Abstract
Purpose
Although lymphedema of the arm is a well-known complication of breast and axillary surgery, breast lymphedema has received scant attention. We sought to prospectively characterize breast lymphedema’s incidence, associated symptoms, clinical course, and impact on quality of life.
Methods
Subjects were enrolled prospectively from a consecutive sample of patients undergoing non-mastectomy breast procedures (excisional biopsy or wide local excision ± lymph node removal) and followed for signs and symptoms of lymphedema in the operated breast. Symptoms and distress were serially assessed with 11-point linear analogue scales. Breast lymphedema was diagnosed independent of symptoms, based on the distribution and degree of edema and erythema.
Results
One hundred twenty-four women were followed for a median of 11 months, and breast lymphedema was diagnosed in 38 (31%) women. Breast lymphedema was more frequent after breast surgery with axillary node removal (49%) compared to breast surgery alone (0%), p<0.0001. Breast lymphedema involved multiple quadrants in most women and was characterized by edema in 100% and erythema in 79%. Patients with breast lymphedema were significantly more likely than women without breast lymphedema to report symptoms of breast heaviness (65% vs 22%, p<0.0001), redness (62% vs 29%, p=0.0006), and swelling (59% vs 22%, p<0.0001), but symptom-associated distress was low overall. Three of 32 breast lymphedema patients with clinical follow-up developed chronic edema.
Conclusions
Breast lymphedema occurs in approximately one-half of women who undergo breast surgery with axillary node removal. The condition is characterized by diffuse skin edema and erythema as well as self-reported symptoms with a low level of distress.
Keywords: breast lymphedema, quality of life, breast edema, symptom
Introduction
Arm lymphedema is a well-known complication of breast cancer treatment [1] but lymphedema can also involve the breast [2, 3]. The associated erythema and edema of breast lymphedema may raise concerns for infection or inflammatory cancer, leading to prolonged antibiotics or overly aggressive biopsies [4]. In addition, sentinel lymph node surgery, by disrupting lymph drainage from the breast, may increase risk of breast lymphedema.
Breast lymphedema warrants attention, as lymphedematous tissues are at increased risk of infection and impaired wound healing, possibly leading to delayed chronic cellulitis [5, 6]. Quality of life (QOL) is compromised with arm lymphedema [7, 8] but remains poorly characterized in breast lymphedema. Although a few studies have addressed breast edema after breast-conserving treatment [2, 3], the existing literature lacks detail on the specific physical findings of breast lymphedema and their distribution within the affected breast, as well as correlation with patient symptoms and QOL. Therefore, the current prospective study of women undergoing breast surgery was conducted to determine the frequency of breast lymphedema and to characterize its physical findings, associated symptoms, clinical course, and impact on QOL.
Methods
Patient population
This prospective study was approved by the Mayo Clinic Institutional Review Board. Women with intact breasts and axillae, defined as an absence of prior mastectomy or axillary surgery, scheduled for unilateral non-mastectomy breast surgical procedures were recruited consecutively for enrollment from the breast surgical practice at Mayo Clinic Rochester from September 2006 to February 2009. Indications for surgery included either benign or malignant disease. Exclusion criteria included a history of DVT or lymphedema in either upper extremity, or lymphoma or malignancy involving axillary nodes. Enrolled individuals who required mastectomy due to extensive disease were later excluded.
Post-operative visits
Follow-up visits were planned at 1, 3, 6, and 12 months postoperatively, with adequate follow-up defined as one or more follow-up visit(s) in the 3–12 month postoperative period.
Clinician-reported outcomes
At each visit, an experienced nurse study coordinator examined the patient and performed a graded assessment (mild/moderate/severe) for edema and erythema for each breast quadrant and the nipple-areolar complex.
Definition of breast lymphedema
A clinical impression of breast lymphedema was judged to be either present or absent at each visit based on physical findings; i.e. edema, erythema, and their distribution, independent of a participant’s present or past symptoms. Cases diagnosed as breast lymphedema were defined as participants with a clinical impression of breast lymphedema beyond the one month visit with either 1) moderate or severe signs at ≥1 visit, or 2) a clinical impression of breast lymphedema with mild signs at ≥2 visits. Medical record review was utilized to identify breast lymphedema cases among participants who had only one study visit (after one month) with mild signs of breast lymphedema. These participants were classified as breast lymphedema cases if non-study care providers documented breast lymphedema at other clinical visits during the 12 month follow-up period.
