Abstract
Background: Perforations of surgical gloves are common and increase with the duration of glove wear. Skin flora, re-grown after pre-operative disinfection of the hands, may contaminate a surgical site. An antimicrobial surgical glove with chlorhexidine on its inner surface has been developed. We hypothesized that by suppressing the re-growth of skin flora during the complete course of a surgical procedure, antimicrobial gloves may reduce the risk of surgical site contamination in the event of an intra-operative glove breach.
Methods: We conducted a randomized, double-blind, single-center trial, to measure any differences in the bacterial skin populations of surgeons' hands during surgical procedures done with antimicrobial and non-antimicrobial surgical gloves [ISRCTN71391952]. In this study, 25 pairs of gloves were retrieved from 14 surgeons who donned them randomly on their dominant or non-dominant hand. The number of bacteria retrieved from glove fluid was measured and expressed as colony forming units (CFU)/mL.
Results: The median cfu/mL of antimicrobial gloves was 0.00 (LQ: 0.00 CFU/mL; UQ: 0.00 cfu/mL), with a mean log10 cfu/mL=0.02 (range: 0.00–0.30). The median CFU/mL of non-antimicrobial gloves was 54.00 (LQ: 3.00 cfu/mL; UQ: 100.00 cfu/mL) with a mean log10 CFU/mL=1.32 (range: 0.00–2.39). After a mean operating time of 112 min, the difference in the log10 CFU/mL was 1.30 (p<0.001).
Conclusions: A new antimicrobial surgical glove suppressed surgeons' hand flora during operative procedures. In the event of a glove breach, the use of such a glove may have the potential to prevent bacterial contamination of a sterile surgical site, thereby decreasing the risk of surgical site infection (SSI) and increasing patient safety. Further clinical studies are needed to confirm this concept.
During the past century, a number of important developments in the field of surgery have made surgical procedures safer and relatively free of the risk of transmission of micro-organisms between surgeon and patient and vice versa. However, absolute numbers of surgical site infections (SSIs) remain high because of the increasing numbers of procedures performed in modern surgical practice, with a substantial morbidity and mortality for patients and escalating costs for health-care systems worldwide [1].
The risk of microbial contamination of the surgical site and the subsequent development of SSI is increased when foreign bodies are implanted in a patient. Reported rates of SSI associated with the implantation of prosthetic devices range from 0.5%–2.0% after total joint replacement to 1%–6% after cardiac valve implantation, 25%–50% with the use of aortic balloon pumps, 5%–41% with cerebrospinal fluid (CSF) shunts, and 1%–20% with mesh implants for treating hernias [2–5]. Infection of peripheral vascular prostheses ranges from 1%–6% [6]. Moreover, although the incidence of aortic endograft infection ranges only from 0.2%–0.7%, it represents a catastrophic complication and a challenging problem in aortic surgery [7].
The reason for these implant-associated SSIs is the ability of micro-organisms to attach to foreign materials, survive on them, and form protective biofilms [8]. Meticulous attention to maintaining aseptic conditions during the insertion of an implant is therefore essential to decreasing the risk of SSI.
Intact surgical gloves act as a physical barrier against the transmission of skin flora from the surgeon's hand to the surgical site. The intact surgical glove is the most important barrier to the bi-directional migration of micro-organisms between the hands of the members of a surgical team and the patient [9,10]. However, several studies have shown that undetected perforations of surgical gloves are common and that the frequency of such defects increases with the duration of glove wear [10–13]. The risk of glove defects is related to the type of surgery being done, ranging from 7% in urologic surgery to 65% in cardiothoracic surgery [14–18].
Because of the possibility of glove breaches, various measures have been developed to reduce the risk of surgical site contamination with micro-organisms originating from the surgeon's hands. A standard practice for decreasing the microbial bio-burden on the hands of surgeons and other surgical team members is pre-operative surgical hand disinfection with an anti-microbial soap (surgical scrub) or an alcohol-based hand disinfectant (surgical rub). Pre-operative surgical hand disinfection can reduce, but not eradicate, the resident flora on the surgeon's hands, and thus does not eliminate the risk of transmission of such organisms into the surgical site in the event of a glove breach. Because of re-growth of skin flora during a surgical procedure, original levels of skin flora on a surgeon's hands can be re-established within 3–6 h, depending on the formulation of the product used to disinfect the hands [19,20].
A novel sterile antimicrobial surgical glove, featuring a proprietary complex coating with 14 ingredients and chlorhexidine (CHG) as an active antimicrobial ingredient on its inner surface (Gammex PF (Powder-Free) with AMT, Ansell, Richmond, Australia), has been developed. Gloves serve as a mechanical protective barrier between the surgeon's hand and the surgical site, but we hypothesised that antimicrobial gloves might also reduce the risk of contamination of the surgical site in the event of an intraoperative glove breach by suppressing the re-growth of skin flora during the course of a surgical procedure.
