Abstract
Significance: Mechanosignaling is vital for maintaining the structural integrity of bone under physiologic conditions. These signals activate and suppress multiple signaling cascades regulating bone formation and resorption. Understanding these pathways is of prime importance to exploit their therapeutic potential in disorders associated with bone loss due to disuse, trauma, or disruption of homeostatic mechanisms. Recent Advances: In the case of cells of the bone, an impressive amount of data has been generated that provides evidence of a complex mechanism by which mechanical signals can maintain or disrupt cellular homeostasis by driving transcriptional regulation of growth factors, matrix proteins and inflammatory mediators in health and inflammation. Mechanical signals act on cells in a magnitude dependent manner to induce bone deposition or resorption. During health, physiological levels of these signals are essential for maintaining bone strength and architecture, whereas during inflammation, similar signals can curb inflammation by suppressing the nuclear factor kappa B (NF-κB) signaling cascade, while upregulating matrix synthesis via mothers against decapentaplegic homolog and/or Wnt signaling cascades. Contrarily, excessive mechanical forces can induce inflammation via activation of the NF-κB signaling cascade. Critical Issues: Given the osteogenic potential of mechanical signals, it is imperative to exploit their therapeutic efficacy for the treatment of bone disorders. Here we review select signaling pathways and mediators stimulated by mechanical signals to modulate the strength and integrity of the bone. Future Directions: Understanding the mechanisms of mechanotransduction and its effects on bone lay the groundwork for development of nonpharmacologic mechanostimulatory approaches for osteodegenerative diseases and optimal bone health. Antioxid. Redox Signal. 20, 970–985.
Introduction
Mechanical signals regulate diverse cellular processes, including cell proliferation, metabolism, homeostasis, differentiation, immune responses, and cell damage through control of anabolic and catabolic activities (75). Bone is an inherently dynamic tissue capable of recognizing and adapting to external forces to meet its functional demands. It responds to the external mechanical forces in the environment by dynamic remodeling of its mass and architecture via bone resorption, synthesis, and apposition (8, 38). The importance of mechanical load is further underscored by the observations that reduced physical demands or disuse of bones leads to rapid bone loss and skeletal fragility and osteopenia generally observed in aging, spinal cord injuries, prolonged bed rest, and microgravity (9, 84, 86, 89, 120, 122). Conversely, physiological loading of the skeleton is anabolic and stimulates osteogenesis; hence, acting as an important therapeutic signal in diseases associated with inflammation, osteopenia, osteoporosis, and arthritis (8, 85, 107, 120, 121). Clinically, it is well documented that appropriate exercise is beneficial not only for arthritic diseases of the joints involving cartilage and bone damage, but also for systemic diseases, such as systemic lupus erythematous and diabetes (76, 109). However, excessive mechanical forces or overload are detrimental and initiate bone pathologies. These observations provide ample evidence that mechanical forces are critical determinants in regulating gene expression, protein synthesis, cell function, and overall skeletal integrity (1, 57, 82). All of these studies point to the fact that bone tissue has mechanosensing networks that direct growth and remodeling; therefore, understanding the molecular mechanisms by which mechanical stimuli on the bone are sensed and translated to biochemical responses is relevant to human health and disease.
Mechanical forces applied to the bone cause deformation. In the bone these are in the form of strain and shear. Strain is a dimensionless unit formally defined as the change in length divided by its original length (ɛ=ΔL/L). Shear stress arises from the interstitial fluid flow through the bone canalicular system and is expressed as force of flow per unit area. While the range of physiological levels of dynamic strain that are osteogenic still remain vague, it is well established that static strain suppresses bone formation (18, 87). Both the magnitude and frequency of mechanical forces are important for bone formation. Being a mineralized tissue, bone perceives relatively low strains (500 μɛ or 0.05%) at frequencies of 1–3 Hz during normal activity (6, 23, 40, 92). In the long bones of humans and animals, these strains have been observed to increase up to 0.2%–0.35% during vigorous activities (6, 12, 91). Strains of ∼0.7% or greater have been shown to be traumatic and lead to bone damage (13, 116, 133). It is now well established that bone cells respond to changes in compressive, tensile, or shear stresses applied by the external mechanical environment. However, because of the complexity of the simultaneous exposure of these forces on the cells, how these forces affect cellular physiology and metabolism disparately is as yet unclear (43, 93, 98). In recent years, several in vivo and in vitro systems have been developed to better understand the responses of bone cells to mechanical forces. Many aspects of mechanosignaling have been elegantly reviewed in recent years (6, 8, 30, 38, 42, 67, 72, 74, 86, 91, 106, 122). The goal of the present review is to elaborate on the major intracellular mechanisms of mechanotransduction that regulate bone homeostasis, bone pathologies, and attenuation of inflammation using in vitro and in vivo models.
Mechanosensitive Cells in Bone
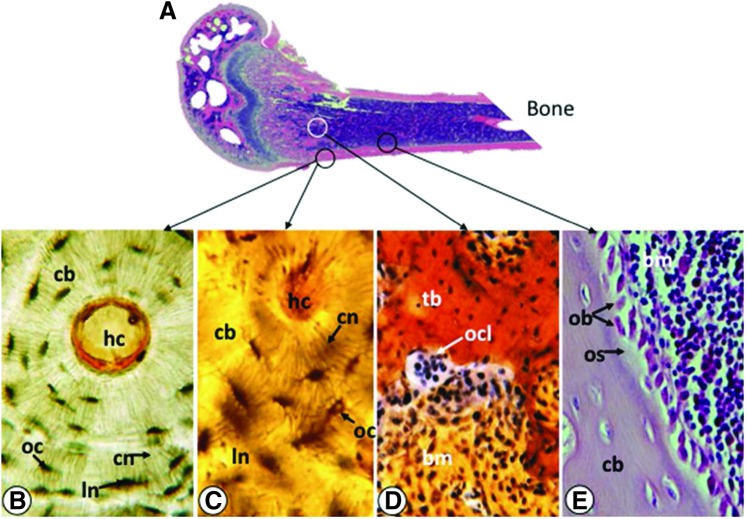
Mechanical forces perceived by bone are translated into biochemical signals that are then integrated into cellular responses via mechanotransduction (39). Bone adapts to mechanical stimuli via the extensively interconnected networks of cells in the bone multicellular unit (BMU) (63, 100). The key cellular components of the BMU are osteocytes, osteoblasts, and osteoclasts (Fig. 1A–E). Osteoblasts and osteocytes are mesenchymal cells that differentiate from stem cells residing in the bone marrow, while osteoclasts originate from monocytes of the hematopoietic cell lineage. Osteocytes reside in cortical (or compact) and trabecular (or spongy) bone matrix (Fig. 1A–C), whereas osteoclasts (Fig. 1D) and osteoblasts (Fig. 1E) occupy the bone marrow lining the bones in their active state. During bone remodeling, a process driven by mechanical loads, mature bone is resorbed by osteoclasts and new bone is deposited by osteoblasts to meet the mechanical demands. The osteocytes, osteoblasts, and osteoclasts are interconnected via intricate cellular networks designed to perceive and transmit mechanical stimuli and facilitate communication between cells within the bone. All of these cells are shown to perceive and respond to mechanical signals in vitro (82, 93). In vivo, osteocytes heavily populating cortical and trabecular bone have long dendritic processes that interconnect neighboring osteoblasts via functional gap junctions and hemichannels. While osteocytes are regarded as the initiator of the bone remodeling process, the collective mechanosensitivity of osteocytes, osteoblasts and osteoclasts appear to be essential for bone remodeling (42, 90). For example, intercellular communication between osteocytes and osteoblasts is essential for the induction of alkaline phosphatase (ALKP) and activation of extracellular signal regulated kinase 1/2 (ERK1/2), both of which are required for bone formation/remodeling (3, 114).
FIG. 1.
Cells of the BMU. (A) Saggital section of bone showing locations of mature osteocytes, osteoclasts and osteoblasts shown in (B–E). (B) Cross-section of cortical bone (cb) showing an osteon of the Haversian system. Each osteon contains concentric layers of lamellae that surround the central Haversian canal (hc). Osteocytes (oc) are located in the lacunar space (ln) and are connected by an extensive network of canaliculi (cn). The cytoplasmic processes of osteocytes located in the canaliculi perceive mechanical signals and respond to them. (C) Enlarged micrograph of osteon showing osteocytes in the lacunar space and interconnected canaliculi. (D) A multinucleated osteoclast (ocl) juxtaposed to trabecular bone (tb), showing bone resorption. Bone marrow (bm) is shown around the trabeculae. (E) Sagittal section at junction of cortical bone and bone marrow showing a layer of osteoblast-like lining cells (ob) depositing osteoid (os) at the endocortical region of the bone. BMU, bone multicellular unit. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mechanosensors on cells
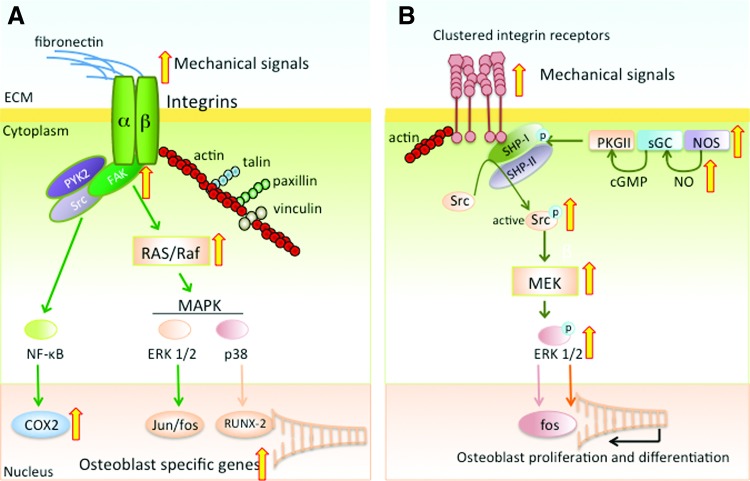
The components of the BMU function as a large mechanosensitive organ by bridging the physical forces and biochemical signals through activation or inhibition of receptors on the cell surface and within the signaling pathways. Evidence thus far strongly suggests that integrins and focal adhesions are the ubiquitous receptors of mechanical forces capable of detecting alterations in the mechanical environment in the extracellular milieu. Heterodimers of α and β subunits of integrins (α5β1) in osteocytes and osteoblasts connect their cytoskeleton to the extracellular matrix (ECM) via arginine-glycine-aspartic acid sequences in fibronectin (Fig. 2A) (112). The sensing of mechanical signals by integrins transmits force through focal adhesions and intracellular stress fibers of F-actin, talin, vinculin, p130Cas, and paxillin (41, 99). When associated with talin and paxillin in focal adhesions, integrins bind to focal adhesion kinase (FAK). FAK also mediates bone regeneration in adult mice and osteoblastic responses to oscillatory fluid shear stress. Furthermore, the absence of FAK function compromises multiple responses to fluid flow, such as ERK1/2 activation, as well as expression of c-Fos, cyclooxygenase 2 (COX2) and osteopontin (OPN) that are required for osteoblast proliferation (46, 130).