Reliability of clinical impression of breast lymphedema
A sub-study was performed to assess inter-observer agreement among 46 patients recruited by letter from the pool of all enrolled patients. At visits that occurred separately from other study visits, physical examinations were performed independently by three clinicians; the research nurse study coordinator (JPM) who performed (>90%) of the study breast lymphedema assessments, a breast surgeon (ACD), and a lymphedema specialist (ALC). Breast lymphedema assessments by the nurse study coordinator showed good agreement with both the breast surgeon and lymphedema specialist, with kappa statistics of 0.76 and 0.75 respectively.
Patient-reported outcomes
Participants rated breast heaviness, discomfort, redness, visible swelling, and associated distress using 11-point numerical rating scales [9, 10]. QOL was evaluated with the FACT-B scale.[11] Arm and shoulder disability were evaluated with the quick DASH.[12] The quick DASH contains 11 items and results in a score ranging from 0 to 100, with higher scores indicating more disability/symptoms.
Electronic medical record (EMR) abstraction
Data abstracted from participants’ EMRs included: body mass index, details of the operative procedure, pathologic features, breast lymphedema signs and symptoms reported by non-study clinicians, referral to the Lymphedema Clinic, breast lymphedema treatments, participants’ adherence to breast lymphedema treatments, and breast lymphedema progression or improvement.
Statistical analysis
A sample size of 150 patients was planned under the assumption that 30% of subjects would develop breast lymphedema. This would provide 80% power to detect an effect size of 0.50 standard deviations or larger when comparing a quantitative variable (such as total number of symptoms or a QOL scale) between two groups based on a two-sided two-sample t-test with alpha = 0.05.
Comparisons between patients with and without breast lymphedema were made using chi-square tests for nominal variables and two-sample t-tests for quantitative variables. Because the number and timing of follow-up visits were heterogeneous, the following plan was designated to define the timepoint of data to be used for analyses. In patients who did not develop breast lymphedema, signs, symptoms, and QOL scores recorded at the earliest available of the 3, 6, or 12 month visits were used. In patients defined as cases of breast lymphedema, data from the earliest of these visits in which breast lymphedema was present was used. Sensitivity analyses were performed and confirmed the robustness of this approach. Additional analyses compared the groups using the worst value during follow-up for each of the measures and the best value during follow-up for each between the two groups. Repeated measures models were also examined. All approaches yielded consistent results, supporting the initial straightforward approach. All tests were two-sided with p-values < 0.05 considered statistically significant. Analysis was performed using SAS (Version 9.2, SAS Institute Inc., Cary, NC).
Results
Study population
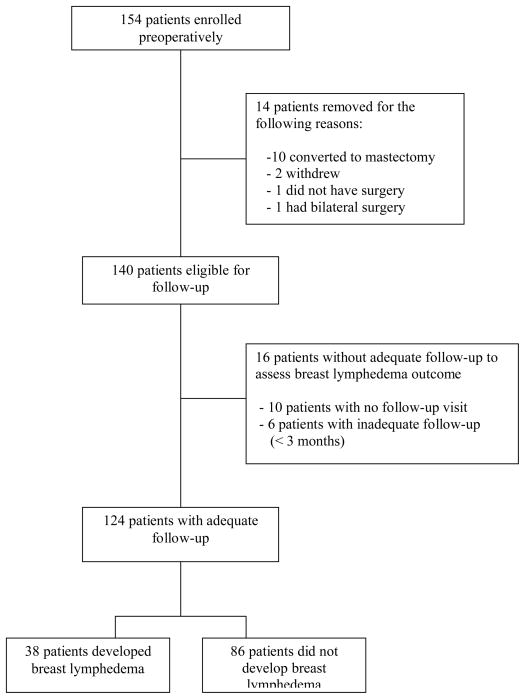
Study enrollment, eligibility, and follow-up are summarized in Figure 1. Although 154 women were consented and enrolled, 30 women were excluded from analysis due to disqualification from eligibility or inadequate followup, leaving 124 as the remaining cohort with adequate followup. Median length of follow-up was 11 months (range: 3 to 14 months); the number of visits that participants had at three months and beyond were: 3 or more visits- 48 subjects (39%), 2 visits- 40 (32%), and one visit- 36 (29%). Median length of follow-up was similar between participants who did, versus did not, meet case definition criteria for breast lymphedema (11.3 and 10.8 months, respectively).
Figure 1.
Patient enrollment and follow-up diagram
Patients and treatment characteristics
Clinical and surgical baseline characteristics are summarized in Table 1. Mean age was 59 years (range 36 to 85 years). In 92 patients (74%), surgery was performed for cancer, with the remaining 32 patients undergoing an excisional biopsy of benign tissue. Of the 92 with cancer, 85% underwent an axillary procedure (either SLNB or ALND) in conjunction with wide local excision (WLE). Among patients with SLNB, a median of 3 lymph nodes were removed, while the median number of lymph nodes removed for ALND patients was 20. Ninety-one of the 92 patients with cancer (99%) underwent radiation therapy, 61 (66%) received endocrine therapy, and 29 (32%) received chemotherapy.