Methods
We designed a randomized, double-blind, single-center study (ISRCTN71391952) to measure the number of colony forming units per mL (CFU/mL) in glove fluid collected under real-time conditions during surgical procedures in the operating theatre and to calculate the differences in the counts of CFU/mL with the use of antimicrobial and non-antimicrobial surgical gloves.
The study was conducted in a department for vascular and endovascular surgery at a 1,106-bed, tertiary care, governmental and university-affiliated hospital in Vienna. The department has 56 beds and performs more than 2,000 surgical procedures annually. Seventeen surgeons and residents were involved in the study. Of these, nine were long-tenure consultant vascular surgeons and the rest were surgical residents. All of the surgeons, with one exception, agreed to participate in the study after appropriate orientation. Two surgeons were involved in four surgical procedures, three surgeons were involved in three procedures, three surgeons were involved in two procedures, and six surgeons participated in one procedure. The median number of participants per surgical procedure was 3 surgeons (interquartile range, 2.75–3.75). All participating surgeons were right handed.
Individuals without visibly healthy skin or with cuts or abrasions, and individuals who had used medicated soap or medicated hand creams within 1 wk before the study were excluded. Surgeons were allowed to re-join the study as participants only after a period of 1 wk had elapsed, to allow full reconstitution of normal skin flora. Patients with existing infection at any site or undergoing re-operation within 30 d after an operation were excluded from the study. There was no directive to surgeons about maximum glove-wearing time. The use of alcohol-based hand rubs with a sustained antimicrobial efficacy within 24 h before testing was done was not an exclusion criterion.
The study was planned in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement and was conducted over the 3-mo period from November 2011–February 2012. Approval for the study was obtained from the ethics committee of the municipality of Vienna (EK 11-201-1111), and written informed consent was obtained from all participating patients. The study was registered with International Standard Randomized Controlled Trial Registry, trial number ISRCTN 71391952.
Experimental procedure
The experimental procedure used in the study was designed to follow the method for assessment of a surgical hand rub as described in European Norm (EN) 12791, which specifies the test method under practical conditions for establishing whether a surgical hand-disinfection regimen reduces the release of hand flora according to the standard's requirements [21]. European Norm 12791 requires the testing of a minimum of 20 healthy volunteers divided randomly into two groups of the same size in a crossover design. The method was modified for the present study so that one volunteer tested both an antimicrobial surgical glove (intervention glove; Gammex PF with AMT) on one hand and a non-antimicrobial surgical glove (reference glove; Gammex PF, Ansell) on the other hand, with the gloves allocated randomly to the dominant and non-dominant hand. The study was designed to test 20 pairs of gloves, consisting of one intervention glove and one reference glove per pair. Instead of comparing the reduction factor of a test hand rub against a reference product, the viable log10 CFU/mL means of the post-values of the two glove groups were compared with each other, resulting in a mean log10 CFU/mL difference. A further modification was the use of the glove fluid collection method [22] instead of the fingertip sampling method as described by EN 12791 to sample the whole hand rather than the fingertips alone.
Before surgery, each surgeon performed surgical hand disinfection (“surgical scrub”) according to the reference surgical hand-disinfection procedure described in EN 12791, but with the procedure extended from the forearms to the elbows. Briefly, the hands and forearms were washed with 10 mL of a non-medicated liquid soap (Lifosan Soft; B. Braun, Melsungen, Germany) for 1 min. During each surgical hand-preparation procedure, fingernails were brushed with a sterile brush. After thorough drying of the hands with a clean, disposable paper towel, hands and forearms were disinfected over a period of 3 min with an alcohol-based hand disinfectant (Sterillium Classic Pure; Paul Hartmann AG, Heidenheim, Germany) [23]. After evaporation of the alcohol, surgeons donned sterile surgical gloves before the surgical procedure in which the gloves were used.
