Abstract
Significance: Ion channels control membrane potential, cellular excitability, and Ca++ signaling, all of which play essential roles in cellular functions. The regulation of ion channels enables cells to respond to changing environments, and post-translational modification (PTM) is one major regulation mechanism. Recent Advances: Many PTMs (e.g., S-glutathionylation, S-nitrosylation, S-palmitoylation, S-sulfhydration, etc.) targeting the thiol group of cysteine residues have emerged to be essential for ion channels regulation under physiological and pathological conditions. Critical Issues: Under oxidative stress, S-glutathionylation could be a critical PTM that regulates many molecules. In this review, we discuss S-glutathionylation-mediated structural and functional changes of ion channels. Criteria for testing S-glutathionylation, methods and reagents used in ion channel S-glutathionylation studies, and thiol modification crosstalk, are also covered. Mechanotransduction, and S-glutathionylation of the mechanosensitive KATP channel, are discussed. Future Directions: Further investigation of the ion channel S-glutathionylation, especially the physiological significance of S-glutathionylation and thiol modification crosstalk, could lead to a better understanding of the thiol modifications in general and the ramifications of such modifications on cellular functions and related diseases. Antioxid. Redox Signal. 20, 937–951.
Introduction
Ion channels play essential roles in virtually all aspects of cellular function including action potential generation in the nervous system and heart; blood vessel contraction; insulin secretion; and many other biological processes involving ion homeostasis, membrane excitability, or calcium signaling. Post-translational modifications (PTMs) are important mechanisms regulating ion channel functions. One of the classical PTM is protein phosphorylation, and a large number of ion channels are found to be regulated by phosphorylation through PKA, PKC, and other protein kinases (30, 57, 109, 112, 113, 139). A variety of different types of PTMs (e.g., Ubiquitylation, SUMOylation, O-glycosylation/O-GlcNAcylation, etc.) exist and are discussed elsewhere (14, 22, 98, 99).
Among all these PTMs, redox-mediated PTM is an important category of PTMs that targets the thiol group of cysteine residues. Recently, redox-mediated PTMs are receiving increasing attention, as they are found in both physiological and pathological conditions including oxidative stress. S-glutathionylation is a major redox-mediated thiol modulation mechanism, involving the addition of a glutathione (GSH) moiety to the protein. Oxidative stress, or reactive oxygen species (ROS), facilitates S-glutathionylation. Protein S-glutathionylation has been extensively discussed in many excellent reviews with a variety of different emphases (31, 44, 45, 75, 76, 88, 93, 100, 107). Over the past few years, S-glutathionylation has been increasingly observed in many ion channels, such as voltage-gated calcium channels, ryanodine receptor (RyR), and ATP-sensitive potassium channels (KATP channels), all of which contribute to critical cellular functions (4, 123, 137, 138). In this review, we introduce redox-mediated thiol modulation first and then focus on S-glutathionylation of ion channels. Criteria for testing S-glutathionylation, methods and reagents used in designing experiments are also discussed. Besides S-glutathionylation, other important thiol modifications that contribute to ion channel modulation (e.g., S-nitrosylation, S-palmitoylation, and S-sulfhydration) are briefly discussed, mainly to elaborate on the possible interplay and crosstalk among these thiol modifications. Finally, we consider how the regulation of mechanosensing would be affected by thiol modifications of the mechanosensitive channels, specifically the KATP channel that is functionally inhibited upon S-glutathionylation.
Thiol Groups (−SH) as a Reactive Center in PTM
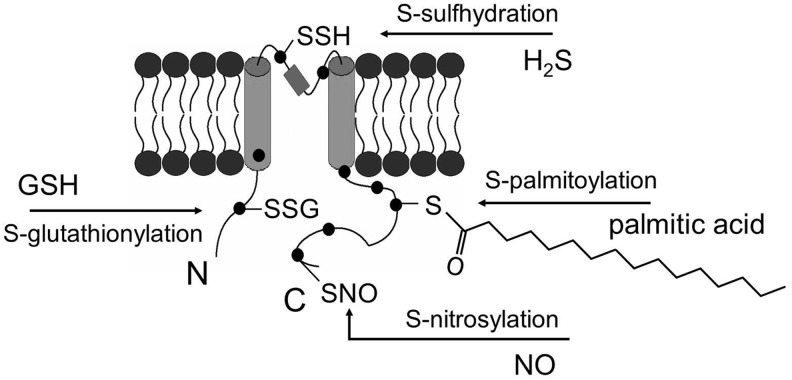
The thiol groups of protein cysteine residues are susceptible to oxidative modification, through which PTMs may occur. Thiol groups could be modified in response to exogenous stimuli or changes in local redox environment, thus affecting the function of the proteins with such accessible thiol groups (80). In the oxidative stress condition, where the redox balance is shifted toward oxidation, the thiol groups could be modified differently depending upon the accessibility of oxidants or other small molecules. For example, (i) If glutathione (GSH) is accessible to the thiol group, oxidation may lead to S-glutathionylation. (ii) In the presence of abundant nitric oxide (NO), S-nitrosylation may occur. (iii) If two cysteine residues are close to each other, oxidation may cause the formation of a disulfide bond between them; either within the protein or between two separate proteins. (iv) Oxidation of cysteine residues may also result in the sequential formation of cysteine radicals (P-S•), followed by sulfenic (PS-OH), sulfinic (PS-O2H), and eventually sulfonic (PS-O3H) acids. In addition, S-palmitoylation and S-sulfhydration can also modify the thiol groups and attach lipid or hydrogen sulfide (H2S) to the cysteine residues, respectively (Fig. 1).
FIG. 1.
Ion channel thiol group (–SH) as a reactive center. Depending on the molecules (e.g., GSH, NO, H2S, or 16-carbon chain palmitate lipid) accessible to a particular −SH group and concomitant modification mechanism, S-glutathionylation, S-nitrosylation, S-sulfhydration, or S-palmitoylation may occur on the ion channel protein. GSH, glutathione; NO, nitric oxide; H2S, hydrogen sulfide.
S-Glutathionylation Overview
S-glutathionylation is a PTM of proteins at the cysteine residues by adding a GSH moiety (31). Protein S-glutathionylation may occur in physiological conditions but is generally facilitated by oxidative stress when excessive ROS are present and GSH is locally available to the target protein (33). The ratio of reduced and oxidized glutathione (GSH/GSSG) and deglutathionylation enzymes also contribute to the protein S-glutathionylation. S-glutathionylation has been found in a large number of proteins, affecting a variety of cellular processes (32, 51). Many different thiol modification mechanisms have been associated with oxidative stress but as GSH is naturally abundant within the cells, GSH is likely to serve as a major electron donor, making S-glutathionylation a preferred mechanism for protein modification during oxidative stress (33, 44).
Glutathione
Glutathione (GSH) exists in virtually all cells in the millimolar concentration range. It is the major non-protein thiol compound that acts as an inherent antioxidant, and works together with oxidized glutathione (or alternatively called glutathione disulfide, GSSG) as an intracellular redox buffer (31). GSH is a tri-peptide, containing a cysteine, a glycine, and a glutamate. Cysteine is attached to glycine via a normal peptide bond, whereas the carboxyl group of the glutamate side-chain is bound to the amine group of cysteine via an unusual peptide bond (80). In mammalian cells, the concentration of GSH ranges from ∼1 to 10 mM depending on the cell type.
Deglutathionylation enzymes
Protein deglutathionylation is catalyzed by specific enzymes. Glutaredoxin (Grx) is suggested to be a major deglutathionylation enzyme in mammalian cells (74, 76). The mammalian cytosolic form of Grx (Grx1) is very selective and effective for protein-SSG compared with other forms of disulfides (e.g., S-S disulfide bond, S-nitrosylation, etc.), and thus is considered as a specific deglutathionylating enzyme (44). Moreover, in Grx1 knockout mice, no deglutathionylating activity is detected (74), further supporting its critical role. Besides Grx1, other enzymes have been implicated in deglutathionylation, for example, sulfiredoxin (39) and many other enzymes are known to contribute to antioxidant defense, including thioredoxins, peroxiredoxins (Prx3/5) (41), thioredoxin reductases, glutathione reductase, and so on. (3).
Specificity of protein S-glutathionylation
The specificity of protein S-glutathionylation is still under wide debate (31, 44, 75). In general, it is believed that not all cysteine residues are equally susceptible to S-glutathionylation upon stimuli. However, no consensus motif has been found that is related to S-glutathionylation site (32). Although enzymes have been suggested to promote glutathionylation (44, 51, 124), this process is still considered as a reaction mainly mediated by oxidants. Several contributing factors conferring specificity have been pointed out (32, 51), including the accessibility of the thiol group, the reactivity of the cysteine residues, and the like.
Criteria for Testing S-Glutathionylation
As various thiol modifications arise from cysteine oxidation, multiple approaches should be employed to test S-glutathionylation specifically and to avoid identifying S-glutathionylation that is artificial or has no functional consequences. Several criteria should be taken into consideration:
(i) Physiological or pathological relevance of the experimental conditions. Experimental conditions that closely mimic the in vivo physiological or pathophysiological situation such as micromolar concentrations of hydrogen peroxide (H2O2) are better than millimolar concentrations of H2O2. Using enzyme systems such as the xanthine oxidase (XO) or NADPH oxidase (NOX) to produce endogenous ROS are also preferred choices.
