Abstract
A fundamental controversy is whether cognitive decline with advancing age can be entirely explained by decreased processing speed, or whether specific neural changes can elicit cognitive decline, independent of slowing. These hypotheses are anchored by studies of healthy older individuals where age is presumed the sole influence. Unfortunately, advancing age is also associated with asymptomatic brain white matter injury. We hypothesized that differences in white matter injury extent, manifest by MRI white matter hyperintensities (WMH), mediate differences in visual attentional control in healthy aging, beyond processing speed differences. We tested young and cognitively healthy older adults on search tasks indexing speed and attentional control. Increasing age was associated with generally slowed performance. WMH was also associated with slowed search times independent of processing speed differences. Consistent with evidence attributing reduced network connectivity to WMH, these results conclusively demonstrate that clinically silent white matter injury contributes to slower search performance indicative of compromised cognitive control, independent of generalized slowing of processing speed.
Keywords: Cognitive control, Visual attention, Aging, Cerebrovascular disease, Cognitive neuroscience, Neuroimaging
1. Introduction
Consistent, gradual differences in cognition are commonly found among elderly individuals even in the absence of clinical diseases such as Alzheimer's disease, and are frequently ascribed to “normal” or cognitively healthy aging (Grady & Craik, 2000; Salthouse, 2009). Researchers have attributed cognitive differences with advancing age to multiple, possibly overlapping factors; some argue that global processing speed reductions explain a majority of cognitive impairment, while others attribute aging-related impairments to declines in prefrontal function and brain network connectivity (Greenwood, 2000; Nordahl et al., 2006; Salthouse, 1996; Salthouse, 2000; West, 1996). These speed and disconnection hypotheses are not mutually exclusive, yet they generate specific predictions regarding the effects of brain structural differences in healthy aging on cognition. If declines in processing speed predominantly explain cognitive differences, little to no residual performance differences should remain once generalized slowing is factored out (Salthouse, 1996; Salthouse, 2000). If some cognitive differences remain independent of speed differences, however, other hypotheses related to connection efficacy and degraded information transfer would contribute explanatory power to cognitive differences in healthy aging.
Advancing age also is associated with a variety of other disease processes, including clinically asymptomatic cerebrovascular disease (CVD), that can impact the results of cognitive tasks aimed at understanding specific differences in brain systems affected with age. White matter abnormalities linked to cardiovascular risk factors and CVD, such as white matter hyperintensities (WMH) seen on brain magnetic resonance images (MRI), increase with age and are correlated with declines in processing speed and cognitive control (DeCarli et al., 1995; DeCarli, Fletcher, Ramey, Harvey, & Jagust, 2005a; Gunning-Dixon & Raz, 2000; Mayda, Westphal, Carter, & DeCarli, 2011); previous research has shown that WMH are associated with frontal lobe and executive dysfunction regardless of where in the brain they are located (Tullberg et al., 2004). Recent research in our laboratory suggests at least some cognitive differences in healthy aging result from asymptomatic CVD contributing to altered connection efficacy and degraded information transfer between prefrontal systems and their cortical targets (Mayda et al., 2011; Nordahl et al., 2006). Yet the specific role of CVD-related degradations in network information transfer in terms of neurobiological and cognitive differences in healthy aging has not been fully explored. Previous aging studies showed correlations of WMH with fluid intelligence or neuropsychological measures (van den Heuvel et al., 2006; Rabbitt et al., 2007; Raz, Rodrigue, Kennedy, & Acker, 2007), but used broad-domain tools assessing global cognitive function rather than detailed cognitive neuroscience methods designed to tease apart distinct components of cognition, or utilized qualitative and semi-quantitative WMH measures. Additional studies have examined visual attentional search performance in relation to white matter integrity with diffusion tensor imaging (DTI), and have found that visual search performance was related to white matter tract integrity, but excluded participants with conditions such as diabetes or hypertension that contribute to white matter pathology, limiting generalizability to the full range of age- and CVD-related cognitive differences (Madden et al, 2004; Bennett, Motes, Rao, & Rypma, 2012).
One possible role of CVD in cognitive aging could be that WMH contribute to cognitive impairments by leading to generalized perceptual and motor slowing, affecting multiple cognitive domains. There is evidence suggesting that WMH affect frontal-subcortical systems associated with balance and motor speed (Poggesi et al., 2013). Other evidence, from previous studies of cognitively healthy older adults (OA), finds that the extent of WMH may affect speed and mediate cognitive differences in aging (Andrews-Hanna et al., 2007; Head, Rodrigue, Kennedy, & Raz, 2008; Madden et al, 2004; Nordahl et al., 2006; O'Sullivan et al., 2001; Smith et al., 2011; Sullivan et al., 2001; Ziegler et al., 2010). Another possibility is that WMH also affect cognition through injury to distributed cortical networks necessary for specific cognitive functions, such as cognitive control, independent of generalized slowing. This latter hypothesis is supported when generalized reductions in processing speed cannot address certain findings, such as associations between impaired structural connectivity (e.g. WMH) and improved cognitive performance or increased task-related activations (Cabeza, 2002; Greenwood, 2007; Mayda et al., 2011). This hypothesis is also consistent with findings that impaired prefrontal connectivity impacts cognitive control across several domains, including visual attention and working memory, in a manner separable from age-related processing speed differences (Braver & Barch, 2002; Rush, Barch & Braver, 2006). The use of WMH as a proxy for injury to connectivity within broadly-distributed cognitive systems is supported by previous findings: WMH may detrimentally affect cognition by impairing neural transmission and intraneuronal connectivity (Gunning-Dixon & Raz, 2000), and WMH are associated with impaired activation of prefrontal systems under cognitive demand and altered connection efficacy of prefrontal systems (Mayda et al., 2011; Nordahl et al., 2006). The specific impacts of CVD-related white matter injury on processing speed and cognitive differences in healthy aging have received little study yet are crucial to understanding mechanisms of cognitive decline. Examining the role of CVD in cognitive decline in healthy aging has public health implications; early and aggressive treatment of vascular risk factors may deter brain injury and cognitive decline, and might suggest cognitive benefits of speed-preserving interventions.
Therefore we conducted a study to directly test whether individual differences in white matter injury in cognitively healthy aging, measured by WMH, contribute to differences in cognitive control independent of age-related generalized slowing. We designed a cognitive control paradigm (a visual search task where attentional control is controlled by working memory) emphasizing coordination of distributed frontoparietal control systems with visual cortex (Corbetta & Shulman, 2002; Kastner & Ungerlieder, 2000). Our approach was designed to dissociate two visual search components: a generalized processing speed component reflecting time to conduct basic bottom-up search sensory and motor processing, and a top-down control component that augments basic search strategies.