Blinding and randomization
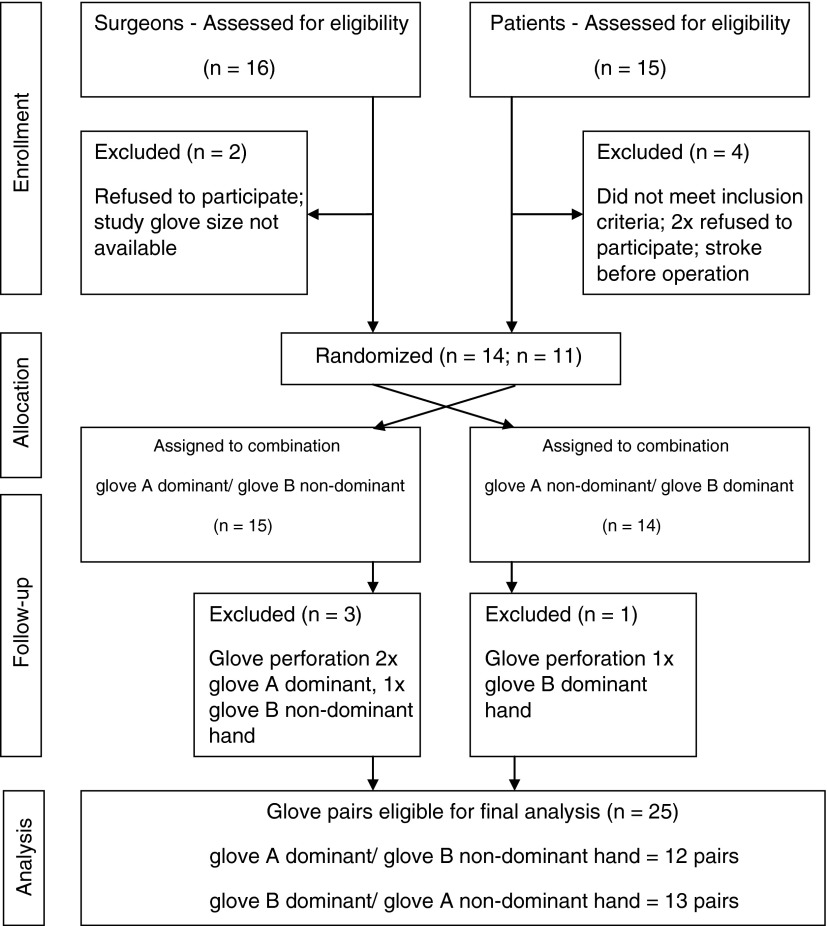
Each surgeon gloved one hand with an antimicrobial sterile surgical latex glove and the other hand with an identical, but non-antimicrobial sterile surgical latex glove. Gloves were provided by the manufacturer and their identity was double-blinded through the use of identical packs distinguished only by the letters “A” or “B.” The decision to wear the antimicrobial or non-antimicrobial glove on the dominant or non-dominant hand was blinded and followed a preset, computer-generated, random table, according to which each surgeon was assigned either the combination of: (1) glove A dominant hand/glove B non-dominant hand, or 2) glove A non-dominant hand/glove B dominant hand (Fig. 1). If a glove breach occurred during a surgical procedure, the entire glove allocation procedure was excluded from the trial. The inclusion target was a minimum of 20 antimicrobial gloves and 20 non-antimicrobial gloves, resulting in a minimum of 20 matched pairs of gloves for statistical analysis. To ascertain the minimum required number of surgical gloves required for further analysis, a glove-puncture frequency of 25% was expected, on the basis of previous studies [10,11]. Therefore, attainment of the needed numbers of matched pairs of gloves without punctures required the use of a minimum of 26 pairs of gloves (n=20 gloves+30%×20) as an objective.
FIG. 1.
Enrollment, randomization, and follow-up according to the Consolidated Standards of Reporting Trials (CONSORT) protocol.
Surgical procedures and data collection
Patients undergoing carotid endarterectomy, aortic reconstruction for aneurysms or occlusive disease, or open surgical bypass for peripheral arterial occlusive disease were included in the study. For each surgical procedure the patient's name, date of birth, gender, underlying disease, names of the surgical team members, and duration of the surgical procedure were recorded. The member of the surgical team wearing the study gloves, the role of that member on the surgical team, the type of surgery being done, and the date and duration of glove-wearing were documented. The duration of surgery was defined as the time from the beginning of incision to final skin closure. All patients received peri-operative antibiotic prophylaxis consisting of 1.5 g cefuroxime administered 30–60 min prior to incision of the patient's skin.
Sampling and microbiologic processing
At the end of each surgical procedure, the glove from one hand of the surgical team member wearing the study gloves was filled carefully with 25 mL of a validated neutralizer (30 mL polysorbate 80, 3 g lecithin, and 1 g histidine) active against chlorhexidine [21] and massaged gently for 1 min, with the procedure then repeated with the glove from the participant's other hand. The neutralizing solution was then collected with a sterile 25 mL syringe (B. Braun), which served as a transport container for the processing laboratory. All gloves were collected and examined for perforations, using the impermeability test described in the European Norm requirements for medical gloves for single use, EN 455-1 [24].