Table 1.
Patient baseline characteristics and surgical variables.
| Variable | N =124 |
|---|---|
|
| |
| Age at surgery, years | |
| Mean (SD) | 58.5 (11.4) |
| Median (Range) | 56.5 (36 to 85) |
|
| |
| BMI | |
| Mean (SD) | 29.1 (6.3) |
| Median (Range) | 28.1 (17.3 to 47.1) |
|
| |
| Type of surgery, n (%) | |
| Excisional biopsy only | 32 (26%) |
| WLE only | 14 (11%) |
| WLE+SLNB | 67 (54%) |
| WLE+ALND | 11 (9%) |
|
| |
| Cancer present in breast, n (%) | |
| N | 32 (26%) |
| Y | 92 (74%) |
|
| |
| T stage, n (%) | |
| Tis | 23/92 (25%) |
| T1 | 59/92 (64%) |
| T2 | 10/92 (11%) |
|
| |
| N stage (among patients with invasive cancer), n (%) | |
| N0 | 51/69 (74%) |
| N1 | 15/69 (22%) |
| N2 | 2/69 (3%) |
| N3 | 1/69 (1%) |
|
| |
| TNM stage, n (%) | |
| 0 | 23/92 (25%) |
| I | 47/92 (51%) |
| II | 19/92 (21%) |
| III | 3/92 (3%) |
WLE – wide local excision, SLNB – sentinel lymph node biopsy, ALND – axillary lymph node dissection
Frequency of breast lymphedema
Among the 124 analysis-eligible participants, 38 (31%) developed breast lymphedema. Only two subjects qualified for breast lymphedema with moderate/severe signs at a single visit, while 36 had milder signs of breast lymphedema observed at more than one visit. All women who developed breast lymphedema underwent either a SLNB or ALND; conversely, breast lymphedema was not observed in any subjects who underwent breast surgery without an axillary procedure. Among the 78 patients who underwent SLNB or ALND, 38/78 (49%) developed breast lymphedema. The incidence of breast lymphedema was not associated with the extent of axillary surgery, since the frequency of breast lymphedema was similar among women who had WLE+ SLNB (33/67, 49%) compared to those with WLE+ALND (5/11, 45%), p = 0.82. Clinical impression of breast lymphedema was first noted at the 3 month visit in 18 patients (47%), at the 6 month visit in 15 (39%), and at the 12 month visit in 5 (13%). Among the 38 breast lymphedema cases, 8 preceded any radiation therapy, 4 occurred during radiation, and 30 occurred at least one month after completing radiation.
Graded physical findings
All 38 women diagnosed as breast lymphedema cases had skin edema (100%) compared to 10/86 (11.6%) who did not meet the breast lymphedema case definition, p<0.0001. Edema in women with breast lymphedema was usually mild (95%) and most often located in the inferior breast (Figure 2). The majority of breast lymphedema cases (74%) had edema in more than one quadrant of the breast, with 19/38 showing edema in two quadrants and 9/38 with edema in three or more quadrants. Breast erythema was observed in 30/38 (79%) of those diagnosed with breast lymphedema compared to 11/86 (13%) without breast lymphedema, p<0.0001. Among the 30 breast lymphedema cases with erythema of the breast skin, it was mild in 29 (97%) and moderate in 1 (3%). When erythema was present in cases of breast lymphedema, it was usually diffuse (23, 83%), involving two quadrants in 9 women and three or more quadrants in 16. In contrast to edema, erythema was observed at similar frequency among superior and inferior breast quadrants (Figure 3).
Figure 2. Location and frequency of edema per quadrant among 38 cases of breast lymphedema.
