Abstract
The majority (~70%) of breast cancers are steroid hormone receptor (SR) positive at the time of diagnosis. Endocrine therapies that target estrogen receptor α (ERα) action (tamoxifen, toremifene, fulvestrant) or estrogen synthesis (aromatase inhibitors: letrozole, anastrozole, exemestane; or ovarian suppression) are a clinical mainstay. However, up to 50% of SR+breast cancers exhibit de novo or acquired resistance to these clinical interventions. Mechanisms of resistance to endocrine therapies often include upregulation and/or activation of signal transduction pathways that input to cell cycle regulation. Cyclin D1, the regulatory subunit of cyclin-dependent protein kinases four and six (CDK4/6) serves as a convergence point for multiple signaling pathways. In a recent paper entitled ‘Therapeutically Activating Retinoblastoma (RB): Reestablishing Cell Cycle Control in Endocrine Therapy-Resistant Breast Cancer’, Thangavel et al. reported maintenance of cyclin D1 expression and RB phosphorylation in the face of ER ablation in multiple breast cancer cell line models of endocrine resistance. RB-dysfunction defined a unique gene signature that was associated with luminal B-type breast cancer and predictive of poor response to endocrine therapies. Notably, a new CDK4/6 inhibitor (PD-0332991) was capable of inducing growth arrest by a mechanism that was most consistent with cellular senescence. In this review, these findings are discussed in the context of SRs as important mediators of cell cycle progression, and the frequent loss of cell cycle checkpoint control that typifies breast cancer progression. These studies provide renewed hope of effectively stabilizing endocrine-resistant breast cancers using available complementary (to endocrine-based therapies) cytostatic agents in the form of CDK4/6 inhibitors.
Introduction
Endocrine-based therapies aimed at blocking estrogen receptor (ER) action are well tolerated and offer a highly effective means of breast cancer therapy. However, breast tumor cells may escape from such front-line therapies and treatment of endocrine resistance remains a significant clinical challenge. Many basic and translational studies are aimed at reversing or preventing this type of resistance. A new article by Thangavel et al. (2011) appearing in the previous issue of Endocrine-Related Cancer suggests an effective means of targeting loss of cell cycle control in endocrine-resistant breast cancers. These investigators noted that a unique gene signature indicative of retinoblastoma (RB) protein loss of function could identify luminal B-type breast cancers most likely to fail on endocrine therapies. Cyclin D1 is a well-studied ER target gene that is required for estrogen-induced cell proliferation (Lukas et al. 1996). Cyclin D1 binds to and activates cell cycle-dependent protein kinases four and six (CDK4/6) important for mediating phospho-RB-induced cell cycle progression at the G1/S boundary or ‘checkpoint’ (Fig. 1). Other well-characterized functions of D-type cyclins include sequestration of cell cycle inhibitors (p21 and p27) from CDK2 and regulation of transcription (Stamatakos et al. 2010). In MCF-7 cell line models of endocrine-resistant breast tumors, cyclin D1 expression and phosphorylation of RB were maintained despite effective ER blockade. PD-0332991, a selective CDK4/6 inhibitor, blocked cell cycle progression of numerous endocrine-resistant breast cancer cell models. The work provides further insight that targeting the downstream mediators of ER action (cyclin D1 and CDK4/6) may provide a viable means to stabilize endocrine-resistant tumors. In this review, we discuss ER/cell cycle crosstalk and other (estrogen independent) regulatory inputs to cyclin D1 expression as they relate to loss of cell cycle checkpoint control and mechanisms of endocrine resistance. Challenges to the translation of these laboratory-based/preclinical find-ings to clinical use are also considered.
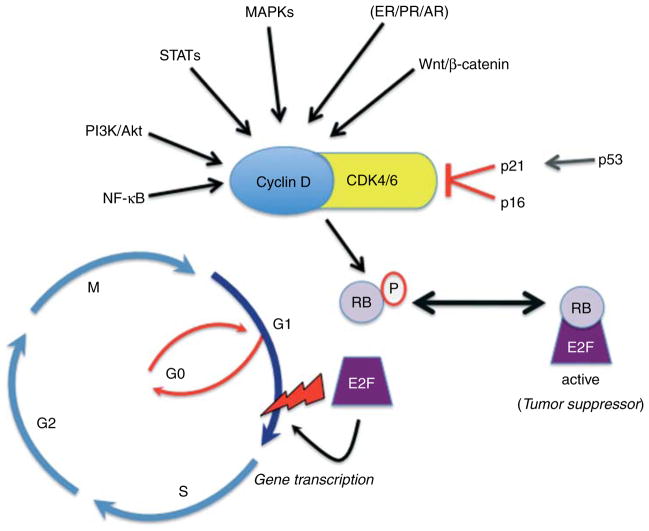
Figure 1.