Samples were transported immediately to the laboratory and processed. Briefly, three serial dilutions of the neutralizing solution from each glove, of 100, 10–1, and 10–2, were vortexed for 30 s at 5,000 rpm. One milliliter of the respective sample was plated on tryptone soya agar plates (TSA plates; Oxoid, Basingstoke, United Kingdom) using a sterile pipette tip and a sterile spreader. The agar plates were incubated for 48 h at 37±1 °C. After incubation, the number of cfu was counted and recorded for each dilution step. The number of CFU/mL of sampling fluid was calculated by multiplying the plate count of cfu by the dilution factor. Counts were obtained from plates growing 15–300 cfu. If suitable counts were obtained in two sequential dilution steps, the weighted arithmetic mean cfu/mL, and the standard deviation (SD) and variance of these counts, were calculated. Viable mean counts were transformed to decimal values of log10. Counts of 0 (log10 0=–∞) were replaced with a value of 1 to avoid an infinite negative log10 value (log10 1=0).
Statistical analysis
Bacterial counts were expressed as medians with inter-quartile ranges (IQR), or were logarithmically transformed and expressed as means±SD before statistical analysis. Viable CFU log10 differences were calculated as log10 CFU/mL of hands that wore glove A minus log10 CFU/mL of hands that wore glove B for each corresponding pair of gloves. The mean log10 difference was calculated as the mean of all separate counts in the form of log10 cfu/mL. The mean log10 CFU/mL values of counts were tested for statistically significant differences with the Wilcoxon matched-pairs signed-ranks test. For continuous variables, means±SD were calculated and compared through use of a paired two-tailed t-test. The duration in minutes of the surgical procedures in which each pair of gloves was used, and the ages of the patients in years, were reported as mean, median, IQR, and range. All tests for significance were run as two-sided tests, with alpha set at the 1% level.
Results
Fourteen primary operating surgeons and assistants operated on 11 patients. The patients' mean age was 72±9 y (range: 60–85 y; median: 71 y; IQR: 66–78 y). Seven patients were male (mean age: 73±8 y; range: 60–84 y; median: 74 y; IQR: 71–78 y), and four patients were female (mean age: 70±11 y; range: 61–85 y; median: 66 y; IQR: 61–75 y). There was no statistically significant difference in the mean ages of male and female patients (p=0.56).
Surgical procedures included carotid endarterectomy (n=4), peripheral bypass surgery (n=4), and revascularization of the common femoral and profunda femoris arteries (n=3). The mean duration of all surgical procedures was 112±43 min, with the shortest duration being 50 min and the longest 185 min (median: 115 min; IQR: 79–140 min). The mean duration of operation was 68±15 min (median: 68 min; IQR: 61–74 min) for carotid arterectomy, 144±37 min (median: 146 min; IQR: 123–166 min) for peripheral bypass surgery, and 129±18 min (median: 130 min; IQR: 116–146 min) for common femoral and profunda artery surgery. There was no statistically significant difference in operating time for patients who underwent common femoral and profunda femoris artery surgery (p=0.34) and peripheral bypass surgery (p=0.21) as compared to the overall operation time for all patients. Procedures for carotid endarterectomy were significantly shorter in duration than the other procedures included in the study (p=0.01).
Twenty-nine glove pairs were obtained. Surgeons randomly wore 29 antimicrobial gloves (A gloves) and 29 non-antimicrobial gloves (B gloves), on either the dominant or the non-dominant hand. In 15 of these instances glove A was worn on the dominant and glove B on the non-dominant hand, and in 14 instances glove A was worn on the non-dominant and glove B on the dominant hand (Fig. 1). A perforation was detected in four gloves (n=4/29; 14%), in three of which the perforation was in the glove worn on the dominant hand (twice involving antimicrobial gloves [A] and once involving a non-antimicrobial glove [B]), and in one case the perforation was detected in a non-antimicrobial glove (B) worn on the non-dominant hand. In three of the four instances of perforation the perforation remained unnoticed by the surgeon. Perforations were located on the index finger of the dominant hand in two cases, on the palm of the non-dominant hand in one case, and on the little finger of the non-dominant hand in one case. Microbiologic results from the sampling of these gloves, together with the results for the corresponding gloves on the non-affected hand, were excluded from further analysis.
A total of 25 antimicrobial/non-antimicrobial glove pairs (12 pairs of antimicrobial gloves on the dominant hand with the corresponding non-antimicrobial glove on the non-dominant hand, and 13 pairs of non-antimicrobial gloves on the dominant hand with the corresponding antimicrobial glove on the non-dominant hand) were eligible for further data analysis (Fig. 1).
After correction for the four perforated gloves describe above, the mean total duration of the surgical procedures included in the study was 112±43 min in 11 patients (male: n=7; female: n=4), in operations done by 14 surgeons. For the antimicrobial gloves in the study (A), the mean log10 CFU/mL was 0.02 (range: 0.00–0.30 cfu/mL) and the median CFU/mL was 0.00 (IQR: 0.00–0.00 CFU/mL). There was no statistically significant difference in the counts of cfu/mL for antimicrobial gloves worn on the dominant (n=12) and those worn on the non-dominant (n=13) in terms of mean log10 CFU/mL (p=0.18) or duration of wear (p=0.68).
