Abstract
Background
We investigated factors associated with positive margins following mastectomy and the impact on outcomes.
Methods
We identified 240 patients with stage I-III invasive breast cancer who underwent mastectomy from 1999-2009. Data included patient and tumor characteristics, pathologic margin assessment, and outcomes. Margin positivity was defined as the presence of in situ or invasive malignancy present at any margin. Descriptive statistics were utilized for data summary and were compared using Chi-square.
Results
Of the 240 patients, 132 (55%) had a simple mastectomy with sentinel lymph node biopsy and 108 (45%) had a modified radical mastectomy. Overall, 21 (9%) patients had positive margins, including 12 (57%) with one positive margin, 3 (14%) with two positive margins, and 6 (29%) with three or more positive margins. The most commonly affected margin was the deep margin (48% of patients). Eight (38%) of the 21 patients received adjuvant chest wall irradiation. There were no differences between patients who had a positive margin versus those who did not with respect to patient age, race, percentage of in situ component, tumor size, tumor grade, lymphovascular invasion, or immunostain profile (p>0.05 for all). None of the patients with positive margins experienced a local recurrence.
Conclusions
Positive margins following mastectomy occurred in nearly 10% of our patients. No specific patient or tumor characteristics predicted a risk for having a positive margin. Despite the finding that only approximately 40% of patients received adjuvant radiation in the setting of a positive margin, no local recurrences have been observed.
Keywords: Breast cancer, Margin status, Mastectomy, Radiation therapy
INTRODUCTION
Although there has been a major increase in the use of breast-conserving surgery in recent years, many patients with invasive breast cancer still require mastectomy for various indications, including locally advanced disease, inflammatory carcinoma, or multicentric disease. Other patients may elect to undergo mastectomy to avoid consequences of lumpectomy such as adjuvant radiotherapy or the potential need for re-excision. Though mastectomy involves removal of all breast tissue, the possibility of positive margins following surgical management still exists, and this has been shown to portend a poorer prognosis in regards to local and systemic disease recurrence. However, the risk factors and natural history of having a positive margin status following mastectomy are less well studied compared to breast-conserving techniques, where several factors such as younger age, larger tumor size, presence of multifocal disease, and percentage of in situ component have been associated with an increased risk of positive margins. [1-3] Furthermore, the definition of adequate margins is still controversial in both mastectomy and breast conservation techniques, and it varies between surgeons and institutions from simply the absence of tumor at the inked margins of the resected specimen (or a clear cell line at the inked margin) to more than 2 mm of normal tissue around the tumor margins. [4-5]
Significant debate also surrounds whether subsequent management involving surgery or radiation therapy is indicated in the cases where a positive margin is identified following mastectomy. The belief that removal of intact pectoral fascia may be adequate to prevent local recurrence has encouraged breast surgeons over the years to opt for close monitoring rather than re-excision in cases where there is a close deep margin, and reliance on adjuvant radiotherapy and systemic chemotherapy has been shown to reduce local recurrence as well. [6-8] Therefore, in this study, our goals were to identify potential specific predictors of having positive margins following mastectomy in patients with stage I-III disease and to evaluate if the presence of positive margins was associated with compromised patient outcomes.
PATIENTS AND METHODS
Institutional review board approval was obtained prior to the commencement of this retrospective study. Written informed consent of patients was not required. Clinical, demographic, and pathologic data from all breast cancer patients treated at our institution are prospectively recorded in a database. We reviewed this database and identified all patients undergoing mastectomy for stage I-III invasive breast cancer from 1999-2009. Inclusion criteria included patients who had invasive ductal carcinoma with or without associated ductal carcinoma in situ (DCIS). All patients included in the study were diagnosed by pre-operative image-guided needle core biopsy. Exclusion criteria were patients with DCIS only (stage 0), invasive lobular carcinoma, lobular carcinoma in situ (LCIS), or diagnosis by excisional biopsy.