(ii) Using specific reactive species. Peroxynitrite (ONOO−) is one of the commonly used reactive species that has significant physiological relevance, however, the application of exogenous peroxynitrite could result in both S-nitrosylation and S-glutathionylation, which need to be further distinguished by other methods.
(iii) The combination of biochemical and functional assays. Biochemical evidence for protein S-glutathionylation is critical, but to rule out non-functional modifications, assays that assess the functional consequence of S-glutathionylation: for example, channel activities measured by patch-clamp electrophysiology or Ca++ level measured by Ca++ imaging, need to be employed.
(iv) Identification of specific cysteine residues responsible for S-glutathionylation. It has been previously shown that for a single protein, some of the cysteine residues are more likely to be S-nitrosylated and others are more likely to be S-glutathionylated (72). Besides, in some proteins only one cysteine residue is modified while for other proteins, the thiol modulation could occur on multiple sites. Moreover, S-glutathionylation may occur on a different protein associated with the target protein being studied. Without identifying the exact residues, it is hard to distinguish all these possibilities and pinpoint the exact modulation mechanism.
(v) Reversibility. S-glutathionylation is considered to be a reversible reaction while some other modifications (e.g., the formation of PS-O2H and PS-O3H) are considered irreversible (32). Using a general reducing agent (e.g., dithiothreitol [DTT]) or specific deglutathionylation enzyme (Grx) to demonstrate the reversibility would aid in distinguishing S-glutathionylation from other kinds of modifications (45).
Oxidative Stress and ROS
Oxidative stress is the condition in which excessive ROS production overwhelm the cellular antioxidant system, leading to an imbalance of the cellular redox state (71). S-glutathionylation is a modification that could be facilitated by both exogenous ROS and ROS producing enzymes.
Reactive oxygen species
ROS that are of importance in biological systems include superoxide (O2•−), hydroxyl radicals (•OH), H2O2, singlet oxygen (1O2), and so on. (94).
H2O2 is well established to participate both in oxidative modifications-mediated damage and in activation of signaling molecules. H2O2 has a relatively long lifespan and can travel in the cytosol or across the membrane to execute its effect (40). H2O2 is a relatively weak oxidizing agent but its cytotoxic effect is potentiated by interaction with a free metal, for example, iron. Because of its stability and relatively long lifespan, H2O2 is often chosen as the primary exogenous source of ROS to perform experiments.
Superoxide is another major endogenous ROS that participates in a limited range of signaling functions (48). Superoxide rapidly reacts with NO or iron-sulfur-containing proteins forming peroxynitrite or hydroxyl radicals. Dismutation of superoxide spontaneously or via catalysis by superoxide dismutase (SOD) is a major source of endogenous H2O2.
Hydroxyl radicals are the most reactive ROS and the strongest oxidizing agent, considered as the most damaging ROS that exist endogenously. However, they have a very short half-life and are in general membrane impermeable.
When ROS and NO are present together, the formation of peroxynitrite (ONOO−) is the dominant reaction as NO and ROS react at an extremely fast rate (8, 116). Therefore, in the presence of ROS, the bioavailability of NO will drastically decrease (20). Moreover, ONOO− is a strong and relatively stable reactive species (86), which could facilitate the formation of both S-nitrosylation and S-glutathionylation.
ROS-producing enzymes
ROS can also be produced by enzymes including NOX and XO. The NOX family generates ROS (mainly O2•−) through electron transfer from NADPH to molecular oxygen (9). NOX activity is regulated by Ca++, which is in turn affected by the membrane excitability of the cells (117). NOX has been found to regulate ion channel function including TWIK-related acid-sensitive potassium channel (63), T-type Ca++ channel and RyR (115). Interestingly, the activation of endothelial NADPH oxidase is also ion channel (KATP channel)-dependent (143). The closure of the KATP channel leads to membrane depolarization, which triggers NOX activation and further generation of ROS (27). These studies suggest a potentially important interplay between ROS signaling and ion channel regulation.
XO was initially found to be involved in metabolizing hypoxanthine, purine degradation, and xanthine degradation to uric acid with the generation of superoxide but has since been widely used in experiments to generate ROS (11).
Methods to Test S-Glutathionylation
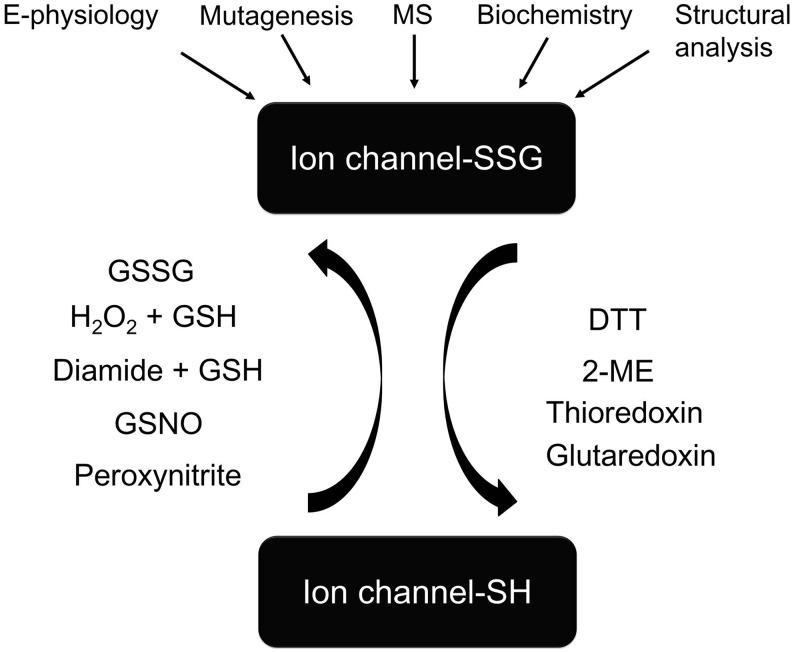
The methods and reagents for testing ion channel S-glutathionylation are illustrated in Figure 2.
FIG. 2.
Induction and detection of ion channel S-glutathionylation. Ion channel S-glutathionylation can be induced by the treatment of GSSG, H2O2+GSH, Diamide+GSH, GSNO, or peroxynitrite and reversed non-selectively by chemical reducing agents DTT and 2-ME or the disulfide-reducing enzyme thioredoxin; or reversed selectively by the deglutathionylation enzyme glutaredoxin 1. Electrophysiology, site-directed mutagenesis, MS, biochemistry, and structural analysis are useful tools for the study of ion channel S-glutathionylation. H2O2, hydrogen peroxide; DTT, dithiothreitol; 2-ME, 2-mercaptoethanol; MS, mass spectrometry.
Induction of S-glutathionylation
H2O2, discussed earlier, is a classic reagent to induce S-glutathionylation, provided the system has sufficient GSH. H2O2 concentrations ranging from 10 μM to 10 mM have been used to induce oxidative stress in different experimental conditions (65, 71, 141). However, it is worth keeping in mind that low concentration of H2O2 applied in an experimental condition is more relevant to physiological or pathophysiological conditions.
Using enzymatic systems (e.g., XO system) to manipulate the endogenous redox system may also be a good choice (13, 34, 56). Xanthine (50 μM) plus XO (50 mU ml−1) are the common ways to generate superoxide. Literature shows that XO (0.75 mU ml−1) and hypoxanthine (125 μM) can not only raise superoxide levels but also increase H2O2 (59). Since SOD decreases superoxide and increases H2O2 while catalase decreases H2O2, using SOD and catalase together with the XO system can provide more specific information to further distinguish the involvement of different ROS.
GSSG (1–10 mM) is often used to induce S-glutathionylation as well (28, 129, 138). The combined treatments of GSH (100 μM–5 mM) with oxidants (e.g., H2O2, 10 μM–1 mM; diamide 100 μM–1 mM) are also commonly used (138, 145). Both of these treatments are relatively specific to S-glutathionylation.
In addition, the application of peroxynitrite (ONOO−) or S-nitrosoglutathione (GSNO, 10–500 μM) to induce S-glutathionylation is also found in the literature (12, 38, 125, 129). The application of peroxynitrite is of high physiological relevance but as peroxynitrite can also induce other modification including S-nitrosylation, additional experiments should be performed to further dissect the exact modification mechanism. Peroxynitrite is generally made with the combination of NO and ROS (e.g., superoxide) (8, 19, 34, 66). NO can be generated using sodium nitroprusside (10 nM) or 3-morpholinosydnonimine (SIN-1, 1 mM) and superoxide can be made using the X/XO system (xanthine, X, 0.1 mM and XO, 0.01 U ml−1). The combination of these compounds thus would lead to the formation of ONOO−.