For non-antimicrobial gloves (B), the mean log10 CFU/mL was 1.32 (range: 0.00–2.39 cfu/mL) and the median CFU/mL was 54.00 (IQR: 3.00–100.00 CFU/mL). There was no statistically significant difference in counts of CFU/mL for non-antimicrobial gloves worn on the dominant hand (n=13) vs. the non-dominant hand (n=12) in terms of mean log10 CFU/mL (p=0.63) or duration of wear (p=0.68).
The overall difference in log10 CFU/mL for antimicrobial vs. non-antimicrobial gloves was 1.30. The difference between the mean cfu/mL counts for all antimicrobial and all non-antimicrobial surgical gloves was statistically significant (p<0.001) regardless of the type of or duration of the procedure in which the gloves were used (Table 1). For gloves sampled ≤90 min after donning, the mean difference in log10 cfu/mL between the antimicrobial and non-antimicrobial gloves was 1.23 (p=0.029), and the mean difference in log10 cfu/mL for gloves sampled later than 91 min after donning was 1.35; p=0.001; Table 2). These differences favored the antimicrobial gloves (A) over the non-antimicrobial gloves (B) in terms of suppression of bacterial re-growth during operative procedures.
Table 1.
Mean Counts of cfu/mL from Glove A and Glove B Stratified by Time and Procedure
| Glove A mean cfu/mL (log10) | Glove B mean cfu/mL (log10) | Log10 diff. | Mean duration (±SD) | p | |
|---|---|---|---|---|---|
| Carotid endarterectomy | 0.00 | 1.23 | 1.23 | 68 (±15 min) | 0.015 |
| Revascularization | 0.10 | 1.65 | 1.55 | 129 (±18 min) | 0.001 |
| Bypass | 0.00 | 1.24 | 1.24 | 144 (±37 min) | 0.021 |
| ≤90 min | 0.00 | 1.43 | 1.43 | 71 (±15 min) | 0.015 |
| 91–130 min | 0.06 | 1.97 | 1.91 | 112 (±12 min) | 0.037 |
| ≥131 min | 0.03 | 1.13 | 1.10 | 159 (±20 min) | 0.003 |
Table 2.
Immediate (<90 min) and Long-Term (≥91 min) Effect of Antimicrobial and Non-Antimicrobial Surgical Gloves Based on Mean Retrieval of Resident Skin Flora from Surgeons' Hands in cfu/mL
| Immediate ≤90 min (log10 cfu/mL) | Long-term ≥91 min (log10 cfu/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Duration (min) | Glove A | Glove B | log10 difference | Duration (min) | Glove A | Glove B | log10 difference |
| 50 | 0.00 | 0.00 | 0.00 | 100 | 0.00 | 2.00 | 2.00 |
| 50 | 0.00 | 2.00 | 2.00 | 100 | 0.00 | 2.00 | 2.00 |
| 65 | 0.00 | 0.48 | 0.48 | 115 | 0.00 | 1.95 | 1.95 |
| 70 | 0.00 | 2.16 | 2.16 | 123 | 0.30 | 2.03 | 1.73 |
| 70 | 0.00 | 1.00 | 1.00 | 123 | 0.00 | 1.85 | 1.85 |
| 70 | 0.00 | 1.96 | 1.96 | 131 | 0.00 | 2.21 | 2.21 |
| 87 | 0.00 | 2.19 | 2.19 | 131 | 0.00 | 0.00 | 0.00 |
| 87 | 0.00 | 0.78 | 0.78 | 149 | 0.00 | 1.18 | 1.18 |
| 87 | 0.00 | 0.48 | 0.48 | 149 | 0.30 | 0.48 | 0.18 |
| 149 | 0.00 | 2.39 | 2.39 | ||||
| 160 | 0.00 | 1.26 | 1.26 | ||||
| 160 | 0.00 | 0.00 | 0.00 | ||||
| 160 | 0.00 | 1.73 | 1.73 | ||||
| 185 | 0.00 | 2.16 | 2.16 | ||||
| 185 | 0.00 | 0.00 | 0.00 | ||||
| 185 | 0.00 | 1.00 | 1.00 | ||||
| mean | 0·00 | 1·23 | 1·23 | 0·04 | 1·39 | 1·35 | |
| Total | 9 | 9 | 9 | 16 | 16 | 16 | |
Discussion
The results of this study support our hypothesis that by suppressing the re-growth of skin flora during the course of a surgical procedure, antimicrobial surgical gloves, coated on their inner side with chlorhexidine, may reduce the risk of contamination of the surgical site in the event of intra-operative glove perforation. Although the design of this study does not allow the establishment of a link between the prevention of SSI and the use of antimicrobial gloves, the hypothesis of such a link is indirectly supported by the demonstration in the study of the suppression of re-growth of the skin flora on hands wearing antimicrobial gloves during surgical procedures. Indeed, after 2 h of glove wear, the mean count of bacterial CFU/mL in the antimicrobial-glove arm of the study was 0.28 (mean log10=0.02), whereas the mean count with a breach in a non-antimicrobial glove was 65 CFU/mL (mean log10=1.32), which would have contaminated the sterile surgical field, with the highest measured colony counts being 245 CFU/mL (log10=2.39).
