Abstract
Activation of the NLRP3 inflammasome by diverse stimuli requires a priming signal from Toll-like receptors (TLRs) and an activation signal from purinergic receptors or pore-forming toxins. Here we demonstrate through detailed analysis of NLRP3 activation in macrophages deficient in key downstream TLR signaling molecules that MyD88 is required for an immediate early phase, whereas TRIF is required for a subsequent intermediate phase of posttranslational NLRP3 activation. Both IRAK1 and IRAK4 kinases are critical for rapid activation of NLRP3 through the MyD88 pathway, but only IRAK1 is partially required in the TRIF pathway. IRAK1 and IRAK4 are also required for rapid activation of NLRP3 by Listeria monocytogenes as deletion of IRAK1 or IRAK4 led to defective inflammasome activation. These findings define the pathways that lead to rapid NLRP3 activation and identify IRAK1 as a critical mediator of a transcription-independent, inflammasome-dependent early warning response to pathogenic infection.
Introduction
Toll-like receptors (TLRs) sense a broad range of microbial ligands leading to the expression of genes involved in inflammation and other immune responses (1). All TLRs associate directly or indirectly with MyD88 except TLR3, which associates with TRIF, to propagate their downstream signaling (2). Signaling downstream of MyD88 requires the association of MyD88 with members of the IL-1R-associated kinase (IRAK) family, which include IRAK1, IRAK2, IRAKM and IRAK4 (3).
NLRP3 assembles an inflammasome complex with ASC and procaspase-1 after receiving a priming signal (signal 1) from TLRs and a second signal (signal 2) from purinergic receptors or pore forming toxins (4). We recently demonstrated that acute stimulation of TLR4 with LPS triggers rapid post-translational priming of NLRP3 independent of NLRP3 upregulation (5). Here, we show that IRAK1 is essential for this immediate early activation of NLRP3, but partially required for a subsequent intermediate phase of NLRP3 activation. IRAK1 is also critical for rapid transcription-independent activation of the NLRP3 inflammasome in response to infection with Listeria monocytogenes. Our results provide evidence for a previously unrecognized role for TLRs and IRAK1 in the non-transcriptional regulation of the inflammasome and innate immunity against pathogenic bacteria.
Materials and Methods
BMDM Culture and treatments
Bone marrow derived cells from wild-type (C57BL/6) and various knockout mice were cultured in DMEM with 10% fetal bovine serum and 20% L929 supernatants. BMDMs were pre-stimulated with the TLR ligands Pam3CSK4 (1 µg/ml), poly I:C (500 ng/ml) or ultrapure LPS (500 ng/ml) for various periods of times followed by stimulation with ATP (5 mM) for 45 min. Infection with Listeria was performed as described previously (6). Caspase-1 activation was assayed by immunoblotting as described previously (7). ASC oligomerization was assayed as described before with minor modifications (8). All results are representative of at least three-five independent experiments.
Confocal microscopy
Cells were grown on cover slips in 12 well plates. After various treatments, cells were fixed with 2% formaldehyde, mounted on slides and then examined using a confocal laser microscope (Nikon C1 plus, Bioimaging Shared Resource of the Kimmel Cancer Center (NCI 5 P30 CA-56036).