Information on tumor histology and surgical margins was obtained from the original pathology reports. Tumor size was assessed microscopically by our dedicated breast pathologists. The tissue specimen was serially sectioned at 3 to 5 mm intervals in quadrant blocks in the anteroposterior plane perpendicular to the mediolateral axis and stained by hematoxylin and eosin. Detailed examination was performed, including documentation of the invasive tumor size and the tumor-margin distance for all six margins (anterior, posterior, medial, lateral, superior, and inferior). Margin positivity was defined as the presence of in situ or invasive malignancy focally or extensively at the ink of any margin. Focal positivity of a margin was defined as a single, small focus of tumor abutting the margin, typically seen only on one slide (i.e., one 3-5 mm slice of the tissue block). Extensively positive margins were defined by a large area of margin positivity with multiple slides involved or multiple sites along the margin involved.
Patients were grouped according to whether they had positive or negative margins after mastectomy. Patient and tumor characteristics, as well as the pathology findings following mastectomy were recorded. Variables included patient age, patient race, tumor size, tumor grade, nodal status, tumor stage, percentage of DCIS in the specimen, presence or absence of lymphovascular invasion (LVI), and biomarker profile of the tumor. All data were transferred to a single spreadsheet (Excel; Microsoft, Redmond, WA). Descriptive statistics were used to assess frequency distributions between the two groups. Categorical variables and continuous variables were compared between the two groups using chi-squared and analysis of variance tests, where appropriate. Statistical analyses were performed using a statistical package SAS (SAS Institutes, Cary, NC). P values <0.05 were considered to be statistically significant.
RESULTS
We identified 240 of 617 patients (39%) who underwent mastectomy for stage I-III invasive breast cancer from 1999-2009 and met the inclusion criteria of the study. Of the 240 patients, 132 (55%) underwent simple mastectomy with sentinel lymph node biopsy and 108 (45%) had a modified radical mastectomy. Skin-sparing and/or nipple-sparing techniques were utilized in 146 (61%) of patients undergoing either simple or modified radical mastectomy. The pathologic stage included 74 (31%) stage I, 108 (45%) stage II, 35 (15%) stage III, and 23 (9%) unknown. Overall, 21 (9%) patients had at least one positive margin on the final mastectomy specimen, while 219 (91%) had negative margins at all six examined margin sites. Neoadjuvant therapy was utilized in 61 (25%) of the 240 patients, including 4 (18%) of the 21 patients with positive margins. Table 1 summarizes the patient and tumor characteristics for both groups. There were no differences between patients who had a positive margin versus those who did not with respect to patient age, race, type of surgical technique (skin-sparing versus total mastectomy), percentage of in situ component, tumor size, tumor grade, lymphovascular invasion, or immunostain profile (p>0.05 for all).
Table 1.
Patient and tumor characteristics for 240 patients who underwent mastectomy for stage I-III invasive breast cancer according to margin status*
| Positive margins N = 21 (9%) |
Negative margins N = 219 (91%) |
Total N = 240 (100%) |
|
|---|---|---|---|
| AGE | |||
| <50 | 11 (52%) | 58 (26%) | 69 (29%) |
| ≥50 | 10 (48%) | 161 (74%) | 171 (71%) |
| RACE | |||
| African- American |
4 (19%) | 50 (23%) | 54 (23%) |
| Caucasian | 17 (81%) | 159 (73%) | 176 (73%) |
| Other | 0 (0%) | 10 (4%) | 10 (4%) |
| % DCIS | |||
| <25 % | 16 (76%) | 188 (86%) | 204 (85%) |
| 26-50 % | 3 (14%) | 14 (6%) | 17 (7%) |
| >50 | 2 (10%) | 17 (8%) | 19 (8%) |
| STAGE | |||
