Abstract
Background
Patients with BD suffer from multifaceted symptoms, including hyperactive and psychomotor agitated behaviors. Previously, we quantified hyperactivity, increased exploration, and straighter movements of patients with BD mania in the human Behavioral Pattern Monitor (BPM). A similar BPM profile is observed in mice that are hyperdopaminergic due to reduced dopamine transporter (DAT) functioning. We hypothesized that dopamine depletion through alpha-methyl-p-tyrosine (AMPT) administration would attenuate this mania-like profile.
Methods
Male and female DAT wild-type (WT; n=26) and knockdown (KD; n=28) mice on a C57BL/6 background were repeatedly tested in the BPM to assess profile robustness and stability. The optimal AMPT dose was identified by treating male C57BL/6 mice (n=39) with vehicle or AMPT (10, 30, or 100 mg/kg) at 24, 20, and 4 h prior to testing in the BPM. Then, male and female DAT WT (n=40) and KD (n=37) mice were tested in the BPM after vehicle or AMPT (30 mg/kg) treatment.
Results
Compared to WT littermates, KD mice exhibited increased activity, exploration, straighter movement, and disorganized behavior. AMPT-treatment reduced hyperactivity and increased path organization, but potentiated specific exploration in KD mice without affecting WT mice.
Limitations
AMPT is not specific to dopamine and also depletes norepinephrine.
Conclusions
KD mice exhibit abnormal exploration in the BPM similar to patients with BD mania. AMPT-induced dopamine depletion attenuated some, but potentiated other, aspects of this mania-like profile in mice. Future studies should extend these findings into other aspects of mania to determine the suitability of AMPT as a treatment for BD mania.
Keywords: AMPT, Bipolar Disorder, mice, mania, dopamine transporter
INTRODUCTION
Dysregulated dopamine neurotransmission is thought to contribute to several psychiatric disorders including bipolar disorder (BD) (Manji et al., 2003; Vawter et al., 2000). Polymorphisms in the dopamine transporter (DAT) gene have been associated with BD (Greenwood et al., 2006; Pinsonneault et al., 2011). These polymorphisms likely result in reduced cell surface expression and hence function of the DAT in patients (Horschitz et al., 2005). Indeed, reduced striatal levels of DAT have been observed in unmedicated patients with BD by using positron emission tomography (Anand et al., 2011) as well as in postmortem tissue (Rao et al., 2012). Hyperdopaminergia caused by reduced DAT function may therefore underlie many of the behavioral abnormalities observed in patients with BD.
Previously, we reported that patients with BD mania are hyperactive, exhibit increased object interactions, and walk in straight paths as quantified by the human Behavioral Pattern Monitor (BPM) (Minassian et al., 2011; Perry et al., 2009). This increased motor and exploratory activity is a cardinal feature of a manic episode and is described in the DSM-IV as an “increase in goal-directed activity” or “psychomotor agitation” (APA, 1994; Perry et al., 2010). Using a cross-species translational approach, we observed consistent behavioral patterns of mice with reduced functional DATs via either pharmacological or genetic manipulations (Perry et al., 2009; Ralph-Williams et al., 2003; Young et al., 2010a, b). Specifically, mice treated acutely with the DAT inhibitor GBR12909 or constitutive DAT knockdown (KD) mice exhibit hyperactivity, increased specific exploration, and straighter paths of movement compared to controls in the mouse BPM. The hyperactivity of each model was attenuated after chronic treatment with the mood-stabilizer valproate (van Enkhuizen et al., 2013a). Moreover, we observed that the mania-like behavior of these mice is not limited to altered motor and exploratory activity since these animals also exhibited increased risk preference in a gambling task (van Enkhuizen et al., 2013b; Young et al., 2011b).
Hence, DAT KD mice have proven to be a useful model for BD mania. The original DAT KD mice were on a mixed 129/S background however, and their phenotype was less stable than that produced by acute administration of GBR12909 to C57BL/6 mice (Young et al., 2010b). Despite the fact that one technique used pharmacological and the other genetic manipulations, we hypothesized that the discrepancy in stability was likely as a result of background strain (Young et al., 2010b). Indeed, the 129/S line of DAT wild-type (WT) and KD mice exhibited lower levels of activity than C57BL/6 mice as expected from these lines (Paulus et al., 1999). To better compare stability of the genetic model to that presented in the C57BL/6 pharmacological model, we have assessed the phenotypic stability of DAT KD mice on a C57BL/6 background.
Better models for BD are required in order to develop treatments targeted at the underlying mechanisms, as opposed to the serendipitous discovery of treatments as has occurred until now. For instance, aberrant motor and exploratory behavior are still observed in patients with BD, despite being on medication for 3 weeks (Minassian et al., 2011). Moreover, euthymic BD patients also still exhibit a hyperexploratory profile (Henry et al., 2013), poor risk learning (Adida et al., 2011), and poor cognitive function, particularly memory difficulties and impaired executive function (Martinez-Aran et al., 2004a; Martinez-Aran et al., 2004b) compared with healthy subjects. Given the reduced DAT levels and hyperdopaminergic state of people with BD, reducing dopamine availability may theoretically normalize their neurochemical state and perhaps their behavior. Dopamine depletion can be induced by administration of alpha-methyl-p-tyrosine (AMPT). AMPT is a competitive inhibitor of tyrosine hydroxylase, the rate limiting enzyme in the synthesis of catecholamines from tyrosine (Booij et al., 2003; Engelman et al., 1968). Supporting this idea, pretreatment with AMPT blocked chlordiazepoxide/(+)-amphetamine-induced hyperactivity in mice without affecting activity of control mice (Davies et al., 1974). Moreover, AMPT treatment can reduce symptoms in patients with BD mania, while an increase in depression was observed in depressed patients treated with AMPT (Bunney et al., 1971). More recently, dopamine depletion with AMPT did not affect mood in patients with BD during treatment, but patients experienced a relapse of hypomanic symptoms post-depletion (Anand et al., 1999).