N-ethylmaleimide is often used to block the S-glutathionylation (129), and reactive disulfides (pyridine disulfides), for example, 2,2′-dithiodipyridine and 2,2-dithiobis-5-nitropyridine are sometimes used to mimic the addition of the GSH moiety to the cysteine residues (137, 138). These reagents, however, are also used for the study of S-nitrosylation (140). Thus, the effects of reactive disulfides should not be directly interpreted as the exclusive effect of S-glutathionylation. Rather, the results of the reactive disulfide should be viewed as supportive evidence in addition to other S-glutathionylation detection and induction methods.
Functional assays
S-glutathionylation often renders the protein active/inactive, thus the protein activity change upon S-glutathionylation should be monitored. For instance, in the case of enzymes such as glyceraldehyde phosphate dehydrogenase, cAMP-dependent protein kinase, creatine kinase, and protein kinase C, the altered enzyme activities by S-glutathionylation could be detected using biochemical assays (16, 35, 53, 60, 79, 96, 130). For ion channels, patch-clamp electrophysiology is the gold standard to test ion channel function and Ca++ imaging is also commonly used to study a variety of Ca++-permeable channels.
Biochemical tests
The most popular method to test S-glutathionylation biochemically is to use BioGEE (a membrane permeable biotinylated glutathione ethyl ester) in western blot experiments (87, 138). In principle, the cells expressing proteins of interest are loaded with BioGEE (100 μM–500 μM) first. The cells are then challenged with oxidants, for example, H2O2 (10 μM–1 mM) or diamide (10 μM–1 mM) for a time period (5 min–a few hours). After that, the cells are lysed and streptavidin-conjugated agarose beads (or magnetic beads) are used to pull down the BioGEE-conjugated protein complex, and the protein of interest is detected by the protein-specific antibodies (145). Alternatively, the BioGEE-protein complex could be immunoprecipitated (IP) first with specific antibodies against the protein of interest and then immunoblotted (IB) with streptavidin or anti-biotin antibody (125). A variation of this experiment is to avoid using BioGEE. Instead, the cells are directly challenged with oxidants and then IP with anti-GSH followed by IB with antibodies against the protein of interest (2) (or vice versa, IP with specific antibodies and IB with anti-GSH) (38). Additionally, the use of Biotin-GSSG (2 mM) is reported (10).
Mass spectrometry
Mass spectrometry (MS) could be employed to detect S-glutathionylation specifically. GSH has a molecular weight (MW) of 307 Da. If the protein of interest were glutathionylated, the MW of the protein would change accordingly and could be detected by MS. If one GSH is attached, then the protein MW would be shifted by 305 Da. If two were attached, then the protein MW would shift by 610 Da (2*305) and so on. By using MS, additional information regarding the number of GSH moieties attached to the target protein could be obtained. Moreover, if the proteins are digested with enzymes (e.g., trypsin or LysC) and followed by tandem mass spectroscopy (MS/MS) analysis, the specific cysteine sites that are S-glutathionylated can also be revealed (10, 28). It is worth noting that obtaining enough ion channel protein in acceptable purity for MS analysis is relatively challenging and this method has been mainly used for soluble protein so far.
Mutagenesis and chimeras
Systemic site-directed mutagenesis is one of the gold standards to test any kind of PTMs including S-glutathionylation. Single, double, triple, or even cysteine-free constructs could be generated with this method. Alanine and serine are the two major substitutions for cysteine. Mutation of cysteine to serine has an advantage over alanine when one considers the structural similarity. However, the substitution of serine may introduce potential unexpected phosphorylation site.
If the proteins of interest have a homology that responds to S-glutathionylation differently, chimeras could also be created using these homologies to determine which domains or motifs are responsible for S-glutathionylation (137).
Structural analysis
The incorporated GSH moiety is likely to interact with the surrounding amino acids, structurally affect the protein conformation, and thus change the protein function. The GSH moiety can affect the protein structure in at least two ways: First, GSH is a tripeptide so its relatively large size may have a steric hindrance effect on proteins that are normally tightly packed. Second, GSH has charged groups that may interact with the charged groups in the protein, either attracting or repelling certain amino acids to cause protein conformational change.
Structural modeling and molecular dynamics are useful tools to study ion channel structure and function (54, 134, 135) including S-glutathionylation-mediated structural changes of ion channels (55, 137). Homologous ion channel crystal structures determined by X-ray are the most common templates for structural modeling. If suitable crystal structure templates are not available, the structural information obtained by other methods, for example, cryoelectron microscopy and nuclear magnetic resonance (NMR) spectroscopy, may also be valuable for modeling.
Reversibility
The S-glutathionylation of protein is reversible. Reducing agents, for example, DTT (1–20 mM), 2-mercaptoethanol (2-ME, 1–10 mM), or specific enzymes like Grx1 (1 μM–10 μM), could be used to test the reversibility of S-glutathionylation. Grx1 is considered as the only specific deglutathionylation enzyme so far (45), so Grx1-mediated recovery is often essential to demonstrate the functional S-glutathionylation reversibility of the protein of interest (129, 138).
S-glutathionylation of Ion channels
Here, we discuss the S-glutathionylation of a variety of ion channels in detail.
S-glutathionylation of KATP channels
KATP channels are modulated by neurotransmitters and changes in the metabolic state thus contribute to the regulation of membrane excitability, which in turn is involved in insulin secretion, vascular tone regulation, shear-sensing, cardioprotection, and so on. (7, 27, 29, 77, 84, 110, 111, 136, 138). KATP channels are made of four pore-forming Kir (inward rectifier K+ channel) subunits and four regulatory sulphonylurea receptors of the ATP-binding cassette (SUR) subunits (102). Different combinations of Kir and SUR have been found in distinct tissues including Kir6.2/SUR1 in β-cells, Kir6.2/SUR2A in cardiac muscle, Kir6.1/SUR2B in smooth muscles, and others. Earlier studies have found that native KATP channel can be modulated by H2O2 and other oxidants but the molecular mechanisms of these modulations are not clear. Recently, Yang et al., reported that the vascular KATP (Kir6.1/SUR2B) channel is inhibited in the oxidative stress condition via S-glutathionylation of the Kir6.1 subunit in a physiologically relevant context (137, 138). Functional studies show that the treatments with a variety of S-glutathionylation inducers including GSH/H2O2, (GSH: GSSG)/H2O2, GSSG, or GSH/diamide lead to functional channel inhibition. The formation of a disulfide bond between the channel protein and GSH was further tested in biochemical experiments with BioGEE. Using site-directed mutagenesis, Cys176 was identified as the primary S-glutathionylation site and Cys43 as a contributing site.
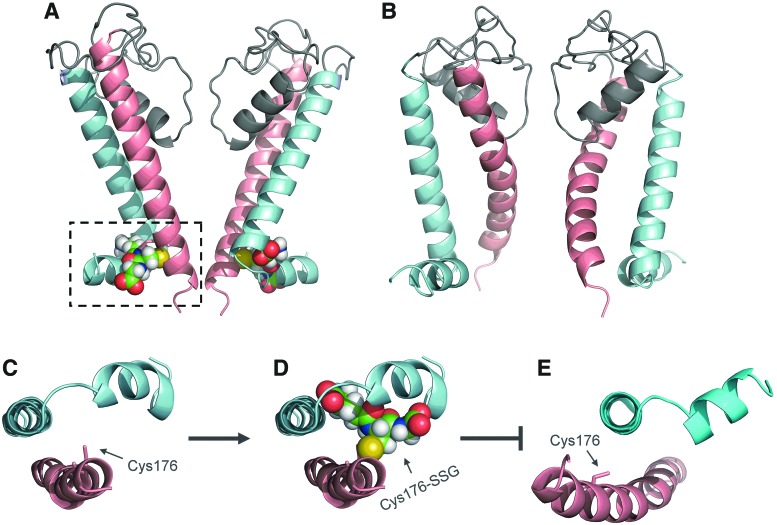
To understand the structural consequence of KATP channel S-glutathionylation, structural modeling of KATP channel has been performed. In particular, the Kir6.1 pore-forming subunit in its closed and open states were modeled, based on the recently crystalized chicken Kir2.2 and the bacterial Kir channel as templates, to study how S-glutathionylation affects the KATP channel gating (137) (Fig. 3A, B). From the model of Kir6.1 channel in its closed state, it was found that Cys176, the primary cysteine site of Kir6.1 for S-glutathionylation, is located in the critical region of the inner helix, close to the activation gate residue Phe178 and the hinge residue Gly175 (residue numbers are based on rat Kir6.1 protein). Phe178 is suggested to form the activation gate and block the ion-conducting pathway when the channel is in its closed state. As the gating hinge, Gly175 is the site where the channel bends to open (61). Cys176 locates between Phe178 and Gly175 and its side chain faces a pocket formed by inner helix, outer helix, and slide helix (Fig. 3C). When the GSH moiety is modeled onto Cys176, it is found that GSH moiety can fit into this pocket nicely after energy minimization (Fig. 3D). The Kir6.1 channel structure in its open state is also modeled and shows that the inner helix undergoes a significant leftward movement (137), resulting in the disruption of the binding pocket for the GSH moiety (Fig. 3E). Therefore, based on these models, it is proposed that incorporation of the GSH moiety into the Kir6.1 subunit prevents the channel from entering its open state, thus retaining the channel in its closed state.
FIG. 3.