| I | 7 (33%) | 67 (31%) | 74 (31%) |
| II | 9 (43%) | 100 (45%) | 109 (45%) |
| III | 5 (24%) | 30 (14%) | 35 (15%) |
| Unknown | 0 (0%) | 22 (10%) | 22 (9%) |
|
TUMOR
SIZE |
|||
| 1 | 10 (47%) | 113 (51%) | 123 (51%) |
| 2 | 8 (38%) | 69 (32%) | 77 (32%) |
| 3 | 1 (5%) | 16 (7%) | 17 (7%) |
| 4 | 2 (10%) | 10 (5%) | 12 (5%) |
| Unknown | 0 (0%) | 11 (5%) | 11 (5%) |
|
NODAL
STATUS |
|||
| 0 | 10 (48%) | 104 (48%) | 114 (47%) |
| 1 | 8 (38%) | 94 (43%) | 102 (43%) |
| 2 | 3 (14%) | 7 (3%) | 10 (4%) |
| 3 | 0 (0%) | 4 (2%) | 4 (2%) |
| Unknown | 0 (0%) | 10 (4%) | 10 (4%) |
| GRADE | |||
| I | 5 (24%) | 41 (19%) | 46 (19%) |
| II | 8 (38%) | 70 (32%) | 78 (33%) |
| III | 8 (38%) | 92 (42%) | 100 (41%) |
| Unknown | 0 (0%) | 16 (7%) | 16 (7%) |
| LVI | |||
| Present | 7 (33%) | 67 (31%) | 74 (31%) |
| Absent | 14 (67%) | 145 (66%) | 159 (66%) |
| Unknown | 0 (0%) | 7 (3%) | 7 (3%) |
|
ER STATUS |
|||
| Positive | 18 (86%) | 142 (65%) | 160 (67%) |
| Negative | 3 (14%) | 72 (33%) | 75 (31%) |
| Unknown | 0 (0%) | 5 (2%) | 5 (2%) |
|
PR
STATUS |
|||
| Positive | 14 (67%) | 128 (58%) | 142 (59%) |
| Negative | 7 (33%) | 86 (40%) | 93 (39%) |
| Unknown | 0 (0%) | 5 (2%) | 5 (2%) |
| HER2/NEU | |||
| Amplified | 8 (38%) | 72 (33%) | 80 (33%) |
| Non- amplified |
7 (33%) | 67 (31%) | 74 (31%) |
| Unknown | 6 (29%) | 80 (36%) | 86 (36%) |
p>0.05 for all comparisons between positive and negative margin groups.
DCIS = ductal carcinoma in situ; LVI = lymphovascular invasion; ER = estrogen receptor; PR = progesterone receptor
For the 21 patients with a positive margin on final pathology, 12 (57%) had only one positive margin, 3 (14%) had two positive margins, and 6 (29%) had three or more positive margins. A total of 37 positive margins were identified in the 21 patients. The deep pectoralis margin was the most commonly affected (48%), followed by the inferior margins (20%). Only one patient in the positive margin group underwent a re-excision for a positive anterior margin and the final re-excision pathology was benign. Table 2 summarizes the positive margin data.
Table 2.
Margin details for the 21 patients with positive margins following mastectomy.
| Number of positive margins |
Patients N = 21 |
|---|---|
| 1 | 12 (57%) |
| 2 | 3 (14%) |
| 3 or more | 6 (29%) |
| Site of positive margin |
Total positive margins N = 37 |
|---|---|
| Posterior (deep) | 18 (48%) |
| Inferior | 7 (20%) |
| Superior | 4 (11%) |
| Other | 8 (21%) |
Eight (38%) of the 21 patients with a positive margin following mastectomy underwent adjuvant chest wall radiation therapy. In 6 of the 8, there were other indications for adjuvant chest wall radiation (T3 tumor prior to neoadjuvant therapy, >4 positive axillary lymph nodes, and/or presence of lymphovascular invasion). However, in 2 of the 8 patients who received adjuvant radiation therapy, the only indication for radiation therapy was the presence of the positive margin following mastectomy. For the 13 patients with a positive margin who did not receive adjuvant chest wall radiation therapy, the positive mastectomy margin was the only risk factor in 10, while 3 had additional risk factors for locoregional recurrence. There were no statistically significant relationships between the use of adjuvant radiation and site of the positive mastectomy margin (p>0.05).
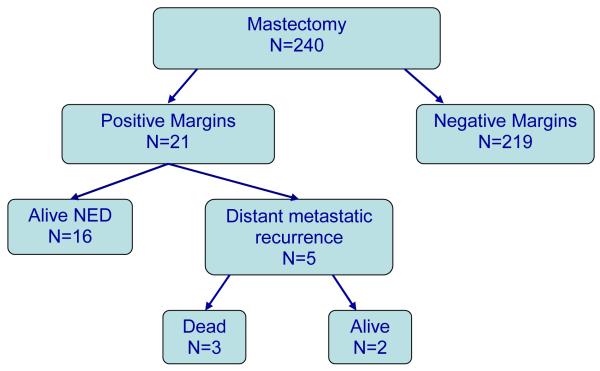
The median follow-up was 74 months (range 7-122 months). At follow-up, none of the patients in the positive margin group had experienced a locoregional recurrence. Overall, of the 21 patients with a positive mastectomy margin, 16 were alive at follow-up without evidence of locoregional or systemic recurrence. The remaining 5 patients suffered distant metastatic recurrences; 3 have died and 2 are living with metastatic disease. Figure 1 illustrates the outcomes for the study patients.
