Abstract
Background
The current study aim was to identify predictors of pathologic complete response (pCR) following neoadjuvant therapy.
Methods
From 2000-2007, 518 breast cancer patients received neoadjuvant therapy. Data were compared using Chi-square, Fisher’s exact test, and MANOVA, where appropriate.
Results
Of 518 breast cancer patients receiving neoadjuvant therapy, 81 (16%) had a pCR [77 of 456 (17%) chemotherapy; 4 of 62 (6%) endocrine therapy; p<0.05]. Four factors were associated with pCR: higher tumor grade (p=0.015), lack of estrogen and progesterone receptor (ER/PR) expression (p<0.0001), HER-2/neu amplification (p=0.025), and negative lymph node status (p<0.0001). On multivariate analysis, ER/PR negativity, HER-2/neu amplification, and negative lymph node status were found to significantly correlate with pCR.
Conclusions
Patients with ER/PR negative and Her-2/neu amplified breast cancer phenotypes are more likely to experience a pCR to neoadjuvant therapy. While a pCR is more frequently observed following neoadjuvant chemotherapy, it is rare following neoadjuvant endocrine therapy.
Keywords: Breast cancer, Neoadjuvant therapy, Pathologic response
INTRODUCTION
Neoadjuvant therapy is the standard of care for the management of locally advanced breast cancer, and is increasingly being used for the treatment of early-stage disease(1). By reducing tumor volume, it has been shown to increase rates of breast conserving surgery, and has additional theoretical advantages including treatment of undetected micrometastatic disease, and allowing early assessment of response to chemotherapy(2).
A small but consistent proportion of patients treated with neoadjuvant therapy have been observed to have a pathologic complete response (pCR) (3, 4). There has, as a result, been much interest in identifying these patients a priori (2, 5-11). The purpose of this study was to identify clinical and pathologic factors associated with a pCR following neoadjuvant therapy in patients with breast cancer treated at our institution. Unique to this study was the inclusion of patients undergoing both neoadjuvant systemic chemotherapy as well as neoadjuvant endocrine therapy.
METHODS
Study Design
Institutional review board approval was obtained prior to the commencement of this retrospective study. Written informed consent of patients was not required. The prospectively maintained surgical database at Washington University/Barnes Jewish Hospital was queried from January 1, 2000 to December 31, 2007 to identify all patients with a diagnosis of Stage I-III [according to American Joint Committee on Cancer guidelines (12)] biopsy-proven invasive breast cancer who underwent neoadjuvant chemotherapy or endocrine therapy (n=603). Patients were excluded if they had incomplete hormone receptor and/or HER-2/neu status information. Thus, 518 patients were included in the analysis.
Pathologic Assessment
Pathologic diagnosis, estrogen receptor (ER) status, progesterone receptor (PR) status, and HER-2/neu status were determined by core biopsy prior to neoadjuvant therapy. ER and PR status were determined by standard immunohistochemical methods. Tumors with greater than 10% stained cells were considered to have positive receptor status. All patients had HER-2/neu status assessed by fluorescence in situ hybridization (FISH). A pCR was determined by microscopic examination of the excised tumor and lymph nodes after completion of therapy, and defined as the disappearance of all invasive disease from the breast and lymph nodes. Patients who had only ductal carcinoma in situ (DCIS) in the breast tissue following neoadjuvant therapy were considered to have a pCR.
Statistical Analyses
Categorical data were compared using Fisher’s exact and chi squared tests. P values <0.05 were considered statistically significant. All P values were two-tailed. Univariate analysis was used to estimate the effects of clinical and pathologic characteristics on response to neoadjuvant therapy. To identify variables independently associated with a pCR, a multivariate analysis using MANOVA was performed. All variables with a P value < 0.20 in the univariate analysis were included in a logistic regression model, allowing for interaction. Overall survival, defined from the date of diagnosis to the date of death from any cause, was estimated with the Kaplan-Meier method. All analyses were performed with SAS version 9 (SAS Institute).