FIG. 2.
Mechanosensors FAK and integrins. (A) Schematic representation of heterodimers of alpha and beta subunits of integrin connecting the ECM to cytoskeleton via binding to fibronectin. After perception of mechanical signals, integrins attach to actin, recruiting paxillin, talin, and vinculin in the focal adhesions and transmit the force to the cytoskeleton. This requires FAK phosphorylation and PYK2 and Src activation. The integrins and focal adhesion complexes lead to activation of RAS/Raf and MAPKs, resulting in ERK1/2 and p38 activation with subsequent transcription of Jun/fos and bone associated transcription factors. Integrin activation also induces NF-κB activation, leading to COX2 production required for osteoblast proliferation. (B) Mechanosome pathway of integrin-mediated mechanotransduction showing that shear stress triggers activation of NOS, sGC and PKGII complex via NO production and cGMP-mediated activation of PKGII, respectively. This leads to phosphorylation of SHP-1 and subsequent activation of Src, which is required for the activation of the MEK pathway activating ERK1/2. ERK is known to activate osteoblast proliferation and differentiation via transcription of fos and other osteogenic genes. The wide (yellow in online version) arrows indicate the known steps affected by mechanosignaling in the integrin and FAK signaling cascades. cGMP, cyclic guanosine monophosphate; COX2, cyclooxygenase 2; ECM, extracellular matrix; ERK1/2, extracellular signal regulated kinase 1/2; FAK, focal adhesion kinase; MAPK, mitogen activated protein kinases; MEK, mitogen activated protein kinase kinase; NF-κB, nuclear factor kappa B; NO, nitric oxide; NOS, nitric oxide synthase; PKGII, protein kinase GII; sGC, soluble guanylyl cyclase; SHP-1, small heterodimer partner-1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Rangaswami et al. have suggested an alternate “mechanosome pathway” of mechanotransduction that is mediated by integrin β3 (80). In this pathway, fluid shear stress from interstitial fluid flow triggers assembly of small heterodimer partner-1 (SHP-1) and SHP-2 with β3 integrins that contain adhesion complexes capable of initiating gene expression and cell proliferation. In this process, nitric oxide synthase (NOS) leads to activation of soluble guanylyl cyclase (sGC) and protein kinase GII (PKGII) via cyclic guanosine monophosphate (cGMP). This activation phosphorylates SHP-1, Src and subsequently mitogen activated protein kinase kinase (MEK) (Fig. 2B). These findings further confirm the role of integrins and FAK in mechanosignaling. Other molecules, such as E-cadherins, calcium (Ca++) channels, connexin 43, and ion channels are also shown to mediate signals generated by shear/compressive strain (5, 51, 73, 78).
Mechanotransduction in healthy bones
Mechanotransduction initiates diverse signaling cascades that collectively regulate functions of various bone cell types. Cells exposed to mechanical strain experience combinations of shear, compressive and tensile forces. Both in vivo and in vitro systems have been used to study mechanotransduction; in vitro studies have utilized primary cultures of osteocytes, osteoblasts, osteoblast-like cells, and osteoclasts in monolayers and 3D scaffolds, while in vivo studies employ experimental animal models (22, 66, 68, 122). It is as yet unclear how mechanosignals perceived by the candidate sensors β-integrin/FAK complexes trigger a vast variety of signaling cascades. Nevertheless, evidence thus far indicates that regulation of bone formation and resorption via mechanotransduction is primarily mediated by pathways used by growth factors and mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), Ca++, mothers against decapentaplegic homolog (SMAD), β-catenin, nuclear factor kappa B (NF-κB) and ERK1/2 cascades, rather than a single “mechanosignaling pathway” (39, 44, 74, 75, 97, 98, 116). Mechanoactivation of these intracellular signaling pathways converge to activate/inhibit osteogenic transcription factors, runt-related transcription factor-2 (RUNX-2), Osterix (OSX) and Msh homeobox-2 (MSX-2), as well as regulators of growth factors and matrix proteins required for osteodifferentiation (76).
Elements of Mechanosignaling
Nitric oxide
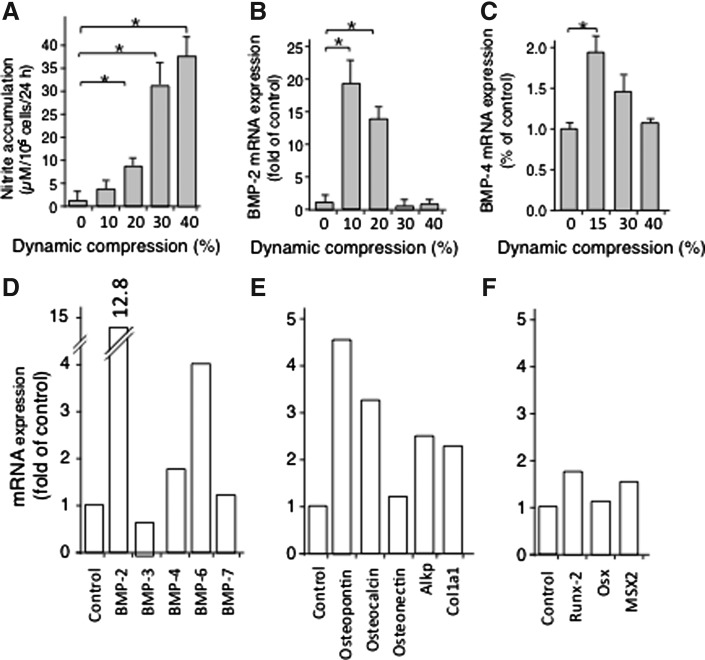
NO induction is a key event during load sensing. It is induced by shear stress and dynamic compression in osteoblasts (82, 125, 132). Relatively small magnitudes of dynamic compression stimulate low levels of inducible NO synthase (iNOS) mRNA expression and synthesis in calvarial cells (82). Nitrite accumulation in response to various magnitudes of dynamic compression has revealed that dynamic compression at relatively low magnitudes (10%) induces low levels of NO production; however, at higher magnitudes of dynamic compression (20%–30%) mechanical signals become traumatic/proinflammatory and induce high levels of NO. NO induction at low magnitudes of mechanical strain is paralleled by induction of osteogenic proteins that drive bone/cartilage formation, such as bone morphogenetic proteins (BMP)-2 and −4, while higher magnitudes (20%–30%) inhibit BMP-2 and −4 expression in osteoblasts and osteoblast-like periodontal ligament cells (Fig. 3A–C) (1, 55, 70). Furthermore, lower dynamic compression (10%) promotes mRNA expression for BMPs, matrix associated proteins and osteogenic transcription factors (Fig. 3D–F) (18, 26, 32, 58). These findings provide evidence for the force magnitude dependent responses of bone cells. Similarly, gentle exercise in vivo leads to the production of BMPs, transcription factors, and matrix-associated proteins which drive osteogenesis.
FIG. 3.
Mechanotransduction in osteoblasts regulates NO and BMP production in a magnitude-dependent manner in vitro. Effects of various magnitudes of dynamic compression on the (A) production of NO, exhibiting lower induction of NO at 10% dynamic compression and higher induction at 20%–40%, (B) mRNA expression for BMP-2, and (C) BMP-4 showing upregulation of both BMP-2 and BMP-4 at 10% dynamic compression and suppression at 20%–40%. Effects of 10% dynamic compression on mRNA expression for (D) BMPs, (E) matrix associated proteins and (F) bone associated transcription factors. The data in (A–F) represent the standard error of the mean of three separate experiments performed in duplicate. *Represents p<0.05. BMP, bone morphogenetic proteins.
Recently, an integral role of NO in osteoblast mechanotransduction has also been described (80). The authors demonstrate that fluid shear stress activates the integrin-Src containing complex through NO-cGMP-PKGII pathway to activate the ERK pathway and induction of several genes (c-fos, fra-1, fra-2, fosB). ERK1/2 activation and induction of these genes are required for multiple cell functions, such as adhesion, migration, proliferation, and cell survival (Fig. 2B).