To assess whether dopamine depletion could rescue some of the cross-species quantified behavioral deficits relevant to BD mania, we examined the effects of AMPT in the DAT KD mouse model for BD mania, which have approximately 10% expression of the DAT and exhibit increased extracellular dopamine compared to control mice (Zhuang et al., 2001). We hypothesized that: (1) DAT KD mice on a C57BL/6 background would exhibit a BD mania-like profile in the BPM consistent with DAT KD mice on a 129/S background; (2) repeated testing of these mice would demonstrate a robust and stable phenotypic profile; and (3) catecholamine depletion by AMPT treatment would attenuate this mania-like behavioral profile.
METHODS
Animals
Male C57BL/6J mice (n=39), DAT KD (male, n=37; female, n=28), and DAT WT (male, n=32; female, n=34) littermate mice were used throughout the three studies. DAT heterozygous breeders backcrossed onto a C57BL/6 background for more than 10 generations were sent to our laboratory from the University of Chicago. Male and female DAT KD and WT mice were generated from heterozygous breeding pairs. All mice were group housed (four/cage) and maintained in a temperature-controlled vivarium (21±1°C) on a reversed day-night cycle (lights on at 7.00 PM, off at 7.00 AM). Mice were 3-6 months old at the time of testing, weighed between 20-40 g, and were tested during the dark phase between 8.00 AM and 5.00 PM. Mice had ad libitum access to water and food (Harlan, Madison, WI, USA). All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Drug treatment
Alpha-methyl-p-tyrosine methyl ester hydrochloride (AMPT) (Sigma-Aldrich, St Louis, MO, USA) was dissolved in saline (10 ml/kg). AMPT or saline was administered to mice in three equal i.p. injections 24, 20, and 4 h prior to testing (Davies et al., 1974). Previous studies have shown that mouse brain dopamine and norepinephrine levels are reduced ~30–40% 4 h after administration of 40–80 mg/kg (i.p.) AMPT (Dobrzanski and Doggett, 1976; Flexner and Goodman, 1975).
Mouse behavioral pattern monitor
Locomotor behavior and exploration were examined using eight mouse BPM chambers (BPM; San Diego Instruments, San Diego, CA) as described previously (Risbrough et al., 2006; Tanaka et al., 2012). Each Plexiglas arena consists of a 30.5 × 61 × 38 cm area with three floor holes and eight wall holes (three along each long side and one in each of the short sides; 1.25 cm in diameter, 1.9 cm from the floor), each equipped with an infrared photobeam to detect holepoking. An outer box with an internal white house-light above the arena (350 lux in the center and 92 lux in the four corners) minimized external light and noise. Activity was obtained from a grid of 12 × 24 infrared photobeams 1 cm above the floor (2.5 cm apart; 24 × 12 X-Y array), recording the location of the mouse every 0.1 s, with its position defined across nine unequal regions (four corners, four walls, and center (Geyer et al., 1986)). Another set of 16 photobeams, placed 2.5 cm above the floor, was used to detect rearing behavior. Mice were placed in the bottom left-hand corner of the arena and the test session started immediately. The primary outcome measures were transitions across the defined regions and center entries (locomotor activity), holepoking and rearing (exploratory behavior), and entropy (h) and spatial d (locomotor patterns). Lower entropy reflects predictable, ordered sequences of activity, while higher entropy indicates a greater disorder of movement. Spatial d quantifies the geometric structure of the locomotor path, where a value of 1 reflects a straight path, and 2 highly circumscribed small-scale movements (Paulus and Geyer, 1991).
Experiment 1
Female (n=11) and male (n=17) DAT KD mice and their female (n=12) and male (n=14) WT littermates were tested in the BPM to assess their exploratory profile. Activity was measured in the BPM for 60 min during the first test (Experiment 1a), then one week later for 60 min (Experiment 1b), and finally 10 days later for 180 min (Experiment 1c).
Experiment 2
The effects of AMPT on the exploratory profile of male C57BL6/J mice were assessed in the BPM for 60 min. Mice received saline or 10, 30, or 100 mg/kg AMPT 24, 20, and 4 h prior to testing (n=10/group).
Experiment 3
The effects of 30 mg/kg AMPT were assessed on the exploratory behavior of female (n=17) and male (n=20) DAT KD mice and their female (n=22) and male (n=18) WT littermates in the BPM for 60 min. These mice were not BPM naïve and had been treated with the antipsychotic risperidone six weeks earlier. The mice were baseline-matched based on transitions, holepoking, and spatial d, and counter-balanced with previous risperidone experience. Mice were treated with AMPT or saline 24, 20, and 4 h prior to testing.