To cause an infection, bacteria must reach an incision; although seeding of surgeon's skin flora may occur through perforation of a glove, [8,9] seeding from the patient's own skin flora is likely to be at least as important. Furthermore, only small numbers of organisms are necessary to cause infections from prosthetic devices, and the higher the bacterial count the more likely is the development of infection [25]. Therefore, anti-infective measures must be targeted at multiple sites. Moreover, foreign bodies, trauma, hematoma, or hypoxemia enhance the pathogenicity of a bacterial inoculum. Van Wijngaerden et al. [26] demonstrated in an experimental rat model that an inoculum of 2.70–3.90log10 CFU/mL of different Staphylococcus epidermidis strains was sufficient to establish an implant infection in every case. This finding confirms that when foreign materials are present, the bacterial inoculum required to cause SSI may be even lower [27]. The presence of a surgical suture decreases the minimum inoculum of S. aureus needed to establish infection from 6log10 to 2log10 CFU/mL, [28] and in the presence of polytetrafluoroethylene (PTFE) vascular grafts, as few as 10 CFU/mL of S. aureus can cause infection [29]. Misteli et al. [13] have shown that perforations of surgical gloves is a risk factor for SSI when prophylactic antibiotics are not administered.
These findings suggest that early intraoperative bacterial contamination of implanted foreign material is a likely mechanism for the pathogenesis of SSIs. To prevent bacterial contamination of the surgical site by micro-organisms derived from the surgeon's hand, various measures have been adopted. One early tactic was antiseptic surgery, introduced by Sir Joseph Lister. He began washing his hands with carbolic acid before operating, and introduced the concept of antimicrobial hand washing [30,31]. Today, pre-operative surgical hand disinfection is regarded as standard practice for decreasing the microbial bio-burden on surgeons' hands. However, because of bacterial re-growth, micro-organisms may contaminate the surgical site if there is glove perforation. To overcome this risk, it has been recommended that surgical gloves be routinely changed every 2–3 h [32]. However, because 80%–85% of all glove perforations remain undetected by the wearer, [10,33–35] this measure may often be taken too late to prevent contamination. Hence, antimicrobial surgical gloves may be an innovative approach to overcoming the issue of bacterial re-growth during surgery and the increasing risk of glove puncture over the course of operative time.
Conclusions
Early bacterial contamination of implanted foreign material from intra-operative glove puncture is a risk for the development of SSI. The use of antimicrobial surgical gloves may prevent bacterial contamination of the surgical site and may therefore indirectly decrease the risk of SSI and thus increase patient safety, particularly when the consequences of an SSI are catastrophic, as in vascular surgery. The present study has shown that a new antimicrobial surgical glove was able to suppress the skin flora of surgeons' hands during operation by a factor of approximately 1.3 log10 CFU/mL. In the event of a glove breach, such a new tactic may have the potential to prevent bacterial contamination of the surgical site, and hence may decrease indirectly the risk of SSI and therefore increase patient safety. Further well-powered clinical studies with SSI as a direct endpoint are needed to confirm this concept. Such studies, however, would require large sample sizes, which are unlikely to be obtained within single centers. One promising option for meeting this need would be the establishment of international registries with standard definitions of SSI [36]. The alternative would be to accept results obtained from well-designed clinical or in vitro studies for evaluating and comparing these concepts under uniform conditions of testing and test methodology [37].
Acknowledgments and Author Disclosure Statement
The sterile, blinded surgical gloves used in the study were provided by Ansell Ltd., Richmond, Australia, which also provided transportation costs for the study. The costs for laboratory consumables was funded by the routine research budget of the Institute for Hospital Hygiene and the Division for Medical-Technical Hygiene of the Institute for Hygiene and Applied Immunology. The authors declare that they used the gloves in the study as a class III medical device in an indication that is not claimed by the manufacturer. The authors have no financial or other conflict of interest to declare and no financial or other relationships leading to conflict of interest. Ojan Assadian and Axel Kramer have received travel compensation and speaker's honovaria in the past from B. Braun Melsungen AG and Paul Hartmann AG. David Leaper received fees for speaking for Ansell Inc. in the past. Ojan Assadian is medical advisory board member of Hutchinsan santé and serves as a part-time consultant for Gersan-Lehrman Group and Quantum Management Services. The content of this paper and the conclusions reached in it are the personal opinion of the authors, based on scientific and professional grounds.