RESULTS
Over the period 2000-2007, 603 patients with breast cancer were treated with neoadjuvant therapy. Of these, 518 had complete hormone receptor and HER-2/neu amplification status, and were therefore included for subsequent analysis. Patient and tumor characteristics are shown in Table 1. The median patient age at the time of diagnosis was 52 years (range: 24-87). The majority of patients were Caucasian (69%), with 178 being African American (29%). Median follow-up was 44 months (range 3-133 months).
Table 1.
Patient and tumor characteristics of 518 patients undergoing neoadjuvant therapy for breast cancer.
| Characteristic | N (%) |
|---|---|
| Age | |
| <50 ≥50 |
261 (50%) 257 (50%) |
|
| |
| Race | |
| Caucasian African-American Other |
357 (69%) 149 (29%) 12 (2%) |
|
| |
| Clinical T stage | |
| T1 T2 T3 T4 Unknown |
59 (11%) 268 (52%) 90 (17%) 80 (16%) 21 (4%) |
|
| |
| Histology | |
| Ductal carcinoma Lobular carcinoma Inflammatory Mixed (ductal+lobular) Other |
379 (72%) 51 (10%) 35 (7%) 37 (7%) 16 (4%) |
|
| |
| Nuclear grade | |
| Grade 1 (well differentiated) Grade 2 (moderately differentiated) Grade 3 (poorly differentiated) Unknown |
51 (10%) 170 (33%) 282 (54%) 15 (3%) |
|
| |
| Estrogen receptor status | |
| Positive Negative |
298 (58%) 220 (42%) |
|
| |
| Progesterone receptor status | |
| Positive Negative |
219 (42%) 299 (58%) |
|
| |
| HER2-neu status | |
| Non-amplification Amplification |
384 (74%) 134 (26%) |
|
| |
| Clinical N status | |
| N0 N1 N2 N3 Unknown |
237 (46%) 210 (41%) 28 (5%) 14 (3%) 29 (5%) |
|
| |
| Clinical AJCC stage | |
| 1 2A 2B 3A 3B 3C 4 Unknown |
32 (6%) 177 (35%) 145 (28%) 52 (10%) 69 (13%) 12 (2%) 13 (3%) 18 (3%) |
|
| |
| Type of neoadjuvant therapy | |
| Chemotherapy only Hormonal therapy |
456 (88%) 62 (12%) |
|
| |
| Type of surgery performed on primary tumor |
|
| Lumpectomy Mastectomy |
207 (40%) 311 (60%) |
|
| |
| Pathologic complete response | |
| Yes No |
81 (16%) 437 (84%) |
AJCC = American Joint Commission on Cancer
The median tumor size was 3.8 cm. The majority of patients had T2 tumors (54%), with 12% having T1 tumors, 18% having T3 tumors, and 16% having T4 tumors. The vast majority of patients had ductal carcinoma (72%), with lobular, mixed and miscellaneous histologies each comprising ≤ 10%. Only 10% of tumors were well-differentiated, with 34% being moderately-well differentiated, and 56% being poorly differentiated. Lymphovascular invasion was present in 19%. Of patients with positive nodal status, most were N1. With regard to clinical stage, 64% of patients were stage 2A or 2B, with 30% of patients being stage 3A or higher.
With regard to hormone receptor status, 58% of patients were ER positive, and 42% were PR positive. The proportion of patients who were therefore double negative (ER negative and PR negative) was 41%. HER-2/neu gene amplification was detected in 148 patients (26%).
The majority of patients received neoadjuvant chemotherapy (88%), and the remaining patients received neoadjuvant endocrine therapy (12%). Overall, following neoadjuvant therapy, 60% of patients underwent mastectomy, with the remaining 40% having breast-conserving surgery. Upon pathologic review of tumor and nodal specimens, 81 of the 518 patients (16%) were found to have had a pCR. Of the 81 patients with a pCR, 77 (95%) occurred following neoadjuvant chemotherapy and 4 (5%) occurred following neoadjuvant endocrine therapy. In the total cohort, there were 155 patients with ER negative, PR negative and HER-2/neu non-amplified breast cancers [“triple negative breast cancer (TNBC)”], 58 patients with HER-2/neu amplified/ER negative breast cancers (“HER-2+”), and 305 patients with ER positive and/or PR positive breast cancers (“luminal” subtype). A pCR occurred in 23% of TNBC, 31% of HER2+, and 10% of luminal subtypes (P<0.0001).