Regulation of cell cycle checkpoint control. Mitogenic signals converge at the level of cyclin D1 upregulation and CDK4/6 association, localization, and kinase activity. CDK4/6 phosphorylates and inactivates RB tumor suppressor proteins, leading to dissociation of E2F transcription factors and transcriptional regulation of genes important for G1/S transition and cell cycle progression through the restriction point (lightning bolt).
Mechanisms of ER-dependent and independent cell cycle regulation
Loss of cell cycle checkpoint control is a hallmark of breast and other cancers. The G1/S restriction point or checkpoint refers to the point in the cell cycle at which exogenous mitogens can be withdrawn and cells will still progress through the remaining cell cycle phases to complete one round of cell division (Johnson & Shapiro 2010). Notably, in contrast to other cyclins, a wide variety of mitogenic signaling pathways (steroid hormones, PI3K/Akt, MAPKs, wnt/β-catenin, STATs, and NF-κB/IKK) converge at the level of cyclin D mRNA and/or protein upregulation (Witzel et al. 2010). D-type cyclins (D1, D2, and D3) are the regulatory subunits of CDK4/6; the assembly, nuclear localization, and catalytic activity of these key kinases is highly regulated (Paternot et al. 2010). They function as master integrators that couple mitogenic signaling cascades to the cell cycle by phosphorylation and inactivation of RB tumor suppressor proteins. CDK4/6-mediated phosphorylation of RB activates E2F transcription factors by causing their release from repressive (active) RB-containing complexes (Fig. 1). Among the genes classically regulated by E2F include DNA polymerase and cyclins A and E, the binding partners of CDK2; cyclin E/CDK2 further maintains RB phosphorylation as cells transition through S phase followed by G2/M (Fig. 1). During stressful conditions that may induce DNA damage, upregulation of p53 leads to increased expression of the cell cycle inhibitor, p21 (WAP1/Cip1). P21 may stabilize or promote assembly of cyclin D/CDK4/6 complexes, but is classically defined as an inhibitor of CDK kinase activity (and substrate recognition), allowing the cell to pause so that DNA can be effectively repaired before S-phase entry when DNA replication occurs (Paternot et al. 2010).
Crosstalk between steroid hormone receptors (SRs) and the cell cycle is well appreciated (Sutherland et al. 1998). Insight to the mechanisms of endocrine resistance has come, in part, from a better understanding of hormone-induced changes in the cell cycle. Estrogen-bound ER induces cell cycle progression via transcriptional upregulation of cyclin D1 mRNA and protein expression (Prall et al. 1998). In addition, membrane-associated ERα molecules induce rapid activation of c-Src and MAPKs and are capable of transactivation of epidermal growth factor (EGFR) in breast cancer cells (Levin 2009). In response to steroid hormones or growth factors, sustained activation of Akt and MAPKs downstream of EGFR serves as a direct input to cyclin D1 upregulation and cell cycle progression; MAPK regulates AP1 (Fos/Jun) complexes that can induce cyclin D1 transcription independently of ER and phosphorylation events also stabilize cyclin D1 mRNA and/or protein (Witzel et al. 2010). ER and cyclin D1 are also known to interact and may function as part of activated transcription complexes at selected ER target genes important for cell proliferation and prosurvival (Bernards 1999). Inhibition of ER action commonly blocks breast cancer cell proliferation in the G0/G1 and/or G2 phases of the cell cycle, causing growth arrest and the induction of apoptosis (Sutherland et al. 1983, Ichikawa et al. 2008). These events are associated with reduced expression of cyclin D1, increased expression of p53 and p21 (WAF1/Cip1), and loss of Bcl-2, a prosurvival protein (Ichikawa et al. 2008).