We hypothesized that, among both young adult (YA) and OA subjects, search slope (the increase in log-normalized reaction time [lnRT] with additional distracters) would increase with task difficulty. In addition, we hypothesized that OA would show higher search intercepts (lnRT in each condition with no distracters present) than YA, representing age-related generalized slowing. We further hypothesized that, among OA, greater extent of WMH would be associated with impaired performance in this cognitive control task beyond generalized slowing (measured by a main effect of WMH volume producing increasingly longer lnRTs, controlling for baseline search intercept), explaining, in part, network disconnection leading to declines in cognitive performance in cognitively healthy older adults.
2. Method
2.1. Participants
Forty cognitively healthy OA and twenty YA were recruited. OA, aged 65-89 years and in stable health, were cognitively healthy controls (free of cognitive impairment or dementia) from the UC Davis (UCD) Alzheimer's Disease Center (ADC) participant pool, who received detailed neuropsychological testing to determine the clinical diagnosis of cognitively healthy or “normal,” as described previously (He et al., 2012). OA participants possessed a range of whole-brain WMH volumes similar to the larger cognitively healthy aging population, enabling examination of the role of white matter injury previously linked to aging and CVD processes in cognition (Carmichael et al., 2010). YA aged 18-30 were recruited from UCD. All participants were right-handed, free of major illness, and not taking medications thought to affect cognition; all had normal or corrected-to-normal visual acuity and color vision, consented to participation, and received compensation. No participants were excluded based on gender, race, or ethnicity. The UCD Institutional Review Board approved the project. Procedures took 60-90 minutes with breaks as necessary. Two OA were excluded who did not complete testing, yielding 38 OA for study. One YA subject was excluded due to task non-compliance, yielding 19 YA.
2.2. Neuropsychological Testing
Participants performed several standard neuropsychological tests reported in Table 1. OA performed more poorly than YA but exhibited no clinically significant cognitive impairment.
Table 1. Summary (Means and Standard Deviations or Frequencies) for Demographics, Neuropsychological Measures, and Brain Measures.
| YA (n = 19) |
OA (n = 38) |
Group Difference |
|
|---|---|---|---|
| Agea | 20.5 (3.26) | 77.5 (5.13) | t(55) = -44.08, p < .001* |
| Years of educationa | 13.5 (1.74) | 14.9 (3.51) | t(55) = -1.69, p = .097 |
| Sex, F/M | 11/8 | 25/13 | χ2(1, N = 57) = 0.08, p = .77 |
| % Minorityb | 68 | 74 | χ2 (1, N = 57) = 0.01, p = .92 |
| Days between MRI and behavioral testing | N/A | 545.9 (401.6) | N/A |
| WMH volume, %TCV | N/A | 0.730 (0.835) | N/A |
| MMSE scorea | 29.6 (0.77) | 27.8 (1.31) | t(55) = 5.34, p < .001* |
| Forward digit spana | 9.1 (1.75) | 7.7 (1.81) | t(55) = 2.66, p = .010* |
| Backward digit spana | 6.4 (1.95) | 5.3 (1.97) | t(55) = 2.00, p = .051 |
| Category fluencya | 24.8 (6.36) | 19.1 (6.71) | t(55) = 3.10, p = .0031* |
Note: TCV = Total cranial volume. WMH = White Matter Hyperintensities.
Denotes significant at p < .05.
Values displayed as M (SD) unless otherwise noted.
Minority defined as subject not identifying as Caucasian Non-Hispanic.
2.3. Cognitive Control Task
Participants performed a computerized cue-guided search task (Carlisle, Arita, Pardo, & Woodman, 2011; Wolfe, Horowitz, Kenner, Hyle, & Vasan, 2004; Woodman, Luck, & Schall, 2007) with 3 conditions requiring varying levels of top-down and bottom-up attentional control (Figure 1), using Presentation® (v14.9, www.neurobs.com). Participants were required to report which of two targets (left- or right-oriented C, each presented in 50% of trials) was present in a set of distracters (up- and down-oriented C's) while reaction time (RT) was measured. Each C was 2.1° in diameter with a 0.41° gap, in an 8.6°-diameter ring. A central fixation cross began each trial (1000 ms), followed by a color cue indicating target color (1000 ms), then the search array (4000 ms). All stimuli were viewed against a black background on a 15.6” screen at a 70 cm distance. There were 3 cue/stimulus colors (red, green, and blue, each presented randomly on 1/3 of trials), and three different conditions (Feature, Mixed, and Identity search).
Figure 1.
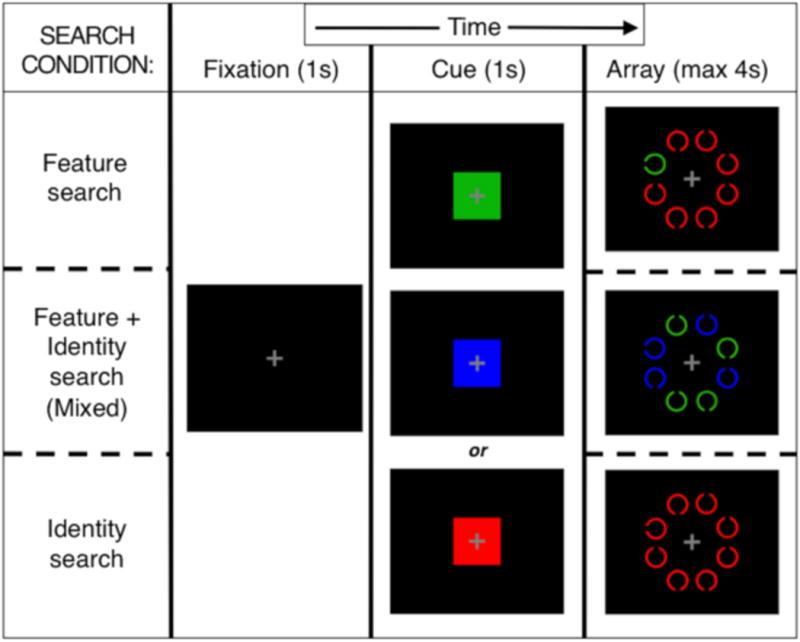
Cue-guided search task conditions: Feature, Mixed (Feature plus Identity), and Identity search. For each condition, an example 8-item array is presented with one possible target-distracter color combination.
2.3.1. Feature search condition
The target appeared in the cued color and all distracters (if present) were in one of the other two colors (randomly determined). Minimal top-down control should be needed, as the target attracted attention automatically and bottom-up signaling primarily drove search (Egeth & Yantis, 1997).