The authors thank Ansell Inc. for generously donating the blinded sterile surgical gloves used in the study and Mr. Eric Boeckmans for his willingness to test a new medical device with an unknown outcome in this study. The authors would also acknowledge gratefully the excellent work-ups of laboratory specimens by Mrs. Martina Weinlich of the Medical University of Vienna. Ojan Assadian, Kenneth Ouriel, Martin Rottman, and Axel Kramer formulated the study hypothesis. Afshin Assadian, Kenneth Ouriel, and David Leaper advised on selection of the surgical field to be investigated. Afshin Assadian supervised patient and participant information and quality of surgical procedures, and obtained informed consent. Ojan Assadian, Miranda Suchomel, and Mary-Louise McLaws designed and performed the statistical analysis and interpreted statistics. Ojan Assadian and Miranda Suchomel collected and processed the microbiologica specimens. All authors were involved in the literature search for the study, drafted the manuscript, and were involved in drafting and processing the study results and in interpreting the study data.
References
- 1.Leaper D, Burman-Roy S, Palanca A, et al. . on behalf of the GDG Prevention and treatment of surgical site infection: summary of NICE guidance. Br Med J 2008;337:1048–1051 [DOI] [PubMed] [Google Scholar]
- 2.Lozier AP, Sciacca RR, Romagnoli MF, et al. . Ventriculostomy-related infections: A critical review of the literature. Neurosurgery 2002;51:170–181 [DOI] [PubMed] [Google Scholar]
- 3.Safdar N, Kluger DM, Maki DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: Implications for preventive strategies. Medicine 2002;81:466–479 [DOI] [PubMed] [Google Scholar]
- 4.Lew DP, Pittet D, Waldvogel FA. Infections that complicate the insertion of prosthetic devices. In: Mayhall G. (ed.): Hospital Epidemiology and Infection Control 3rd ed. Baltimore: Lippincott Williams & Wilkins; 2004:1181–1205 [Google Scholar]
- 5.Knoll BM, Baddour LM, Wilson WR. Prosthetic valve endocarditis. In: Mandell GL, Bennett JE, Dolin R. (eds): Principles and Practice of Infectious Diseases. 7th ed. St Louis. MO: Churchill–Livingstone Elsevier, 2010:1113–1126 [Google Scholar]
- 6.Leroy O, Meybeck A, Sarraz-Bournet B, et al. L. Vascular graft infections. Curr Opin Infect Dis 2012;25:154–158 [DOI] [PubMed] [Google Scholar]
- 7.Hobbs SD, Kumar S, Gilling-Smith GL. Epidemiology and diagnosis of endograft infection. J Cardiovasc Surg 2010;51:5–14 [PubMed] [Google Scholar]
- 8.Peters G, Locci R, Pulverer G. Microbial colonization of prosthtic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zbl Bakt Mikrobiol Hyg [B] 1981;173:293–299 [PubMed] [Google Scholar]
- 9.Hübner NO, Goerdt AM, Stanislawski N, et al. . Bacterial migration through punctured surgical gloves under real surgical conditions. BMC Infect Dis 2010;10:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harnoss J, Partecke LI, Heidecke CD, et al. . Concentration of bacteria passing through puncture holes in surgical gloves. Am J Infect Control 2010;38:154–158 [DOI] [PubMed] [Google Scholar]
- 11.Partecke LI, Goerdt AM, Langner I, et al. . Incidence of microperforation for surgical gloves depends on duration of wear. Infect Control Hosp Epidemiol 2009;30:409–414 [DOI] [PubMed] [Google Scholar]
- 12.Eklund AM, Ojajarvi J, Laitinen K, et al. . Glove punctures and postoperative skin flora of hands in cardiac surgery. Ann Thorac Surg 2002;74:149–153 [DOI] [PubMed] [Google Scholar]
- 13.Misteli H, Weber WP, Reck S, et al. . Surgical glove perforation and the risk of surgical site infection. Arch Surg 2009;144:553–558 [DOI] [PubMed] [Google Scholar]
- 14.Laine T, Kaipia A, Santavirta J, et al. . Glove perforations in open and laparoscopic abdominal surgery: The feasibility of double gloving. Scand J Surg 2004;93:73–76 [DOI] [PubMed] [Google Scholar]
- 15.Brough SJ, Hunt TM, Barrie WW. Surgical glove perforations. Br J Surg 1988;75:317. [DOI] [PubMed] [Google Scholar]