Results and Discussion
MyD88 and TRIF independently prime the NLRP3 inflammasome
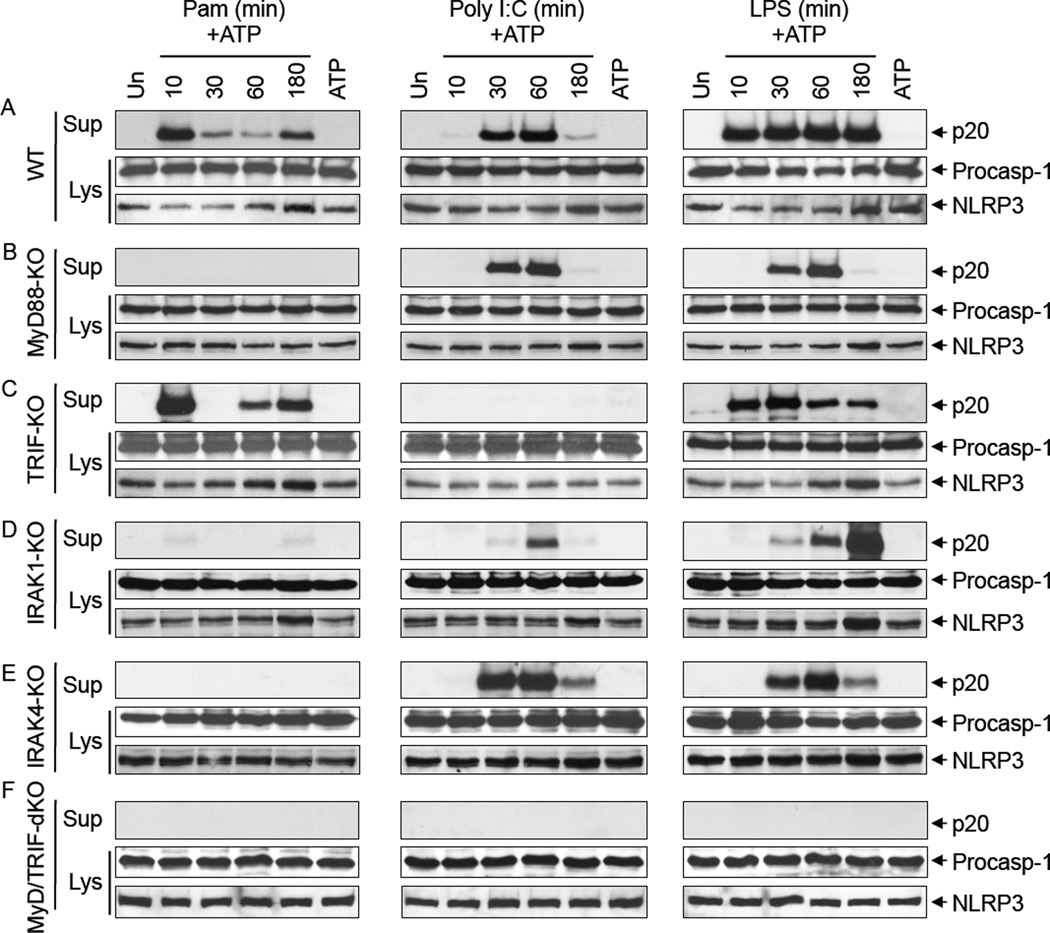
To define the TLR signaling pathways involved in the posttranslational priming of NLRP3 (5), we performed detailed analysis of NLRP3 activation in response to priming with different TLR ligands in macrophages deficient in the key TLR signaling components MyD88, TRIF, IRAK4, IRAK1 and TBK1 (Fig. 1A–F and Supplemental Fig. 1A–E). In WT macrophages, priming with Pam3CSK4 which signals through TLR2 and MyD88 caused a biphasic activation of caspase-1 with maximum activation observed in the early phase at 10 min and a substantially reduced activation in the late phase at 180 min after Pam3CSK4 treatment (Fig. 1A, left panels). In contrast, priming with poly I:C which signals through TLR3 and TRIF induced a monophasic activation of caspase-1 with maximum activation observed at 30–60 min (intermediate phase) after poly I:C treatment (Fig. 1A, middle panels). Priming with LPS which signals through TLR4, MyD88 and TRIF resulted in robust caspase-1 activation at all time points (Fig. 1A, right panels). These results indicate that priming through the MyD88 pathway (TLR2, TLR4) induces an immediate early phase (0–10 min) and a late phase (180 min) of NLRP3 activation, whereas priming through the TRIF pathway (TLR3, TLR4) induces an intermediate phase (30–60 min) of NLRP3 activation. Consistent with this, priming of MyD88-KO macrophages with Poly I:C or LPS induced only an intermediate monophasic (30–60 min) activation of caspase-1 (Fig. 1B, middle and right panels), whereas priming with Pam3CSK4 failed to induce any activation in these cells (Fig. 1B, left panels). In contrast, priming of TRIF-KO macrophages with Pam3CSK4 or LPS induced an immediate early phase (0–10 min) of caspase-1 activation (Fig. 1C, left panels), whereas priming with Poly I:C failed to induce any activation in these cells (Fig. 1C, middle panels). All three ligands failed to induce caspase-1 activation in the MyD88/TRIF-double knockout macrophages (Fig. 1F). Together our results indicate that TLRs use both MyD88 and TRIF pathways to priming and activate the NLRP3 inflammasome.