Table 2 is a summary of the relationship between clinico-pathologic factors and pCR. On univariate analysis, factors associated with an increased percentage of pCR include high tumor grade (p=0.015), double negative hormone receptor status (p< 0.0001), HER-2/neu gene amplification (p=0.025), and N0 nodal status pre-therapy (p<0.001). On multivariate analysis, only hormone receptor status (odds ratio [OR] 2.8, p=0.012), HER-2/neu amplification (OR 2.2, p=0.022) and N0 nodal status pre-therapy (OR 0.2, p<0.0001) were independent predictors of complete pathologic response.
Table 2.
Univariate Predictors of Pathologic Complete Response to Neoadjuvant Therapy for Breast Cancer
| Characteristic | P value |
|---|---|
| Race | 0.287 |
| Increasing tumor grade | 0.015 |
| Clinical T stage | 0.231 |
| ER / PR negative status | 0.00002 |
| HER-2/neu amplification | 0.025 |
| Clinical N0 nodal status | < 0.0001 |
| Use of chemotherapy vs. endocrine therapy | 0.081 |
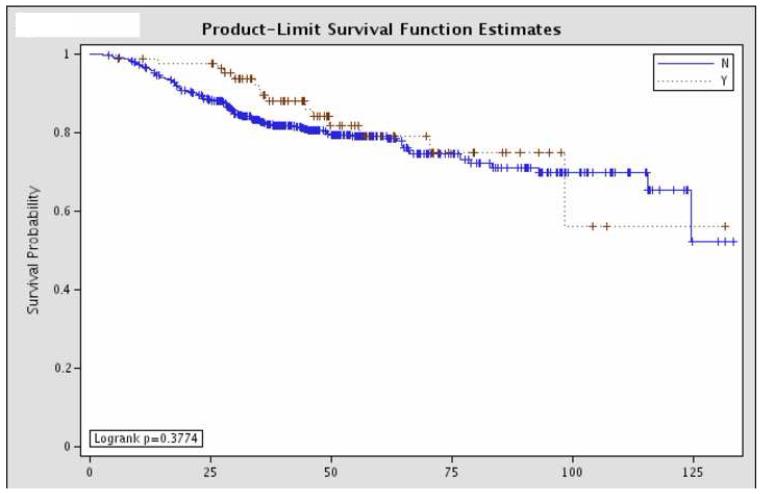
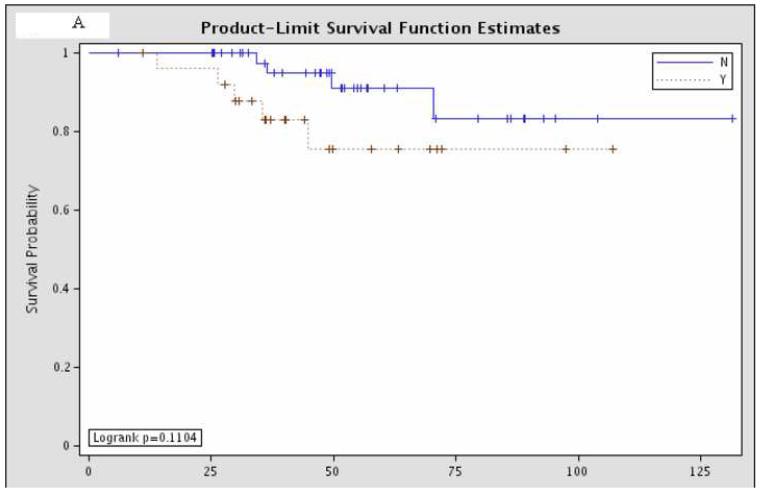
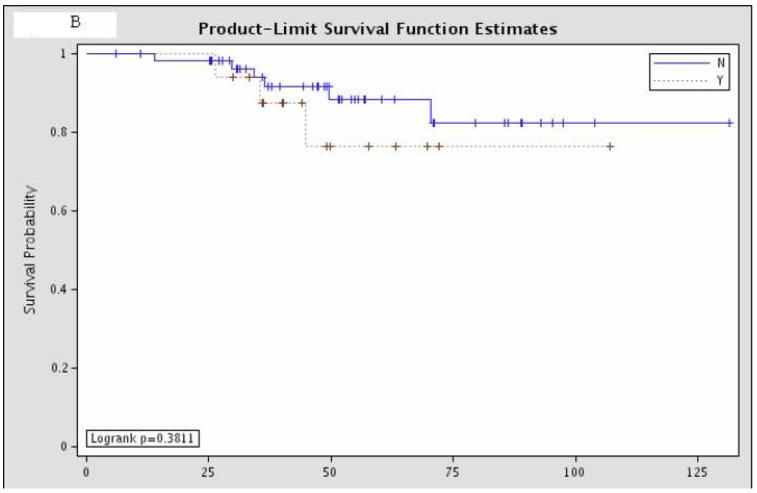
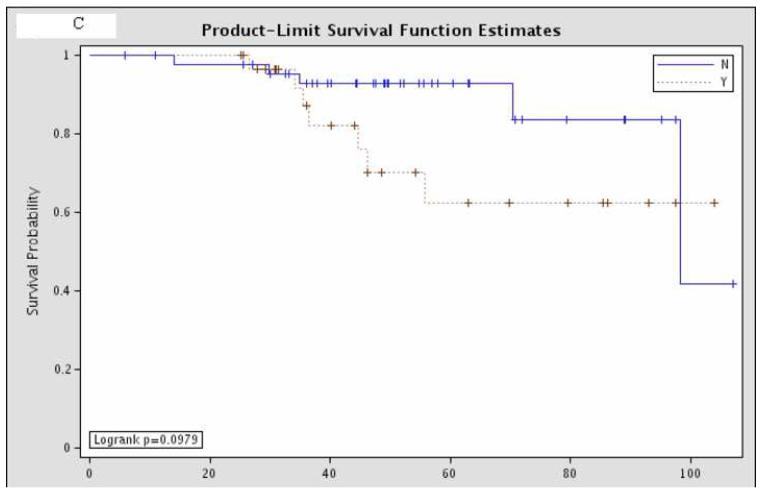
Overall survival was not significantly different between the 81 patients with pCR and the remaining 437 patients with residual disease (Figure 1). Further sub-group analysis of the overall survival of the patients with pCR showed that there was no significant difference according to hormone status or HER-2/neu amplification status (Figure 2).
Figure 1.