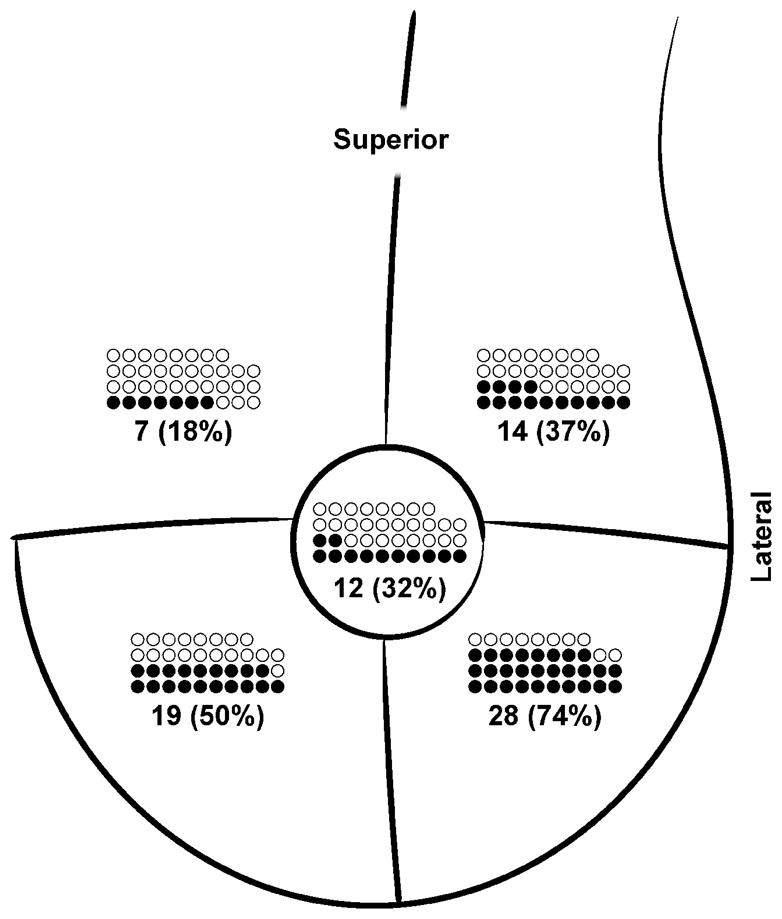
Closed circle (●) represents case of breast lymphedema with edema in that quadrant; open circle (○) represents case of breast lymphedema without edema in that quadrant.
Figure 3. Location and frequency of erythema per quadrant among 38 cases of breast lymphedema.
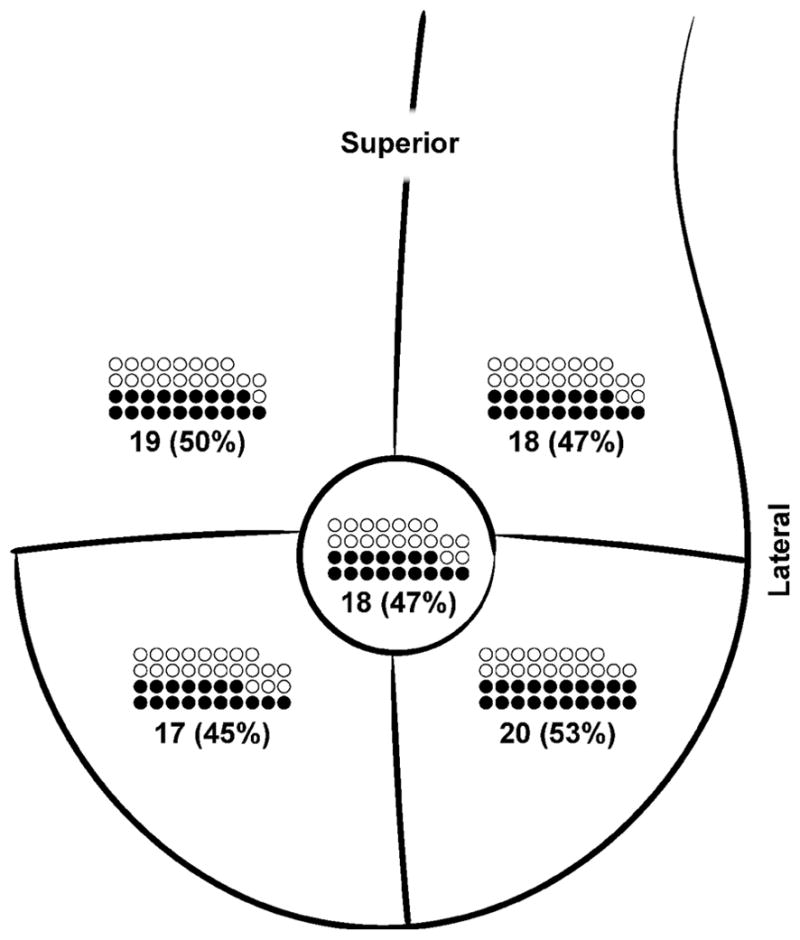
Closed circle (●) represents case of breast lymphedema with erythema in that quadrant; open circle (○) represents case of breast lymphedema without erythema in that quadrant.