Figure 1.
Flow diagram illustrating the outcomes for the 240 patients undergoing mastectomy for stage I-III invasive breast cancer.
DISCUSSION
The standard of care for patients with breast cancer for over half of the 20th century was the radical mastectomy introduced by Halsted. [5] Gradually replaced by the less morbid modified radical mastectomy and more recently by skin-sparing and nipple/areola-sparing mastectomy in selected cases, these procedures still include the total removal of the mammary gland and the pectoral fascia, which is now commonly accepted as the marker for the deep margin in modern mastectomy. [5] The removal of this layer has traditionally been considered a key factor in assuring adequacy of resection in mastectomy. Challenging this practice, a Swedish randomized controlled trial evaluated the necessity of removing the pectoral fascia. [8] Consistent with previous studies, the results of this trial showed that the majority of chest wall recurrences occurred within 5 years of surgery. However, they concluded that neither chest wall recurrence rate nor breast cancer-specific survival was significantly influenced by the preservation of pectoralis fascia; they observed 18 chest wall recurrences in the pectoralis fascia preservation group and 10 in the removal group. [8] It is routine practice at our institution to remove the pectoralis fascia en bloc with the breast and all patients in the current study would have undergone this standard approach. However, nearly half of our patients with a positive margin following mastectomy had a positive deep pectoralis margin, raising the possibility of inadequate or incomplete fascial excision.
Following mastectomy, locoregional recurrence may occur in up to 20% of patients with operable breast cancer within 10 years of surgery. [5, 9-11] A critical prognostic factor after initial surgical management, locoregional recurrence has been shown to result in a 5-year overall survival rate of 40-60% and is associated with a distant metastasis rate that can reach up to 80%. [9-11] In the great majority of the patients affected, the chest wall is the site of failure, and treatment usually requires surgical excision when possible as well as radiation therapy and cytotoxic or hormonal therapy if the patient is an appropriate candidate. [9-11]
Several studies have investigated the outcomes for patients who suffer a chest wall recurrence following mastectomy with respect to various clinical endpoints as well as patient and/or tumor characteristics, surgical therapy, pathologic assessment, and hormone receptor status. [9, 12-14] One factor which has been shown to be of significance is the disease-free interval from original diagnosis to chest wall recurrence. [9] In a series of 113 patients who were treated for chest wall recurrence and followed for 10 years, Haffty et al. [9] identified that a longer time to local recurrence from the initial operation and a positive progesterone receptor status were both associated with a higher distant metastasis-free rate and with increased long-term survival. Evaluating for other favorable clinical indicators of increased overall or disease-free survival following chest wall recurrence, other studies have shown that a single recurrence, a recurrence measuring ≤ 1 cm, or a disease-free interval of >24 months from initial mastectomy are associated with better outcomes. [9, 12-14]
Margin status and the definition of a positive or close surgical margin following surgical therapy for breast cancer has been a subject of substantial debate. [15-16] Positive margin status has been found to translate into a higher incidence of systemic recurrence, and the presence specifically of a positive deep margin after mastectomy has been associated with an increased local recurrence. [17] In a study by Freedman et al. [17], 789 patients with T1-2 tumors were studied. Within the series, they identified 34 patients who underwent modified radical mastectomy and who had a tumor margin between 0-6 mm from the deep resection margin, including two patients with tumor present at the margin. With a median interval of 26 months, five chest wall recurrences developed, three of which were associated with distant metastasis. In the analysis of patient and tumor characteristics, the study also found that younger patients (age ≤50) with positive or close margins were at the highest risk for chest wall recurrence (28% at 8 years) and they recommended consideration of adjuvant radiation therapy in this high-risk group. [17] Other factors associated with chest wall failure have been shown to include tumor size ≥ 5cm, 4 or more positive axillary lymph nodes, extracapsular extension of the involved lymph nodes, negative estrogen receptor status, Her2/neu amplification, and high grade DCIS. [18, 19]