Overall survival comparing patients with a pathologic complete response (“Y”, n=81) and those with residual disease (“N”, n=437).
Figure 2.
Sub-group analysis of 81 patients with pCR, comparing overall survival according to (A) estrogen receptor status (“Y” = estrogen receptor positive; “N” = estrogen receptor negative; p=0.11); (B) progesterone receptor status (“Y” = progesterone receptor positive; “N” = progesterone receptor negative; p=0.38); and (C) HER-2/neu amplification status (“Y” = amplified; “N” = non-amplified; p=0.098).
DISCUSSION
This study reports outcomes of patients with breast cancer treated with neoadjuvant therapy from a large, single-institution series. An analysis was performed to identify clinico-pathologic factors associated with complete pathologic response. Three independent factors were found: hormone receptor status, HER-2/neu amplification status and nodal status. No survival benefit of pCR was observed over the median 44 month follow-up.
In clinical trials of neoadjuvant chemotherapy, the pCR has ranged from 6-26% (4, 13-16). In our study, the pCR for patients treated with neoadjuvant chemotherapy was found to be 16%, which is the same as for another large, single-institution retrospective analysis by Liedtke et al (3). In contrast, it appears that pCRs occur infrequently with neoadjuvant endocrine therapy. The pCR rates for tamoxifen and letrozole in the P024 trial were <1%, despite much higher clinical objective response rates (17). Four of the sixty-two patients in our study who were treated with neoadjuvant endocrine therapy demonstrated pCR.