2.3.2. Mixed search condition
Half the items were in the cued color (targets were always in this group) and the other half (all distracters) were in one remaining color. A working memory representation of cued color could be used to eliminate half the items from search. Individuals with poor top-down control will end up searching uncued-color items, leading to slowed RTs (Gold, Fuller, Robinson, Braun, & Luck, 2007).
2.3.3. Identity search condition
The target and all distracters were in the cued color. Target detection relied entirely upon top-down-controlled search processes, since no salient bottom-up features distinguished the target from distracters.
2.3.4. Task difficulty and presentation
Set size was varied (1, 4, and 8 items) so we could separately measure the intercept and slope. The intercept reflects the basic search component, or processes occurring before and after search including sensory processing and motor programming (see Fuller et al., 2006), and the search slope reflects implementation of the cognitive control component, with increasing difficulty (set size and search condition). We predicted search slope would increase with task difficulty, with negligible slope for Feature search, and progressively steeper slopes for Mixed and Identity search. In addition, we predicted OA would show higher intercepts than YA, and amongst OA, higher WMH volumes would lead to increasingly longer RTs and therefore progressively steeper slopes for Mixed and Identity search conditions.
Task presentation was blocked by condition, with set size randomly determined for each trial; each search condition block was divided into 3 mini-blocks to provide rest, with counterbalanced mini-block ordering. Task parameters were piloted to ensure high performance. Participants were instructed to indicate target direction quickly and accurately using a left or right index finger button press. There were 42 trials for each of 9 set size*search condition combinations. Participants practiced the task prior to the experiment; none complained of difficulty performing the task.
For RT analyses, we removed incorrect response trials, excluded RTs under 200 ms as anticipation responses, and log-transformed the remainder, yielding a primary dependent variable termed trimmed correct lnRTs. Slower lnRT thus served as a marker of impaired cognitive function, likely a combination of speeded processes and components of cognitive control (goal maintenance, top-down and bottom-up attentional signaling). Logarithmic transformations convert multiplicative factors into additive factors (Cui, Kerr & Churchill, 2003; Sokal & Rohlf, 1995; van den Berg, Hoefsloot, Westerhuis, Smilde, & van der Werf, 2006); this enabled us to examine the impact of independent variable interactions, which when observed using lnRT as a dependent variable, cannot be explained by generalized slowing. This also normalizes the data. We considered alternative variable normalization, including inverse RT (speed not time), as the outcome measure because of modest heteroscedasticity, but results did not change materially.
2.4. MRI Acquisition and Processing
OA brain MRI data were obtained on a 1.5T GE Signa scanner on separate visits as part of an ongoing study. High-resolution T1-weighted (TR/TE: 9/2.9 ms, slice thickness: 1.5 mm, 128 slices, FOV: 25 × 25 cm, matrix: 256 × 256) and FLAIR (TR/TE/TI = 11000/144/2250 ms, slice thickness: 3 mm, 48 slices, FOV: 22 × 22 cm, matrix: 256 × 192) sequences were acquired.
Total intracranial volume (TCV) and WMH volume were measured from FLAIR images, by operators blind to participant age and gender, using the Quanta package of software routines produced in-house (DeCarli et al., 2005a; DeCarli et al., 1992; DeCarli, Murphy, Teichberg, Campbell, & Sobering, 1996). First, non-brain elements were manually removed from the image by tracing of dura mater within the cranial vault including middle cranial fossa, but excluding posterior fossa and cerebellum. The volume of the traced region was defined as the TCV. Tissues outside the traced cranial vault were removed from the image. To identify brain matter, image intensity nonuniformities were removed, with the corrected image modeled as a mixture of two Gaussian probability functions corresponding to brain and non-brain tissue. The segmentation threshold between brain and non-brain intensities was located at the minimum probability between these distributions (DeCarli et al., 1992; DeCarli et al., 1996), with the volume of voxels on the brain side of the threshold defined as brain volume (BV). Erosion of two exterior image pixels was applied to the BV image to remove the effects of partially-volumed CSF pixels on WMH detection. A single Gaussian distribution was fitted to the intensity distribution of remaining BV voxels; all voxels with intensity greater than 3.5 standard deviations above the mean BV intensity were defined as WMH (DeCarli et al., 2005a). A rigorous protocol ensured validity across analysts (Carmichael et al., 2010). We corrected for head size by expressing WMH volume as a percentage of TCV, and log-transformed these values for normality, to yield log-normalized WMH volumes (lnWMH); lnWMH values were z-transformed for statistical modeling. Representative images of brain WMH, as well as a WMH frequency map for this OA sample, are illustrated in Figure 2. We additionally measured WMH and TCV volumes in a separate sample of 40 cognitively healthy YA (aged 25.3 ± 2.55 years) to demonstrate that YA WMH volumes can be expected to be categorically zero.
Figure 2.
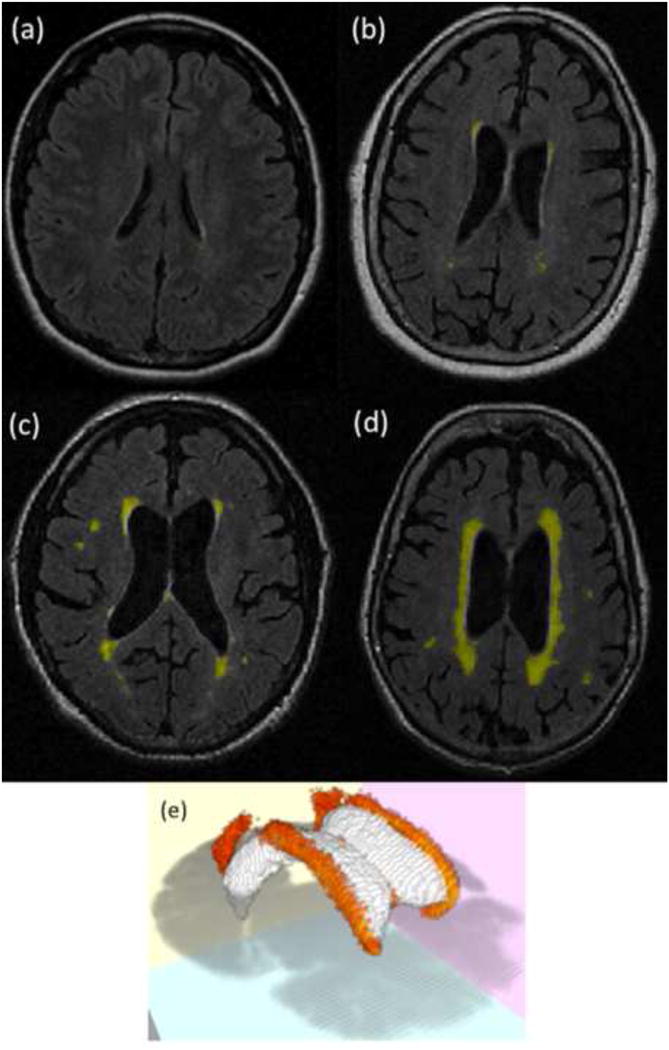
WMH within the brain. These figures illustrate characteristic slices from FLAIR scans of a young adult (a; acquired in a separate study that did not measure task performance), as well as older adult individuals in this study who had low (b), moderate (c), and severe (d) values of WMH volume. In addition, a WMH frequency map (e) thresholded at 20% of subjects demonstrates the location of WMH throughout the white matter of OA subjects in this study (ventricles are presented in white, along with an axial slice, for reference).