Symptom scores
Reported symptom data were recorded at similar times postoperatively in patients with (mean 5.5 months) and without breast lymphedema (mean 5.4 months, p = 0.82). Patients with breast lymphedema had significantly higher scores for breast heaviness, redness, and swelling (Table 2). They also had a significantly higher total number of symptoms reported (possible range 0–4), mean 2.6 versus mean of 1.3 in women without breast lymphedema (p < 0.0001). In the subset of patients who reported at least one symptom, average scores regarding how much they were bothered by the symptom were consistently low in both groups for each symptom (means ranging from 1–2 on a scale from 0–10), with no significant differences between those with and without breast lymphedema (Table 2). Evidence of a “dose-response” correlation of symptom scores was observed for edema specifically, with higher levels of distress (mean 2.7 versus 1.0, p=0.03) corresponding to edema characterized as moderate versus mild.
Table 2.
Patient reported symptoms by breast lymphedema status.
| Breast lymphedema | ||||
|---|---|---|---|---|
| Symptoms at follow-up* | Breast lymphedema (n=38) N (%) |
No breast lymphedema (n=86) N (%) |
P-value | |
| Breast appears/feels swollen | Missing | 1 | <0.0001 | |
| No | 15 (41%) | 67 (78%) | ||
| Yes | 22 (59%) | 19 (22%) | ||
| How much does it bother you? | 0.49 | |||
| Mean (SD) | 1.6 (2.2) | 2.1 (2.1) | ||
| Breast feels heavy | Missing | 1 | <0.0001 | |
| No | 13 (35%) | 67 (78%) | ||
| Yes | 24 (65%) | 19 (22%) | ||
| How much does it bother you? | 0.39 | |||
| Mean (SD) | 1.1 (2.1) | 1.7 (2.0) | ||
| Breast more reddened | Missing | 1 | 0.0006 | |
| No | 14 (38%) | 61 (71%) | ||
| Yes | 23 (62%) | 25 (29%) | ||
| How much does it bother you? | 0.29 | |||
| Mean (SD) | 1.4 (2.3) | 0.8 (1.6) | ||
| Pain/discomfort in breast | Missing | 2 | 0.10 | |
| No | 11 (31%) | 40 (47%) | ||
| Yes | 25 (69%) | 46 (53%) | ||
| How much does it bother you? | 0.74 | |||
| Mean (SD) | 2.1 (2.1) | 2.0 (1.9) | ||
| Number of symptoms present | Missing | 2 | <0.0001 | |
| 0 | 4 (11%) | 28 (33%) | ||
| 1 | 3 (8%) | 28 (33%) | ||
| 2 | 8 (22%) | 14 (16%) | ||
| 3 | 10 (28%) | 11 (13%) | ||
| 4 | 11 (31%) | 5 (6%) | ||
| Mean (SD) | 2.6 (1.3) | 1.3 (1.2) | ||
Follow-up measure reported is from the earliest study visit (later than 1 month) in those without breast lymphedema and, in those with breast lymphedema, from the earliest study visit (later than 1 month) where breast lymphedema was present. The mean time from surgery to the date of the reported measure was 5.4 months postop in those without breast lymphedema and 5.5 months postop in those with breast lymphedema.
Quality of life and function scores
Quality of life measures are summarized in Table 3 and are from the same visit for which symptom data were reported. FACT-B subscale and total scores during follow-up did not differ significantly between patients with and without breast lymphedema. The DASH score was added to our study assessments after the study was underway and is thus available at baseline in only 117/124 patients. Baseline, follow-up, and change from baseline to follow-up DASH scores did not differ significantly between subjects with and without breast lymphedema.
Table 3.
Quality of life and functional scales
| Breast lymphedema | |||
|---|---|---|---|
| Breast lymphedema (n=38) | No breast lymphedema (n=86) | P-value | |
| FACT-B measures at follow-up* [Mean (SD)] | |||
| Physical well-being | 23.4 (4.0) | 24.2 (4.8) | 0.36 |
| Social/family well-being | 25.9 (3.0) | 24.6 (4.5) | 0.11 |
| Emotional well-being (missing n=1 no breast lymphedema) | 20.9 (2.5) | 20.1 (3.6) | 0.22 |
| Functional well-being (missing n=1 no breast lymphedema) | 23.5 (4.3) | 23.6 (4.9) | 0.93 |
| Additional concerns | 27.8 (5.3) | 28.7 (5.2) | 0.41 |
| FACT-B total score (missing n=1 no breast lymphedema) | 121.5 (16.2) | 121.2 (17.6) | 0.92 |
| Quick DASH disability/symptom score at baseline [Mean (SD)] | 9.5 (11.2) | 6.6 (10.5) | 0.18 |
| Missing | 1 | 6 | |
| Quick DASH disability/symptom score at follow-up* [Mean (SD)] | 12.3 (12.6) | 7.6 (11.7) | 0.05 |
| Missing | 2 | 6 | |
| Change in Quick DASH disability/symptom score from baseline [Mean (SD)] | 2.5 (14.5) | 0.9 (11.4) | 0.52 |
| Missing | 2 | 6 | |
Follow-up measure reported is from the earliest study visit (later than 1 month) in those without breast lymphedema and, in those with breast lymphedema, from the earliest study visit (later than 1 month) where breast lymphedema was present. The mean time from surgery to the date of the reported measure was 5.4 months postop in those without breast lymphedema and 5.5 months postop in those with breast lymphedema.