Statistics
The primary measures for each experiment were analyzed using two- or three-way analyses of variance (ANOVA), with sex, genotype, or drug as between-subject variables. In experiment 3, an ANCOVA was used to assess whether prior risperidone treatment affected the outcome of the study. Statistically significant or relevant interactions and main effects were analyzed using Tukey's post hoc analyses where applicable. The data were analyzed for 60 min testing periods using the BMDP statistical software (Statistical Solutions Inc., USA). The level was set to 0.05.
RESULTS
Experiment 1a: Stability of DAT WT and KD exploration in the BPM
Because no sex by genotype interactions were observed for any measures, male and female data were pooled and analyzed together.
Locomotor activity
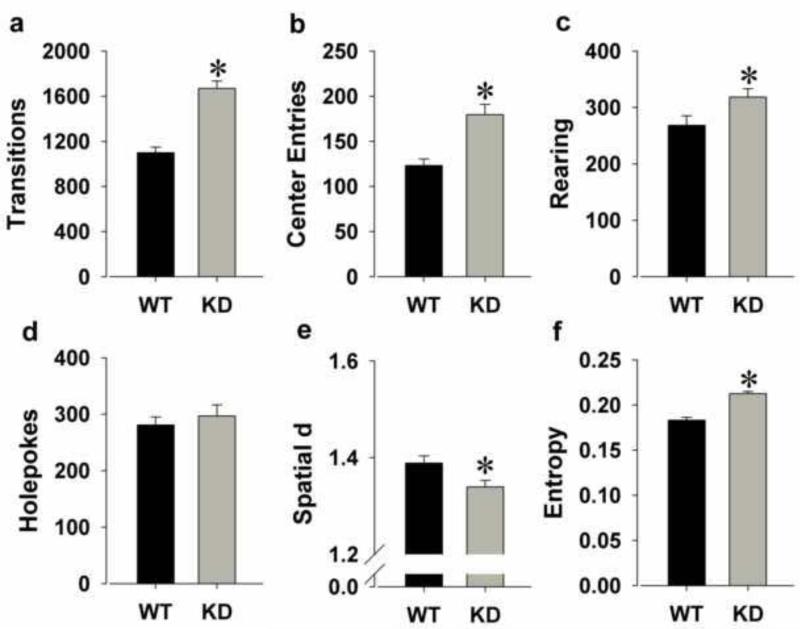
KD mice were hyperactive as reflected by increased transitions (F(1,52)=46.3, p<0.0001; Fig. 1a) and center entries (F(1,52)=16.8, p<0.0001; Fig. 1b).
Figure 1.
The exploratory profile of DAT KD and WT mice on a C57BL/6J background in the BPM. KD mice were hyperactive compared to WT mice as measured by increased transitions (a) and more center entries (b). KD mice also exhibited increased exploration compared to WT mice as reflected by increased rearing (c), but not holepoking (d). KD mice moved in straighter paths than WT mice as measured by a lower spatial d (e) and exhibited disordered movement organization as measured by higher entropy (f). Data are presented as mean + S.E.M. *p<0.05 when compared to WT mice.
Exploratory behavior
KD mice exhibited more exploration as reflected by increased rearing (F(1,52)=4.9, p<0.05; Fig. 1c), but not holepoking (F<1, ns; Fig. 1d).
Locomotor patterns
KD mice moved in straighter lines as reflected by reduced spatial d (F(1,52)=6.0, p<0.05; Fig. 1e) and exhibited greater disorder of movement as reflected by higher entropy (F(1,52)=52.4, p<0.0001; Fig. 1f).
Experiment 1b: Repeated testing of DAT WT and KD mice in the BPM
The exploratory profiles of mice from experiment 1a were assessed in the BPM one week later.
Locomotor activity
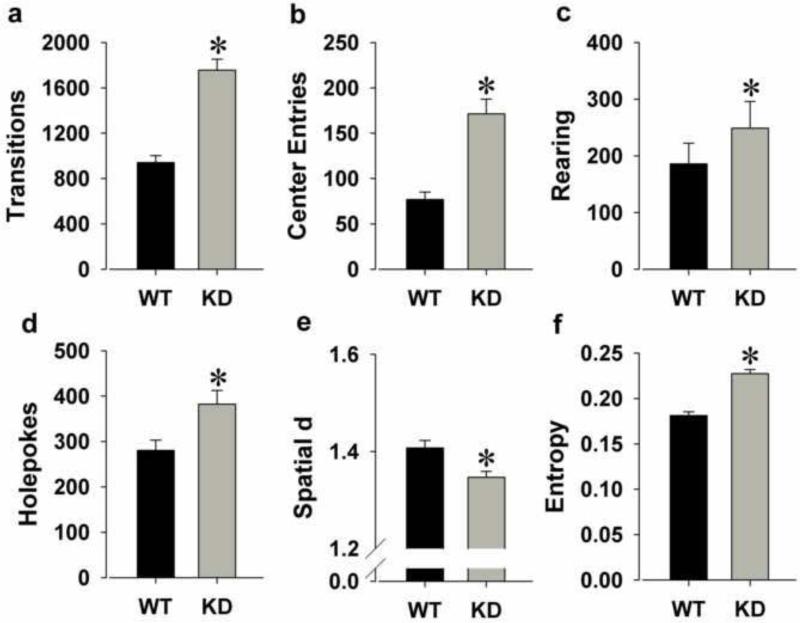
KD remained hyperactive as reflected by increased transitions (F(1,52)=49.3, p<0.0001; Fig. 2a) and center entries (F(1,52)=25.4, p<0.0001; Fig. 2b).