Figure 1. Post-translational activation of the NLRP3 inflammasome by MyD88 and TRIF signaling.
A–C, immunoblots of caspase-1 in the culture supernatants (Sup) or cell lysates (Lys) of mouse macrophages derived from wildtype (WT) (A), MyD88-KO (B), TRIF-KO (C) MyD88/TRIF-dKO (D), IRAK1-KO (E) or IRAK4-KO (F) mice, primed with Pam3CSK4 (Pam), Poly I:C or LPS for the indicated times (min) followed by stimulation with ATP for 45 min. The bottom panels show NLRP3 in the same cell lysates.
IRAK1 and IRAK4 are required for rapid activation of the NLRP3 inflammasome
Similar experiments in macrophages deficient in IRAK1 or IRAK4 which signal downstream of MyD88, or TBK1, which signals downstream of TRIF (1–3), revealed that IRAK1 and IRAK4 are essential for the immediate early phase of NLRP3 activation (Fig. 1D, and E, left panels), while IRAK1, but not IRAK4, is partially required for the subsequent intermediate phase of NLRP3 activation (Fig. 1D, and E, middle panels). Caspase-1 activation kinetics by all three ligands in the TBK1-KO macrophages was similar to that of the WT macrophages (Data not shown), indicating that TBK1 is not involved in NLRP3 priming. Notably, the IRAK1-KO macrophages showed a small amount of caspase-1 activation after 10 min stimulation with Pam3CSK4 (Fig. 1D), compared to MyD88-KO and IRAK4-KO macrophages, which completely lost their response to Pam3CSK4 (Fig. 1B, and E). This suggests that another downstream signaling molecule might also participate in the immediate early phase of NLRP3 activation. Intriguingly, the amount of caspase-1 activation at 180 min in response to LPS was increased in IRAK1-KO, but was diminished in IRAK4-KO (Fig. 1D, and E, right panel, 5th lane) compared to WT macrophages. This indicates that IRAK4 is required for the late phase of NLRP3 activation, but IRAK1 is not. It is possible that IRAK4 exerts this late effect on NLRP3 through another downstream kinase such as IRAK2. Indeed, IRAK2 has been shown to be critical for the late-phase TLR responses (9). Altogether, these results indicate that IRAK1 is required for the immediate early phase and partially required for the intermediate phase, whereas IRAK4 is required for the immediate early and late phases of NLRP3 activation. The results also suggest that IRAK1 deficiency might have very little consequences on NLRP3 activation by prolonged TLR4 stimulation.
Listeria induces rapid activation of NLRP3 through the MyD88-IRAK4-IRAK1 pathway
During infection with some bacterial pathogens such as Listeria monocytogenes (6, 10–12) or Staphylococcus aureus (13, 14) it is likely that their PAMPs (signal 1) and pore-forming toxins (signal 2) stimulate TLRs and NLRP3 simultaneously, to induce rapid activation of caspase-1 through the MyD88-IRAK4-IRAK1 pathway. Supporting this, infection of NLRP3-reconstituted N1–8 macrophages which express basal level of NLRP3 (5) with Listeria, induced rapid caspase-1 activation within 30–45 min after infection without prior TLR priming (Supplemental Fig. 1F). Infection of the parental NLRP3-KO macrophages with Listeria did not activate caspase-1 indicating that NLRP3 is required for caspase-1 activation by Listeria. As the expression of NLRP3 in N1–8 macrophages is not regulated transcriptionally by TLR signaling, this indicates that Listeria infection activates NLRP3 by a non-transcriptional mechanism. Activation of the NLRP3 inflammasome by Listeria was blunted in MyD88-KO, IRAK4-KO and IRAK-1-KO macrophages (Supplemental Fig. 1G). These results indicate that Listeria can simultaneously provide signal 1, likely through binding of its lipoproteins to TLR2 (12), and signal 2 likely through the release of its pore-forming toxin listeriolysin O (11), to rapidly activate the NLRP3 inflammasome through the immediate early phase, which is dependent on the MyD88-IRAK4-IRAK1 signaling pathway.