Clinical course
Among 38 subjects with breast lymphedema, 32 (84%) were evaluated in the Lymphedema Clinic, and 30/32 received one or more treatment recommendation(s). The recommended interventions included compression bra (n = 29), manual lymphatic drainage (MLD, n = 22), and compression wrap (n = 5), with more than one of these interventions recommended in 21/30 patients. Three other patients received treatment recommendations from providers outside in the Lymphedema Clinic (1 compression bra only, 1 MLD only, and 1 compression bra plus MLD). Among the 33 patients with treatment recommended, 29 (88%) appeared compliant with treatment recommendations per provider documentation. Clinical status of breast lymphedema at last follow-up was improved in 23, stable in 4, fluctuating in 6, progressive in 3, and unclear in 2. Overall, the median length of clinical follow-up subsequent to diagnosis of breast lymphedema was 11 months (range 0 to 37 months). The 3 cases that progressed experienced this outcome despite complying with recommended treatments of compression bra plus MLD (n = 2) and compression bra/MLD/chest compression wrap (n = 1).
Discussion
In this prospective study, breast lymphedema occurred in approximately one half of women following breast surgery with concomitant axillary surgery, whereas breast lymphedema was not observed after excisional breast biopsies or lumpectomies without lymph node removal. Rates of breast lymphedema were similar whether the nodal surgery was limited (SLNB) or more extensive (ALND). These findings support a mechanism of breast lymphedema risk due to disruption of the primary axillary lymphatic drainage pathway.
This study’s findings agree with the few previous prospective studies examining breast lymphedema incidence but none of these studies offered detailed data on location of physical findings, patient symptoms, or quality of life. Clarke et al reported in 1982 on 74 patients with a minimum of one-year follow-up after lumpectomy and radiation [2]. They observed breast edema in 41% of patients with a breast and axillary procedure and breast radiation, similar to the frequency found in the present study. A prospective study by Ronka et al reported breast lymphedema in 34% of 160 breast conservation patients at one year after surgery [3]. More recently, Goffman et al retrospectively identified breast lymphedema in 11% of 206 women after breast-conserving treatment for cancer [13], acknowledging that the retrospective approach increased the likelihood of underestimation.
Similar to Goffman et al, the current study found no difference in the frequency of breast lymphedema after ALND versus SLNB [13]. In contrast, Clarke et al reported breast edema in 79% of those who underwent ALND compared to 25% of those with axillary sampling [2]. Ronka et al also found that breast lymphedema was more common after axillary dissection (48% in node-positive patients and 35% in node-negative) compared to sentinel node biopsy (23%) [3]. The reason for this discrepancy in findings across studies is unknown but may be related to lack of standardized diagnostic criteria for breast lymphedema and sample size issues, with relatively small numbers of women with breast lymphedema within subgroups by axillary surgery.
The current prospectively conducted study prospectively enhances the published literature by providing a combined evaluation of breast lymphedema and correlation with patients’ symptoms, limb function, and QOL. Patient reported symptoms correlated strongly with signs of breast lymphedema. In most cases, signs and symptoms were mild, but women with more severe edema reported a higher level of distress. Although breast lymphedema occurs frequently after axillary surgery, most affected women have mild symptoms and very little distress, which likely explains why this condition has not been the focus of much systematic study. Two reports in the late 1980’s mentioned breast edema after breast-conserving cancer treatment as a possible source of dissatisfaction [14, 15].
The relative contributions of surgery, radiation therapy, and systemic drug therapy in the etiology of breast lymphedema remains to be defined [16]. In one study, persistent breast edema was associated with radiation [17]. Clarke et al observed that approximately one-third of breast lymphedema cases occurred before starting radiation, the majority developed during radiation, and approximately 10% occurred later [2]. In the Goffman study, 12% of breast lymphedema cases occurred prior to radiation, with the majority during or after [13]. The present study similarly found that approximately 20% of breast lymphedema cases occurred prior to radiation and the majority within a few months after completion of radiation. Most likely, surgical disruption of primary draining lymphatic pathways is sufficient to result in clinical edema in some cases, but in other cases the tissue lymphatics in the postoperative state are functioning near capacity, and the secondary insult of radiation crosses the threshold to overload, yielding clinical edema.