Figure 2.
The exploratory profile of DAT KD and WT mice on a C57BL/6J background tested in the BPM a second time, one week after their initial testing. KD mice remained hyperactive compared to WT mice upon repeated testing as measured by increased transitions (a) and more center entries (b). KD mice remained more exploratory than WT mice as measured by increased rearing (c), and now also increased holepoking (d). KD mice still moved in straighter paths than WT mice as measured by a lower spatial d (e) and exhibited disordered movement organization as measured by higher entropy (f). Data are presented as mean + S.E.M. *p<0.05 when compared to WT mice.
Exploratory behavior
KD mice exhibited more exploration as reflected by increased rearing (F(1,52)=5.8, p<0.05; Fig. 2c) and now holepoking (F(1,52)=7.1, p<0.05; Fig. 2d).
Locomotor patterns
KD mice moved in straighter lines as reflected by reduced spatial d (F(1,52)=9.6, p<0.005; Fig. 2e) and exhibited greater disorder of movement as reflected by higher entropy (F(1,52)=56.2, p<0.0001; Fig. 2f).
Experiment 1c: Prolonged testing for three hours of DAT WT and KD mice in the BPM
Mice from experiment 1b were tested 10 days later for three hours and data were analyzed in three 60 min time bins.
Locomotor activity
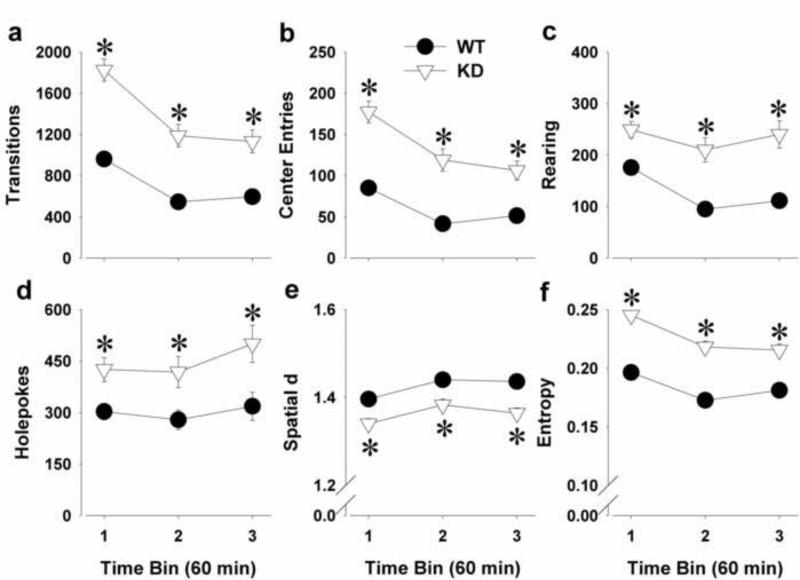
KD remained hyperactive as reflected by increased transitions (F(1,52)=46.0, p<0.0001; Fig. 3a) and center entries (F(1,52)=36.3, p<0.0001; Fig. 3b). There was a main effect of time and a genotype by time bin interaction for transitions [F(2,104)=50.7, p<0.0001 and F(2,104)=3.4, p<0.05 respectively] and center entries [(F(2,104)=36.8, p<0.0001 and F(2,104)=3.4, p<0.05 respectively]. Post hoc analyses revealed that KD mice had increased transitions and center entries compared to WT mice at each time bin (p<0.0005) and both WT and KD mice decreased activity over time (p<0.0001).
Figure 3.
The exploratory profile of DAT KD and WT mice on a C57BL/6J background tested in the BPM a third time, 10 days after their second testing. The profile of mice was assessed in the BPM for a prolonged period of 3-h and divided in three 60-min time bins. Although both WT and KD mice habituated over time, KD mice remained hyperactive throughout the 3-h period as measured by increased transitions (a) and more center entries (b). While WT mice decreased rearing over time, KD mice did not and exhibited increased rearing at each time bin (c). WT mice did not change in holepoking behavior over time, while KD mice exhibited increased holepoking behavior compared to WT mice and over time (d). Although both WT and KD mice exhibited increased spatial d and reduced entropy over time, KD mice moved in straighter paths (e) and exhibited disordered movement organization (f) compared to WT mice throughout the 3-h period. Data are presented as mean ± S.E.M. *p<0.05 when compared to WT mice.
Exploratory behavior
KD mice remained more explorative as reflected by increased rearing (F(1,52)=23.5, p<0.0001; Fig. 3c) and holepoking (F(1,52)=8.2, p<0.01; Fig. 3d). There was a main effect of time for holepoking (F(2,104)=5.1, p<0.01) and rearing (F(2,104)=12.7, p<0.0001) but only a trend towards a genotype by time bin interaction for rearing (F(2,104)=2.9, p<0.1). Post hoc analyses revealed that WT mice decreased rearing over time (p<0.0001), but made similar amount of holepokes over time. In contrast, KD mice increased holepoking over time (p<0.05), while their rearing behavior did not change.