Confocal imaging studies in NLRP3-KO macrophages that were stably reconstituted with a GFP-tagged WT NLRP3 showed that infection of these cells with Listeria for 45 min induces the oligomerization of NLRP3 into one or two NLRP3 specks per cell (Supplemental Fig. 1H, lower panels). This was associated with robust activation of caspase-1 (Supplemental Fig. 1J). In contrast, infection of Walker A/B mutant NLRP3-GFP-expressing cells with Listeria caused the mutant NLRP3 to form many small non-functional puncta throughout the cytosol which failed to activate caspase-1 (Supplemental Fig. 1I and J). These results provided further evidence that NLRP3 is rapidly activated by Listeria infection and indicate that Listeria induces clustering of NLRP3 into one or two functional speckles per cell through activation of its ATPase activity.
Further studies showed that simultaneous stimulation of TLR2 or TLR4, but not TLR3, and P2×7 receptor (signal 2) was able to induce robust caspase-1 activation and the release of the alarmin HMGB1 in WT, TRIF-KO and TBK1-KO macrophages, but not in MyD88-KO, IRAK4-KO or IRAK-1-KO (Supplemental Fig. 2A). These results support the observations with Listeria infection and indicate that stimulation of the MyD88-IRAK4-IRAK1 pathway can rapidly prime the NLRP3 inflammasome even in the presence of signal 2, whereas stimulation of the TRIF pathway in the presence of signal 2 induces little or no activation of NLRP3.
IRAK1 and IRAK4 are required for NLRP3 and ASC oligomerization
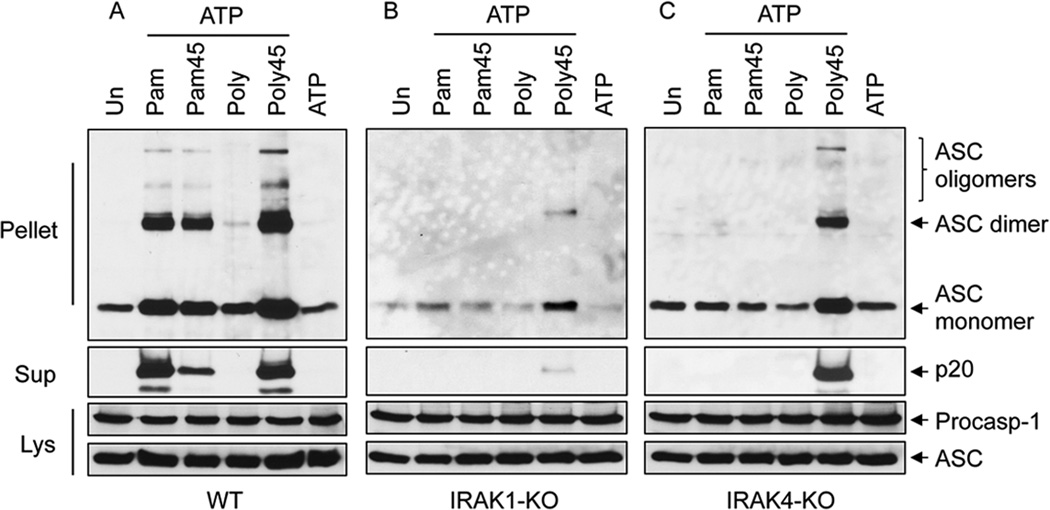
Analysis of ASC oligomerization in WT macrophages showed that co-stimulation with Pam3CSK4 and ATP or 45 min pre-stimulation with poly I:C followed by ATP induced strong ASC oligomerization (Fig. 2A). This was evident by the large increase in the amount of ASC in the NP40-insoluble pellets from these cells and the formation of dimeric and oligomeric ASC species in their pellets after DSS-crosslinking (Fig. 2A). This was also associated with robust caspase-1 activation (Fig. 2A, second panel from top). In contrast, simultaneous stimulation of IRAK1-KO or IRAK4-KO macrophages with Pam3CSK4 and ATP failed to induce either ASC oligomerization or caspase-1 activation (Fig. 2B and C). However, 45 min pre-stimulation with poly I:C followed by ATP induced strong ASC oligomerization and caspase-1 activation in IRAK4-KO macrophages (Fig. 2C), but very weak ASC oligomerization and caspase-1 activation in IRAK1-KO macrophages (Fig. 2B).