Prostaglandin E2
Mechanical signals are shown to induce transient secretion of PGE2 as an osteogenic signal (15). This increase in PGE2 is paralleled by the induction of COX2 (50) that drives PGE2 synthesis. In vivo, loading induces upregulation of PGE2 in humans and COX2 in rats (21, 117). COX2 inhibitors, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or NS-398 prevent loading induced bone synthesis, suggesting an important role for PGE2 in mechanosensitive osteogenesis (21). Furthermore, fluid flow leads to the expression of prostaglandins and ATP through connexin 43 expression on the cell surface (3). Prostaglandins thus, act on osteoblasts and osteoclasts via autocrine and paracrine signaling to provoke osteogenic responses to mechanical stimuli (42).
Interestingly, in comparison to wild type COX2+/+ mice, the null mutation of COX2 (COX2−/−) results in reduced femoral bone mass, altered architecture, and inferior mechanical properties. However, the COX2−/− genotype does not influence the responses of bone to mechanotransduction, suggesting that a functional COX2 gene may not be required for mechanical load-induced osteogenesis. COX2−/− mice show an increased expression of COX1 in response to mechanical loading, likely to compensate for the absence of COX2 (2).
Ca++ and ion channels
Ca++ plays an integral role in osteogenesis. Therefore, it is not surprising that Ca++ mobilization is upregulated by mechanotransduction. Intracellular Ca++ is increased in response to mechanical loading of osteoblasts that is inhibitable by gadolinium, a stretch activated ion channel blocker. Verapamil and nifedipine, which block L-type Ca++ channels, also suppress loading induced bone formation, further suggesting a role of Ca++ mobilization in mechanotransduction (51). Influx of Ca++ has been linked to many mechanosensitive signaling pathways, such as NO, protein kinase A, NF-κB, PGE2, mitogen activated protein kinases (MAPK), c-fos, and phosphoinositide 3-kinase (116).
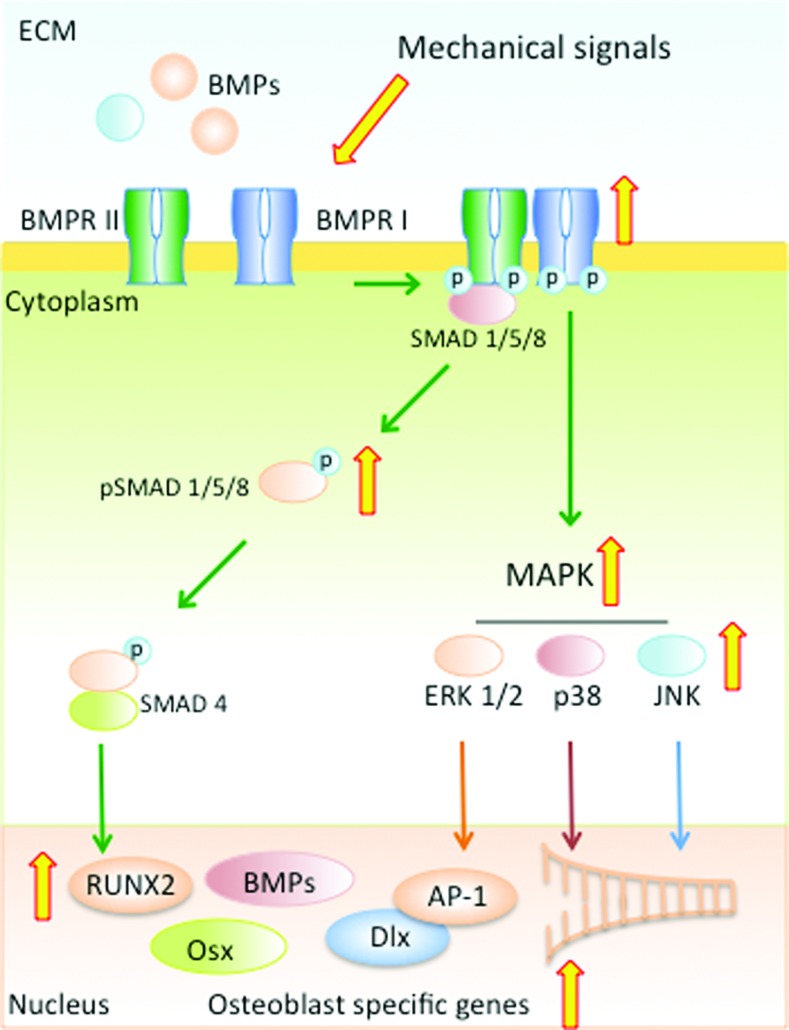
SMAD signaling
SMAD signaling plays an important role in bone formation and is required for induction of bone specific transcription factors, BMPs and bone matrix proteins (Fig. 4). SMADs are a family of transcription factors that are regulated by the members of the transforming growth factor-β (TGF-β) family of molecules, TGF-β and BMPs. So far eight different SMADs have been identified and these work through a myriad of pathways to regulate bone formation. BMP regulated SMADs, such as SMADs 1/5/8 that are activated via BMP binding (to type I and type II receptors) activate BMP target genes, such as RUNX-2 and OSX that are required for osteogenesis. The regulatory SMAD 4 or inhibitory SMADs 6/7 also work via SMAD/1/5/8 by regulating the nuclear translocation and gene transcription activity of the activated/phosphorylated SMAD 1/5/8 complexes. BMP receptor and SMAD 1/5/8 complexes can also activate the MAPK pathway to activate genes involved in osteoblast differentiation (4, 71). In contrast to the BMP activated SMADs, TGF-β regulatory SMADs, such as SMAD 2/3 work synergistically and antagonistically to regulate osteogenesis and when activated by TGF-β, SMAD3 can inhibit osteocalcin (OCN) expression by repressing RUNX-2 (45, 104).
FIG. 4.
SMAD signaling cascade. Mechanoactivation of murine osteoblasts by dynamic compression and fluid flow results in SMAD 1/5/8 phosphorylation via association and activation of BMP receptors. Phosphorylated SMAD 1/5/8 coupled with the regulatory SMAD-4 translocate to the nucleus and activate osteogenic transcription factors and proteins, such as RUNX-2, OSX, DLX and BMPs. Simultaneously, activation of BMP-receptors activates MAPK, ERK1/2, p38, and JNK and subsequently their response elements. The wide (yellow in online version) arrows indicate the known steps utilized by mechanosignaling in the SMAD and MAPK signaling cascades. DLX, distal-less homeobox; JNK, c-JUN N-terminal kinases; OSX, osterix; RUNX-2, runt-related transcription factor-2; SMAD, Mothers against decapentaplegic homolog. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
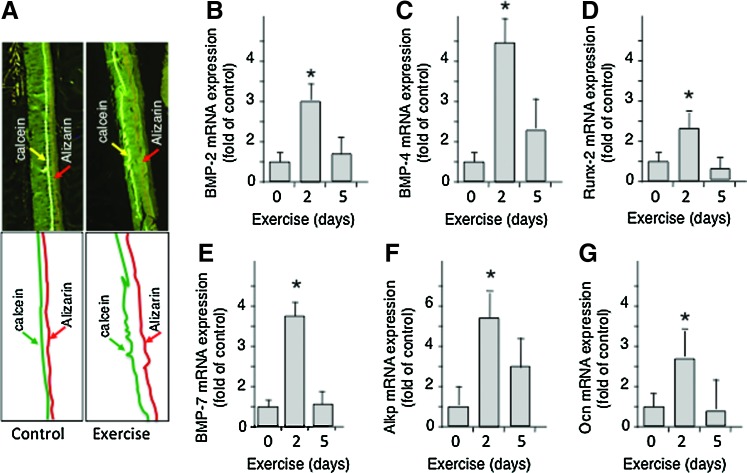
Mechanical signals are potent activators of bone formation via activation of the SMAD signaling cascade. Activation of murine osteoblasts by dynamic compressive forces or fluid flow strain results in SMAD 1/5/8 phosphorylation, which mediates the BMP signaling cascade (48, 81). Furthermore, treatment of osteoblasts with Dorsomorphin, an inhibitor of BMP receptor 1 (BMPR1), downregulates dynamic compression induced SMAD 1/5/8 phosphorylation in osteoblasts, suggesting that BMPR1 may be activated by mechanotransduction (81, 82). Activation of SMAD 1/5/8 by mechanical forces is paralleled by the induction of RUNX-2, BMP2, MSX-2, OSX, as well as bone matrix proteins, such as, OPN, OCN, ALKP, and Collagen type 1α1 (COL-1α1) in osteoblasts (Fig. 3D–F) (82, 128). The observation that inactivation of RUNX-2 in transgenic mice results in complete lack of ossification further supports the fact that mechanical signals have osteogenic potential (47). Not only does fluid flow strain induce osteogenic transcription factors, but it also synergizes with BMP-2 to induce expression of BMP-2, −4 and −7, as well as distal-less homeobox (DLX)-4 and DLX-5 homeotic genes involved in osteogenesis (48). Similar to the direct effects of mechanical loading on osteoblasts, distraction osteogenesis or reconstruction surgery are also shown to induce SMAD 1/5/8 induction and BMP-2 and −4 expression in sheep mandible (20). Similarly, physical activity is also a potent osteogenic signal in vivo, as evident by incorporation of Ca++ binding fluorescent dyes in bone and formation of osteoid at the endosteal and periosteal sides of long bones (Fig. 5A) (122). This bone formation is paralleled by induction of BMP-2, −4, −7, RUNX-2, ALKP, and OCN in trabecular bone (Fig. 5B–G) (47, 61, 122).