Locomotor patterns
KD mice moved in straighter paths as reflected by reduced spatial d (F(1,52)=10.6, p<0.005; Fig. 3e) and exhibited greater disorder of movement as reflected by higher entropy (F(1,52)=64.1, p<0.0001; Fig. 3f). There was a main effect of time for spatial d (F(2,104)=24.6, p<0.0001) and entropy (F(2,104)=83.8, p<0.0001) and a genotype by time bin interaction for entropy (F(2,104)=6.2, p<0.005). Post hoc analyses revealed that spatial d increased and entropy decreased over time in both WT and KD mice (p<0.0001).
Experiment 2: Dose-response study of AMPT in C57BL6/J mice
The effects of sub-chronic AMPT on the exploratory profile of male C57BL6/J mice (n=39) were investigated for 60 min in the BPM.
Locomotor activity
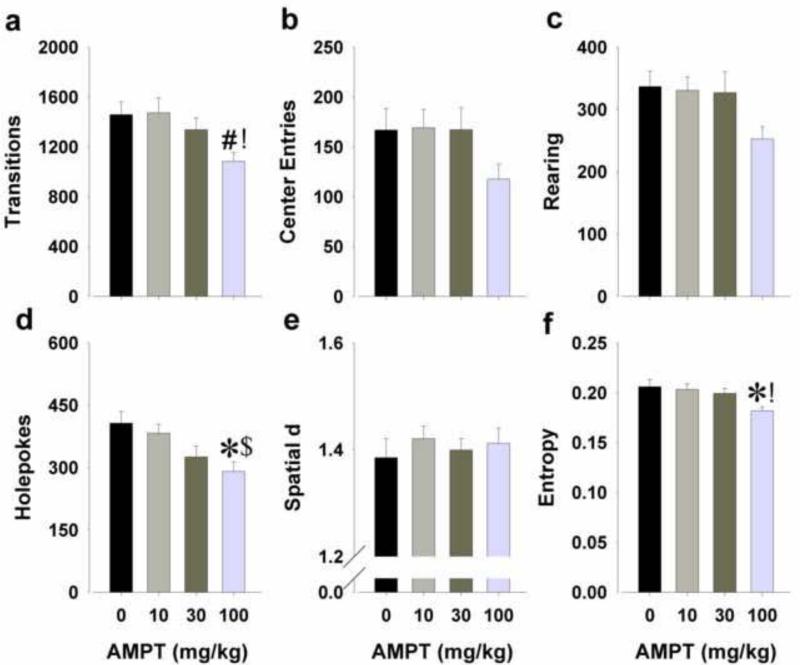
There was a significant main effect of AMPT on transitions (F(3,35)=3.4, p<0.05; Fig. 4a), but not on center entries (Fig. 4b). Post hoc analyses revealed that the highest dose of AMPT reduced activity compared to the lowest dose (p<0.05) and tended to compared to saline (p<0.1).
Figure 4.
Effects of AMPT on exploratory behavior in male C57BL6/J mice in the BPM. The tyrosine hydroxylase inhibitor AMPT (10, 30, and 100 mg/kg) was administered to mice at 24, 20, and 4 h prior to their assessment in the BPM. AMPT at the highest dose reduced activity as measured by reduced transitions (a), but not center entries (b). AMPT at the highest dose reduced exploratory behavior as measured by a non-significant drop in rearing (c) and a significant drop in holepoking (d). AMPT did not affect spatial d (e), but reduced entropy at the highest dose (f). Data are presented as mean + S.E.M. * p<0.05 and # p<0.1 when compared to saline; ! p<0.05 and $ p<0.1 when compared to AMPT 10 mg/kg.
Exploratory behavior
A main effect of AMPT was observed on exploration, specifically on holepoking (F(3,35)=4.5, p<0.01; Fig. 4d) and a trend effect on rearing (F(3,35)=2.4, p<0.1; Fig. 4c). Post hoc analyses revealed that the highest dose of AMPT reduced holepoking compared to saline (p<0.05) and tended to compared to the lowest dose (p<0.1).
Locomotor patterns
AMPT treatment affected entropy (F(3,35)=4.2, p<0.05; Fig. 4f) but not spatial d (Fig. 4e). Post hoc analyses indicated that the highest dose of AMPT resulted in more ordered sequences of activity (lower entropy) compared to saline and the lowest dose of AMPT (p<0.05).
Experiment 3: Low dose AMPT in DAT WT and KD mice
The effects of 30 mg/kg AMPT (a dose that was ineffective in C57BL/6 mice; Experiment 2a) were assessed on the exploratory behavior of WT and KD mice in the BPM. Since there were no effects of or interactions with sex for any of the measures, male and female data were pooled and analyzed together. Moreover, covarying for prior risperidone experience did not affect the statistical findings.