The importance of a pCR lies in the fact that large clinical trials of neoadjuvant chemotherapy have demonstrated that patients with pCR have an improved disease-free survival (DFS) and overall survival (OS) compared to those with residual disease. In Protocol B-18 of the National Surgical Adjuvant Breast and Bowel Project (NSABP), patients who achieved a pCR had DFS and OS of 75% and 85%, respectively, at median follow-up of 9 nine years, compared with 58% and 73% for patients with residual disease (18). Similar results have been demonstrated in NSABP Protocol B-27 (4). This has led to interest in using pCR as a surrogate clinical endpoint for the testing of new chemotherapeutic regimens, thereby shortening the required duration of follow-up for analysis of these trials (19). In our study, the absence of a survival advantage for patients with pCR versus those with residual disease may be due to small sample size, short follow-up, and the inclusion of patients treated with neoadjuvant endocrine therapy.
There have been a number of reports analyzing factors associated with pCR after neoadjuvant therapy. Prior to the era of routine testing for hormone receptors, both NSABP B-18 and B-27 demonstrated a strong association (p<0.0001) between pathologic response and nodal status, with the group of patients with pCR being more frequently node-negative (4, 20). This was corroborated by the current study. Kuerer et al. (21) found that patients with a smaller clinical T size prior to chemotherapy had a higher rate of pCR. Clinical T size was not a significant predictive factor for pCR in our patient population. The reasons for this are unclear. In the Kuerer et al. (21) cohort, 30% of patients had a clinical T3 and 39% of patients had a clinical T4 breast cancer at diagnosis. This is dramatically different from our cohort where 11% of patients had T1 tumors and 52% of patients had T2 tumors. This predominance of smaller T1/T2 lesions in our study may account for this difference. It is also possible that our inclusion of neoadjuvant endocrine therapy in addition to neoadjuvant chemotherapy contributed to this finding.
Subsequently, multiple studies, both clinical trials and retrospective analyses, have shown a negative association between hormone receptor expression and pCR (13, 16, 22-25). For example, in the European Cooperative Trial in Operable breast cancer (ECTO), on multivariate analysis, ER status was found to be the only independent variable associated with achieving a pCR (odds ratio 5.77, p<0.0001) (22). These findings were confirmed in a large study by Berry et al (26). Further, Colleoni et al (6) demonstrated that patients with ER and PR negative tumors more commonly achieved pCR than those with ER and/or PR positive tumors (23% vs. 7%, respectively; P = 0.04).
While HER-2/neu amplification is well-known to be a negative prognostic factor, there are conflicting reports concerning its role as a predictive factor for response to neoadjuvant chemotherapy (7, 8, 11, 27-29). For example, in evaluating anthracycline and taxane neoadjuvant chemotherapy, Tiezzi et al (11) were unable to demonstrate an association between HER-2/neu amplification and pathologic response, whereas studies by Rouzier et al (28) and Rody et al (8) found that HER-2/neu amplification was highly predictive of pathologic response. The current study supports the finding that patients with HER-2/neu amplified breast cancers are more likely to experience a pCR than patients with HER-2/neu non-amplified breast cancers. This finding is likely dependent on the use of a trastuzumab-containing neoadjuvant regimen, though we are unable to confirm that all patients received such treatment in this retrospective analysis.
At the same time, however, we observed that those patients with TNBC [also termed “basal-like”(30)] are more likely to achieve pCR than non-TNBC (23% versus 10%, respectively). This is consistent with recent work by other groups (3, 5). In particular, Carey et al (5), in their study of 107 patients, found that it was two hormone receptor-negative subtypes (TNBC and HER2+) that displayed much higher rates of pCR (27% and 36%, respectively), compared to the luminal subtype, which had a pCR rate of 7% (P=0.01). These findings concur very closely with those reported here. The TNBC and HER2+ breast cancer subtypes are characterized by a high proliferation index which may account for an increased sensitivity to chemotherapy.