FIG. 5.
Mechanical signals generated by gentle exercise up-regulate bone remodeling and expression of osteogenic growth factors. (A) Sagittal sections of femurs endogenously stained with calcein and alizarin in control and exercised mice (upper panels), showing bone growth in mouse femur subjected to gentle exercise for 10 days and compared to femur from nonexercised controls. Lower panels are schematic representations of calcein and alizarin incorporation in the micrographs. Expression of mRNA for (B) BMP-2, (C) BMP-4, (D) RUNX-2, (E) BMP-7, (F) ALKP, and (G) OCN in rat femurs after 0, 2, or 5 days of exercise at 12 M/min for 45 min/day. Each bar represents five rats. *Represents p<0.05. ALKP, alkaline phosphatase; OCN, osteocalcin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Wnt (wingless) signaling
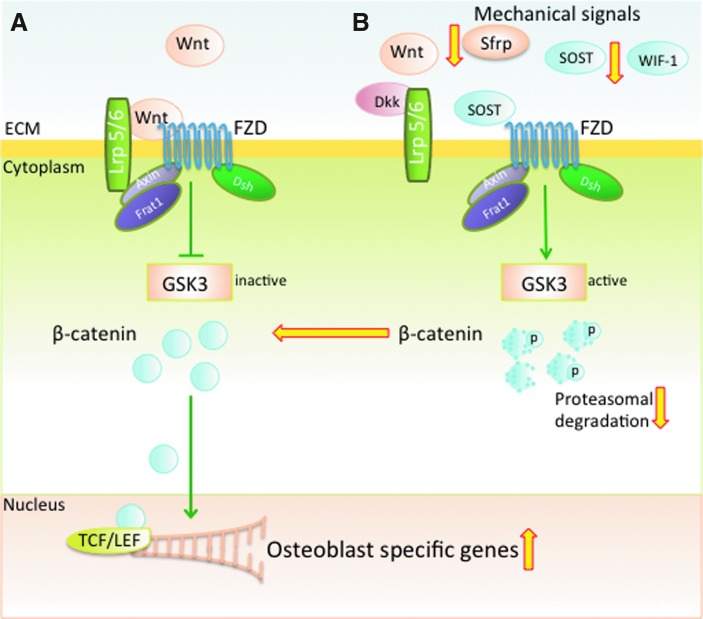
Wnt/β-catenin signaling plays a critical role in osteogenic differentiation and maintenance of bone (49, 77, 124). This pathway is initiated by Wnt ligands binding to the receptor complex formed by low-density lipoprotein receptor-related protein 5 or 6 (Lrp 5/6) and the 7-transmembrane domain spanning frizzled (Fzd) receptor (16, 34, 37). β-catenin a key component of this pathway; lies downstream of Wnt, and is stabilized by Wnt activation. Transduction of Wnt signals leads to downstream inhibition of cytoplasmic glycogen synthase kinase 3 (GSK3), effectively preventing proteasomal degradation of β-catenin. This leaves β-catenin free to accumulate in the cytoplasm and reach levels that promote nuclear translocation where it regulates gene transcription of osteoblastogenic mediators, such as RUNX-2 (24, 28). During cellular differentiation, the presence of β-catenin is required to commit mesenchymal stem cells (MSCs) to an osteoblastic lineage and to prevent adipogenic or chondrocytic differentiation of MSCs (29, 36). Several antagonists tightly regulate Wnt signaling in bone. For example, sclerostin (SOST) and Dickkopf related protein (Dkk) take a major role in inhibiting Lrp 5/6 activity, whereas secreted frizzled related protein (Sfrp) and Wnt inhibitory factor-1 (WIF-1) prevent interactions between Wnt and Fzd receptors (Fig. 6) (10, 53, 62, 102, 119). As discussed below several of the key players in the Wnt pathway are regulated by mechanical forces.
FIG. 6.
Mechanical signals up-regulate Wnt signaling to promote bone formation. (A) Binding of Wnt to Fzd and Lrp 5/6 inhibits GSK3 via activation of Frat1, Axin and Dsh. Inactivation of GSK3 allows β-catenin to accumulate in the cytoplasm and translocate into the nucleus. In the nucleus, the binding of β-catenin to TCF/LEF and transcriptional coactivators allows transcription of osteoblast specific genes. (B) Inhibitors of Wnt signaling (SOST, Sfrp, Dkk, WIF-1) can block the interactions of Wnt with Lrp 5/6 and Fzd. Blockage of this complex allows phosphorylation of β-catenin by active GSK3 and stimulates β-catenin degradation. Mechanical signals inhibit SOST, Sfrp and WIF-1, enabling Wnt to bind to Fzd and Lrp 5/6. This binding prevents β-catenin degradation, allowing for nuclear translocation and subsequent transcriptional activity. The wide (yellow in online version) arrows indicate the known steps in mechanotransduction that up or down regulate Wnt signaling cascades. Dkk, Dickkopf related protein; Dsh, disheveled; Fzd, frizzled; GSK3, glycogen synthase kinase 3; Lrp 5/6, lipoprotein receptor-related protein 5 or 6; Sfrp, secreted frizzled related protein; SOST, sclerostin; WIF-1, Wnt inhibitory factor-1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mechanical loading promotes Wnt/β-catenin canonical signaling in osteoblasts subjected to cyclic strain in vitro and after tibial loading (35, 83). Loading controls β-catenin levels by regulating phosphorylation of GSK3, thereby inhibiting β-catenin degradation and increasing its levels in the cytoplasm; thus, driving osteoblastic differentiation (14, 103). The Wnt signals are reported to be transduced through the Lrp 5/6 receptor; thus, mutations in this receptor results in a constitutive “ON” signal, leading to a state of high bone mass (10, 55, 69), while loss of function mutations in the Lrp5 receptor lead to poor mechanical response to loading (70, 101).
Mechanical signals prevent SOST expression in osteocytes, thereby preventing SOST-induced inhibition of the Wnt signaling pathway (88, 119). SOST is secreted primarily by osteocytes and binds to Lrp 5/6, inhibiting the Wnt signaling pathway and impeding osteoblast proliferation and differentiation (53, 102, 123). The relationship between osteogenesis and SOST is demonstrated through the loss of function mutations in the Sost gene, which leads to sclerosteosis, a disease characterized by high bone mass (7, 102). The SOST−/− mice are also resistant to mechanical unloading induced bone loss and exhibit higher bone mass compared to wild type mice (54). Thus, upregulation of β-catenin and downregulation of SOST by mechanical signals emphasize the relationship between osteoblastic activity and mechanical strain (Fig. 6) (79, 122).
Mechanotransduction in osteoclast formation and function
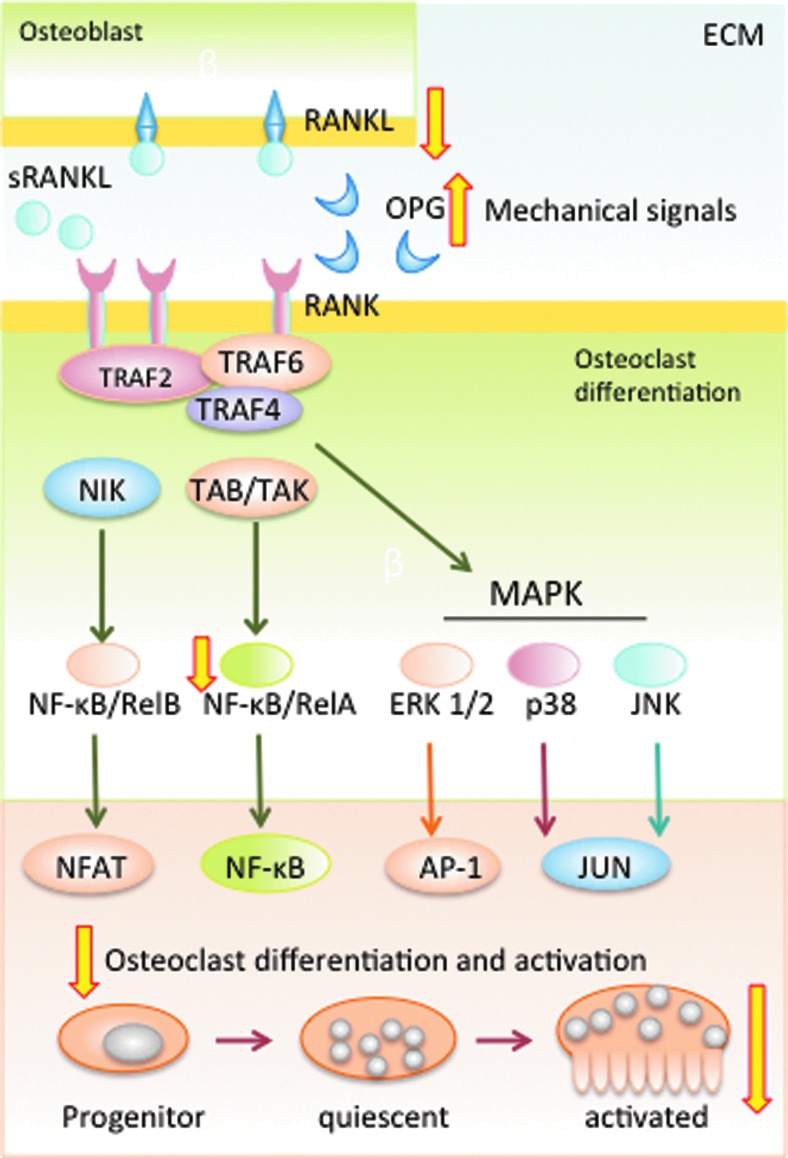
Dynamic bone remodeling preserves bone mass and architecture through the balanced activity of osteoclasts that resorb bone and osteoblasts that produce new bone. Thus, stringently controlled osteoclastic activity is integral in preserving the structural integrity of bone as osteoblasts. Fully differentiated osteoclasts are multinucleated bone resorptive cells found on the surfaces of bone. These cells originate from the hematopoietic (monocyte/macrophage) lineage, but their differentiation is regulated by a group of factors mainly elicited by osteoblasts and osteocytes. The factors involved in the differentiation and maturation of osteoclasts are the receptor activator of NF-κB (RANK/TNFS11), RANK ligand (RANKL/TNFRS11A), and osteoprotegerin (OPG/TNFRS11B). The role of the RANK/RANKL signaling cascade is to regulate bone turnover by stimulating osteoclasts differentiation and activation. Binding of soluble or membrane-bound RANKL to RANK present on the pre-osteoclast surface triggers lineage commitment and osteoclastic differentiation, while continued RANKL/RANK signaling is required for mature osteoclast function. OPG, a soluble protein expressed by osteoblasts, as well as many other cell types, acts as a decoy receptor for RANKL, thereby inhibiting osteoclast differentiation and activation (11, 105, 115, 118, 126). Binding of RANKL to RANK activates both canonical and noncanonical NF-κB signaling cascades, which, along with the transcription factor activator protein-1 (AP-1), leads to activation of calcinurin dependent NF of activated T-cells c1 (NFATc1), which is required for terminal differentiation of osteoclasts (Fig. 7) (113).