Limitations
A potential limitation of our study is the subjectivity of the breast lymphedema diagnosis that was based on the clinical impression of a care provider. This approach has been used in other published studies because there are no established diagnostic criteria for this condition; furthermore this approach requires no special equipment and appeared consistent across clinicians in our study. Several approaches were used to validate this study’s case definition. First, findings from the 1 month postoperative visit were excluded in establishing the diagnosis of breast lymphedema case definition to reduce confounding from post-surgical edema. Second, an interobserver sub-study was conducted to examine agreement of the clinical impression of breast lymphedema between the study nurse and two physicians who saw the patient on the same day. Finally, medical records of all patients meeting our case definition of breast lymphedema were examined to corroborate the study nurse’s impression with the clinical observations and diagnoses of providers separate from the study. Recent preliminary data [3, 18, 19] suggest that both ultrasound and bioimpedance may be useful modalities in the diagnosis of breast lymphedema. Further evaluation is ongoing to compare these two tools with clinical exams and patient reported outcomes.
Another limitation of the current study is that it did not discern the natural history of breast lymphedema because all those judged to have breast lymphedema were recommended to undergo evaluation and treatment. Clarke et al reported that most patients showed clinical improvement by two years and complete resolution by three years, although treatments in that trial were not specified [2]. Most women with breast lymphedema in the current study were treated and improved, although they might have improved without treatment. Goffman et al reported improvement of both arm and breast lymphedema with manual lymphatic drainage [13]. Manual lymphatic drainage and exercise can improve swelling and quality of life for arm lymphedema [20–23], but their efficacy in the breast has not been formally investigated. Regardless, the available evidence suggests that the majority of breast lymphedema cases improve. Two other limitations include the fact that this was a single-institution study and the absence of data on patient weight gain and its possible influence on breast lymphedema. Rates of breast lymphedema might vary among surgeons due to differences in surgical technique that could impact lymphatic disruption, making it possible that the results from our institution will not generalize well to other settings. Patient weight gain is an important potential risk factor that will be included in a future report assessing risk factors for development of breast lymphedema in these patients.
In summary, breast lymphedema occurred commonly after breast-conserving surgery with axillary node removal. Breast lymphedema was characterized by diffuse mild edema and erythema in more than one quadrant, and most affected women have noticeable symptoms but a low level of distress from this condition. However, improved awareness of the condition of breast lymphedema may help to avoid unnecessary use of antibiotics and to assist patients with symptom management. Breast lymphedema usually improves with time, and specific treatment interventions may be beneficial although their efficacy remains unproven.
Acknowledgments
This publication was made possible by the Mayo Clinic CTSA through grant number UL1RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Amy C. Degnim is also supported by the CA90628-08 Paul Calabresi Award for Clinical-Translational Research (K12) via the Mayo Clinic Cancer Center. We also graciously thank the women who participated in this study.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. doi: 10.1002/1097-0142(20010915)92:6<1368::AID-CNCR1459>3.0.CO;2-9. [pii] [DOI] [PubMed] [Google Scholar]
- 2.Clarke D, Martinez A, Cox RS, Goffinet DR. Breast edema following staging axillary node dissection in patients with breast carcinoma treated by radical radiotherapy. Cancer. 1982;49(11):2295–2299. doi: 10.1002/1097-0142(19820601)49:11<2295::aid-cncr2820491116>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Ronka RH, Pamilo MS, von Smitten KA, Leidenius MH. Breast lymphedema after breast conserving treatment. Acta Oncol. 2004;43(6):551–557. doi: 10.1080/02841860410014867.RB3FV353P5W91974. [pii] [DOI] [PubMed] [Google Scholar]