Structural insights of KATP channel S-glutathionylation. (A) Side view of structural model of Kir6.1 (pore-forming domain) of the KATP channel in its closed state (two opposing monomers are shown). Outer helix is colored cyan and inner helix is colored red. Cys176 residue of inner helix is S-glutathionylated and boxed. (B) Side view of structural model of Kir6.1 pore-forming domain in its open state. (C) Intracellular view of outer and inner pore-forming helices in closed state (connecting loops are omitted for clarity). Cys176 residue is shown as a stick. (D) Same view as C but Cys176 residue is bound to the gluthionyl moiety via disulfide bond. (E) Intracellular view of pore-forming helices in open state. The inner helix is bent leftward, disrupting the binding pocket for the glutathionyl moiety. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
S-glutathionylation of Kir4-Kir5 channel
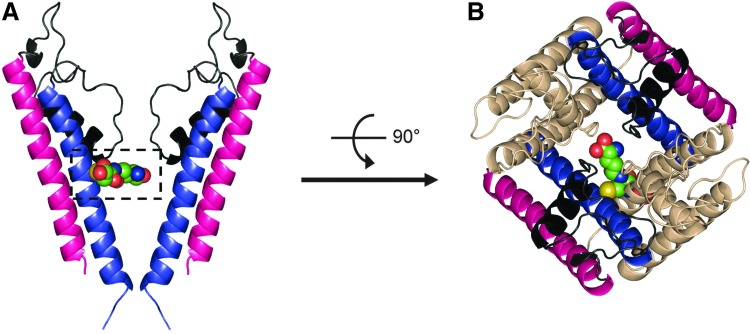
Kir4.1 channel is found in the kidney, retina, and central nervous system as a homodimeric or heterodimeric (together with the Kir5.1) channel, acting as a K+ transporter or a pH-dependent membrane potential regulator (50, 62). Jin et al. has found that the presence of Kir5.1 in the Kir4-Kir5 heterodimeric channel enables channel sensitivity to S-glutathionylation. Using diamide or H2O2 together with GSH, it was found that the Kir4-Kir5 channel could be inhibited via S-glutathionylation while the homodimeric Kir4.1 channel is not sensitive to this modification (55). Further study showed that Cys158 in the S6 helix of Kir5.1 is the major residue for S-glutathionylation (Fig. 4A, B). In addition, it was demonstrated that one GSH moiety is sufficient to block the channel activity and S-glutathionylation occurs when the channel is in its open state (55).
FIG. 4.
Structural insights of Kir4.1-Kir5.1 channel S-glutathionylation. (A) Side view of structural model of Kir4.1–5.1 (pore-forming domain) in the closed state (two opposing Kir5.1 monomers are shown). Outer helix of Kir5.1 is colored magenta and inner helix of Kir5.1 is colored blue. A glutathionyl moiety is bound to one Cys158 residue of Kir5.1 via disulfide bond. (B) Extracellular view of glutathionyl moiety adducted to the Cys158 residue of the inner helix in Kir4.1-Kir5.1 channel. The two Kir4.1 subunits are colored wheat. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
S-glutathionylation of ryanodine receptor
The RyR is the first ion channel found to be S-glutathionylated, and S-nitrosylated (4, 118, 119). Expressed on the endoplasmic reticulum (ER), the RyR is responsible for Ca++ release from ER to cytosol. The RyR protein has ∼100 cysteine residues and is highly sensitive to oxidation. Both S-glutathionylation and S-nitrosylation could occur on RyR channels with S-nitrosoglutathione (GSNO) treatment (5). Further study has demonstrated that certain cysteine residues (Cys1040 and Cys1303) are exclusively S-nitrosylated, some (Cys1593 and Cys3193) are selectively S-glutathionylated, and others can undergo both reactions (4).
S-glutathionylation of cystic fibrosis transmembrane conductance regulator
Cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel belonging to the ABC transporter family (43). CFTR regulates salt and water transportation across epithelial membranes in lung and gut. Mutations of the CFTR gene can lead to cystic fibrosis and congenital bilateral absence of vas deferens (108). During inflammation, extensive production of ROS could target the epithelial cells and modulate the plasma membrane-bound CFTR. Indeed, Wang et al., found that several oxidized forms of glutathione including GSSG (10–20 mM), and nitrosylated glutathione/S-nitrosoglutathione (GSNO, 50–200 μM) can inhibit the channel activity markedly in excised patch recordings (129). In addition, the combined application of GSH and diamide (5–100 μM, equimolar) can also cause significant channel inhibition. These reactions could be rescued by the reducing agent DTT (20 mM) and the deglutathionylation enzyme Grx (4 μM). Moreover, biochemical experiments further demonstrate that diamide (100 μM) facilitates the incorporation of biotin-GSH (125 μM) into CFTR protein. Using site-directed mutagenesis, Cys344 located in the NBD2 domain was identified as the primary site for S-glutathionylation. This modulation could potentially affect the nucleotide binding and disrupt the ATP-dependent channel opening. Although a schematic diagram has been provided to explain the structure-function of CFTR S-glutathionylation (129), detailed structural insight has not been reported, presumably due to the lack of an accurate template for modeling.
S-glutathionylation of CRAC channel
Ca++ release-activated Ca++ (CRAC) channels belong to store-operated channels, which involve in a variety of signaling cascades of non-excitable cells, including T lymphocytes and mast cells. Recent studies have identified Orai1 (CRAC modulator) as the pore-forming subunit of the CRAC channel while stromal-interacting molecule 1 (STIM1) serves as the ER Ca++ sensor (15, 91, 95). Hawkins et al. reported that oxidative stress, induced by lipopolysaccharide (LPS) (1.5 μg/ml) and H2O2 (100 μM), alters the Ca++ signaling in B-lymphocytes through the modification of the CRAC channels (47). They further identified by pull-down experiment that the ER-resident STIM1 is S-glutathionylated. A conserved cysteine residue (Cys56) was revealed as the target of S-glutathionylation. The incorporation of GSH to STIM1 was confirmed by MS. The S-glutathionylation of STIM1 results in a constitutively activated CRAC channel.
S-glutathionylation of voltage-gated Ca++ channels and channels for Ca++ homeostasis
Intracellular Ca++ concentration and Ca++ oscillations play important roles in endothelial cells, including the regulation of vascular permeability, inflammation, and so on. The disruption of Ca++ homeostasis has been associated with oxidative stress, whereas the molecules that are directly targeted by ROS are not clear. Lock et al. demonstrated that diamide, a thiol-oxidizing agent, could increase the intracellular Ca++ concentration and oscillations (68). The authors showed that this modulation is related to GSH. It is further identified that IP3 receptors and the plasmalemmal Ca++-ATPase pump are the molecules targeted by ROS via S-glutathionylation using pull-down experiments with BioGEE (69).
Disruption of calcium homeostasis also contributes to cardiac hypertrophy and cardiac failure. Tang et al. found that the L-type Ca++ channel (CaV1.2), a main channel for calcium influx into cardiac myocytes, could be regulated by S-glutathionylation (123). The open probability of CaV1.2 increases with GSSG and biochemical evidence shows that the CaV1.2 channel is glutathionylated both in H2O2 treatment and in ischemic human heart.
S-Glutathionylation of Ion Transporters
Besides ion channels, ion transporters (or ion pumps) are another group of molecules that move ions across cellular membrane, but at the expense of energy consumption (e.g., ATP or the concentration gradient of another ion) (42). Many ion transporters are regulated by S-glutathionylation as well (93).
For example, sarco/endoplasmic reticulum Ca++-ATPase [SERCA], is a calcium ATPase that transfers Ca++ from cytosol to the lumen of the sarcoplasmic reticulum. It has been reported that SERCA can be activated by NO via peroxynitrite-mediated S-glutathionylation at the Cys674 residue (1). Na+/K+-ATPase (sodium-potassium adenosine triphosphatase) is another ion transporter that is found to be modulated by S-glutathionylation (38). Peroxynitrite, among other stimuli, can facilitate S-glutathionylation of β-1 subunit of Na+/K+-ATPase at the Cys46 residue (38). S-glutathionylation also occurs on the catalytic α subunit of Na+/K+-ATPase, affecting the ATP binding (92). The crosstalk between S-nitrosylation and S-glutathionylation has also been observed on Na+/K+ ATPase, indicating that a switch between S-glutathionylation and S-nitrosylation contributes to the oxygen-induced regulation of Na+/K+-ATPase (132).
Ion Channel S-Nitrosylation and Its Relationship with S-Glutathionylation
S-nitrosylation is referred to as the addition of an NO moiety to the thiol group of a cysteine residue (46). Like S-glutathionylation, no enzyme that can facilitate the formation of protein S-nitrosylation has been found yet. However, enzymes like S-nitrosoglutathione reductase (GSNOR) have been shown to mediate the denitrosylation (72). Many ion channels (or their regulators) (46), including the RyR channel (4), voltage-gated sodium channel (97), the voltage-gated potassium channel (6), the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (103, 104), the TRP channel (140), and the KATP channel (21, 58, 64, 67, 120), are found to be regulated by S-nitrosylation or NO.