Locomotor activity
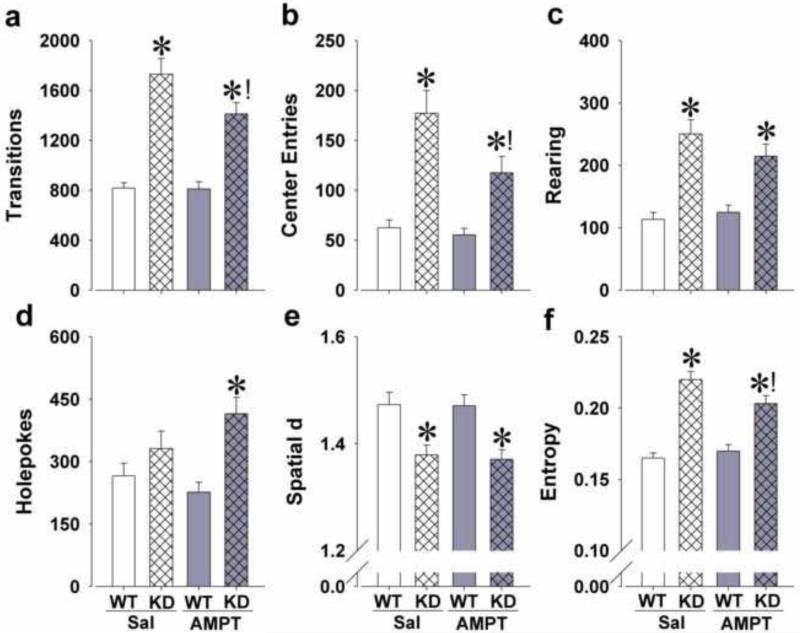
Significant genotype effects were observed for transitions (F(1,73)=83.3, p<0.0001; Fig. 5a) and center entries (F(1,73)=36.0, p<0.0001; Fig. 5b). A significant effect of AMPT was observed for center entries (F(1,73)=5.1, p<0.05) and tended to affect transitions (F(1,73)=3.9, p<0.1). AMPT treatment and genotype tended to interact on transitions (F(1,73)=3.5, p<0.1) and center entries (F(1,73)=3.2, p<0.1). Post hoc analyses revealed that AMPT did not affect activity in WT mice, but significantly reduced transitions and center entries in KD mice compared with saline (p<0.05).
Figure 5.
Effects of 3 × 30 mg/kg AMPT on the mania-like profile of DAT KD mice in the BPM. Hyperactivity of KD mice compared to WT mice was attenuated, but not completely abolished, by AMPT treatment as measured by reduced transitions (a) and less center entries (b). KD mice exhibited increased exploration compared to WT mice as measured by increased rearing without an effect of AMPT (c). AMPT potentiated specific exploration as measured by increased holepoking in the KD mice compared to WT mice (d). KD mice exhibited straighter paths of movement compared to WT mice, which was unaffected by AMPT treatment (e). Disordered movement organization of KD mice compared to WT mice was attenuated by AMPT treatment as measured by a reduction in entropy (f). Data are presented as mean + S.E.M. * p<0.05 when compared to WT mice; ! p<0.05 when compared to saline.
Exploratory behavior
Main effects of genotype were observed for holepoking (F(1,73)=13.6, p<0.0005; Fig. 5d) and rearing (F(1,73)=45.7, p<0.0001; Fig. 5c). AMPT did not affect exploration alone (F<1, ns), but tended to interact with genotype on holepoking (F(1,73)=3.2, p<0.1). Interestingly, post hoc analyses revealed that while saline-treated KD mice did not differ from saline-treated WT mice in holepoking, AMPT-treated KD mice made significantly more holepokes compared to AMPT-treated WT mice (p<0.05), but not compared to saline-treated KD mice.
Locomotor patterns
Significant effects of genotype were observed for spatial d (F(1,73)=23.1, p<0.0001; Fig. 5e) and entropy (F(1,73)=79.2, p<0.0001; Fig. 5f). There were no main effects of AMPT on these variables (F<1, ns), but there was a significant AMPT by genotype interaction for entropy (F(1,73)=4.8, p<0.05). Post hoc analyses revealed that AMPT did not affect entropy in WT mice, but significantly reduced entropy in KD mice compared with saline-treated KD mice (p<0.05).
DISCUSSION
DAT KD mice on a C57BL/6J background exhibit a robust BD mania-like profile in the BPM, which did not wane with repeated testing. Furthermore, AMPT-induced dopamine depletion attenuated some, but potentiated one other, mania-like behaviors, without affecting control animals. Consistent with previous reports, KD mice exhibited hyperactivity, increased exploration, and disrupted behavioral organization in the BPM similar to patients with BD (Perry et al., 2009; Young et al., 2011b). Increased exploration was driven more by increased rearing compared to holepoking behavior, consistent with GBR12909-induced exploration in C57BL/6 mice (Young et al., 2010a). Interestingly, DAT KD mice on a 129/S background primarily exhibited increased holepoking behavior (Perry et al., 2009), replicated in 129/SvJ mice administered GBR12909 (Young et al., 2010a). With the prolonged testing period, the DAT KD mice on the C57BL/6 background also exhibited increased holepokes compared with WT controls. As hypothesized, the mania-like phenotype of KD mice was consistent even after repeated testing, reflecting a more robust and stable phenotype when compared with KD mice on the 129/S background. Taken together, the behavioral profile of KD mice observed here matches the effects of acutely administered GBR12909 in C57BL/6J mice and that of BD mania patients in the human BPM.