Figure 2. IRAK1 signaling is required for ASC oligomerization.
A–C, immunoblots of DSS cross-linked ASC in the NP40-insoluble pellets of WT (A), IRAK1-KO (B) and IRAK4-KO (C) macrophages after co-stimulation with Pam3CSK4 (Pam) or poly I:C (Poly) and ATP for 45 min, or pre-stimulation for 45 min with these ligands (Pam45, Poly45) followed by ATP for an additional 45 min as indicated. Immunoblots of caspase-1 and ASC in the culture supernatants (Sup) or cell lysates (Lys) of the corresponding samples are shown underneath as indicated.
Confocal imaging studies in the WT NLRP3-GFP-reconstituted macrophages showed that acute stimulation with TLR ligands plus nigericin induces the formation of one or two NLRP3-GFP specks per cell (Supplemental Fig. 2B). Quantification of these specks (Supplemental Fig. 2C) revealed similar results as observed with ASC oligomerization in WT macrophages. Together these results indicate that the TLR priming signals act upstream or at the level of NLRP3-ASC oligomerization and further confirm that IRAK1 is an essential signaling molecule required for early NLRP3-ASC oligomerization and activation.
TLR signaling primes NLRP3 by a non-transcriptional ROS-dependent mechanism
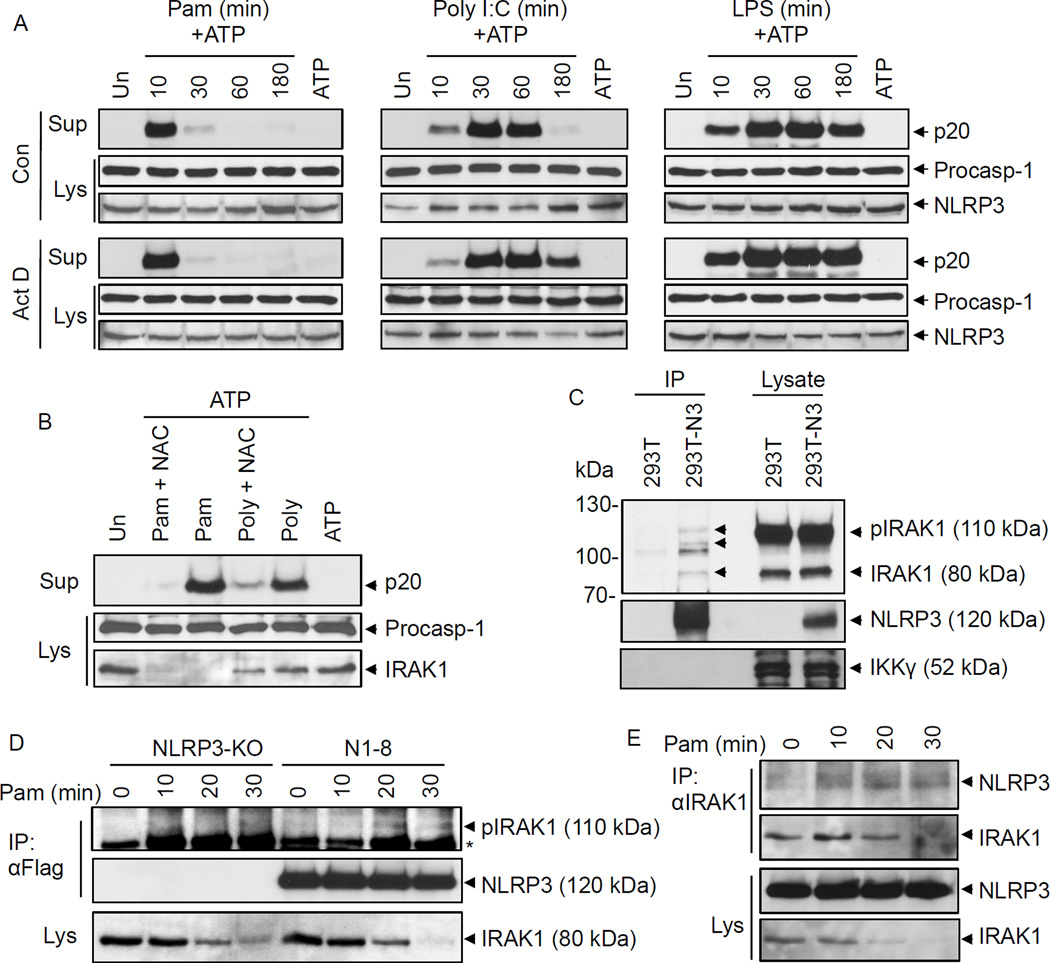
To further demonstrate that TLR signaling primes NLRP3 by a non-transcriptional ROS-dependent mechanism, we primed WT macrophages with TLR ligands in the presence of the transcription inhibitor actinomycin D or the ROS scavenger NAC. Actinomycin D did not reduce caspase-1 activation in the early, intermediate or late phases (Fig. 3A and supplemental Fig. 2D), although it completely inhibited TLR-induced upregulation of NLRP3 and pro-IL-1β (Supplemental Fig. 2E). In contrast, NAC inhibited caspase-1 activation in response to TLR signaling (Fig. 3B). NAC did not affect modification of IRAK1 in