- 4.Loprinzi CL, Okuno S, Pisansky TM, Sterioff S, Gaffey TA, Morton RF. Postsurgical changes of the breast that mimic inflammatory breast carcinoma. Mayo Clin Proc. 1996;71(6):552–555. doi: 10.4065/71.6.552. [DOI] [PubMed] [Google Scholar]
- 5.Staren ED, Klepac S, Smith AP, Hartsell WF, Segretti J, Witt TR, Griem KL, Bines SD. The dilemma of delayed cellulitis after breast conservation therapy. Arch Surg. 1996;131(6):651–654. doi: 10.1001/archsurg.1996.01430180077016. [DOI] [PubMed] [Google Scholar]
- 6.Baddour LM. Breast cellulitis complicating breast conservation therapy. J Intern Med. 1999;245(1):5–9. doi: 10.1046/j.1365-2796.1999.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(12 Suppl American):2817–2820. doi: 10.1002/(SICI)1097-0142(19981215)83:12B+<2817::AID-CNCR32>3.0.CO;2-2. [pii] [DOI] [PubMed] [Google Scholar]
- 8.Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137(11):1253–1257. doi: 10.1001/archsurg.137.11.1253. soa2024 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. doi: 10.1002/1097-0142(20001001)89:7<1634: AID-CNCR29>3.0.CO;2-V. [pii] [DOI] [PubMed] [Google Scholar]
- 10.Buchanan DR, O’Mara AM, Kelaghan JW, Minasian LM. Quality-of-life assessment in the symptom management trials of the National Cancer Institute-supported Community Clinical Oncology Program. J Clin Oncol. 2005;23(3):591–598. doi: 10.1200/JCO.2005.12.181. 23/3/591 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 12.Hayes S, Battistutta D, Newman B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res Treat. 2005;94(1):1–10. doi: 10.1007/s10549-005-5991-z. [DOI] [PubMed] [Google Scholar]
- 13.Goffman TE, Laronga C, Wilson L, Elkins D. Lymphedema of the arm and breast in irradiated breast cancer patients: risks in an era of dramatically changing axillary surgery. Breast J. 2004;10(5):405–411. doi: 10.1111/j.1075-122X.2004.21411.xTBJ21411. [pii] [DOI] [PubMed] [Google Scholar]
- 14.Patterson MP, Pezner RD, Hill LR, Vora NL, Desai KR, Lipsett JA. Patient self-evaluation of cosmetic outcome of breast-preserving cancer treatment. Int J Radiat Oncol Biol Phys. 1985;11(10):1849–1852. doi: 10.1016/0360-3016(85)90044-6. [DOI] [PubMed] [Google Scholar]
- 15.McCormick B, Yahalom J, Cox L, Shank B, Massie MJ. The patients perception of her breast following radiation and limited surgery. Int J Radiat Oncol Biol Phys. 1989;17(6):1299–1302. doi: 10.1016/0360-3016(89)90540-3. [DOI] [PubMed] [Google Scholar]
- 16.Meek AG. Breast radiotherapy and lymphedema. Cancer. 1998;83(12 Suppl American):2788–2797. doi: 10.1002/(SICI)1097-0142(19981215)83:12B+<2788::AID-CNCR27>3.0.CO;2-I. [pii] [DOI] [PubMed] [Google Scholar]
- 17.Senofsky GM, Moffat FL, Jr, Davis K, Masri MM, Clark KC, Robinson DS, Sabates B, Ketcham AS. Total axillary lymphadenectomy in the management of breast cancer. Arch Surg. 1991;126(11):1336–1341. doi: 10.1001/archsurg.1991.01410350026004. discussion 1341–1332. [DOI] [PubMed] [Google Scholar]
- 18.Wratten CR, O’Brien PC, Hamilton CS, Bill D, Kilmurray J, Denham JW. Breast edema in patients undergoing breast-conserving treatment for breast cancer: assessment via high frequency ultrasound. Breast J. 2007;13(3):266–273. doi: 10.1111/j.1524-4741.2007.00420.x. TBJ420[pii] [DOI] [PubMed] [Google Scholar]
- 19.Moseley A, Piller N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat Res Biol. 2008;6(2):85–87. doi: 10.1089/lrb.2008.1002. [DOI] [PubMed] [Google Scholar]
- 20.Williams AF, Vadgama A, Franks PJ, Mortimer PS. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. Eur J Cancer Care (Engl) 2002;11(4):254–261. doi: 10.1046/j.1365-2354.2002.00312.x. 312 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Moseley AL, Piller NB, Carati CJ. The effect of gentle arm exercise and deep breathing on secondary arm lymphedema. Lymphology. 2005;38(3):136–145. [PubMed] [Google Scholar]
- 22.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361(7):664–673. doi: 10.1056/NEJMoa0810118. 361/7/664 [pii]. [DOI] [PubMed] [Google Scholar]
- 23.Turner J, Hayes S, Reul-Hirche H. Improving the physical status and quality of life of women treated for breast cancer: a pilot study of a structured exercise intervention. J Surg Oncol. 2004;86(3):141–146. doi: 10.1002/jso.20065. [DOI] [PubMed] [Google Scholar]