In breast cancer, a non-functional RB pathway, characterized by loss of RB or lack of concordance between RB phosphorylation and cell proliferation, is associated with tamoxifen-resistance (Lehn et al. 2011). Furthermore, cyclin D1 expression is required for tamoxifen-induced cell cycle progression (Kilker & Planas-Silva 2006), suggesting a role for CDK4/6 activity. Indeed, Finn et al. (2009) reported that breast cancer cell lines most likely to respond to CDK4/6 inhibitors (PD-0332991) contain high levels of cyclin D1 and phosphorylated RB, and low levels of CDKN2A (p16; the cell cycle inhibitor of CDK4/6). Thangavel et al. (2011) have extended these findings by testing numerous endocrine-resistant breast cancer cell (MCF-7) models with the same inhibitor and performing detailed cell cycle analyses to get at the mechanisms of cytostasis induced by CDK4/6 inhibition relative to tamoxifen-induced blockade of cell cycle progression. Upon inhibition of ER in serum-starved MCF-7 cells using pure antagonists (ICI 182,780), they observed greatly reduced RB phosphorylation and decreased expression of cyclins D1 and A, while p27/Kip1 levels increased; reactivated RB (dephosphorylated) associated with Sin3B and formed repressive complexes at target genes (DNA polymerase and cyclin A), as measured by ChIP assays. On the contrary, numerous MCF-7-derived endocrine-resistant models exhibited elevated Akt and/or MAPK activities (i.e. ER-independent inputs to cyclin D1 expression), and contained phosphorylated RB and elevated cyclins D1 and A, indicative of high CDK4/6 activity, although ER activity and/or expression was effectively eliminated. As predicted, these cells were only weakly affected by endocrine therapies, but were highly sensitive to CDK4/6 inhibition.
While the results of these studies are perhaps quite predictable (cancer cells easily escape from endocrine control and myriad ER-independent inputs to cyclin D1 expression and/or CDK4 activation exist; Fig. 1), they remind us of the need to more seriously consider targeting pathways well downstream of signaling inputs to cyclin D1; the nuts and bolts of the cell cycle may be a good place to start (Dalvai & Bystricky 2010). Indeed, recent studies suggest that many cancer cells might be addicted to high CDK4/6 activity (Paternot et al. 2010). Interestingly, Thangavel et al. (2011) found that in contrast to cytostasis induced by anti-estrogens (i.e. in sensitive cells), endocrine-resistant cells treated with PD-0332991 appeared to exit the cell cycle (i.e. were blocked in G0) and enter a state of senescence, as measured by expression of senescence-associated β-galactosidase. The authors concluded that cytostasis (by fundamentally distinct mechanisms from those that are ER-regulated) may be achieved in endocrine-resistant cases. While the idea that blocking the cell cycle in different ways may be a viable strategy to treat resistant cancers is clearly expressed in the paper, the mechanisms of how or why CDK4/6-inhibited cells actually undergo senescence were not explored in any depth. In truth, mechanisms of cellular senescence and especially, cancer cell senescence are grossly understudied relative to what is known about regulation of proliferation or apoptosis (Lanigan et al. 2011). In any case, the induction of senescence, wherein cells are irreversibly growth arrested but capable of long-term (years) survival, often involves altered (increased) expression of one or more cell cycle mediators, such as p21/Cip1 or p16/INK4A, events predicted to hyperactivate (dephosphorylate) RB and repress E2F (Jung et al. 2010, Niu et al. 2011). Perhaps targeting senescence (a highly regulated cell fate) as an approach to cancer therapy is a useful new future direction. Because of multiple or accumulated defects, cancer cells are generally vulnerable to any form of ‘tinkering’ with the cell cycle (Sherr 1996). Going forward, a detailed understanding of how cells stably exit the cell cycle and if such (cancer) cells can then be easily killed seems warranted.
Challenges to translation of promising research findings to realistic clinical use
While these studies (Thangavel et al. 2011) suggest that multiple regulatory pathways can bypass ER function, how can the utility of CDK4/6 inhibitors ever be proven outside of model systems? An obvious way to validate preclinical findings is to perform clinical trials designed to test the proposed mechanisms of resistance. To date, there are no definitive clinical data suggesting that any combination of signal transduction pathway inhibitors with anti-ER strategies results in improved clinical benefit. Given the role for CDK4/6 and RB in mediating growth factor regulated tumor biology, an obvious application would be to test dual inhibition of growth factor and ER signaling. In patients with ER-positive, HER2-amplified tumors, the combination of the anti-HER2 therapies (trastuzumab or lapatinib) and an aromatase inhibitor was superior to the aromatase inhibitor alone in metastatic breast cancer (Johnston et al. 2009, Kaufman et al. 2009). While this might appear to suggest the dual combination is superior, this trial failed to include a trastuzumab arm alone or test the sequence of an aromatase inhibitor followed by trastuzumab. Thus, the trial design did not test the necessity of blocking both pathways. Indeed, the combination of an EGFR tyrosine kinase inhibitor with an aromatase inhibitor showed no benefit (Smith et al. 2007). A recently reported trial examining an insulin-like growth factor 1 receptor (IGF1R) inhibitor plus an anti-ER therapy also suggested no benefit to the combination of drugs versus ER-blockade alone (Kaufman et al. 2010) Similarly, trials with rapamycin analogs (targeting mTOR signaling) have not clearly shown benefit for the combination of this drug with endocrine therapy (Johnston et al. 2007).