In the current study, none of our patients with positive margins following mastectomy suffered a locoregional recurrence, though five patients did develop distant metastases. There were no specific patient or tumor characteristics which predicted the risk for a positive margin. The rate of positive margins (9%) in our cohort was relatively small and may account for the inability to identify any predictors associated with margin positivity. Despite utilizing skin-sparing techniques in over 60% of the patients, our rate of positive margins was similar to that reported for patients undergoing traditional total mastectomy. [20] The rate of positive or close margins (within 2 mm) following mastectomy has been reported to be significantly higher when skin-sparing mastectomy techniques are performed (29%) versus traditional total mastectomy (12%). [20] Patients with multiple ipsilateral tumors and/or upper inner quadrant disease have been associated with a significantly higher risk for positive margins following skin-sparing mastectomy. [20] Patients undergoing skin-sparing mastectomy have also been shown to be at greater risk for having positive superficial margins. [21] In one series, the superficial margin of the specimen was positive in 38% of the cases. [21] Although this study only investigated the status of the superficial margin, preliminary results also supported a higher rate of local recurrence (10% vs. 4%) in patients with a positive resection margin compared to those with a negative margin. [21] We previously reported our own experience with skin-sparing mastectomy techniques, demonstrating a low local recurrence rate of 5.3%. [22] The majority of local recurrences occurred in the quadrant of the original tumor; we similarly concluded that the most important factor contributing to this may be inadequate excision and we recommended taking additional tissue margins directly over the tumor site. [22] Whether this technique, which we have widely adopted at our institution, led to our low rates of positive margins is unclear, but unlikely given the overlap in the two study time periods.
The best adjuvant therapy for patients with a positive margin following mastectomy has also been examined in the literature. Radiation therapy delivered to the chest wall and regional lymph nodes, including the supra- and infraclavicular regions and the internal mammary nodes, has proven to be of benefit in preventing relapse in the chest wall and supraclavicular fossa for high-risk patients. [23-25] The American Society of Clinical Oncology guidelines state that adjuvant radiotherapy is suggested following mastectomy in patients with four or more involved axillary lymph nodes and in patients with T3 tumors. [26] Additional professional guidelines support the use of post-mastectomy radiation therapy in cases where there is skin, pectoral or chest wall invasion, but no clear guidelines with respect to margin status are defined. [26, 27] Regarding the effect of multimodal therapy, multiple trials have found a decrease in locoregional recurrence and increase in survival rate: in a study of 1375 postmenopausal women with stage IIIII disease treated with mastectomy, local recurrence decreased from 35% to 8%, and both disease-free survival (36% vs. 24%) and overall survival (45% vs. 36%) at 10 years increased when radiotherapy was used with tamoxifen versus when tamoxifen was used alone. [24]
The data regarding the utility of adjuvant radiation therapy for patients with positive margins and without any other significant risk factors for local recurrence are somewhat conflicting. Truong et al. [28] excluded all patients with clear indications for radiation therapy following mastectomy (i.e., T3-4 or node-positive disease) and examined 94 patients with positive margins following modified radical mastectomy. They were compared in relation to adjuvant therapies, relapse rate, and clinical outcomes. Though there was an overall trend towards increased locoregional recurrence in the patients who did not receive post-operative radiotherapy, no significant association was found for any endpoint (local or distant relapse, disease-free or overall survival). They concluded that the small benefit in absolute reduction in locoregional recurrence with post-operative radiotherapy did not clearly outweigh the possible therapy-related morbidities. [28] However, in the presence of other predictors for recurrence, such as young age (≤50), T2 or greater tumor size, grade III histology, or lymphovascular invasion, the authors recommended consideration of adjuvant radiotherapy as their analysis of these specific subgroups demonstrated a higher locoregional recurrence rate (20% vs. 0%) in comparison with patients with none of these additional factors. [28] These conflicting, and difficult-to-interpret data likely contributed to the observed rate of <40% use of adjuvant radiation therapy in our patients with positive margins.