FIG. 7.
Mechanical signals suppress osteoclast differentiation. The RANK-RANKL signaling cascade controls differentiation and activation of osteoclasts. RANKL, bound to osteoblast membranes or as soluble RANKL, binds to and activates RANK. RANK-RANKL binding stimulates activation of TRAF6 to activate NF-κB and NFAT transcription factors. TRAF activation also activates the MAPK cascade. NF-κB, NFAT and MAPKs are required for osteoclast differentiation and activation. A competitive inhibitor of RANKL, OPG, prevents osteoclast differentiation by blocking RANKL binding to RANK. Mechanical signals suppress RANKL expression, as well as upregulate OPG expression; thus, inhibiting the initial step in osteoclast differentiation and activation. Mechanical signals, shown to suppress NF-κB activation, may also prevent osteoclast activation. The wide (yellow in online version) arrows indicate the known steps in mechanotransduction that up or down regulate RANK-RANKL signaling cascades. NFAT, NF of activated T-cells; OPG, osteoprotegerin; RANK, receptor activator of NF-κB; RANKL, RANK ligand. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mechanical loading was originally shown to inhibit osteoclastogenesis, a key step to osteoclast differentiation, in murine marrow cells in vitro by Rubin et al. (94). Since then, studies have shown that physiological strain inhibits key steps during osteoclast differentiation. iNOS and NO that are increased by fluid flow strain and dynamic compression inhibit osteoclast differentiation by upregulating OPG; thus, decreasing RANKL activity (19, 27, 33, 60). Mechanical strain (of 2% at 0.16 Hz) reduces vitamin 1,25(OH)2D3− induced osteoclast formation by 50% in murine bone marrow stromal cell cultures in vitro. This suppression in osteoclast differentiation is paralleled by a reduction in RANKL expression by 60% as compared to untreated cells (96). These independent findings suggest that mechanical signals may induce OPG and suppress RANKL to collectively inhibit osteoclast differentiation (Fig. 7). Cyclic stretch at 10% elongation and 0.5 Hz also reduces the total number of differentiated osteoclasts, nuclei/cell and expression of osteoclast-specific genes tartrate-resistant acid phosphatase (TRAP), matrix metalloproteinases (MMP)-9, cathepsin-K, and calcitonin receptors in murine RAW 264.7 preosteoclasts (111).
Mechanotransduction in response to trauma
Deformation of bone above strains of 0.7% could cause traumatic bone damage (13, 116). Acute or chronic cartilage/bone injuries (sterile or nonsterile) can cause bone trauma and is considered to be a major etiological factor for initiation of diseases like osteoarthritis (31, 129). Mechanosensitive osteoblasts and osteoblast-like cells perceive trauma as an external insult. It is as yet unclear how bone cells perceive and differentiate responses to two drastically opposite magnitudes of mechanical signals, that is, those of physiological magnitudes that initiate regenerative responses and of traumatic signals that initiate bone damage and resorption. Knowledge regarding the receptors involved in relaying traumatic signals from the extracellular surface of the bone cells to the intracellular milieu also remains unclear. Traumatic signals are perceived by all bone cells and initiate proinflammatory signaling cascades via activation of NF-κB transcription factors. NF-κB activation leads to the production of high levels of NO and superoxide that mediate both bone damage and matrix degradation. Additionally, elaboration of proinflammatory mediators, such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β) and MMPs plays a key role in the pathogenesis of inflammatory bone diseases and injuries (1, 31, 57, 108). These proinflammatory mediators amplify inflammation and perpetuate tissue damage. Concurrently, the inhibition of growth factors and matrix synthesis by inflammation further exacerbates tissue damage (17, 58, 129).
Disuse or reduced loading of bone inhibits osteoblast-mediated bone formation and accelerates osteoclast-mediated bone resorption. This leads to a coarse trabecular pattern and thinning of cortical bone. Bone loss in disuse is accompanied by NF-κB activation that promotes RANKL production by osteoblasts and neighboring immune cells to trigger osteoclast differentiation and activation (131). Collectively, these processes result in tissue breakdown and loss of bone matrix and structure (Fig. 7).
Mechanotransduction and repair in inflammation
Mechanotransduction at low magnitudes is a potent anti-inflammatory signal. Inflammation activates multiple pathways to counter cellular insults; mechanical signals also utilize similar pathways, particularly the NF-κB signaling cascade, to resolve inflammation. In fact, mechanotransduction, while dampening inflammatory signaling cascades to suppress cytokine production, simultaneously restores the synthesis of BMPs and ECM proteins to repair the damaged tissue.
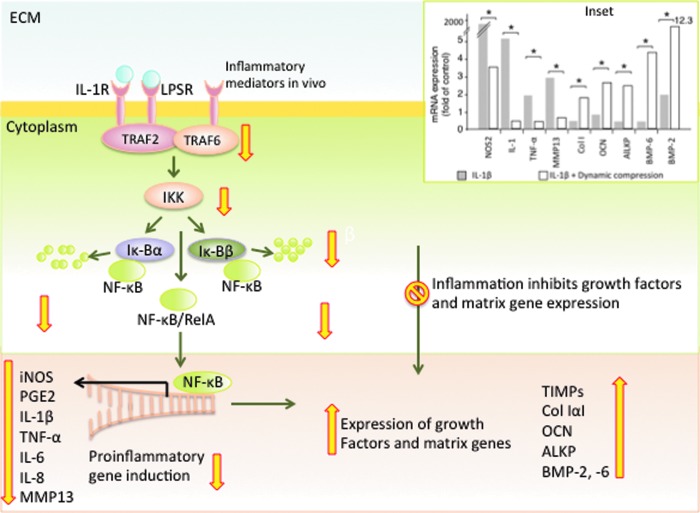
In general, trauma, infections, injury, and many bone diseases involve some degree of inflammation. Inflammatory stimuli sensed by cells activate the rapid response NF-κB signaling cascade that controls transcription of over 150 target genes, making it one of the central mediators of inflammation and immune response (26). Both NF-κB signaling pathways, canonical and noncanonical, are activated by signals associated with stress, growth factors, cytokines and inflammatory mediators (44). The canonical NF-κB signaling cascade involves activation of multiple intracellular adaptor proteins, including myeloid differentiation primary response gene 88 (MYD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), TAK, TAB, and TRAF6. Phosphorylated TRAF6 triggers NF-κB activation via IκB kinase complex (IKK), comprised of IKKα and IKKβ catalytic units and the IKKγ regulatory unit. In the cytoplasm, inactive NF-κB is sequestered by the inhibitor of NF-κB (IκB). Upon activation, the IKK complex phosphorylates IκBα and IκBβ proteins on specific serine residues that promote its proteasomal degradation. The free NF-κB then translocates to the nucleus and initiates NF-κB dependent gene transcription capable of eliciting inflammation, innate immune responses, adaptive immune responses and osteoclastogenesis (Fig. 8) (25, 44, 110).
FIG. 8.
