dev Biol. 1973 Jan;30(1):217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- Le Douarin N., Fontaine J., Le Lièvre C. New studies on the neural crest origin of the avian ultimobranchial glandular cells--interspecific combinations and cytochemical characterization of C cells based on the uptake of biogenic amine precursors. Histochemistry. 1974 Mar 13;38(4):297–305. doi: 10.1007/BF00496718. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. Particularites du noyau interphasique chez la caille Japonaise (Coturnix coturnix japonica) Bull Biol Fr Belg. 1969;103(3):435–452. [PubMed] [Google Scholar]
- MAYOR H. D., HAMPTON J. C., ROSARIO B. A simple method for removing the resin from epoxy-embedded tissue. J Biophys Biochem Cytol. 1961 Apr;9:909–910. doi: 10.1083/jcb.9.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden D. M. An analysis of migratory behavior of avian cephalic neural crest cells. Dev Biol. 1975 Jan;42(1):106–130. doi: 10.1016/0012-1606(75)90318-8. [DOI] [PubMed] [Google Scholar]
- Norr S. C. In vitro analysis of sympathetic neuron differentiation from chick neural crest cells. Dev Biol. 1973 Sep;34(1):16–38. doi: 10.1016/0012-1606(73)90336-9. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Obata K., Claude P., Furshpan E. J., Potter D. D. Evidence for cholinergic synapses between dissociated rat sympathetic neurons in cell culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. II. Developmental aspects. Dev Biol. 1977 Oct 15;60(2):473–481. doi: 10.1016/0012-1606(77)90144-0. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The influence of non-neuronal cells on catecholamine and acetylcholine synthesis and accumulation in cultures of dissociated sympathetic neurons. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3607–3610. doi: 10.1073/pnas.71.9.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Reichardt L. F., Chun L. L. Biochemical studies on the development of primary sympathetic neurons in cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:389–397. doi: 10.1101/sqb.1976.040.01.037. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Rost F. W., Fontaine J., Le Lièvre C., Le Douarin N. Demonstration of the neural crest origin of type I (APUD) cells in the avian carotid body, using a cytochemical marker system. Histochemie. 1973;34(3):191–203. doi: 10.1007/BF00303435. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F., Patterson P. H. Neurotransmitter synthesis and uptake by isolated sympathetic neurones in microcultures. Nature. 1977 Nov 10;270(5633):147–151. doi: 10.1038/270147a0. [DOI] [PubMed] [Google Scholar]
- Ross D., Johnson M., Bunge R. Development of cholinergic characteristics in adrenergic neurones is age dependent. Nature. 1977 Jun 9;267(5611):536–539. doi: 10.1038/267536a0. [DOI] [PubMed] [Google Scholar]
- Smith J., Cochard P., Le Douarin N. M. Development of choline acetyltransferase and cholinesterase activities in enteric ganglia derives from presumptive adrenergic and cholinergic levels of the neural crest. Cell Differ. 1977 Oct;6(3-4):199–216. doi: 10.1016/0045-6039(77)90016-1. [DOI] [PubMed] [Google Scholar]
- UNGEWITTER L. H. A urea silver nitrate method for nerve fibers and nerve endings. Stain Technol. 1951 Apr;26(2):73–76. doi: 10.3109/10520295109113182. [DOI] [PubMed] [Google Scholar]
- Unsicker K. Chromaffin, small granule-containing and ganglion cells in the adrenal gland of reptiles: A comparative ultrastructural study. Cell Tissue Res. 1976 Jan 28;165(4):477–508. doi: 10.1007/BF00224477. [DOI] [PubMed] [Google Scholar]
- Wechsler W., Schmekel L. Elektronenmikroskopische Untersuchung der Entwicklung der vegetativen (Grenzstrang-) und spinalen Ganglien bei Gallus domesticus. Acta Neuroveg (Wien) 1967;30(1):427–444. doi: 10.1007/BF01239924. [DOI] [PubMed] [Google Scholar]