response to Pam3CSK4 stimulation, as evidenced by the disappearance of the IRAK1 band (Fig. 3B, lower panel), indicating that NAC inhibits NLRP3 activation downstream of IRAK1. The apparent disappearance of IRAK1 band could be due to a shift into higher molecular weight species resulting from phosphorylation and ubiquitination of IRAK1 in response Pam3CSK4. Together our results indicate that transcriptional induction of NLRP3 or other proteins is not required, but ROS production is required for inflammasome activation by TLR signaling.
Figure 3. Transcription-independent ROS-dependent priming of the NLRP3 inflammasome.
A, B, immunoblots of caspase-1 in the culture supernatants (Sup) or cell lysates (Lys) of mouse WT macrophages primed with Pam3CSK4 (Pam), Poly I:C or LPS for the indicated times (min) followed by stimulation with ATP for 45 min in the absence (Con) or presence of actinomycin D (Act D, 0.5 µg/ml) (A), or primed with Pam3CSK4 (Pam for 10 min) or poly I:C (Poly for 45 min) followed by stimulation with ATP for 45 min in the absence or presence of N-acetyl cysteine (NAC, 25 µM) (B) as indicated. The bottom panels show NLRP3 (A) or IRAK1 (B) in the same cell lysates. C, immunoblots showing NLRP3 associates with IRAK1 when NLRP3 was immunoprecipitated (IP) from 293T-NLRP3 cells (293T-N3) but not 293T cells. 293T and 293T-N3 were transfected with WT IRAK1 plasmid. The bands marked with arrows indicate interacting IRAK1 isoforms. The bottom panel shows IKKγ as a negative control. D, E, immunoblots showing NLRP3 associates with endogenous IRAK1 when NLRP3 (D) or IRAK1 (E) were immunoprecipitated (IP) from macrophages following stimulation with Pam3CSK4 for the indicated times. N1–8 represents macrophages stably reconstituted with Flag-NLRP3. NLRP3-KO cells were used as control. Asterisk indicates a non-specific band.