Recent studies suggest that the NO-mediated pathway and the ROS-mediated pathway may have extensive crosstalk (37, 93, 132); the reaction between NO and superoxide gives rise to peroxynitrite, which can be a potent mediator of S-glutathionylation in the presence of GSH; NO may form protein-SNO, which can serve as a precursor to protein-SSG. And in the case of RyR channels, S-nitrosylation and S-glutathionylation may compete for the same cysteine residues (4).
In addition, S-nitrosylation and S-glutathionylation share lots of common features (72): they are both facilitated by oxidants; they both target the thiol group and attach a moiety to the cysteine residues. Therefore, in experimental design, reactive disulfides can be used to mimic both S-nitrosylation and S-glutathionylation (72, 137, 140). Moreover, studies have shown that for a given protein, both S-nitrosylation and S-glutathionylation could occur depending on the accessibility of the cysteine, specific local environment around the cysteine, the redox state of the proteins, and other unidentified factors. It is still elusive to predict if a given cysteine may be selective for S-glutathionylation of S-nitrosylation. Why S-nitrosylation and S-glutathionylation selectively occur on different cysteine residues is not known. In some cases, S-nitrosylation and S-glutathionylation occur on the same cysteine residues, but how S-nitrosylation competes with S-glutathionylation or serves as a precursor to S-glutathionylation for the same residue is not clear either. Future studies are needed to understand the intertwining relationship between S-glutathionylation and S-nitrosylation.
Ion Channel S-Palmitoylation and S-Sulfhydration
Besides S-glutathionylation and S-nitrosylation, other thiol modulation mechanisms, such as S-palmitoylation and S-sulfhydration, have been reported to modulate ion channels. S-palmitoylation refers to the formation of a reversible thioester linkage of a palmitoyl lipid to cysteine residue. This modification has been shown to control the maturation, trafficking, and regulation of ligand- and voltage-gated ion channels (114).
S-sulfhydration refers to H2S-mediated PTM (81, 89). H2S is a gaseous messenger molecule that is generated in vivo from L-cysteine through the enzymes cystathionine β-synthase and cystathionine γ-lyase (CSE) (127). S-sulfhydration shares many common features with S-nitrosylation, as both are mediated by gasotransmitters (H2S and NO) (82, 106). Endogenous H2S could modulate proteins by converting the −SH group of cysteine to an −SSH group (81, 133). The KATP channel reported elsewhere to be mechanosensitive (23, 27) is regulated by H2S (83, 121, 144). Besides the KATP channel, other channels including L- and T-type Ca++ channel, TRPA/V channels, Cl− channels, and so on, are also subjected to the modulation of H2S (90, 122). Readers who are interested in these modification mechanisms are directed to other excellent reviews covering these topics (89, 90, 122, 128).
Possible Crosstalk Among Different Thiol Modifications
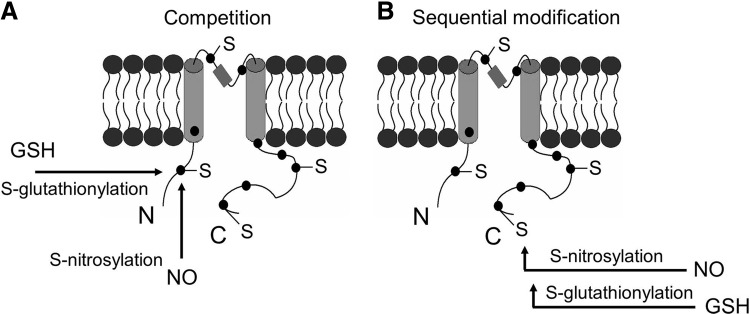
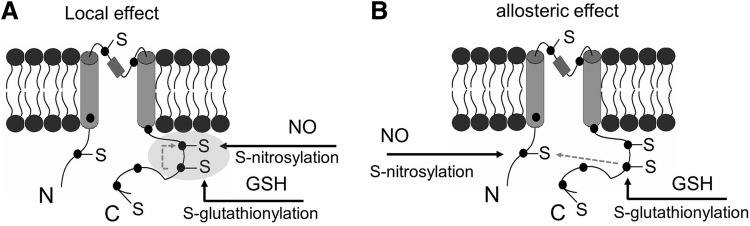
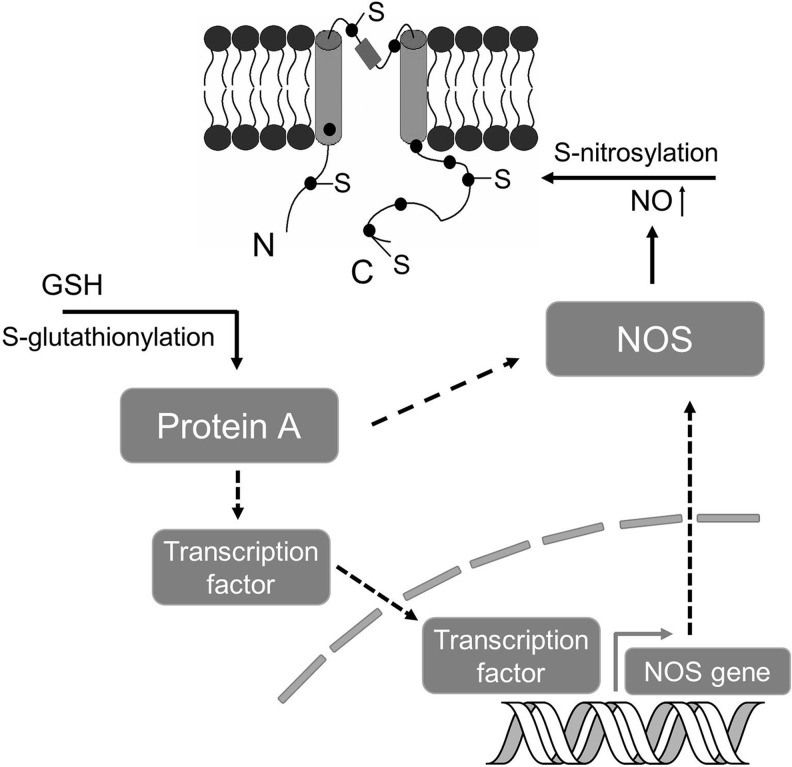
The crosstalk or interplay among various types of cysteine modifications is particularly interesting. The crosstalk could happen in many ways directly or indirectly, including, (i) Different thiol modification mechanisms may compete for the same cysteine residues (Fig. 5A), for example, in the case of RyR channel. (ii) Different modifications may occur sequentially, for example, S-nitrosylation may serve as a precursor to S-glutathionylation in certain conditions (Fig. 5B). (iii) The modification of a cysteine residue by one mechanism may alter the local environment so that the reactivity of a nearby residue (e.g., cysteine, phosphorylation site) may be altered (Fig. 6A). (iv) The modification of a cysteine residue in the protein may have an allosteric effect so that the reactivity of another cysteine residue in a distinct region of that protein or in another protein of a multiple protein complex may be altered (Fig. 6B). (v) Thiol modification of a protein may affect the activity or production of key elements in another thiol modification cascade, for example, S-glutathionylation of certain proteins (CSE, nitric oxide synthase [NOS] or their regulators/transcription factors) may lead to increased activities of CSE or NOS, or lead to the production of more CSE, NOS proteins through gene regulation (Fig. 7).
FIG. 5.
Possible crosstalk between S-nitrosylation and S-glutathionylation targeting the same cysteine residue. (A) S-glutathionylation and S-nitrosylation complete for the same cysteine thiol group. (B) Two thiol modification mechanisms happen sequentially: S-nitrosylation serves as a precursor of S-glutathionylation.
FIG. 6.
Possible crosstalk between S-nitrosylation and S-glutathionylation targeting different cysteine residues. (A) Modification of one cysteine by S-glutathionylation affects the local environment for a second cysteine, thus modulating the reactivity of the second cysteine residue to S-nitrosylation. (B) The modification of one cysteine by S-glutathionylation has an allosteric effect on another cysteine residue at a different place, modulating its reactivity to S-nitrosylation.
FIG. 7.
A hypothetical indirect modulation mechanism of S-nitrosylation pathway by S-glutathionylation. S-glutathionylation of a hypothetical protein (e.g., a kinase) may regulate the activity of an NOS protein (e.g., eNOS), affecting the production of NO molecule and S-nitrosylation. Or, a hypothetical protein may regulate a transcription factor of a NOS gene, leading to the production of more NOS protein, generating additional NO molecule for S-nitrosylation. NOS, nitric oxide synthase; eNOS, endothelial NOS.