2.5. Statistical Modeling
We used multivariate linear mixed-effects (LME) regression to model single-trial trimmed correct lnRTs among OA, and their associations with continuous predictors such as age and WMH volume. LME models (Pinheiro & Bates, 2000) allow for systematic differences, not accounted for by predictors, in intercept and variation in lnRT (subject-specific random effects). LME models also assume individual trial observations might differ from the person's general performance by a quantity drawn independently from a Gaussian distribution with zero mean and constant “noise” variance. LME models effectively assess general trends in cognitive performance data and their modification by predictors, and characterize between- and within-person variation from general trends (Carmichael et al., 2010; Wilson et al., 2002). Our approach allowed missing observations (variable numbers of correct trials) and possible correlation between the reference level of cognitive measurement for an individual and variation with predictors. LME models were fitted using nlme routines in R 3.1 (Ihaka & Gentleman, 1996). Model assumptions were validated graphically and by examining alternative models to investigate violations. For OA, we centered education at 12 years and age at 65 years. Sex and education variables represented the influence of early childhood experiences and genetics on performance.
We developed four LME search task contrasts of interest, representing hypothesized tests for differences in search performance, to assess how different search task attributes contributed to trial lnRT. Contrasts were designed to treat Feature search condition and set size 1 as reference levels, controlling for subjects' own baseline processing speed and allowing comparison of different effects of predictors on basic cognitive processing speed and more complex cognitive control. We defined processing speed this way to control for those stages of task performance occurring before and after search, including sensory processing, response selection, and motor programming, which are the basic perceptual-motor processes necessary for responding with a button press to a visual target in isolation. In extant literature this is often referred to as a “choice reaction time” measure of generalized processing speed (Salthouse, 2000), utilizing simple manual keypress responses to basic visual stimuli, to combine perceptual speed, response selection, and psychomotor speed into a measure of processing speed. Using performance on the Feature search conditions and set size 1 from the same task, as a measure of generalized slowing of processing speed, additionally controls for differences in visual stimuli and response mapping that might occur if a separate task were used.
The contrast set level tested for linear trends with increasing trial set size (1, 4, 8). Set nonlinearity tested for nonlinear performance differences with increasing set size. A Top-down control contrast assessed whether subjects were slowed in more difficult conditions (Mixed and Identity) requiring additional top-down attentional control relative to the reference Feature search condition (which was more reliant on bottom-up signaling). Selective vs. nonselective directly contrasted performance on the Mixed and Identity search conditions.
To estimate relations between age and search performance among OA, we constructed an LME model to predict single-trial trimmed correct lnRTs, with random effects of participant, and fixed effects of age and the 4 search task contrasts. We additionally modeled all 2-way age*contrast interactions. To test whether WMH and other variables predicted OA search performance, we constructed an LME model to predict single-trial trimmed correct lnRTs, with random effects of participant and fixed effects of the 4 search task contrasts, plus effects of WMH volume, sex, education, and days between testing and brain MRI. We also tested specific set level*WMH, top-down control*WMH, and set level*top-down control interactions, aiming to understand effects of WMH not only on basic search speed but also on performance as difficulty and top-down control increased (we also examined 3-way interactions, but results were similar). As mentioned, any effects or interactions with WMH on trial lnRT in this model cannot be explained by generalized slowing. Our model specifically tested the effect of WMH on modifying performance in difficult task scenarios with high cognitive control demand (Mixed and Identity search, larger set sizes) relative to an easier reference (Feature search; set size 1). To assess the contribution of age in the WMH-search relationship, we constructed an LME model identical to the previous one, adding age.
We identified a number of potential confounding variables representing search task parameters that could relate to aspects of task performance, such as stimulus color and fatigue, which did not pertain to our hypotheses. Such nuisance variables included target direction, target color with green as reference, target side of screen, and trial order within each block and across the experiment. Nuisance variables were added stepwise to models and retained if p < .1. Our aim was to control for the contribution of such nuisance variables to performance, in order to be more confident that any additional results were not due to factors such as stimulus differences or fatigue.
We used univariate linear regression models, t-tests, and ANOVA as necessary to test whether interrelations between age and other variables might influence our analyses, therefore requiring inclusion of age in full models. We also constructed mixed ANOVAs examining effects of predictors like age group or search condition on dependent variables including search intercept, slope, and mean RT at each set size*search condition level. These analyses were done to allow comparison with previous attention studies, but lack sensitivity compared with LME models regarding trial-to-trial performance variability, as data are averaged. We used log-transformed slope as this converts multiplicative into additive effects.
3. Results
3.1. Participant Characteristics
OA were older and marginally more educated than YA participants, with significantly lower scores on most neuropsychological measures (Table 1). OA also had a range of brain WMH consistent with previously described non-demented community based populations (DeCarli et al., 2005b), illustrated in Figure 2. As YA in this study did not undergo MRI and have no WMH volumes for analysis, we performed WMH quantification on a separate sample of 40 cognitively healthy young adults for whom identical structural imaging sequences were available. In this separate sample there was no significant relation between age and WMH volume (R2 = .04; p = .2), and all YA WMH volumes fell within or below the lowest decile of OA WMH volumes; the WMH burden of YA performing this task was therefore assumed to be near zero.
3.2. Speed-accuracy Tradeoff
Search task accuracy was high, at 99% for YA and 96% for OA, averaged across conditions and set sizes. Mean accuracy for each search condition*set size combination was >90% for YA and OA; the lowest accuracy was 92% for OA for Identity search set size 8. For each search condition*set size*participant combination, we constructed linear regression models for YA and OA to assess relations between mean trimmed correct lnRT and errors. There was no association between lnRT and errors in YA (p = .51). In OA, longer lnRT was associated with significantly more errors (R2 = .31; p < .001); as lnRT and error rates were positively correlated, we conclude our results were likely not confounded by speed-accuracy trade-off.