Physiologic levels of mechanical signaling inhibit the NF-κB signaling cascade. Inflammatory molecules such IL-1 or LPS in vitro and inflammatory mediators in vivo activate their respective receptors to initiate a chain of activation and deactivation of signaling and adaptive proteins, leading to phosphorylation of TRAFs. TRAFs activate IKK that activates IκBα and IκBβ, leading to IκB proteasomal degradation and subsequent nuclear translocation of NF-κB. NF-κB in the cytoplasm is sequestered by IκBα and IκBβ. IKK induced phosphorylation and degradation of IκB allows NF-κB nuclear translocation. Subsequently, NF-κB binds to the promoters of a plethora of proinflammatory cytokines and mediators to initiate inflammation. Mechanical signals suppress TRAF-6 activation, leading to IKK and IκB inactivation, with subsequent inhibition of NF-κB nuclear translocation and suppression of proinflammatory gene induction. Simultaneously, inflammation inhibits matrix synthesis. Mechanical signals counteract this blockage of matrix synthesis and upregulate expression of growth factors and matrix associated genes. Inset. Effects of 10% dynamic compression on IL-1 induced mRNA expression of salient proinflammatory and matrix associated genes, showing that 10% dynamic compression suppresses proinflammatory gene (NOS2, IL-1, TNF-α, MMP13) induction and upregulates matrix associated gene expression (Col-IαI, OCN, ALKP, BMP-6, BMP-2) suppressed by inflammation. *Represents p<0.05. The wide (yellow in online version) arrows indicate the known steps in mechanotransduction that up or down regulate NF-κB signaling cascades and matrix synthesis. IκB, inhibitor of NF-κB; IKK, IκB kinase complex; IL-1, interleukin-1; LPS, lipopolysaccharide; MMP, matrix metalloproteinases; TNF-α, tumor necrosis factor alpha. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mechanical signals (tensile, compressive, and shear) of low magnitudes are powerful anti-inflammatory signals that suppress expression of several proinflammatory moieties, such as IL-1β induced NOS2, NO, COX2, PGE2, cytokines (IL-1β and TNF-α), and MMPs in osteoblasts and osteoblasts like periodontal ligament cells in vitro. Simultaneously, these signals upregulate the expression of growth factors COL-1α1, BMPs, OCN and ALKP that are inhibited during inflammation (inset in Fig. 8) (1, 57). Several anti-inflammatory cytokines (IL-10) and tissue inhibitors of metalloproteinases (TIMPs) that are inhibited during inflammation are upregulated by mechanical signals. For instance IL-10 and TIMP-II synthesized by low magnitudes of mechanical signals can suppress inflammation and matrix breakdown in osteoblast and osteoblast-like cells (1, 56, 127).
Investigations focused on the mechanisms of mechanotransduction in IL-1β treated osteoblast-like cells have shown that mechanical signals dramatically inhibit NF-κB nuclear translocation within the initial 10 min of the signal to inhibit proinflammatory gene induction (1). At present, it is unclear at what stage mechanotransduction intercepts the NF-κB signaling cascade within osteoblasts. However, similar studies in chondrocytes have revealed that mechanotransduction inhibits TRAF6 phosphorylation and subsequent activation of the IKK complex that in turn fails to phosphorylate IκBα and IκBβ and their eventual proteasomal degradation. This consequently inhibits nuclear translocation of NF-κB and its binding to consensus sequences and subsequent transcriptional activity (Fig. 8) (17, 59, 64, 127). Since the NF-κB pathway is ubiquitously present in all mammalian cells, it is activated by more than 150 different ligands/signals and follows the same sequential activation, making it likely that mechanotransduction in osteoblasts follows the same events as those observed in chondrocytes.
Mechanical signals are shown to inhibit osteoclast differentiation and maturation via suppression of RANKL expression and induction of OPG by osteoblasts in vitro (95, 116). Bone resorption in osteoclasts is heightened during inflammation through NF-κB activation and induction of RANKL (Fig. 7). Hence, suppression of NF-κB activity in osteoblasts by mechanical signals may also suppress osteoclast differentiation.
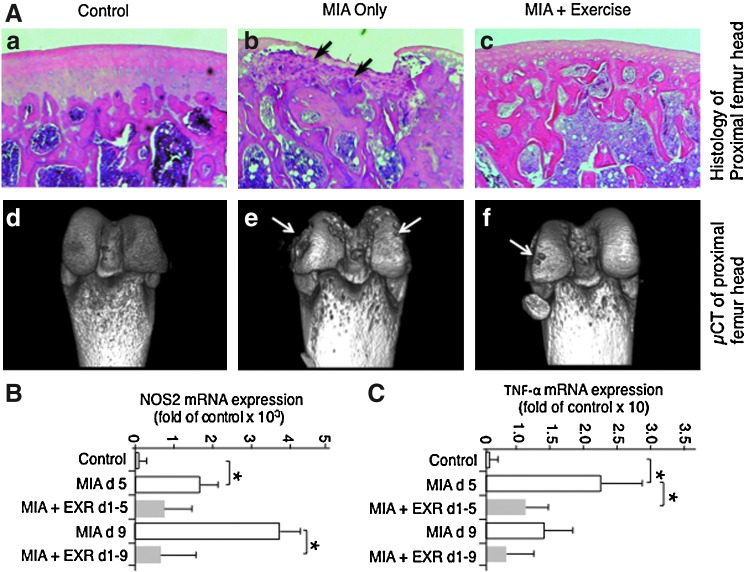
Chronic systemic or local inflammatory disorders are invariably associated with bone loss. These observations have prompted investigations to examine the anti-inflammatory effects of mechanical loading in experimental models of knee osteoarthritis (32, 66). According to these investigations, exercise effectively suppresses inflammation of cartilage and bone. In an experimental model of monoiodoacetate-induced osteoarthritis (MIA) of rat knees, progression of cartilage degradation is paralleled by bone loss and its replacement by inflammatory cells and fibrocartilagenous tissue. However, inflammation and bone loss induced by MIA is markedly prevented by gentle treadmill walking (used as exercise) (Fig. 9). The inhibition of bone loss by exercise corresponds with inhibition of NOS2 and TNF-α mRNA expression in the trabecular bone in comparison to bones showing successive progression of MIA. The inhibition of the progression of MIA in response to exercise demonstrates the potential of mechanosignaling in preferentially targeting the NF-κB signaling cascade to suppress catabolic activities in inflamed tissue (65, 66).
FIG. 9.
Signals generated by gentle exercise suppress bone damage in osteoarthritic lesions. (A) Histological sections showing (a) control distal femoral head with intact bone structures, and (b) bone damage induced by MIA lesions at distal femoral condyles of rat knees on day 21 post-MIA induction. Black arrows indicate formation of massive bone degradation and fibrocartilage formation. (c) MIA afflicted rat knees exposed to exercise (12 m/min for 45 min/day) after MIA induction showing minimal bone damage and normal histology. Lower panels showing μCT analysis of bone in (d) control with no bone damage, and (e) extensive bone damage on the femoral head of MIA afflicted knees. White arrows indicate bone lesions. (f) Minimal bone damage on the femoral head of bone exposed to MIA but exercised daily for 21 days. (B, C) Effects of exercise on MIA induced mRNA expression of NOS2 and TNF-α in control bones, those exposed to MIA only for 5 and 9 days and those exposed to MIA and exercise simultaneously for 5 and 9 days, showing their significant down-regulation by exercise. *Represents p<0.05. μCT, micro computerized tomography; MIA, monoiodoacetate-induced osteoarthritis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Conclusions
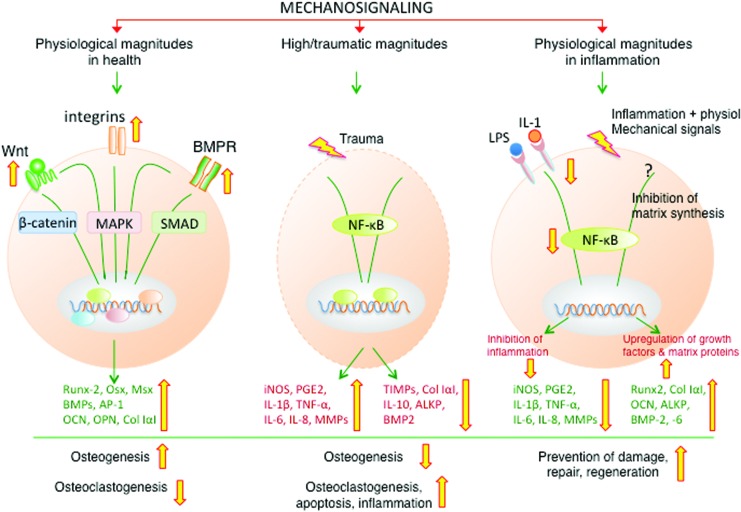
This review illustrates the multifaceted aspects of mechanotransduction initiated signaling in maintenance of bone structure and function. The magnitude of mechanical force is a critical determinant of the outcomes of mechanotransduction (Fig. 10). In healthy bones, mechanotransduction induced by physiologic magnitudes of mechanical forces is anabolic and interacts with cellular signaling networks collectively for optimal deposition and strengthening of bone. At higher magnitudes of mechanical forces, mechanotransduction is directed towards activation of NF-κB signaling cascade and proinflammatory responses. In inflamed bone, mechanotransduction targeted pathways are completely distinct from homeostatic pathways and are focused towards dampening the inflammation and abrogating the repression of growth factors and matrix synthesis caused by inflammation. However, the magnitude of mechanical signals is only one of the many factors that are important to the regulation of multiple signaling cascades that impact bone remodeling. The cellular responses of bone are also influenced by the number of loading cycles per unit of time, frequency and the dynamic versus static nature of the strain. The bone age may also dictate the mechanotransduction pathways regulated by mechanical signals in young versus aging skeleton. Nevertheless, understanding of bone mechanotransduction is important for exploiting its potential to develop new strategies for bone tissue engineering. It is also important to develop mechanical stimulation regimens to combat inflammation at the site of bone damage and create the right environment for bone repair and homeostasis. These initial findings may also provide a foundation for future development of nonpharmacologic treatments of osteodegenerative diseases and maintenance of bone health during the lifetime.
FIG. 10.