- 16.Kojima Y, Ohashi M. Unnoticed glove perforation during thoracoscopic and open thoracic surgery. Ann Thorac Surg 2005;80:1078–1080 [DOI] [PubMed] [Google Scholar]
- 17.Pitten FA, Herdemann G, Kramer A. The integrity of latex gloves in clinical dental practice. Infection 2000;28:388–392 [DOI] [PubMed] [Google Scholar]
- 18.Manjunath AP, Shepherd JH, Barton DP, et al. . Glove perforations during open surgery for gynaecological malignancies. Br J Obstet Gynecol 2008;115:1015–1019 [DOI] [PubMed] [Google Scholar]
- 19.Peterson AF, Rosenberg A, Alatary SD. Comparative evaluation of surgical scrub preparations. Surg Gynecol Obstet 1978;146:63–65 [PubMed] [Google Scholar]
- 20.Rotter ML, Kampf G, Suchomel M, et al. . Population kinetics of the skin flora on gloved hands following surgical hand disinfection within 3 propanol-based hand rubs: A prospective, randomized, double-blinded trial. Infect Control Hosp Epidemiol 2007;28:346–350 [DOI] [PubMed] [Google Scholar]
- 21.European Norm EN 12791: Chemical disinfectants and antiseptics Surgical hand disinfection. Test method and requirement (phase 2/step 2). Brussels: European Committee for Standardization; 2005 [Google Scholar]
- 22.Federal Register Tentative final monograph for healthcare antiseptic products; proposed rule. Fed Regist 1994;59:31401–31452 [Google Scholar]
- 23.Kampf G, Ostermeyer C. A 1-minute hand wash does not impair the efficacy of a propanol-based hand rub in two consecutive surgical hand disinfection procedures. Eur J Clin Microbiol Infect Dis 2009;28:1357–1362 [DOI] [PubMed] [Google Scholar]
- 24.European Committee for Standardization EN 455: Medical gloves for single use. Brussels: European Committee for Standardization, 2000 [Google Scholar]
- 25.Archibald LK, Hierholzer WJ. Principles of infectious disease epidemiology. In: Mayhall CG. (ed): Hospital Epidemiology and Infection Control, 3rd ed. Baltimore: Lippincott Williams & Wilkins, 2004:7–8 [Google Scholar]
- 26.Van Wijngaerden E, Peetermans WE, Vandersmissen J, et al. . Foreign body infection: A new rat model for prophylaxis and treatment. J Antimicrob Chemother 1999;44:669–674 [DOI] [PubMed] [Google Scholar]
- 27.James RC, MacLeod CJ. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br J Exp Pathol 1961;42:266–277 [PMC free article] [PubMed] [Google Scholar]
- 28.Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man: A study of the problems of wound infection. Br J Exp Pathol 1957;38:573–586 [PMC free article] [PubMed] [Google Scholar]
- 29.Arbeit RD, Dunn RM. Expression of capsular polysaccharide during experimental focal infection with Staphylococcus aureus. J Infect Dis 1987;156:947–952 [DOI] [PubMed] [Google Scholar]
- 30.Lister J. On the antiseptic principle in the practice of surgery. Lancet 1867;90:353–356 [Google Scholar]
- 31.Lister J, Hair P. On the use of carbolic acid. Lancet 1867;90:502 [Google Scholar]
- 32.The German Working Group for Hospital Hygiene (AWMF) Hand disinfection and hand hygiene. Hyg Med 2008;33:300–313 [Google Scholar]
- 33.Thomas S, Agarwal M, Mehta G. Intraoperative glove perforation: Single-versus double-gloving in protection against skin contamination. Postgrad Med J 2001;77:458–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caillot JL, Paparel P, Arnal E, et al. . Anticipated detection of imminent surgeon–patient barrier breaches: A prospective randomized controlled trial using an indicator underglove system. World J Surg 2006;30:134–138 [DOI] [PubMed] [Google Scholar]
- 35.Tanner J. Choosing the right surgical glove: An overview and update. Br J Nurs 2008;17:740–744 [DOI] [PubMed] [Google Scholar]
- 36.Teebken OE, Bisdas T, Assadian O, et al. . Recommendations for reporting treatment of aortic graft infections. Eur J Vasc Endovasc Surg 2012;43:174–181 [DOI] [PubMed] [Google Scholar]
- 37.Ricco JB, Assadian O. Antimicrobial silver grafts for prevention and treatment of vascular graft infection. Semin Vasc Surg 2011;24:234–241 [DOI] [PubMed] [Google Scholar]