The dose-response study of AMPT in C57 mice revealed that 3 doses of 100 mg/kg decreased activity, exploration, and behavioral organization, while the other doses had no effect. Previously however, Davies et al. reported that pretreatment with 3 doses of 100 mg/kg AMPT did not reduce the holepoking activity of female control mice (Porton strain), but completely abolished increased holepoking induced by a mixture of chlordiazepoxide and (+)-amphetamine (Davies et al., 1974). The strain difference or the fact that only female mice were used by Davies et al. may have caused this difference in dose-response. When administered to female WT and KD mice, AMPT (3 × 100 mg/kg) also reduced activity in the BPM independent of genotype (unpublished observations). Hence, we attempted to reverse the mania-like behavior of KD mice using 30 mg/kg under the hypothesis that this dose would preferentially affect KD and not WT mice in accordance with the requirement for treatment development (Young et al., 2011a).
Indeed, catecholamine depletion after 3 × 30 mg/kg AMPT did not affect exploratory behavior in WT mice, consistent with the effects observed in C57BL/6J mice. In DAT KD mice however, this dose of AMPT attenuated some facets of their mania-like behavior, including activity levels (transitions and center entries) and movement organization (entropy h). AMPT potentiated the increased holepoking observed in KD mice however. Perhaps importantly, holepoking behavior in the mouse BPM correlated modestly with risk preference in the mouse Iowa Gambling Task (Young et al., 2011b). Moreover, increased object interactions in the human BPM (human analogue for specific exploration) correlated with impaired performance in the Wisconsin Card Sorting Task (Henry et al., 2011). AMPT may therefore negatively affect aspects of cognitive functioning. In fact, several studies have recently demonstrated that AMPT treatment can impair reward processing and probabilistic learning in patients with remitted major depressive disorder (Hasler et al., 2009a; Hasler et al., 2009b) and bulimia nervosa (Grob et al., 2012). Given that cognitive performance correlates with a patients’ ability to live independently (Green, 2006), any treatment that negatively impacts cognition may not be useful therapeutically (Young et al., 2011a).
There are some limitations to this study that warrant attention. First, the generalized effects of AMPT may relate to its lack of specificity for dopamine. As was shown previously, AMPT can also deplete norepinephrine (Dobrzanski and Doggett, 1976; Flexner and Goodman, 1975; Widerlov and Lewander, 1978). Thus, although studies suggest that AMPT causes slightly more dopamine than norepineprhine depletion in rodents (Flexner and Goodman, 1975) and humans (Booij et al., 2003), it will be important to test whether selective dopamine depletion affects cognition. Ultimately, although catecholamine depletion attenuated some facets of the mania-like behavior in KD mice, further investigation of the neurocognitive effects of AMPT are required.
In humans, AMPT is also being used in order to elucidate catecholaminergic pathways underlying brain disorders and as a potential therapeutic in clinical research (Bloemen et al., 2008; Booij et al., 2003). Although AMPT has only been approved as a therapeutic agent to treat the symptoms of phaeochromocytoma, beneficial effects have been studied in various neuropsychiatric disorders, including dystonia, dyskinesia, Huntington's disease, substance abuse, and schizophrenia (Bloemen et al., 2008). Interestingly, some studies have investigated the utility of AMPT as a treatment in BD mania. In one study, AMPT rapidly decreased manic symptoms in patients, while it increased depression symptoms in patients with unipolar depression (Bunney et al., 1971). In another study, AMPT did not affect mood in euthymic BD patients on lithium therapy (Anand et al., 1999). However, soon after catecholamine depletion ceased, all patients experienced a relapse of hypomanic symptoms. Rebound synthesis of catecholamines and/or postsynaptic dopamine receptor supersensitivity after administration of AMPT (Trugman and James, 1992) may have lead to these relapses of mania. Cognitive performance was not assessed in any of these studies however, and would be very useful in future studies. Additionally, it is important to note that AMPT can produce serious dose-related side effects, including crystalluria and acute dystonia (Abi-Dargham et al., 2000; Voruganti et al., 2001). Future studies should therefore also investigate low-dosage regimens, which have been demonstrated to be a suitable alternative that is free from severe side effects (Boot et al., 2008). Thus, it is possible to conduct cross-species relevant studies in animals and humans with previous studies supporting our findings that AMPT can reduce mania-like behavior.
In summary, DAT KD mice on a C57BL/6J background exhibited a robust and stable mania-like phenotype in the BPM. Catecholamine depletion attenuated some facets of mania-like behavior in KD mice, but also potentiated abnormal exploration. These studies shine new light on a forgotten putative treatment for BD mania. Future studies should investigate whether AMPT in low doses could potentially reduce symptoms in patients with BD mania as well as determine its neurocognitive effects in patients and animal models.
Acknowledgements
We thank Richard Sharp, Mahalah Buell, and Dr. Berend Olivier for their support, as well as Dr. Xiaoxi Zhuang for supplying us with the original DAT heterozygous breeding pairs.
Role of the funding source
These studies were supported by NIH grants R01-MH071916, and R01-DA002925, as well as by the Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors JvE, MAG, ALH, and JWY designed the study and wrote the protocol. Authors JvE, ALH, and JWY undertook the experiments. Author JvE managed the literature searches, performed the statistical analyses, and wrote the first draft of the manuscript. Author XZ supplied us with the animals. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors report no conflict of interest.