IRAK1 associates with NLRP3
Considering that IRAK1 is required for the rapid activation of NLRP3 during the immediate early and intermediate phases, we investigated whether IRAK1 can interact with NLRP3 to modulate its activation. Our immunoprecipitation studies revealed that the unmodified 80 kDa and modified (phosphorylated/ubiquitinated) 105–110 kDa forms of IRAK1 were distinctly present only in immunoprecipitates from a stable NLRP3-expressing 293T cell line (293T-NLRP3-Flag) but not in the immunoprecipitate from a control 293T cells that do not express NLRP3 (Fig. 3C). A similar modified IRAK1 band was also seen when NLRP3 was immunoprecipitated from N1–8, but not from NLRP3-KO, macrophages after Pam3CSK4 stimulation (Fig. 3D). Immunoprecipitation of endogenous IRAK1 also resulted in co-precipitation of NLRP3, with notable increase of NLRP3 in the immunoprecipitates after stimulation with Pam3CSK4 (Fig. 3E). However, because of the apparent weak interaction of IRAK1 with NLRP3, it remains possible that IRAK1 associates indirectly with NLRP3 through a novel intermediate protein upstream of NLRP3, or through complex interactions of the myddosome (15), which contains MyD88, IRAK4 and IRAK1/IRAK2, with NLRP3. The later possibility is more likely since IRAK1 dissociates from the myddosome after its phosphorylation by IRAK4 to associate with Traf6, which might explain the rapid decline in NLRP3 inflammasome activity after extended stimulation of TLR2 with Pam3CSK4. This might also explain the apparent incomplete loss of the immediate early activation of NLRP3 in IRAK1-KO macrophages, perhaps because IRAK2 might also participate in these interactions. Future characterization of the interaction between NLRP3 and the myddosome, whether the kinase activity of IRAK1 is required for NLRP3 activation and whether IRAK2 can also modulate NLRP3 activation should provide additional insights into the mechanism of activation of NLRP3 by TLR signaling.
In conclusion, this work provides new insights into the regulation of NLRP3 inflammasome activation by TLR signaling and strengthens the role of TLR signaling in the posttranslational assembly and regulation of the NLRP3 inflammasome. Although TLR signaling is thought to regulate the innate immune responses to pathogenic infections by inducing transcription of genes involved in inflammation and other innate immune responses, our work provides clear evidence for a previously unrecognized non-transcriptional role for TLR signaling in inflammasome activation. It also identifies IRAK1 as a critical regulator of a rapid posttranslational activation mechanism of the NLRP3 inflammasome. Based on our observations with Listeria, this mechanism is likely important for rapid killing of infected cells and as an early warning system for the release of preexisting alarmins and other early pro-inflammatory mediators.
Supplementary Material
Acknowledgements
We thank Lisa Waggoner and Zhaozhao Jiang for help with preparation of BMDMs. We also thank Maria Yolanda Covarrubias for assistance with the confocal microscopy.
Footnotes
This work was supported by NIH grant (AR055398 to ESA).
References
- 1.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunological reviews. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 3.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Molecular cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. The Journal of biological chemistry. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. Journal of clinical immunology. 2010;30:693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes-Alnemri T, Yu J, Juliana C, Solorzano L, Kang K, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel C, Alnemri ES. The AIM2 inflammasome is critical for innate immunity against F.tularensis. Nature Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nature immunology. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, Hornung V. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. European journal of immunology. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, N'Guessan P, Witzenrath M, Netea MG, Chakraborty T, Suttorp N, Opitz B. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010;184:922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 12.Machata S, Tchatalbachev S, Mohamed W, Jansch L, Hain T, Chakraborty T. Lipoproteins of Listeria monocytogenes are critical for virulence and TLR2-mediated immune activation. J Immunol. 2008;181:2028–2035. doi: 10.4049/jimmunol.181.3.2028. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PloS one. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay NJ, Gangloff M, O'Neill LA. What the Myddosome structure tells us about the initiation of innate immunity. Trends in immunology. 2011;32:104–109. doi: 10.1016/j.it.2010.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.