This study has a number of limitations. It is a retrospective analysis, with median follow-up of less than 4 years. The patient sample is heterogeneous with regard to stage, histology, and neoadjuvant protocol utilized. The patients underwent many variable neoadjuvant chemotherapy or endocrine therapy regimens based on individual patient and tumor characteristics. Further, the extended time period over which the patients were accrued to the neoadjuvant protocols could contribute to the pCR rate as improvements in chemotherapy and hormonal therapy have been observed over the study period. We are unable to control for these variations in the current retrospective analysis. These limitations may explain the lack of an observed difference in overall survival between the patients with a pCR and those with residual disease. In addition, we are unable to determine the decisions that resulted in our patients undergoing neoadjuvant therapy. At our institution, approximately 50% of patients undergoing neoadjuvant therapy have locally advanced disease and are not considered candidates for breast-conserving procedures, regardless of response to therapy. The remaining 50% undergo neoadjuvant therapy in hopes of achieving breast conservation. Based on the clinical T stages prior to therapy, approximately 60% of patients had T1/T2 lesions and breast conservation would be expected. Only 40% of the total group, however, underwent breast-conserving surgery. We cannot account for the patient factors and surgeon recommendations that may account for this discrepancy in this retrospective study.
In spite of this lack of some potentially relevant information, the current study represents a large, single-institution cohort analysis of patients undergoing neoadjuvant therapy for breast cancer. The fact that hormone receptor status, HER-2/neu amplification status and nodal status remain independent predictors of a pCR, despite the heterogeneity of the patient population, emphasizes their importance.
SUMMARY.
Patients with estrogen and progesterone receptor negative breast cancers with or without HER-2/neu amplification are more likely to experience a pathologic complete response to neoadjuvant chemotherapy. A pathologic complete response following neoadjuvant endocrine therapy is less common.
Table 3.
Multivariate Predictors of Pathologic Complete Response to Neoadjuvant Therapy for Breast Cancer
| Odds Ratio | ||||
|---|---|---|---|---|
| Characteristic |
|
P value | ||
| Point estimate | 95% confidence limits | |||
| ER / PR negative status |
2.80 | 1.25 | 6.29 | 0.012 |
|
| ||||
| HER-2/neu amplification |
2.21 | 1.12 | 4.35 | 0.022 |
|
| ||||
| N0 nodal status | 0.18 | 0.093 | 0.341 | < 0.0001 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W, Lebeau A, Loibl S, Miller W, Seeber S, Semiglazov V, Smith R, Souchon R, Stearns V, Untch M, von Minckwitz G. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–1949. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 2.Tewari M, Krishnamurthy A, Shukla HS. Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg Oncol. 2008;17:301–311. doi: 10.1016/j.suronc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 5.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 6.Colleoni M, Minchella I, Mazzarol G, Nole F, Peruzzotti G, Rocca A, Viale G, Orlando L, Ferretti G, Curigliano G, Veronesi P, Intra M, Goldhirsch A. Response to primary chemotherapy in breast cancer patients with tumors not expressing estrogen and progesterone receptors. Ann Oncol. 2000;11:1057–1059. doi: 10.1023/a:1008334404825. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Sanchez M, Gamboa-Dominguez A, Uribe N, Garcia-Ulloa AC, Flores-Estrada D, Candelaria M, Arrieta O. Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med Oncol. 2006;23:171–183. doi: 10.1385/MO:23:2:171. [DOI] [PubMed] [Google Scholar]
- 8.Rody A, Karn T, Gatje R, Ahr A, Solbach C, Kourtis K, Munnes M, Loibl S, Kissler S, Ruckhaberle E, Holtrich U, von Minckwitz G, Kaufmann M. Gene expression profiling of breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide within the GEPARTRIO trial: HER-2, but not topoisomerase II alpha and microtubule-associated protein tau, is highly predictive of tumor response. Breast. 2007;16:86–93. doi: 10.1016/j.breast.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, Buzdar AU, Garbay JR, Spielmann M, Mathieu MC, Symmans WF, Wagner P, Atallah D, Valero V, Berry DA, Hortobagyi GN. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–8339. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- 10.Rouzier R, Pusztai L, Garbay JR, Delaloge S, Hunt KK, Hortobagyi GN, Berry D, Kuerer HM. Development and validation of nomograms for predicting residual tumor size and the probability of successful conservative surgery with neoadjuvant chemotherapy for breast cancer. Cancer. 2006;107:1459–1466. doi: 10.1002/cncr.22177. [DOI] [PubMed] [Google Scholar]