A schematic representation of the actions of mechanical signals on the regulation of distinct signaling pathways in health and inflammation. In healthy bones, physiological levels of mechanical signals act as anabolic signals, and promote bone formation via activation of the SMAD 1/5/8 signaling pathway that is required for the induction of BMPs, osteogenic transcription factors (RUNX-2) and proteins necessary for bone formation (ALKP, COL-IαI, OCN). Simultaneously, mechanotransduction suppresses SOST induction to allow bone formation via the Wnt signaling pathway. During inflammation, physiological levels of mechanical signals suppress the NF-κB signaling cascade to block expression of NO, cytokines and MMPs, and inhibit catabolic actions of proinflammatory mediators. Simultaneously, mechanical signals upregulate the synthesis of osteogenic molecules that are suppressed during inflammation to initiate repair. The wide (yellow in online version) arrows indicate the known steps that up or down regulate mechanosignaling in response to various magnitudes of strains. COL-1α1, collagen type 1α1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Abbreviations Used
- μCT
micro computerized tomography
- ALKP
alkaline phosphatase
- AP-1
activator protein-1
- BMP
bone morphogenetic proteins
- BMPR1
BMP receptor 1
- BMU
bone multicellular unit
- Ca++
calcium
- cGMP
cyclic guanosine monophosphate
- COL-1α1
collagen type 1α1
- COX2
cyclooxygenase 2
- Dkk
Dickkopf related protein
- DLX
distal-less homeobox
- Dsh
dishevelled
- ECM
extracellular matrix
- ERK1/2
extracellular signal regulated kinase 1/2
- FAK
focal adhesion kinase
- Fzd
frizzled
- GSK3
glycogen synthase kinase 3
- IKK
IκB kinase complex
- IL-1β
interleukin-1 beta
- iNOS
inducible NOS
- IκB
inhibitor of NF-κB
- JNK
c-JUN N-terminal kinases
- LPS
lipopolysaccharide
- Lrp 5/6
lipoprotein receptor-related protein 5 or 6
- MAPK
mitogen activated protein kinases
- MEK
mitogen activated protein kinase kinase
- MIA
monoiodoacetate-induced osteoarthritis
- MMP
matrix metalloproteinases
- MSC
mesenchymal stem cells
- MSX-2
Msh homeobox-2
- MYD88
myeloid differentiation primary response gene 88
- NFATc1
NF of activated T-cells c1
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- NOS
nitric oxide synthase
- NSAIDs
nonsteroidal anti-inflammatory drugs
- OCN
osteocalcin
- OPG
osteoprotegerin
- OPN
osteopontin
- OSX
osterix
- PGE2
prostaglandin E2
- PKGII
protein kinase GII
- RANK
receptor activator of NF-κB
- RANKL
RANK ligand
- RUNX-2
runt-related transcription factor-2
- Sfrp
secreted frizzled related protein
- sGC
soluble guanylyl cyclase
- SHP-1
small heterodimer partner-1
- SMAD
mothers against decapentaplegic homolog
- SOST
sclerostin
- TGF-β
transforming growth factor-β
- TIMPs
tissue inhibitor of metalloproteinases
- TNF-α
tumor necrosis factor alpha
- TRAP
tartrate-resistant acid phosphatase
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- WIF-1
Wnt inhibitory factor-1
Acknowledgments
The authors are indebted to Dr. Rudy Melfi for providing bone histology slides. This work was supported by Grant No. AR48781 from National Institute of Arthritis, Musculoskeletal and Skin Disease, and DE105399 and DE014320 from National Institute of Craniofacial and Dental Research at National Institutes of Health, Bethesda, MD.
References
- 1.Agarwal S, Long P, Seyedain A, Piesco N, Shree A, and Gassner R. A central role for the nuclear factor-kappaB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J 17: 899–901, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam I, Warden SJ, Robling AG, and Turner CH. Mechanotransduction in bone does not require a functional cyclooxygenase-2 (COX-2) gene. J Bone Miner Res 20: 438–446, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Alford AI, Jacobs CR, and Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism small star, filled. Bone 33: 64–70, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Anderson GJ. and Darshan D. Small-molecule dissection of BMP signaling. Nat Chem Biol 4: 15–16, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Arnsdorf EJ, Tummala P, and Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4: e5388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astrid D Bakker. and Jenneke Klein-Nulend. Role of osteocytes in the adaptation of bone to mechanical loading. Future Rheum 3: 571, 2008 [Google Scholar]
- 7.Balemans W. and Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250: 231–250, 2002 [PubMed] [Google Scholar]
- 8.Bergmann P, Body JJ, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, Kaufman J, Reginster JY, and Rozenberg S. Loading and skeletal development and maintenance. J Osteoporos 2011: 786752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikle DD. and Halloron BP. The response of bone to unloading. J Bone Miner Metab 17: 233–244, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, and Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Boyle WJ, Simonet WS, and Lacey DL. Osteoclast differentiation and activation. Nature 423: 337–342, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, and Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone 18: 405–410, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Carter DR, Caler WE, Spengler DM, and Frankel VH. Fatigue behavior of adult cortical bone: the influence of mean strain and strain range. Acta Orthop Scand 52: 481–490, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Case N. and Rubin J. Beta-catenin—a supporting role in the skeleton. J Cell Biochem 110: 545–553, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, and Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell 16: 3100–3106, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, and Robling AG. Lrp5 functions in bone to regulate bone mass. Nat Med 17: 684–691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dossumbekova A, Anghelina M, Madhavan S, He L, Quan N, Knobloch T, and Agarwal S. Biomechanical signals inhibit IKK activity to attenuate NF-kappaB transcription activity in inflamed chondrocytes. Arthritis Rheum 56: 3284–3296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrlich PJ. and Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int 13: 688–700, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Fan X, Roy E, Zhu L, Murphy TC, Ackert-Bicknell C, Hart CM, Rosen C, Nanes MS, and Rubin J. Nitric oxide regulates receptor activator of nuclear factor-kappaB ligand and osteoprotegerin expression in bone marrow stromal cells. Endocrinology 145: 751–759, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Farhadieh RD, Gianoutsos MP, Yu Y, and Walsh WR. The role of bone morphogenetic proteins BMP-2 and BMP-4 and their related postreceptor signaling system (Smads) in distraction osteogenesis of the mandible. J Craniofac Surg 15: 714–718, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 11: 1688–1693, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Forwood MR. Physical activity and bone development during childhood: insights from animal models. J Appl Physiol 105: 334–341, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Fritton SP, McLeod KJ, and Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech 33: 317–325, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, and Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280: 33132–33140, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Ghosh G, Wang VY, Huang DB, and Fusco A. NF-kappaB regulation: lessons from structures. Immunol Rev 246: 36–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh S. and Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol 8: 837–848, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, and Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Glass DA., 2nd and Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology 148: 2630–2634, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37: 1974–1984, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Guadalupe-Grau A, Fuentes T, Guerra B, and Calbet JA. Exercise and bone mass in adults. Sports Med 39: 439–468, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol 25: 815–823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzman RE, Evans MG, Bove S, Morenko B, and Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol 31: 619–624, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Hall CL, Kang S, MacDougald OA, and Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem 97: 661–672, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Hay E, Faucheu C, Suc-Royer I, Touitou R, Stiot V, Vayssiere B, Baron R, Roman-Roman S, and Rawadi G. Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J Biol Chem 280: 13616–13623, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, and Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res 20: 1103–1113, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, and Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum 52: 794–799, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, and Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 19: 2033–2040, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Huang C. and Ogawa R. Mechanotransduction in bone repair and regeneration. FASEB J 24: 3625–3632, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Kamm RD, and Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol 287: C1–C11, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Huang RP, Rubin CT, and McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci 54: B352–B357, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Hughes DE, Salter DM, Dedhar S, and Simpson R. Integrin expression in human bone. J Bone Miner Res 8: 527–533, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Jacobs CR, Temiyasathit S, and Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng 12: 369–400, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, and Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech 31: 969–976, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanarek N. and Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunol Rev 246: 77–94, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Kang JS, Alliston T, Delston R, and Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J 24: 2543–2555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JB, Leucht P, Luppen CA, Park YJ, Beggs HE, Damsky CH, and Helms JA. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone 41: 39–51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, and Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Kopf J, Petersen A, Duda GN, and Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol 10: 37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan V, Bryant HU, and Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 116: 1202–1209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Burr DB, and Turner CH. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int 70: 320–329, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Li J, Duncan RL, Burr DB, and Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res 17: 1795–1800, 2002 [DOI] [PubMed] [Google Scholar]
- 52.This reference has been deleted.