In a recent systematic review, data were pooled from 25 studies and included 18,863 patients with non-inflammatory breast cancer treated with radical or modified radical mastectomy. [29] Analysis of post-operative findings showed that a positive margin was identified in 2.5%, a close margin in 8.0%, and muscle or fascia invasion in 7.2%. Of note, across the studies included, a variety of definitions for close margins were used and ranged from a margin of ≤2 mm to 4-10 mm. In a meta-analysis of five studies of non-inflammatory breast cancer without radiotherapy, the review found that local recurrence was significantly increased by an involved or close margin (relative risk 2.6, p<0.00001). In contrast, margin status had no effect on the rate of relapse (relative risk 0.84, p= 0.77) in a subset analysis of patients who received post-operative radiotherapy. After combining data from all studies in the review, including those involving inflammatory breast cancer and skin-sparing mastectomies, a relative risk of 2.6 (p<0.00001) was observed for relapse when close or involved margins were present, emphasizing the importance of margin status across a heterogenous group of patients. [29]
The results of our single-institution study must be interpreted in light of its limitations. Limitations of our study include its retrospective analysis approach, the limited number of patients in the positive margin cohort precluding multivariate analysis, and the variability in types and methods of local and systemic adjuvant therapies. Another limitation of our study was the heterogeneity of our patient population. Wide variations in tumor stage, grade, and size certainly could play a significant role in local recurrence rates. Inclusion of patients treated by multiple surgeons may also play a factor with respect to mastectomy technique and flap thickness. We analyzed only patients with true positive margins (tumor present microscopically at the margin) for this reason, but we recognize that the definition of a positive margin varies from institution to institution. In addition, pathologist interpretation of margins is a subjective one that may vary by institution. The patients underwent many variable adjuvant treatment regimens based on individual patient and tumor characteristics. The types of regimens and delivery methods may have changed during the relatively long study period. We are unable to control for these variations in the current retrospective analysis. Finally, as time passes and longer followup is available for these patients, we may see further trends regarding predisposing characteristics for patients developing local recurrences.
Despite these limitations, the current study highlights the difficulty surrounding the management of patients with positive margins following mastectomy. Our observed rate of positive margins was relatively low (9%) and none of those patients experienced a locoregional recurrence, though five suffered distant recurrences. Further study is needed to determine the role of positive margins in the setting of various biologic factors predictive for local recurrence to determine whether certain subsets of patients may be more predisposed for local recurrence. This will help to further inform guidelines for post-operative radiation therapy in future randomized clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.O’Sullivan MJ, Li T, Freedman G, Morrow M. The effect of multiple reexcisions on the risk of local recurrence after breast conserving surgery. Ann Surg Oncol. 2007;14(11):3133–40. doi: 10.1245/s10434-007-9523-4. [DOI] [PubMed] [Google Scholar]
- 2.Dillon MF, Maguire AA, McDermott EW, et al. Needle core biopsy characteristics identify patients at risk of compromised margins in breast conservation surgery. Mod Pathol. 2007;21(1):39–45. doi: 10.1038/modpathol.3800975. [DOI] [PubMed] [Google Scholar]
- 3.Melstrom LG, Melstrom KA, Wang EC, et al. Ductal carcinoma in situ: size and resection volume predict margin status. Am J Clin Oncol. 2010;33(5):438–42. doi: 10.1097/COC.0b013e3181b9cf31. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 6.Ahlborn TN, Gump FE, Bodian C, Habif DV, Kister S. Tumor to fascia margin as a factor in local recurrence after modified radical mastectomy. Surg Gynecol Obstet. 1988;166(6):523–6. [PubMed] [Google Scholar]
- 7.Mentzer SJ, Osteen RT, Wilson RE. Local recurrence and the deep resection margin in carcinoma of the breast. Surg Gynecol Obstet. 1986;163(6):513–7. [PubMed] [Google Scholar]
- 8.Dalberg K, Krawiec K, Sandelin K. Eleven-year follow-up of a randomized study of pectoral fascia preservation after mastectomy for early breast cancer. World J Surg. 2010;34(11):2539–44. doi: 10.1007/s00268-010-0737-4. [DOI] [PubMed] [Google Scholar]