- 11.Tiezzi DG, Andrade JM, Ribeiro-Silva A, Zola FE, Marana HR, Tiezzi MG. HER-2, p53, p21 and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combination. BMC Cancer. 2007;7:36. doi: 10.1186/1471-2407-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer . In: AJCC Cancer Staging Manual. Springer, editor. New York: 2002. pp. 221–240. [Google Scholar]
- 13.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Dieras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, Guastalla JP, Pujade-Lauraine E, Kerbrat P, Maillart P, Penault-Llorca F, Buyse M, Pouillart P. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol. 2004;22:4958–4965. doi: 10.1200/JCO.2004.02.122. [DOI] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Costa SD, Raab G, Blohmer JU, Eidtmann H, Hilfrich J, Merkle E, Jackisch C, Gademann G, Tulusan AH, Eiermann W, Graf E, Kaufmann M. Dose-dense doxorubicin, docetaxel, and granulocyte colony-stimulating factor support with or without tamoxifen as preoperative therapy in patients with operable carcinoma of the breast: a randomized, controlled, open phase IIb study. J Clin Oncol. 2001;19:3506–3515. doi: 10.1200/JCO.2001.19.15.3506. [DOI] [PubMed] [Google Scholar]
- 16.von Minckwitz G, Raab G, Caputo A, Schutte M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann H, Lampe D, Jackisch C, du BA, Kaufmann M. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 17.Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, Mauriac L, Ellis M, Lassus M, Chaudri-Ross HA, Dugan M, Borgs M. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001;12:1527–1532. doi: 10.1023/a:1013128213451. [DOI] [PubMed] [Google Scholar]
- 18.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 19.Hanrahan EO, Hennessy BT, Valero V. Neoadjuvant systemic therapy for breast cancer: an overview and review of recent clinical trials. Expert Opin Pharmacother. 2005;6:1477–1491. doi: 10.1517/14656566.6.9.1477. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Jr., Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 21.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 22.Gianni L, Baselga J, Eiermann W, Guillem P, V, Semiglazov V, Lluch A, Zambetti M, Sabadell D, Raab G, Llombart CA, Bozhok A, Martinez-Agullo A, Greco M, Byakhov M, Lopez Lopez JJ, Mansutti M, Valagussa P, Bonadonna G. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clin Cancer Res. 2005;11:8715–8721. doi: 10.1158/1078-0432.CCR-05-0539. [DOI] [PubMed] [Google Scholar]
- 23.Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F, Middleton L, Hortobagyi GN, Gonzalez-Angulo AM. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 24.Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91:2012–2017. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Minckwitz G, Blohmer JU, Raab G, Lohr A, Gerber B, Heinrich G, Eidtmann H, Kaufmann M, Hilfrich J, Jackisch C, Zuna I, Costa SD. In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol. 2005;16:56–63. doi: 10.1093/annonc/mdi001. [DOI] [PubMed] [Google Scholar]
- 26.Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozzetti C, Musolino A, Camisa R, Bisagni G, Flora M, Bassano C, Martella E, Lagrasta C, Nizzoli R, Personeni N, Leonardi F, Cocconi G, Ardizzoni A. Evaluation of HER-2/neu amplification and other biological markers as predictors of response to neoadjuvant anthracycline-based chemotherapy in primary breast cancer: the role of anthracycline dose intensity. Am J Clin Oncol. 2006;29:171–177. doi: 10.1097/01.coc.0000204405.96572.f9. [DOI] [PubMed] [Google Scholar]
- 28.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Yang Y, Smith T, Kau SW, McConathy JM, Esteva FJ, Kuerer HM, Symmans WF, Buzdar AU, Hortobagyi GN, Pusztai L. Correlation between HER-2 expression and response to neoadjuvant chemotherapy with 5-fluorouracil, doxorubicin, and cyclophosphamide in patients with breast carcinoma. Cancer. 2003;97:1758–1765. doi: 10.1002/cncr.11245. [DOI] [PubMed] [Google Scholar]
- 30.Perou CM, Sorlie T, Eisen MB, van de, Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular Portraits of Human Breast Tumors. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]