So why are all these clinical trials negative? First, as suggested by the authors (Thangavel et al. 2011), the right inhibitor may not have been tested yet in a clinical trial. Their data show direct inhibition of CDK4/6 was necessary to have effects on cell biology. None of the trials tested above directly addressed inhibition of CDK4/6; molecular targets tested in these studies also function as part of signaling pathways that lie well upstream of the CDK4/6 ‘node’. Secondly, there are many types of ‘endocrine-resistant’ breast cancer. The authors have utilized cell lines derived from the highly estrogen-dependent MCF-7 cell line. These cells, selected for tamoxifen resistance, display a heavy dependence on CDK4/6 function. In patients, there are many types of endocrine-resistant breast cancers. Some patients express ER and never respond to anti-endocrine therapies. Some patient’s relapse while taking tamoxifen, but more commonly we now see patients who relapse after optimal estrogen deprivation via aromatase inhibitors (probably by different mechanism(s) than occurs under tamoxifen relapse). Still other patients relapse months to years after ‘completing’ a standard course of endocrine therapy. The molecular mechanisms responsible for all these varied types of ‘resistance’ are likely to be different. Thirdly, in intact organisms, signal transduction or growth factor inhibitors can disrupt feedback loops that may interfere with their mechanism of action. This effect is clearest with monoclonal antibodies directed against IGF1R. Administration of these drugs disrupts negative feedback resulting in increased levels of GH, insulin, and IGF1 (Haluska et al. 2007). Thus, the receptor inhibitor upregulates serum endocrine factors that act to oppose the inhibition.
Is it thus pointless to clinically test signal transduc-tion inhibitors with endocrine therapies in breast cancer? A clinical trial testing the efficacy of an aromatase inhibitor (letrozole) with or without PD-0332991 is currently underway (NCT00721409) in first-line treatment of SR-positive advanced breast cancer. To gain the most information from this study, the type of endocrine resistance must be considered in the trial design. This trial excludes patients who have previously been treated for advanced breast cancer. Thus, the population has not been determined to be endocrine resistant. This trial design could potentially test two possibilities: CDK4/6 inhibition reverses de novo resistance or CDK4/6 inhibition delays development of acquired resistance. Neither of these possibilities was directly tested in the cell line models used by Thangavel et al. (2011); however, distinguishing between these two possibilities is not simple in the clinic. Ideally, tissue would be biopsied before initiation of therapy to examine and detect the RB gene signature that is indicative of activated CDK4/6 signaling pathways outlined in this preclinical work; non-invasive imaging techniques or capture of circulating tumor cells to measure CDK4/6 activity and ER expression would be even better. Once researchers have ascertained that the tumor has the appropriate ‘on’ signals (i.e. an inactive RB pathway), then the trial should test each inhibitor by itself with a mandated crossover to the opposite arm if the tumor progresses. Certainly, this is the way we would proceed in the laboratory, but this is too burdensome a trial design to ever be performed in the clinic. Unfortunately, the ability to obtain and coordinate high-quality tumor specimens in a multinational trial is also extraordinarily difficult. However, capturing biospecimens in the existing, well-designed ‘conventional’ trial design could be used to identify subsets of ‘RB-inactive’ ER-positive breast cancers that could benefit from inhibition of both CDK4/6 and ER. Encouraging preclinical studies such as those discussed in this review (Thangavel et al. 2011) provide a strong rationale for overcoming these hurdles with the goal of improving relapse-free survival from endocrine-resistant breast cancer.