- 9.Haffty BG, Hauser A, Choi DH, et al. Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer. 2004;100(2):252–63. doi: 10.1002/cncr.11915. [DOI] [PubMed] [Google Scholar]
- 10.Bedwinek JM, Lee J, Fineberg B, Ocwieza M. Prognostic indicators in patients with isolated local-regional recurrence of breast cancer. Cancer. 1981;47(9):2232–5. doi: 10.1002/1097-0142(19810501)47:9<2232::aid-cncr2820470921>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Borner M, Bacchi M, Goldhirsch A, et al. First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase III multicenter study comparing systemic treatment with observation after excision and radiation. Swiss Group for Clinical Cancer Research. J Clin Oncol. 1994;12(10):2071–7. doi: 10.1200/JCO.1994.12.10.2071. [DOI] [PubMed] [Google Scholar]
- 12.Freedman GM, Fowble BL. Local recurrence after mastectomy or breast-conserving surgery and radiation. Oncology (Williston Park) 2000;14(11):1561–81. discussion 1581-2, 1582-4. [PubMed] [Google Scholar]
- 13.Van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–50. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 14.Pisansky TM, Ingle JN, Schaid DJ, et al. Patterns of tumor relapse following mastectomy and adjuvant systemic therapy in patients with axillary lymph node-positive breast cancer. Impact of clinical, histopathologic, and flow cytometric factors. Cancer. 1993;72(4):1247–60. doi: 10.1002/1097-0142(19930815)72:4<1247::aid-cncr2820720418>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Meric F, Mirza NQ, Vlastos G, et al. Positive surgical margins and ipsilateral breast tumor recurrence predict disease-specific survival after breast-conserving therapy. Cancer. 97(4):926–33. doi: 10.1002/cncr.11222. [DOI] [PubMed] [Google Scholar]
- 16.Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18(8):1668–75. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 17.Freedman GM, Fowble BL, Hanlon AL, et al. A close or positive margin after mastectomy is not an indication for chest wall irradiation except in women aged fifty or younger. Int J Radiat Oncol Biol Phys. 1998;41(3):599–605. doi: 10.1016/s0360-3016(98)00103-5. [DOI] [PubMed] [Google Scholar]
- 18.Feigenberg SJ, Price-Mendenhall N, Benda RK, Morris CG. Postmastectomy radiotherapy: patterns of recurrence and long-term disease control using electrons. Int J Radiat Oncol Biol Phys. 2003;56(3):716–25. doi: 10.1016/s0360-3016(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 19.Rashtian A, Iganej S, Amy-Liu IL, Natarajan S. Close or positive margins after mastectomy for DCIS: pattern of relapse and potential indications for radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1016–20. doi: 10.1016/j.ijrobp.2008.06.1954. [DOI] [PubMed] [Google Scholar]
- 20.Sheikh F, Rebecca A, Pockaj B, et al. Inadequate Margins of Excision When Undergoing Mastectomy for Breast Cancer: Which Patients are at Risk? Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1406-4. [DOI] [PubMed] [Google Scholar]
- 21.Cao D, Tsangaris TN, Kouprina N, et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. 2008;15(5):1330–40. doi: 10.1245/s10434-007-9795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughan A, Dietz JR, Aft R, et al. Patterns of local breast cancer recurrence after skin-sparing mastectomy and immediate breast reconstruction. Am J Surg. 2007;194(4):438–443. doi: 10.1016/j.amjsurg.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Rangan AM, Ahern V, Yip D, Boyages J. Local recurrence after mastectomy and adjuvant CMF: implications for adjuvant radiation therapy. Aust N Z J Surg. 2000;70(9):649–55. doi: 10.1046/j.1440-1622.2000.01919.x. [DOI] [PubMed] [Google Scholar]
- 24.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. The Lancet. 1999;353(9165):1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 25.Hehr T, Classen J, Huth M, et al. Postmastectomy Radiotherapy of the Chest Wall. Comparison of electron-rotation technique and common tangential photon fields. Strahlenther Onkol. 2004;180(10):629–636. doi: 10.1007/s00066-004-1264-8. [DOI] [PubMed] [Google Scholar]
- 26.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539–69. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 27.Truong PT, Olivotto IA, Whelan TJ, et al. Clinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapy. CMAJ. 2004;170(8):1263–73. doi: 10.1503/cmaj.1031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truong PT, Olivotto IA, Speers CH, et al. A positive margin is not always an indication for radiotherapy after mastectomy in early breast cancer. Int J Radiat Oncol Biol Phys. 2004;58(3):797–804. doi: 10.1016/S0360-3016(03)01626-2. [DOI] [PubMed] [Google Scholar]
- 29.Rowell NP. Are mastectomy resection margins of clinical relevance? A systematic review. Breast. 2010;19(1):14–22. doi: 10.1016/j.breast.2009.10.007. [DOI] [PubMed] [Google Scholar]