3.3. Age Differences in Search Performance
Visual search performance data (lnRTs as proxy of cognitive performance) are presented in Figure 3. We constructed a 3-way ANOVA examining effects of age group (Young; Old), set size (1; 4; 8 items) and search condition (Feature; Mixed; Identity) on mean trimmed correct lnRT for each search condition*set size*participant combination. As expected from previous results (Madden, 2007; Salthouse, 2009), advancing age was associated with prolonged lnRT: there was a main effect of age (p < .001) related to prolonged response time across all conditions. In addition, increasing task difficulty (assessed by set size or search condition) was associated with increasing lnRT (condition, set size, and condition*set size p's < .001). However, there was no effect modification of age on task difficulty (condition or set size) as seen by the parallel lines in Figure 3 for YA and OA, suggesting age was associated with a fixed increase of response time independent of task difficulty. These results were similar when untransformed data were used (data not shown).
Figure 3.
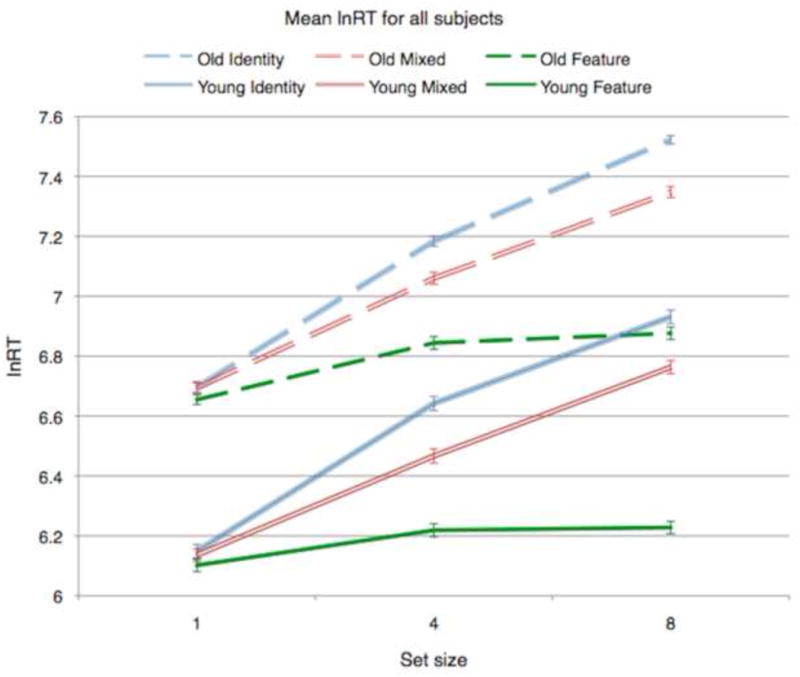
Mean lnRT values, for each search condition and set size, for young (solid lines) and older adults (dashed lines).
Similarly, in a 2-way ANOVA examining effects of age group and search condition on log(slope), there was a significant search condition main effect (F(2, 162) = 141.3, p < .001) but no group or group*condition effects, and in a 2-way ANOVA examining effects of age group and search condition on intercept, only the group main effect was significant (p < .05), again suggesting age was associated with a fixed RT increase, or generalized slowing, independent of task difficulty.
3.4. Univariate Associations of Age with WMH and Search Performance within OA
Among OA, WMH tended to increase with age, but this was not significant (p = .089; Figure 4). Furthermore, age in OA was not significantly associated in univariate models with other measures that may influence performance, such as education (regression p = .56) and sex (t-test p = .098). Age was also not associated with number of correct trials for different nuisance variables (experimental factors such as target color ANOVA p = .99; target direction t-test p = .92; target side of screen t-test p = .99; experiment trial order regression p = .75; block trial order regression p = .98).
Figure 4.
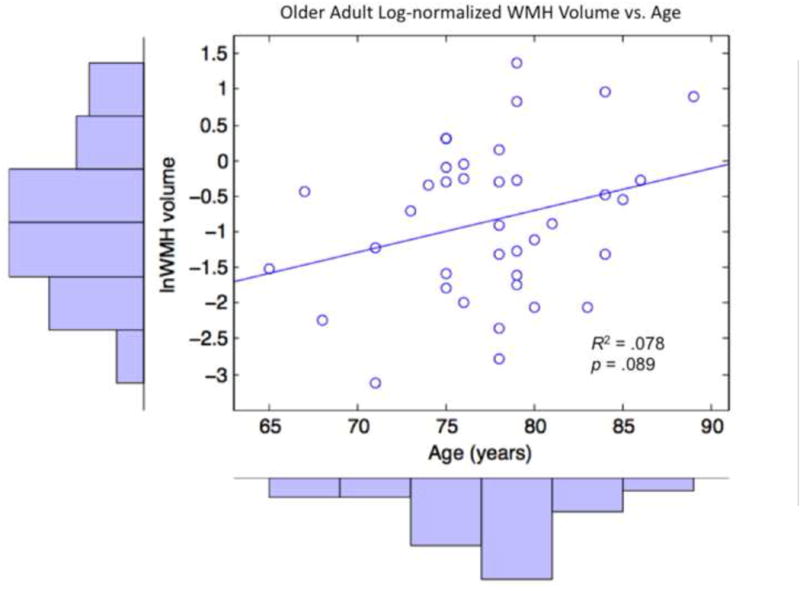
Older adult log-normalized WMH volumes plotted against age, with corresponding histograms and line of best fit.
In an LME model (Table 2), age was not significantly associated with search performance among OA (p = .15), although there was a trend toward longer RTs with advancing age. Having demonstrated that age did not show statistically significant associations with any of our predictor variables in a mediation analysis, we analyzed the relation between WMH and search performance in OA without adjusting for age.
Table 2. Age and Search Task Performance in Older Adults.
| Predictor | Effect of Predictor on lnRT | |||
|---|---|---|---|---|
| β | SE | DF | p | |
| Age | 0.0098 | 0.0067 | 36 | .15 |
| Set Level | 0.3635 | 0.0537 | 13755 | < .001 |
| Set Nonlinearity | -0.0675 | 0.0310 | 13755 | .029 |
| Top-down control | 0.1930 | 0.0927 | 13755 | .037 |
| Selective vs. nonselective | -0.1157 | 0.0538 | 13755 | .032 |
| Age * Set Level | -0.0011 | 0.0007 | 13755 | .12 |
| Age * Set Nonlinearity | 0.0006 | 0.0004 | 13755 | .144 |
| Age * Top-down control | 0.0013 | 0.0012 | 13755 | .29 |
| Age * Selective vs. nonselective | 0.0021 | 0.0007 | 13755 | .002 |
Note: lnRT = log-normalized reaction time on search task. SE = Standard error. DF = Degrees of freedom. Bold denotes significant at p < .05.