Summary
Therapy of breast cancer was simpler when there were only two types: ER-positive and negative. Now that we are beginning to understand the molecular subtypes of breast cancer, we are starting to appreciate that this disease is a collection of orphans. Perhaps each orphan needs its own therapy. There is also a need to understand and target cell fates other than proliferative signaling (i.e. induced senescence). Thangavel et al. (2011) have shown a CDK4/6-driven type of ER-positive breast cancer that responds well to selective kinase inhibitors (PD-0332991). Now, the challenge is to prove that this phenotype exists in the clinic and is responsive to RB reactivation by drugging it to death.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant number R01 CA123763; formerly R01 DK53825 to C A Lange; R01CA 74285 and P30 CA 077598).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Bernards R. CDK-independent activities of D type cyclins. Biochimica et Biophysica Acta. 1999;1424:M17–M22. doi: 10.1016/s0304-419X(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Dalvai M, Bystricky K. Cell cycle and anti-estrogen effects synergize to regulate cell proliferation and ER target gene expression. PLoS ONE. 2010;5:e11011. doi: 10.1371/journal.pone.0011011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Research. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-7 51 871 in patients with refractory solid tumors. Clinical Cancer Research. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- Ichikawa A, Ando J, Suda K. G1 arrest and expression of cyclin-dependent kinase inhibitors in tamoxifen-treated MCF-7 human breast cancer cells. Human Cell. 2008;21:28–37. doi: 10.1111/j.1749-0774.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- Johnson N, Shapiro GI. Cyclin-dependent kinases (cdks) and the DNA damage response: rationale for cdk inhibitor-chemotherapy combinations as an anticancer strategy for solid tumors. Expert Opinion on Therapeutic Targets. 2010;14:1199–1212. doi: 10.1517/14728222.2010.525221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SR, Martin LA, Leary A, Head J, Dowsett M. Clinical strategies for rationale combinations of aroma-tase inhibitors with novel therapies for breast cancer. Journal of Steroid Biochemistry and Molecular Biology. 2007;106:180–186. doi: 10.1016/j.jsbmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmeno-pausal hormone receptor-positive metastatic breast cancer. Journal of Clinical Oncology. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signalling. 2010;22:1003–1012. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. Journal of Clinical Oncology. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- Kaufman PA, Ferrero JM, Bourgeois H, Kennecke H, De Boer R, Jacot W, McGreivy J, Suzuki S, Loh E, Robertson J. A randomized, double-blind, placebo-controlled, phase 2 study of AMG 479 with exemestane (E) or fulvestrant (F) in postmenopausal women with hormone-receptor positive (HR+) metastatic (M) or locally advanced (LA) breast cancer (BC) Cancer Research. 2010;70(Supplement 24):76s. [Google Scholar]
- Kilker RL, Planas-Silva MD. Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. Cancer Research. 2006;66:11478–11484. doi: 10.1158/0008-5472.CAN-06-1755. [DOI] [PubMed] [Google Scholar]
- Lanigan F, Geraghty JG, Bracken AP. Transcriptional regulation of cellular senescence. Oncogene. 2011 doi: 10.1038/onc.2011.34. in press. [DOI] [PubMed] [Google Scholar]
- Lehn S, Ferno M, Jirstrom K, Ryden L, Landberg G. A non-functional retinoblastoma tumor suppressor (RB) pathway in premenopausal breast cancer is associated with resistance to tamoxifen. Cell Cycle. 2011;10:956–962. doi: 10.4161/cc.10.6.15074. [DOI] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends in Endocrinology and Metabolism. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Molecular and Cellular Biology. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Chen T, Han L, Wang P, Li N, Tong T. Transcriptional activation of the senescence regulator Lsh by E2F1. Mechanisms of Ageing and Development. 2011;132:180–186. doi: 10.1016/j.mad.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–699. doi: 10.4161/cc.9.4.10611. [DOI] [PubMed] [Google Scholar]
- Prall OW, Rogan EM, Sutherland RL. Estrogen regulation of cell cycle progression in breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 1998;65:169–174. doi: 10.1016/S0960-0760(98)00021-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Smith IE, Walsh G, Skene A, Llombart A, Mayordomo JI, Detre S, Salter J, Clark E, Magill P, Dowsett M. A phase II placebo-controlled trial of neoadjuvant anastro-zole alone or with gefitinib in early breast cancer. Journal of Clinical Oncology. 2007;25:3816–3822. doi: 10.1200/JCO.2006.09.6578. [DOI] [PubMed] [Google Scholar]
- Stamatakos M, Palla V, Karaiskos I, Xiromeritis K, Alexiou I, Pateras I, Kontzoglou K. Cell cyclins: triggering elements of cancer or not? World Journal of Surgical Oncology. 2010;8:111. doi: 10.1186/1477-7819-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RL, Green MD, Hall RE, Reddel RR, Taylor IW. Tamoxifen induces accumulation of MCF 7 human mammary carcinoma cells in the G0/G1 phase of the cell cycle. European Journal of Cancer & Clinical Oncology. 1983;19:615–621. doi: 10.1016/0277-5379(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Sutherland RL, Prall OW, Watts CK, Musgrove EA. Estrogen and progestin regulation of cell cycle progression. Journal of Mammary Gland Biology and Neoplasia. 1998;3:63–72. doi: 10.1023/A:1018774302092. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, Clarke R, Knudsen ES. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocrine-Related Cancer. 2011;18:333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel II, Koh LF, Perkins ND. Regulation of cyclin D1 gene expression. Biochemical Society Transactions. 2010;38:217–222. doi: 10.1042/BST0380217. [DOI] [PubMed] [Google Scholar]