- 53.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, and Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, and He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24: 1651–1661, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, and Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70: 11–19, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long P, Hu J, Piesco N, Buckley M, and Agarwal S. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res 80: 1416–1420, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long P, Liu F, Piesco NP, Kapur R, and Agarwal S. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone 30: 547–552, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madhavan S, Anghelina M, Rath-Deschner B, Wypasek E, John A, Deschner J, Piesco N, and Agarwal S. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthritis Cartilage 14: 1023–1032, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madhavan S, Anghelina M, Sjostrom D, Dossumbekova A, Guttridge DC, and Agarwal S. Biomechanical signals suppress TAK1 activation to inhibit NF-{kappa}B transcriptional activation in fibrochondrocytes. J Immunol 179: 6246–6254, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mak KK, Chen MH, Day TF, Chuang PT, and Yang Y. Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 133: 3695–3707, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Mantila Roosa SM, Liu Y, and Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res 26: 100–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, and Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417: 664–667, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Mi LY, Basu M, Fritton SP, and Cowin SC. Analysis of avian bone response to mechanical loading. Part two: development of a computational connected cellular network to study bone intercellular communication. Biomech Model Mechanobiol 4: 132–146, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Nam J, Aguda BD, Rath B, and Agarwal S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: experiments and modeling. PLoS One 4: e5262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam J, Perera P, Liu J, Rath B, Deschner J, Gassner R, Butterfield TA, and Agarwal S. Sequential alterations in catabolic and anabolic gene expression parallel pathological changes during progression of monoiodoacetate-induced arthritis. PLoS One 6: e24320, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nam J, Perera P, Liu J, Wu LC, Rath B, Butterfield TA, and Agarwal S. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate-induced arthritis. Arthritis Rheum 63: 1613–1625, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam J, Perera P, Rath B, and Agarwal S. Dynamic regulation of bone morphogenic proteins in engineered osteochondral constructs by biomechanical stimulation. Tissue Eng 19: 783–792, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nam J, Rath B, Knobloch TJ, Lannutti JJ, and Agarwal S. Novel electrospun scaffolds for the molecular analysis of chondrocytes under dynamic compression. Tissue Eng Part A 15: 513–523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niziolek PJ, Farmer TL, Cui Y, Turner CH, Warman ML, and Robling AG. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone 49: 1010–1019, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niziolek PJ, Warman ML, and Robling AG. Mechanotransduction in bone tissue: The A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone 51: 459–465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nohe A, Keating E, Knaus P, and Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal 16: 291–299, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Nomura T, Ueyama T, Ashihara E, Tateishi K, Asada S, Nakajima N, Isodono K, Takahashi T, Matsubara H, and Oh H. Skeletal muscle-derived progenitors capable of differentiating into cardiomyocytes proliferate through myostatin-independent TGF-beta family signaling. Biochem Biophys Res Commun 365: 863–869, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Onyia JE, Bidwell J, Herring J, Hulman J, and Hock JM. In vivo, human parathyroid hormone fragment (hPTH 1–34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone 17: 479–484, 1995 [DOI] [PubMed] [Google Scholar]
- 74.Papachristou DJ, Papachroni KK, Basdra EK, and Papavassiliou AG. Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays 31: 794–804, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, and Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med 15: 208–216, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Petersen AM. and Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Piters E, Boudin E, and Van Hul W. Wnt signaling: a win for bone. Arch Biochem Biophys 473: 112–116, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Plotkin LI. and Bellido T. Beyond gap junctions: connexin43 and bone cell signaling. Bone 52: 157–166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price JS, Sugiyama T, Galea GL, Meakin LB, Sunters A, and Lanyon LE. Role of endocrine and paracrine factors in the adaptation of bone to mechanical loading. Curr Osteoporos Rep 9: 76–82, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Rangaswami H, Schwappacher R, Marathe N, Zhuang S, Casteel DE, Haas B, Chen Y, Pfeifer A, Kato H, Shattil S, Boss GR, and Pilz RB. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci Signal 3: ra91, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rath B, Nam J, Deschner J, Schaumburger J, Tingart M, Grassel S, Grifka J, and Agarwal S. Biomechanical forces exert anabolic effects on osteoblasts by activation of SMAD 1/5/8 through type 1 BMP receptor. Biorheology 48: 37–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rath B, Nam J, Knobloch TJ, Lannutti JJ, and Agarwal S. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J Biomech 41: 1095–1103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, and Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281: 31720–31728, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Robling AG, Burr DB, and Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol 204: 3389–3399, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Robling AG, Burr DB, and Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact 1: 249–262, 2001 [PubMed] [Google Scholar]
- 86.Robling AG, Castillo AB, and Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8: 455–498, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, and Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone 29: 105–113, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, and Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283: 5866–5875, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Robling AG. and Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19: 319–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rochefort GY, Pallu S, and Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 21: 1457–1469, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Rubin CT. Skeletal strain and the functional significance of bone architecture. Calcif Tissue Int 36Suppl 1: S11–S18, 1984 [DOI] [PubMed] [Google Scholar]
- 92.Rubin CT. and Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol 107: 321–327, 1984 [DOI] [PubMed] [Google Scholar]
- 93.Rubin J. Regulation of skeletal remodeling by biomechanical input. Osteoporos Int 14Suppl 5: S43–S45, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Rubin J, Biskobing D, Fan X, Rubin C, McLeod K, and Taylor WR. Pressure regulates osteoclast formation and MCSF expression in marrow culture. J Cell Physiol 170: 81–87, 1997 [DOI] [PubMed] [Google Scholar]
- 95.Rubin J, Fan X, Biskobing DM, Taylor WR, and Rubin CT. Osteoclastogenesis is repressed by mechanical strain in an in vitro model. J Orthop Res 17: 639–645, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Rubin J, Murphy T, Nanes MS, and Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol 278: C1126–C1132, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Rubin J, Murphy TC, Rahnert J, Song H, Nanes MS, Greenfield EM, Jo H, and Fan X. Mechanical inhibition of RANKL expression is regulated by H-Ras-GTPase. J Biol Chem 281: 1412–1418, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Rubin J, Rubin C, and Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene 367: 1–16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salter DM, Robb JE, and Wright MO. Electrophysiological responses of human bone cells to mechanical stimulation: evidence for specific integrin function in mechanotransduction. J Bone Miner Res 12: 1133–1141, 1997 [DOI] [PubMed] [Google Scholar]
- 100.Santos A, Bakker AD, and Klein-Nulend J. The role of osteocytes in bone mechanotransduction. Osteoporos Int 20: 1027–1031, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, and Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281: 23698–23711, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Semenov M, Tamai K, and He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280: 26770–26775, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Sen B, Styner M, Xie Z, Case N, Rubin CT, and Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem 284: 34607–34617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi X, Bai S, Li L, and Cao X. Hoxa-9 represses transforming growth factor-beta-induced osteopontin gene transcription. J Biol Chem 276: 850–855, 2001 [DOI] [PubMed] [Google Scholar]
- 105.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, and Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997 [DOI] [PubMed] [Google Scholar]
- 106.Skerry T. Mechanostat function during skeletal development. J Musculoskelet Neuronal Interact 8: 12, 2008 [PubMed] [Google Scholar]
- 107.Srinivasan S, Ausk BJ, Prasad J, Threet D, Bain SD, Richardson TS, and Gross TS. Rescuing loading induced bone formation at senescence. PLoS Comput Biol 6: e1000924, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, and Tannenbaum SR. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics 8: 1475–1489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strombeck B. and Jacobsson LT. The role of exercise in the rehabilitation of patients with systemic lupus erythematosus and patients with primary Sjogren's syndrome. Curr Opin Rheumatol 19: 197–203, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev 246: 125–140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suzuki N, Yoshimura Y, Deyama Y, Suzuki K, and Kitagawa Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med 21: 291–296, 2008 [PubMed] [Google Scholar]
- 112.Takagi J. Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochem Soc Trans 32: 403–406, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, and Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901, 2002 [DOI] [PubMed] [Google Scholar]
- 114.Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, and Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol 292: C545–C552, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Theill LE, Boyle WJ, and Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20: 795–823, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Thompson WR, Rubin CT, and Rubin J. Mechanical regulation of signaling pathways in bone. Gene 503: 179–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thorsen K, Kristoffersson AO, Lerner UH, and Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J Clin Invest 98: 2446–2449, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, and Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 234: 137–142, 1997 [DOI] [PubMed] [Google Scholar]
- 119.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, and Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50: 209–217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Turner CH. and Robling AG. Exercise as an anabolic stimulus for bone. Curr Pharm Des 10: 2629–2641, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Turner CH. and Robling AG. Mechanisms by which exercise improves bone strength. J Bone Miner Metab 23Suppl: 16–22, 2005 [DOI] [PubMed] [Google Scholar]
- 122.Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, Danzig J, and Robling AG. Mechanobiology of the skeleton. Sci Signal 2: pt3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, and Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199: 805–814, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Noort M, Meeldijk J, van der Zee R, Destree O, and Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem 277: 17901–17905, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Vatsa A, Smit TH, and Klein-Nulend J. Extracellular NO signalling from a mechanically stimulated osteocyte. J Biomech 40Suppl 1: S89–S95, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Wada T, Nakashima T, Hiroshi N, and Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Wang L, Li JY, Zhang XZ, Liu L, Wan ZM, Li RX, and Guo Y. Involvement of p38MAPK/NF-kappaB signaling pathways in osteoblasts differentiation in response to mechanical stretch. Ann Biomed Eng 40: 1884–1894, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Wang L, Zhang X, Guo Y, Chen X, Li R, Liu L, Shi C, Guo C, and Zhang Y. Involvement of BMPs/Smad signaling pathway in mechanical response in osteoblasts. Cell Physiol Biochem 26: 1093–1102, 2010 [DOI] [PubMed] [Google Scholar]
- 129.Weinberg JB, Fermor B, and Guilak F. Nitric oxide synthase and cyclooxygenase interactions in cartilage and meniscus: relationships to joint physiology, arthritis, and tissue repair. Subcell Biochem 42: 31–62, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Young SR, Gerard-O'Riley R, Kim JB, and Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res 24: 411–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu M, Qi X, Moreno JL, Farber DL, and Keegan AD. NF-kappaB signaling participates in both RANKL- and IL-4-induced macrophage fusion: receptor cross-talk leads to alterations in NF-kappaB pathways. J Immunol 187: 1797–1806, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zaman G, Pitsillides AA, Rawlinson SC, Suswillo RF, Mosley JR, Cheng MZ, Platts LA, Hukkanen M, Polak JM, and Lanyon LE. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 14: 1123–1131, 1999 [DOI] [PubMed] [Google Scholar]
- 133.Zhang D, Weinbaum S, and Cowin SC. Estimates of the peak pressures in bone pore water. J Biomech Eng 120: 697–703, 1998 [DOI] [PubMed] [Google Scholar]