3.5. Associations between WMH and Search Performance
In an LME model, increased WMH volume was significantly associated with longer lnRT among OA (p = .02; Table 3), suggesting impaired cognitive performance associated with greater WMH burden. Target color and direction also significantly affected performance, and there were significant effects of the 4 search contrasts and the WMH*top-down control and WMH*set level interactions. The WMH effect, however, remained significant (p = .04) after covarying for age, as did all other significant effects and interactions (data not shown), suggesting that age does not play a strong role in modifying the effect of WMH on cognitive performance among OA. The main effect of WMH, and the interactions of WMH with the search contrasts—particularly the WMH*set level interaction, which demonstrates an increasing search slope with greater WMH volume—suggest that WMH have an effect on the cognitive control component of search independent of generalized slowing.
Table 3. WMH and Search Task Performance in Older Adults.
| Predictor | Effect of Predictor on lnRT | |||
|---|---|---|---|---|
| β | SE | DF | p | |
| Set Level | 0.1071 | 0.0057 | 13750 | < .001 |
| WMH Volume | 0.0826 | 0.0334 | 33 | .019 |
| Set Nonlinearity | -0.0217 | 0.0019 | 13750 | < .001 |
| Top-down control | 0.0311 | 0.0090 | 13750 | < .001 |
| Selective vs. nonselective | 0.0490 | 0.0033 | 13750 | < .001 |
| Days between test and scan | -0.0001 | 0.0001 | 33 | .20 |
| Sex | 0.0001 | 0.0699 | 33 | .99 |
| Education | -0.0221 | 0.0098 | 33 | .032 |
| Set Level * WMH | 0.0082 | 0.0034 | 13750 | .015 |
| WMH * Top-down control | -0.0123 | 0.0058 | 13750 | .034 |
| Set Level * Top-down control | 0.2616 | 0.0070 | 13750 | < .001 |
Note: lnRT = log-normalized reaction time on search task. SE = Standard error. DF = Degrees of freedom. WMH = White Matter Hyperintensities (corrected by total cranial volume, log-normalized and z-transformed). All effects are controlling for significant nuisance variables (DF = 13750 for each) including target color (green faster than blue [p < .001] and red [p < .001]), target direction (right-facing faster, p < .001), target side of screen (right-sided faster, p = .0008), and target order within each block and across the experiment (both p < .001, with later trials faster). Bold denotes significant at p < .05.
Figure 5 illustrates how varying the value of WMH volume modifies search performance (lnRT). Each graph represents model-predicted performance for an average female OA participant. The three lines per plot represent estimated trajectories of an average female OA with differing levels of log-normalized WMH volume: the sample mean (0.43% of head size), the mean plus one standard deviation (1.3%) and the mean minus one standard deviation (0.15%). This figure illustrates the significant main effect of WMH volume, as well as the significant WMH*set level interaction effect (progressively higher slopes in the blue, red, and green lines).
Figure 5.
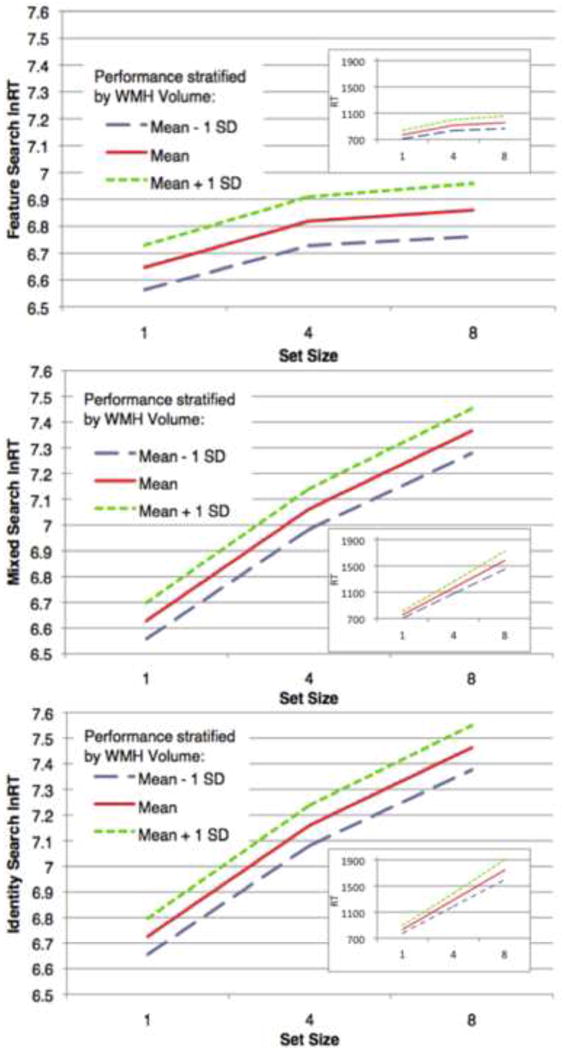
These graphs illustrate how varying WMH volume modifies search performance amongst older adults. The graphs represent predicted performance, on the Feature, Mixed, and Identity search conditions for each set size, for participants whose log normalized WMH volume takes on the mean value and the mean +/- one standard deviation. Each line represents model-predicted performance for a female with mean education (14.9 years) and average length between testing and MRI (545.9 days), for red targets pointing right on the right side of the screen, for the middle trial of the experiment (overall trial 190, block trial 22). The three lines per graph represent the estimated trajectories of an individual that exhibits these characteristics along with differing levels of log-normalized WMH volume: the sample mean (-0.85, or 0.428% head size), the mean plus one standard deviation (1.25%) and the mean minus one standard deviation (0.146%). Larger graphs represent lnRT values (those tested in statistical analyses); insets represent untransformed RT (in ms) for visual comparison.
4. Discussion
The goal of this study was to investigate the contributions of clinically silent small vessel CVD to declines in processing speed and visual attentional control in cognitively healthy aging. We hypothesized that white matter injury indexed by WMH volume, known from previous research to increase with age and CVD, would result in impaired attentional control, measured as slowed search performance beyond the effect of cumulative generalized slowing (i.e., slowed lnRT controlling for baseline performance, distinct from age-related speed shifts). The present results suggest that increased WMH is associated with reduced search performance in older adults, and that WMH exert effects on controlled search performance not explained by simple, generalized perceptual-motor slowing. Critically, we referenced participant performance data to their own baselines to control for differences in response speed and log-transformed our cognitive dependent variable; as logarithmic transformations convert multiplicative factors into additive factors, this allowed us examine the impact of WMH effects and interactions independent of effects of generalized slowing.