Certain possible crosstalk among different PTMs has been previously discussed in reviews (49, 114). Recent research from many labs, especially Dr. Snyder's group, provided more evidence for some interesting possibilities. Ho et al. reported that S-palmitoylation and S-nitrosylation can regulate the principal protein of postsynaptic densities (PSD-95) via competitive modification of cysteine 3 and 5 residues (52). In another study, Sen et al. found that H2S can lead to S-sulfhydration of the p65 subunit of NF-κB at the Cys38 residue, where S-nitrosylation can also occur in a competitive manner (105). More recently, Vandiver et al. showed that S-nitrosylation and S-sulfhydration (sulphydration) might be reciprocal events for parkin, an E3 ubiquitin ligase involving in Parkinson's disease (126). Sulfhydration occurs physiologically and enhances the catalytic activity of parkin, whereas S-nitrosylation inactivates parkin, suggesting a neuroprotective effect and therapeutic implications of H2S donor (126). AMPA receptors are subjected to many types of PTM (70). S-nitrosylation of GluA1 (at Cys875), a regulatory subunit of AMPA receptors, facilitates phosphorylation (at Ser831) and enhances AMPA receptor channel conductance (104). This type of crosstalk was also seen in endothelial NOS (eNOS) from an earlier study (36). In addition, it is also known that NO may affect H2S level in vascular tissues via increasing CSE activity. As CSE has many cysteine residues, S-nitrosylation of CSE has been the subject of speculation (128). With the exception of the RyR channel mentioned above, whether crosstalk or interplay of these thiol modifications may occur in other ion channel proteins is still unknown and would be an exciting new direction in the field.
Ion Channels in Mechanotransduction and Possible Contribution of S-Glutathionylation
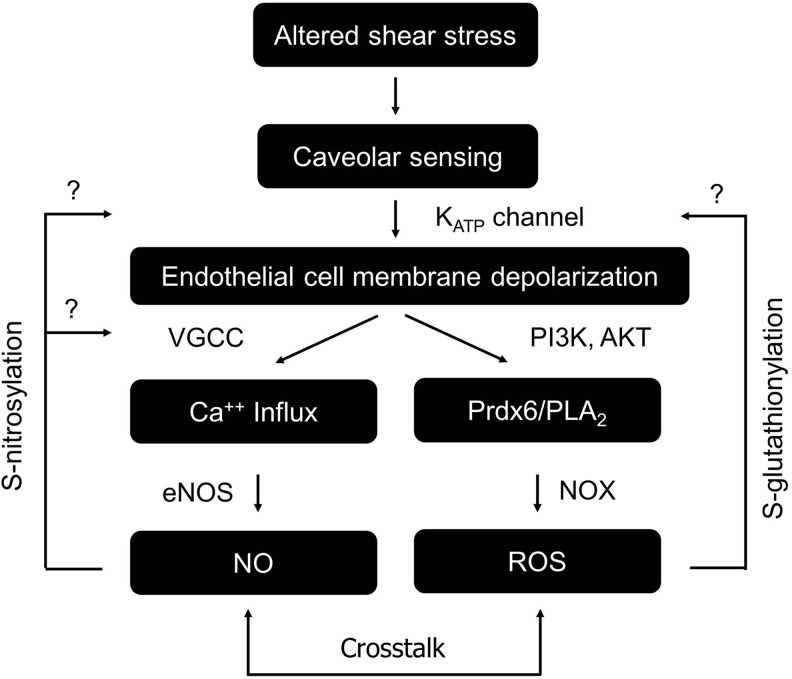
Mechanotransduction is a process through which cells sense altered physical forces and convert them into biological signals (18). The response of endothelium to dynamic fluid shear stress is one example of mechanotransduction (25, 78). Interestingly, the activities of many ion channels could be modulated in response to altered shear stress in this process (18, 27, 131). These channels sense mechanical forces and transduce them into electrical/biochemical signals. In the context of flow and its cessation, K+ channels have been reported to respond to alterations of flow. An inward rectifying K+ channel was initially found to be activated with onset of flow (85) and later studies identified KATP channel as the inward rectifying K+ channel that is closed on cessation of flow (27). The closure of the KATP channel depolarizes the endothelial cell plasma membrane, triggers many downstream signaling cascades (PI3K-Akt), and eventually causes activation of NOX to produce ROS (24, 27). NOX generates superoxide into the extracellular side of the cellular membrane and the extracellular superoxide could flux across the plasma membrane through chloride channel-3, induce intracellular Ca++ release, and activate further mitochondrial superoxide generation (48). The depolarization of endothelial cells could also trigger the activation of eNOS to produce NO in a Ca++-dependent manner (Fig. 8). Collectively, the activation of these signaling cascades thus could lead to vasodilation, cell proliferation, injury, or other consequences (17, 26, 73, 142). As ROS and NO are able to modulate thiol groups of many ion channels (1, 27, 101) including the KATP channel (21, 64, 138), which are potentially involved in membrane potential regulation and downstream signaling, we propose that a feedback loop may exist linking membrane potential, ion channel regulation (by S-glutathionylation or S-nitrosylation), and ROS and/or NO production in mechanotransduction of endothelium (Fig. 8).
FIG. 8.
Potential interplay between ROS/NO production and ion channel modulation in mechanotransduction cascades. In this working model, altered shear stress induced by decrease of flow could be sensed by caveolae, leading to KATP channel inhibition. Closure of KATP channel depolarizes the cell membrane, triggering a variety of downstream events including the activation of eNOS and NOX, generating NO and ROS, respectively. Here, we propose that the production of NO and ROS in these signaling cascades may in turn modulate ion channels including KATP channels, VGCC, and other channels via S-glutathionylation and/or S-nitrosylation, forming a feedback loop in mechanotransduction cascades. Note that the NO and ROS signaling pathways may also have extensive crosstalk, which are outlined in the main text. PI3K, phosphatidyl inositol-3 kinase; Akt, protein kinase B; Prdx6, peroxiredoxin 6; PLA2, phospholipase A2; ROS, reactive oxygen species; NOX, NADPH oxidase; VGCC, voltage-gated calcium channel.
The mechanosensitive cascade initiated by the KATP channel hinges on channel activities and the subsequent S-glutathionylation of KATP channel by ROS would lead to channel inhibition, and presumably affect the ability of KATP channel to sense stopped flow. However, it is not clear if and to what extent deglutathionylation in vivo would restore channel function and mechanosensing. As S-glutathionylation is a relatively new concept in ion channel and mechanotransduction field, the modifications of mechanosensitive channels by S-glutathionylation have not been clearly reported yet. Regardless, based on the strong link between mechanotransduction and oxidative stress, especially in vasculature, we propose that S-glutathionylation could be an important mechanism that regulates mechanosensitive channels during oxidative stress.
Conclusions and Perspectives
Thiol modifications, especially S-glutathionylation, have received increasing recognition as a major mechanism that regulates ion channel structure and function. Especially, in vasculature (93), redox-mediated ion channel modulations seem to be complex phenomena, affecting not merely channel functions but possibly forming feedback loops. On one hand, ROS modify ion channels via S-glutathionylation, S-nitrosylation, and/or other PTMs and affect channel functions and downstream signaling cascades. On the other hand, mechanical and other stimuli may alter ion channel activities, leading to signaling cascades that produce ROS, NO, or other molecules, which in turn, modulate ion channels. A balance among these modulations might be required for vascular homeostasis and other important cellular functions.
Nevertheless, with the advances of research tools and standardized protocols, the list of ion channels that could be regulated by S-glutathionylation would grow rapidly. However, certain outstanding challenges and questions are still awaiting answers from the research community. They are as follows:
(i) Although it is pretty clear that oxidative stress could promote S-glutathionylation and S-nitrosylation, the exact physiological or pathological roles of these modulations are not well understood. Especially, the functional consequences of S-glutathionylation in tissue and organ level, as well as in whole animals have not been explored extensively. Would S-glutathionylation of certain ion channels represent a protection mechanism against oxidative stress or simply represent a consequence of oxidative stress? Would S-glutathionylation serve as a critical signaling mechanism similar to protein phosphorylation contributing to signal transduction?
(ii) With the growing list of newly crystallized ion channel structures and the improvement of structural modeling tools, more studies may be performed to tackle ion channel thiol modification from a structural perspective, addressing how GSH, NO, H2S, or other molecules may affect the local or global conformation of ion channels to affect channel functions.
(iii) As it is known that many PTMs (e.g., S-glutathionylation, S-nitrosylation, S-palmitoylation, and S-sulfhydration) target the thiol group of cysteine residues, investigating the intriguing relationships among these mechanisms would provide critical insights for the understanding of thiol modulation in general. Why are different cysteine residues preferably targeted by specific mechanisms? Could prediction algorithms be developed to provide information regarding which cysteine is sensitive to which modification mechanism under which condition? Indeed, it is very fascinating that cells need such a complicated system to modulate the cysteine residues. Understanding the physiological significance and how the cells fine-tune all these thiol modulations could lead to major breakthroughs.
We anticipate that the understanding of all these basic questions on S-glutathionylation of ion channels may provide a platform to further explore therapeutic approaches to treat many oxidative stress-related devastating diseases.