REFERENCES
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol. Psychiatry. 2011;70:357–365. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, Hutchins GD, Normandin MD, Yoder KK. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disorder. 2011;13:406–413. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Anand A, Darnell A, Miller HL, Berman RM, Cappiello A, Oren DA, Woods SW, Charney DS. Effect of catecholamine depletion on lithium-induced long term remission of bipolar disorder. Biol. Psychiatry. 1999;45:972–978. doi: 10.1016/s0006-3223(98)00293-5. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC.: 1994. [Google Scholar]
- Bloemen OJN, de Koning MB, Boot E, Booij J, van Amelsvoort TAMJ. Challenge and therapeutic studies using alpha-methyl-para-tyrosine (AMPT) in neuropsychiatric disorders: a review. Cent Nerv Syst Agents Med Chem. 2008;8:249–256. [Google Scholar]
- Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: review. Mol. Psychiatry. 2003;8:951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- Boot E, Booij J, Hasler G, Zinkstok JR, de Haan L, Linszen DH, van Amelsvoort TA. AMPT-induced monoamine depletion in humans: evaluation of two alternative [123I]IBZM SPECT procedures. Eur J Nucl Med Mol Imaging. 2008;35:1350–1356. doi: 10.1007/s00259-008-0739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney WE, Jr., Brodie HK, Murphy DL, Goodwin FK. Studies of alpha-methyl-para-tyrosine, L-dopa, and L-tryptophan in depression and mania. Am. J. Psychiatry. 1971;127:872–881. doi: 10.1176/ajp.127.7.872. [DOI] [PubMed] [Google Scholar]
- Davies C, Sanger DJ, Steinberg H, Tomkiewicz M, U'Prichard DC. Lithium and alpha-methyl-p-tyrosine prevent “manic” activity in rodents. Psychopharmacologia. 1974;36:263–274. doi: 10.1007/BF00421808. [DOI] [PubMed] [Google Scholar]
- Dobrzanski S, Doggett NS. Studies in mice on the antagonism of (+)-amphetamine anorexia by alpha-methyl-p-tyrosine methyl ester HCl. Neuropharmacology. 1976;15:619–623. doi: 10.1016/0028-3908(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Engelman K, Jequier E, Udenfriend S, Sjoerdsma A. Metabolism of alpha-methyltyrosine in man: relationship to its potency as an inhibitor of catecholamine biosynthesis. J. Clin. Invest. 1968;47:568–576. doi: 10.1172/JCI105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner LB, Goodman RH. Studies on memory: inhibitors of protein synthesis also inhibit catecholamine synthesis. Proc. Natl. Acad. Sci. U. S. A. 1975;72:4660–4663. doi: 10.1073/pnas.72.11.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol. Biochem. Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J. Clin. Psychiatry 67 Suppl. 2006;9:3–8. discussion 36-42. [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol. Psychiatry. 2006;11:125–133. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Grob S, Pizzagalli DA, Dutra SJ, Stern J, Morgeli H, Milos G, Schnyder U, Hasler G. Dopamine-related deficit in reward learning after catecholamine depletion in unmedicated, remitted subjects with bulimia nervosa. Neuropsychopharmacology. 2012;37:1945–1952. doi: 10.1038/npp.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol. Psychiatry. 2009a;66:201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Mondillo K, Drevets WC, Blair JR. Impairments of probabilistic response reversal and passive avoidance following catecholamine depletion. Neuropsychopharmacology. 2009b;34:2691–2698. doi: 10.1038/npp.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, Geyer MA, Perry W. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. J. Affect. Disord. 2013 doi: 10.1016/j.jad.2013.05.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology (Berl) 2011;215:697–707. doi: 10.1007/s00213-011-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Mol. Psychiatry. 2005;10:1104–1109. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, Zarate CA. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugue E, Daban C, Salamero M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004a;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry. 2004b;161:262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. Public Library of Science One. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Geyer MA. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178:84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch. Gen. Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644–1655. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol. Psychiatry. 2003;53:352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J. Affect. Disord. 2012;136:63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav. Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugman JM, James CL. Rapid development of dopaminergic supersensitivity in reserpine-treated rats demonstrated with 14C-2-deoxyglucose autoradiography. J. Neurosci. 1992;12:2875–2879. doi: 10.1523/JNEUROSCI.12-07-02875.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int J Neuropsychopharmacol. 2013a;16:1021–1031. doi: 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task : Relevance to mania. Psychopharmacology (Berl) 2013b;225:661–674. doi: 10.1007/s00213-012-2854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol. Psychiatry. 2000;48:486–504. doi: 10.1016/s0006-3223(00)00978-1. [DOI] [PubMed] [Google Scholar]
- Voruganti L, Slomka P, Zabel P, Costa G, So A, Mattar A, Awad AG. Subjective effects of AMPT-induced dopamine depletion in schizophrenia: correlation between dysphoric responses and striatal D(2) binding ratios on SPECT imaging. Neuropsychopharmacology. 2001;25:642–650. doi: 10.1016/S0893-133X(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Widerlov E, Lewander T. Inhibition of the in vivo biosynthesis and changes of catecholamine levels in rat brain after alpha-methyl-p-tyrosine; time- and dose-response relationships. Naunyn. Schmiedebergs Arch. Pharmacol. 1978;304:111–123. doi: 10.1007/BF00495547. [DOI] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacol. Biochem. Behav. 2010b;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br. J. Pharmacol. 2011a;164:1263–1284. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J. Psychopharmacol. (Oxf) 2011b;25:934–943. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]