We replicated the large age differences found in previous studies of visual search performance (Madden, 2007; Salthouse, 2009), and also demonstrated a significant association between WMH and performance among cognitively normal elders beyond the effect of age. These results suggest to us that WMH may exert effects more on complex search behavior and attentional control than on the more simple response speed. The significant interactions of WMH with the search contrasts suggest that the contribution of WMH to search becomes more apparent with increasing difficulty, and WMH strongly affect task performance at levels of higher search difficulty (4 and 8 items in particular), consistent with theories attributing cognitive decline with advancing age to impaired connectivity of a frontally-mediated cognitive control network (Braver & Barch, 2002), described below. However, as noted below, WMH may somewhat affect task performance even when minimal top-down and bottom-up attentional signaling are required.
It is plausible that white matter injury could result in reduced cognitive network efficiency: cognitive neural networks are widely distributed in the brain, and axons are a requisite component of intraneuronal connections. Altered connection efficacy within distributed brain networks, caused by white matter deterioration in healthy aging, is hypothesized to lead to cognitive changes in healthy aging (O'Sullivan et al., 2001; Sullivan et al., 2001). These results provide theoretical support for the idea that white matter tract alterations in cognitively healthy aging may degrade network information transmission leading to cognitive dysfunction. Therefore, altered information flow through visual attention networks due to WMH might degrade information transfer required by higher cognitive processes, such as working memory and top-down and bottom-up attentional signaling, and result in visual search impairment which likely places greater demand on these widely distributed systems. To determine whether differences in white matter injury affect information flow into the frontal processing stream, and how this is mediated by decreased processing speed, future studies should assess the impact processing speed differences may have on cognitive network functional connectivity using fMRI.
Within healthy elders, whole-brain WMH volume was associated with impaired search performance, particularly as difficulty increased. These results are inconsistent with a model that increased white matter injury in cognitively healthy aging leads simply to a generalized slowing as the cause for associated reductions in search performance; rather, they appear consistent with a model where WMH also affect another cognitive control component through degrading network connectivity (or efficacy), including those mechanisms engaged at higher levels of task difficulty.
The significant positive WMH*set level interaction suggests that OA with clinically asymptomatic white matter injury expressed increasingly greater difficulty with an increasing number of distracters. This greater susceptibility to distracters, particularly when distracters were of a cued, salient color (in the Mixed and Identity conditions) as suggested by the significant set level*top-down control interaction, is also consistent with theories of reduced inhibitory control in aging (Chao & Knight, 1997), and is inconsistent with a model that would posit WMH as being solely associated with generalized speed reductions in older adults. The significant negative WMH*top-down control interaction suggests that while both WMH and the harder conditions (Mixed and Identity) are associated with increased response time, the effect of WMH on the harder conditions relative to Feature search is slightly less for each additional unit of WMH. However, the interaction effect size is small, such that at all biologically plausible values of WMH the effect of both WMH and the harder conditions is to increase OA subject response time. The negative interaction term appears largely due to the substantial effect that WMH volume already has on the easiest (Feature) condition, where WMH already strongly impairs performance, and increased WMH is associated with increased response time. In harder conditions, subjects with high WMH volume already start slower (i.e., their baseline is already higher) and WMH increase does not produce the same magnitude of slowing relative to baseline. An overall slowing of response time in the harder conditions with increased WMH is still present, but slowing due to increasing WMH volume is not as dramatic as slowing associated with WMH in the easier condition. Visually, this can be appreciated by the spread between the green and red lines in Figure 5, which is larger in Feature than Mixed and Identity search. Nonetheless, WMH in cognitively healthy aging clearly have an effect on search lnRT, these effects are modulated by load (particularly by set size), and they are separable from age-related generalized slowing, suggestive of a link between WMH volume and impaired cognitive control.
In addition, we found significant effects of several nuisance variables, such as target color, target direction and location, and trial order, which unsurprisingly indicate that task parameters had measurable effects on OA performance. For example, subjects were fastest when the target was green, faced right on the right side, and appeared later in the experiment or block. This last finding in particular suggests that fatigue did not impair OA task performance. Given that our objective was to observe a significant WMH main effect even when the model included these nuisance variables, in addition to the high statistical power for finding such effects significant (due to high degrees of freedom), we do not heavily weight these nuisance variable results in our interpretation. We plan to remove green cues and targets from future task versions to avoid a performance bias.
Our results agree with previous findings that impaired prefrontal connectivity impacts cognitive control across domains of working memory and attention, and that cognitive control declines are distinct from age-related processing speed differences. In the context processing theory of cognitive aging (Braver & Barch, 2002; Rush et al., 2006), normal cognitive impairments in aging may be due to declines in dorsolateral prefrontal cortex (DLPFC) function and connectivity. Braver and colleagues argue that a healthy DLPFC and intact connections are important for successful cognitive control, and diverse cognitive functions impose a heavy load on control. Specifically, attention and working memory tax internal representation, maintenance, and updating of context information—such as goal representations that bias processing in task- relevant neural networks—to control cognition. In our study, particularly in Mixed and Identity conditions, successful maintenance and use of goal representations (color and target identity) are critical to successful performance; impaired goal maintenance and updating associated with WMH likely drives poorer search in aging. Our findings support the context processing theory and more general frontal lobe hypotheses attributing aging-related impairments to declines in prefrontal function and connectivity (West, 1996). White matter injury may affect cognition through altered connection efficacy and degraded information transfer of distributed, frontally anchored cortical networks essential to context processing and top-down attentional control, independent of generalized slowing.
Some results in the literature report significant effects of age on more difficult search conditions, for example, on conjunction relative to feature search (Bennett et al., 2012; Madden et al., 2002), which initially appear discordant with the current study; however, there are several possible explanations for such discrepancies. For example, in the current study we used exclusively target-present trials; some studies include both target-present and target-absent trials, and older adults may be less efficient in target-absent trials (Madden et al., 2007). Furthermore, as discussed above, few studies separately explore the contributions of aging and white matter injury to visual search; many studies either exclude subjects with vascular risks or do not measure WMH, which may also affect results. The range of WMH volumes in this study may have also been higher than in other studies, and differences in effects across WMH burden levels are not well understood.
One of the strengths of this study is the broad range of OA WMH volumes enabling generalizability to the cognitively healthy OA population. Additionally, the various task difficulty levels tested increase confidence that effects of WMH volume on search performance in aging, independent of generalized slowing, represent real differences. However, the absence of YA WMH data, and a lack of an independent processing speed measure, may constrain interpretability of our results; future efforts should include YA MRI scans and independent processing speed tasks. Future work should also include a middle-aged group (e.g. 50-64 years old); indeed, middle-aged adults may be the most important group of all to study in this regard since they are likely the optimal target for interventions that prevent or slow WMH accrual.