Abbreviations Used
- 2-ME
2-mercaptoethanol
- Akt
protein kinase B
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BioGEE
biotinylated glutathione ethyl ester
- CFTR
cystic fibrosis transmembrane conductance regulator
- CRAC channel
Ca++ release-activated Ca++ channel
- CSE
cystathionine γ-lyase
- DTT
dithiothreitol
- eNOS
endothelial nitric oxide synthase
- Grx
glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide/oxidized glutathione
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- IB
immunoblotted
- IP
immunoprecipitated
- Kir
inward rectifier K+ channel
- MS
mass spectrometry
- MW
molecular weight
- NO
nitric oxide
- NOX
NADPH oxidase
- O2•−
superoxide
- •OH
hydroxyl radicals
- ONOO−
peroxynitrite
- PI3K
phosphatidyl inositol-3 kinase
- PLA2
phospholipase A2
- Prdx6
peroxiredoxin 6
- PTM
post-translational modification
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarco/endoplasmic reticulum Ca++-ATPase
- SOD
superoxide dismutase
- STIM1
stromal-interacting molecule 1
- VGCC
voltage-gated calcium channel
- XO
xanthine oxidase
Acknowledgments
C.J. and X.J. are supported by NIH (1R21HD060959 and 1R01NS073875). Y.Y. was a B&B fellow at GSU and is currently supported by the Connecticut Stem Cell Research Grants Program. We thank Dr. Yun Shi, Dr. Weiwei Shi, Anuhya S. Konduru of Georgia State University, Dr. Mark Estacion, and Dr. Andrew Tan of Yale University for critical reading of the article. We thank Dr. Aron B. Fisher and Dr. Shampa Chatterjee of University of Pennsylvania, Dr. Rui Wang of Lakehead University and Dr. Michael Shipston of University of Edinburgh for comments and discussions. We thank Dr. Solomon H. Snyder and Dr. Bindu D. Paul of Johns Hopkins University for sharing preprint materials and comments on this article.
References
- 1.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, and Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, and Janssen-Heininger YMW. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol 184: 241–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreyev AY, Kushnareva YE, and Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200–214, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C, and Hamilton SL. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem 281: 40354–40368, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Aracena P, Sanchez G, Donoso P, Hamilton SL, and Hidalgo C. S-glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J Biol Chem 278: 42927–42935, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Asada K, Kurokawa J, and Furukawa T. Redox- and calmodulin-dependent S-nitrosylation of the KCNQ1 channel. J Biol Chem 284: 6014–6020, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Aziz Q, Thomas AM, Khambra T, and Tinker A. Regulation of the ATP-sensitive potassium channel subunit, Kir6.2, by a Ca2+-dependent protein kinase C. J Biol Chem 287: 6196–6207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckman JS. and Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bedhomme M, Zaffagnini M, Marchand CH, Gao XH, Moslonka-Lefebvre M, Michelet L, Decottignies P, and Lemaire SD. Regulation by glutathionylation of isocitrate lyase from Chlamydomonas reinhardtii. J Biol Chem 284: 36282–36291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry CE. and Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555: 589–606, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibert S, Liu CC, Figtree GA, Garcia A, Hamilton EJ, Marassi FM, Sweadner KJ, Cornelius F, Geering K, and Rasmussen HH. FXYD proteins reverse inhibition of the Na-K pump mediated by glutathionylation of its {beta}1 subunit. J Biol Chem 286: 18562–18572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bielefeldt K, Whiteis CA, Sharma RV, Abboud FM, and Conklin JL. Reactive oxygen species and calcium homeostasis in cultured human intestinal smooth muscle cells. Am J Physiol 272: G1439–G1450, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Bimboese P, Gibson CJ, Schmidt S, Xiang W, and Ehrlich BE. Isoform-specific regulation of the inositol 1,4,5-trisphosphate receptor by O-linked glycosylation. J Biol Chem 286: 15688–15697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, and Niemeyer BA. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal 3: ra24, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Borges CR, Geddes T, Watson JT, and Kuhn DM. Dopamine biosynthesis is regulated by S-glutathionylation. Potential mechanism of tyrosine hydroxylast inhibition during oxidative stress. J Biol Chem 277: 48295–48302, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Browning E, Wang H, Hong N, Yu K, Buerk DG, Debolt K, Gonder D, Sorokina E, Patel P, Deleon D, Feinstein SI, Fisher AB, and Chatterjee S. Mechanotransduction drives post ischemic revascularization through KATP channel closure and production of reactive oxygen species. Antioxid Redox Signal 20: 872–886, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Browning EA, Chatterjee S, and Fisher AB. Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. Annu Rev Physiol 74: 403–424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, and Elliott SJ. Peroxynitrite reversibly inhibits Ca(2+)-activated K(+) channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 278: H1883–H1890, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Cai H. and Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Chai Y. and Lin Y-F. Dual regulation of the ATP-sensitive potassium channel by activation of cGMP-dependent protein kinase. Pflügers Arch 456: 897–915, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekhar KD, Lvov A, Terrenoire C, Gao GY, Kass RS, and Kobertz WR. O-glycosylation of the cardiac I(Ks) complex. J Physiol 589: 3721–3730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, and Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 285: C959–C967, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee S, Browning EA, Hong N, DeBolt K, Sorokina EM, Liu W, Birnbaum MJ, and Fisher AB. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol 302: H105–H114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee S, Chapman KE, and Fisher AB. Lung ischemia: a model for endothelial mechanotransduction. Cell Biochem Biophys 52: 125–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D, and Fisher AB. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem 286: 11696–11706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee S, Levitan I, Wei Z, and Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 13: 633–644, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, and Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark R. and Proks P. ATP-sensitive potassium channels in health and disease. Adv Exp Med Biol 654: 165–192, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Dai S, Hall DD, and Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev 89: 411–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, and Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal 10: 445–473, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, and Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci 34: 85–96, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, and Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med 43: 883–898, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Duprat F, Girard C, Jarretou G, and Lazdunski M. Pancreatic two P domain K+ channels TALK-1 and TALK-2 are activated by nitric oxide and reactive oxygen species. J Physiol 562: 235–244, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eaton P, Wright N, Hearse DJ, and Shattock MJ. Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J Mol Cell Cardiol 34: 1549–1560, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Erwin PA, Lin AJ, Golan DE, and Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 280: 19888–19894, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Evangelista AM, Thompson MD, Weisbrod RM, Pimental DR, Tong X, Bolotina VM, and Cohen RA. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxid Redox Signal 17: 1099–1108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figtree GA, Liu CC, Bibert S, Hamilton EJ, Garcia A, White CN, Chia KK, Cornelius F, Geering K, and Rasmussen HH. Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circ Res 105: 185–193, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, and Tew KD. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res 66: 6800–6806, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher AB. Redox signaling across cell membranes. Antioxid Redox Signal 11: 1349–1356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A activities. Antioxid Redox Signal 15: 831–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadsby DC. Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol 10: 344–352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gadsby DC. and Nairn AC. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev 79: S77–S107, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Gallogly MM. and Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol 7: 381–391, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Gallogly MM, Starke DW, and Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal 11: 1059–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez DR, Treuer A, Sun QA, Stamler JS, and Hare JM. S-Nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol 54: 188–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, and Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol 190: 391–405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawkins BJ, Madesh M, Kirkpatrick CJ, and Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell 18: 2002–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess DT. and Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem 287: 4411–4418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, and Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Hill BG. and Bhatnagar A. Protein S-glutathiolation: redox-sensitive regulation of protein function. J Mol Cell Cardiol 52: 559–567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, and Snyder SH. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 71: 131–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humphries KM, Juliano C, and Taylor SS. Regulation of cAMP-dependent protein kinase activity by glutathionylation. J Biol Chem 277: 43505–43511, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Jensen MO, Jogini V, Borhani DW, Leffler AE, Dror RO, and Shaw DE. Mechanism of voltage gating in potassium channels. Science 336: 229–233, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Jin X, Yu L, Wu Y, Zhang S, Shi Z, Chen X, Yang Y, Zhang X, and Jiang C. S-Glutathionylation underscores the modulation of the heteromeric Kir4.1-Kir5.1 channel in oxidative stress. J Physiol 590(Pt 21): 5335–5348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juncos R. and Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Kamp TJ. and Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87: 1095–1102, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Kawano T, Zoga V, Kimura M, Liang MY, Wu HE, Gemes G, McCallum JB, Kwok WM, Hogan QH, and Sarantopoulos CD. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Mol Pain 5: 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, and Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med 48: 493–498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klatt P. and Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: 4928–4944, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, and Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300: 1922–1926, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, and Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Lee Y, Kim B, Chun Y, So I, Choi H, Kim M, and Park J. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Lin YF, Raab-Graham K, Jan YN, and Jan LY. NO stimulation of ATP-sensitive potassium channels: involvement of Ras/mitogen-activated protein kinase pathway and contribution to neuroprotection. Proc Natl Acad Sci U S A 101: 7799–7804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y. and Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol 29: 305–311, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Terata K, Chai Q, Li H, Kleinman LH, and Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res 91: 1070–1076, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Ljubkovic M, Shi Y, Cheng Q, Bosnjak Z, and Jiang MT. Cardiac mitochondrial ATP-sensitive potassium channel is activated by nitric oxide in vitro. FEBS Lett 581: 4255–4259, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Lock JT, Sinkins WG, and Schilling WP. Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am J Physiol Heart Circ Physiol 300: H493–H506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lock JT, Sinkins WG, and Schilling WP. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J Physiol 590: 3431–3447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu W. and Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol 22: 470–479, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madamanchi NR, Vendrov A, and Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Ruiz A. and Lamas S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res 75: 220–228, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Matsuzaki I, Chatterjee S, Debolt K, Manevich Y, Zhang Q, and Fisher AB. Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. Am J Physiol Heart Circ Physiol 288: H336–H343, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Meyer Y, Buchanan BB, Vignols F, and Reichheld J-P. Thioredoxins and Glutaredoxins: Unifying Elements in Redox Biology. Ann Rev Genet 43: 335–367, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Mieyal JJ. and Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: focus on s-glutathionylation. Antioxid Redox Signal 16: 471–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, and Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miki T. and Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol 38: 917–925, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, and Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohr S, Hallak H, de Boitte A, Lapetina EG, and Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 274: 9427–9430, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Moran LK, Gutteridge JM, and Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem 8: 763–772, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, and Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mustafa AK, Gadalla MM, and Snyder SH. Signaling by gasotransmitters. Sci Signal 2: re2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, and Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Olesen SP, Clapham DE, and Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331: 168–170, 1988 [DOI] [PubMed] [Google Scholar]
- 86.Pacher P, Beckman JS, and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park JW, Mieyal JJ, Rhee SG, and Chock PB. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem 284: 23364–23374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pastore A. and Piemonte F. S-Glutathionylation signaling in cell biology: progress and prospects. Eur J Pharm Sci 46: 279–292, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Paul BD. and Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Peers C, Bauer CC, Boyle JP, Scragg JL, and Dallas ML. Modulation of ion channels by hydrogen sulfide. Antioxid Redox Signal 17: 95–105, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, and Cahalan MD. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456: 116–120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petrushanko IY, Yakushev S, Mitkevich VA, Kamanina YV, Ziganshin RH, Meng X, Anashkina AA, Makhro A, Lopina OD, Gassmann M, Makarov AA, and Bogdanova A. S-glutathionylation of the Na,K-ATPase catalytic alpha subunit is a determinant of the enzyme redox sensitivity. J Biol Chem 287: 32195–32205, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pimentel D, Haeussler DJ, Matsui R, Burgoyne JR, Cohen RA, and Bachschmid MM. Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system. Antioxid Redox Signal 16: 524–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Powers SK. and Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, and Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Reddy S, Jones AD, Cross CE, Wong PS, and Van Der Vliet A. Inactivation of creatine kinase by S-glutathionylation of the active-site cysteine residue. Biochem J 347Pt 3: 821–827, 2000 [PMC free article] [PubMed] [Google Scholar]
- 97.Renganathan M, Cummins TR, and Waxman SG. Nitric oxide blocks fast, slow, and persistent Na+ channels in C-type DRG neurons by S-nitrosylation. J Neurophysiol 87: 761–775, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Rengifo J, Gibson CJ, Winkler E, Collin T, and Ehrlich BE. Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation. J Neurosci 27: 13813–13821, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rougier JS, Albesa M, and Abriel H. Ubiquitylation and SUMOylation of cardiac ion channels. J Cardiovasc Pharmacol 56: 22–28, 2010 [DOI] [PubMed] [Google Scholar]
- 100.Sabens Liedhegner EA, Gao XH, and Mieyal JJ. Mechanisms of altered redox regulation in neurodegenerative diseases—focus on S—glutathionylation. Antioxid Redox Signal 16: 543–566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanchez G, Escobar M, Pedrozo Z, Macho P, Domenech R, Hartel S, Hidalgo C, and Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc Res 77: 380–386, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol 61: 337–362, 1999 [DOI] [PubMed] [Google Scholar]
- 103.Selvakumar B, Huganir RL, and Snyder SH. S-nitrosylation of stargazin regulates surface expression of AMPA-glutamate neurotransmitter receptors. Proc Natl Acad Sci U S A 106: 16440–16445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Selvakumar B, Jenkins MA, Hussain NK, Huganir RL, Traynelis SF, and Snyder SH. S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc Natl Acad Sci U S A 110: 1077–1082, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, and Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sen N. and Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends Neurosci 33: 493–502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shelton MD. and Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells 25: 332–346, 2008 [PMC free article] [PubMed] [Google Scholar]
- 108.Sheppard DN. and Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79: S23–S45, 1999 [DOI] [PubMed] [Google Scholar]
- 109.Shi W, Cui N, Shi Y, Zhang X, Yang Y, and Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol 293: R191–R199, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Shi W, Cui N, Wu Z, Yang Y, Zhang S, Gai H, Zhu D, and Jiang C. Lipopolysaccharides up-regulate Kir6.1/SUR2B channel expression and enhance vascular KATP channel activity via NF-kappaB-dependent signaling. J Biol Chem 285: 3021–3029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi WW, Yang Y, Shi Y, and Jiang C. K(ATP) channel action in vascular tone regulation: from genetics to diseases. Sheng Li Xue Bao 64: 1–13, 2012 [PMC free article] [PubMed] [Google Scholar]
- 112.Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, Jiang C, and Harrison RW. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem 283: 7523–7530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, and Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 293: R1205–R1214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shipston MJ. Ion channel regulation by protein palmitoylation. J Biol Chem 286: 8709–8716, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, and Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci 30: 10763–10772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Squadrito GL. and Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med 25: 392–403, 1998 [DOI] [PubMed] [Google Scholar]
- 117.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Sun J, Xu L, Eu JP, Stamler JS, and Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem 276: 15625–15630, 2001 [DOI] [PubMed] [Google Scholar]
- 119.Sun J, Xu L, Eu JP, Stamler JS, and Meissner G. Nitric oxide, NOC-12, and S-nitrosoglutathione modulate the skeletal muscle calcium release channel/ryanodine receptor by different mechanisms. An allosteric function for O2 in S-nitrosylation of the channel. J Biol Chem 278: 8184–8189, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Sunouchi T, Suzuki K, Nakayama K, and Ishikawa T. Dual effect of nitric oxide on ATP-sensitive K+ channels in rat pancreatic beta cells. Pflugers Arch 456: 573–579, 2008 [DOI] [PubMed] [Google Scholar]
- 121.Tang G, Wu L, Liang W, and Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 68: 1757–1764, 2005 [DOI] [PubMed] [Google Scholar]
- 122.Tang G, Wu L, and Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753–763, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Tang H, Viola HM, Filipovska A, and Hool LC. Ca(v)1.2 calcium channel is glutathionylated during oxidative stress in guinea pig and ischemic human heart. Free Radic Biol Med 51: 1501–1511, 2011 [DOI] [PubMed] [Google Scholar]
- 124.Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, and Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem 284: 436–445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Hutchens S, and Tew KD. Nitrosative stress-induced S-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res 69: 7626–7634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Seok Ko H, Il Lee Y, Dawson VL, Dawson TM, Sen N, and Snyder SH. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun 4: 1626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 129.Wang W, Oliva C, Li G, Holmgren A, Lillig CH, and Kirk KL. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol 125: 127–141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ward NE, Stewart JR, Ioannides CG, and O'Brian CA. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry 39: 10319–10329, 2000 [DOI] [PubMed] [Google Scholar]
- 131.Wei Z, Manevich Y, Al-Mehdi AB, Chatterjee S, and Fisher AB. Ca2+ flux through voltage-gated channels with flow cessation in pulmonary microvascular endothelial cells. Microcirculation 11: 517–526, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Yakushev S, Band M, Tissot van Patot MC, Gassmann M, Avivi A, and Bogdanova A. Cross talk between S-nitrosylation and S-glutathionylation in control of the Na,K-ATPase regulation in hypoxic heart. Am J Physiol Heart Circ Physiol 303: H1332–H1343, 2012 [DOI] [PubMed] [Google Scholar]
- 133.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, and Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Y, Dib-Hajj SD, Zhang J, Zhang Y, Tyrrell L, Estacion M, and Waxman SG. Structural modeling and mutant cycle analysis predict pharmacoresponsiveness of a NaV1.7 mutant channel. Nat Commun 3: 1186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang Y, Estacion M, Dib-Hajj SD, and Waxman SG. Molecular Architecture of a sodium channel S6 Helix: Radial Tuning Of The Voltage-Gated Sodium Channel 1.7 activation gate. J Biol Chem 288: 13741–13747, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang Y, Li S, Konduru AS, Zhang S, Trower TC, Shi W, Cui N, Yu L, Wang Y, Zhu D, and Jiang C. Prolonged exposure to methylglyoxal causes disruption of vascular KATP channel by mRNA instability. Am J Physiol Cell Physiol 303: C1045–C1054, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, and Jiang C. Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem 286: 9298–9307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y, Shi W, Cui N, Wu Z, and Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem 285: 38641–38648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Zhu D, and Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta 1778: 88–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, and Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2: 596–607, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Zha XM, Wang R, Collier DM, Snyder PM, Wemmie JA, and Welsh MJ. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc Natl Acad Sci U S A 106: 3573–3578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Q, Chatterjee S, Wei Z, Liu WD, and Fisher AB. Rac and PI3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxid Redox Signal 10: 679–689, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Zhang Q, Matsuzaki I, Chatterjee S, and Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol 289: L954–L961, 2005 [DOI] [PubMed] [Google Scholar]
- 144.Zhao W, Zhang J, Lu Y, and Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zmijewski JW, Banerjee S, and Abraham E. S-glutathionylation of the Rpn2 regulatory subunit inhibits 26 S proteasomal function. J Biol Chem 284: 22213–22221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]