Relatively low subject numbers and limited age variance in the OA sample may also confound our results. The small OA sample size (n = 38), and the number of individuals per decade of life, are relatively small compared to other studies of WMH and cognition in aging populations, such as Raz et al. (2007) which assessed OA ranging from 45-77, or compared to studies explicitly designed to measure the effects of age on cognitive performance, such as Bucur & Madden (2010) and Grady et al. (2006) which each assessed 3 groups including middle-aged and older adults spanning 41-79 and 40-87 years of age, respectively. Our sample is especially small compared with those arising from epidemiological cohorts (e.g. Wilson et al., 2002, which examined 694 OA aged 65-96). This reduced age variance may have impaired our ability to fully examine associations between age, WMH volume, and cognition, in particular our ability to measure the effect of age on cognitive performance. However, the current experiment was not designed from the outset to measure the effect of age on cognitive performance or to test effects across a wide age range; rather, it was to test cognitive performance across a WMH volume range that is characteristic of what is found in cognitively healthy elders. In addition, for studies that use novel cognitive neuroscience paradigms to acquire fine-grained performance measures in specific domains, our sample size and age range are fairly typical (e.g. Head et al., 2008; Ziegler et al., 2010), and while participants only sparsely sampled the 65-89 age range (particularly the lower and upper ends of this age range), they do represent a distribution of WMH volumes highly similar to those observed in prior epidemiological samples (DeCarli et al., 2005b), supporting the generalizability of the findings.
In conclusion, increased age is associated with impaired visual attention measured by slowed search performance. Amongst elders, WMH volume is associated with impaired visual attentional control independent of generalized slowing, particularly as search difficulty increases, exacerbating age-related differences. These data support a model whereby WMH exert specific effects on brain network connectivity and cognition measured by visual search performance in healthy aging, suggesting at least some performance on this task commonly ascribed to advancing age is the consequence of clinically silent vascular brain injury.
Highlights.
We examine differences in visual search performance among younger and older adults.
Aging is associated with a fixed decrease in processing speed.
Among elders, WMHs are associated with search independent of generalized slowing.
Control differences in aging are associated with vascular-related network degradation.
Acknowledgments
This work was supported by NIH Grants R01AG021028 (CD), P30AG010129 (CD), and K01AG030514 (OC). The authors would like to thank the older and younger adult subjects for their participation, and Elizabeth Disbrow for helpful comments and support on earlier drafts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Samuel N. Lockhart, Email: snlockhart@ucdavis.edu.
Alexandra E. Roach, Email: aroach@ucdavis.edu.
Steven J. Luck, Email: sjluck@ucda.edu.
Joy Geng, Email: jgeng@ucdavis.edu.
Laurel Beckett, Email: labeckett@phs.ucdavis.edu.
Owen Carmichael, Email: ocarmichael@ucdavis.edu.
Charles DeCarli, Email: charles.decarli@ucdmc.ucdavis.edu.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Motes MA, Rao NK, Rypma B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiology of Aging. 2012;33:433.e421–433.e431. doi: 10.1016/j.neurobiolaging.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 2002;26(7):809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ. Effects of adult age and blood pressure on executive function and speed of processing. Experimental aging research. 2010;36(2):153–168. doi: 10.1080/03610731003613482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carlisle NB, Arita JT, Pardo D, Woodman GF. Attentional templates in visual working memory. Journal of Neuroscience. 2011;31:9315–9322. doi: 10.1523/JNEUROSCI.1097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski Farias S, Reed BR, DeCarli C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiology of Aging. 2010;33(1):83–95. doi: 10.1016/j.neurobiolaging.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cerebral Cortex. 1997;7(1):63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cui X, Kerr MK, Churchill GA. Transformations for cDNA microarray data. Statistical Applications in Genetics and Molecular Biology. 2003;2(1) doi: 10.2202/1544-6115.1009. art. 4. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005a;36(1):50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. Journal of Computer Assisted Tomography. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Wolf P. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiology of aging. 2005b;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. Journal of Magnetic Resonance Imaging. 1996;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, Rapoport SI. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annual Review of Psychology. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. Journal of Abnormal Psychology. 2006;115(2):266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophrenia Research. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Current opinion in neurobiology. 2000;10(2):224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of cognitive neuroscience. 2006;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society. 2000;6(6):705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21(6):657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- He J, Carmichael O, Fletcher E, Singh B, Iosif A, Martinez O, DeCarli C. Influence of functional connectivity and structural MRI measures on episodic memory. Neurobiology of Aging. 2012 doi: 10.1016/j.neurobiolaging.2011.12.029. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22(4):491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. Journal of computational and graphical statistics. 1996;5(3):299–314. [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual review of neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Aging and Visual Attention. Current directions in psychological science. 2007;16(2):70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychology and Aging. 2002;17(1):24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21(3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mayda AB, Westphal A, Carter CS, DeCarli C. Late life cognitive control deficits are accentuated by white matter disease burden. Brain. 2011;134(6):1673–1683. doi: 10.1093/brain/awr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of cognitive neuroscience. 2006;18(3):418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-Effects Models in S and S-Plus. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- Poggesi A, Gouw A, Flier W, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, Langhorne P, O'Brien JT, Visser MC, Wahlund LO, Waldemar G, Wallin A, Scheltens P, Inzitari D, Pantoni L. Cerebral white matter changes are associated with abnormalities on neurological examination in non-disabled elderly: the LADIS study. Journal of Neurology. 2012;260(4):1014–1021. doi: 10.1007/s00415-012-6748-3. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Lunn M, Thacker N, Lowe C, Pendleton N, Horan M, Jackson A. White matter lesions account for all age-related declines in speed but not in intelligence. Neuropsychology. 2007;21:363–370. doi: 10.1037/0894-4105.21.3.363. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Rush B, Barch D, Braver T. Accounting for cognitive aging: context processing, inhibition or processing speed? Aging, Neuropsychology, and Cognition. 2006;13(3):588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biological psychology. 2000;54(1-3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Salat DH, Jeng J, McCreary CR, Fischl B, Schmahmann JD, Greenberg SM. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492–1499. doi: 10.1212/WNL.0b013e318217e7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry, the principles and practice of statistics in biological research. 3rd. W. H. Freeman; 1995. [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas DM, Reed BR, Harvey DJ, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC genomics. 2006;7:142–156. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, Olofsen H, Bollen EL, Jolles J, Murray HM, Blauw GJ, Westendorp RG, van Buchem MA. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS, Kenner N, Hyle M, Vasan N. How fast can you change your mind? The speed of top-down guidance in visual search. Vision Research. 2004;44:1411–1426. doi: 10.1016/j.visres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ, Schall JD. The role of working memory representations in the control of attention. Cerebral Cortex. 2007;17:i118–i124. doi: 10.1093/cercor/bhm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiology of aging. 2010;31(11):1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


