Abstract
Background
Use of in silico bioinformatics analyses has led to important leads in the complex nature of alcoholism at the genomic, epigenomic, and proteomic level, but has not previously been successfully translated to the development of effective pharmacotherapies. In this study, a bioinformatics approach led to the discovery of neuroimmune pathways as an age-specific druggable target. Minocycline, a neuroimmune modulator, reduced high ethanol drinking in adult, but not adolescent, mice as predicted a priori.
Methods
Age and sex-divergent effects in alcohol consumption were quantified in FVB/NJ × C57BL/6J F1 mice given access to 20% alcohol using a 4 hr/day, 4-day Drinking-In-Dark (DID) paradigm. In silico bioinformatics pathway over-representation analysis for age-specific effects of alcohol in brain was performed using gene expression data collected in control and DID-treated, adolescent and adult, male mice. Minocycline (50 mg/kg i.p., once daily) or saline alone was tested for an effect on ethanol intake in the F1 and C57BL/6J (B6) mice across both age and gender groups. Effects of minocycline on the pharmacokinetic properties of alcohol were evaluated by comparing the rates of ethanol elimination between the saline and minocycline treated F1 and B6 mice.
Results
Age and gender differences in DID consumption were identified. Only males showed a clear developmental increase difference in drinking over time. In silico analyses revealed neuroimmune-related pathways as significantly over-represented in adult, but not adolescent, male mice. As predicted, minocycline treatment reduced drinking in adult, but not adolescent, mice. The age effect was present for both genders, and in both the F1 and B6 mice. Minocycline had no effect on the pharmacokinetic elimination of ethanol.
Conclusions
Our results are a proof of concept that bioinformatics analysis of brain gene expression can lead to the generation of new hypotheses and a positive translational outcome for individualized pharmacotherapeutic treatment of high alcohol consumption.
Keywords: Alcoholism, Drinking-In-Dark, FVB/NJ × C57BL/6J F1 Mouse, Medications Development, Minocycline, Bioinformatics
Alcoholism is a complex trait involving the interaction of numerous biological and environmental factors. In the past several decades, researchers have convincingly elucidated that, for a disease of such complexity as alcoholism, no single molecular target underlies a particular associated phenotype. Instead, many interconnected molecular and cellular targets of small effect size underlie alcohol use disorders (AUD) and many remain poorly characterized. This multi-factorial nature of alcoholism has limited the currently available treatment options and resulted in their disparate efficacy across AUD subtypes.
Global gene expression profiling has proven to be a valuable tool to predict the molecular components leading to predisposition to high drinking (Edenberg et al., 2005, Tabakoff et al., 2003, Mulligan et al., 2008, Mulligan et al., 2006), and to uncover ethanol-induced transcriptome changes in vivo and in vitro (Lewohl et al., 2000, Daniels and Buck, 2002, Mulligan et al., 2011). In silico analysis of gene expression data coupled with the use of bioinformatics programs has detected alcohol-related loci, and functional networks (Daniels and Buck, 2002, Kerns and Miles, 2008 and others to numerous to list). Genomic data, including the use of in silico bioinformatics analyses from our laboratories, has led to the identification of a new neuroimmune-targeted pharmacotherapy for the treatment of high alcohol consumption (Blednov et al., 2012).
The purpose of our study was two-fold. First, we sought to determine whether a commonly used, high drinking, isogenic F1 mouse, FVB/NJ × C57BL/6J, would show age and gender differences in binge drinking. Second, a translational approach that included in silico bioinformatics analysis of brain gene expression was used to identify and test targets for pharmacotherapeutic treatment of high alcohol consumption. The Drinking-In-Dark (DID) paradigm of voluntary ethanol consumption was used to best model binge drinking (Rhodes et al., 2005) in C57BL/6J (B6) and its F1 hybrid FVB/NJ × C57BL/6J (F1) mice, which are well-characterized mouse models (Blednov et al., 2005b).
Age of an individual at the time of onset of alcohol consumption is an important risk factor that affects alcohol-related problems later on in life (Grant and Dawson, 1997, Brown and Tapert, 2004). Age-differential responses to alcohol are confounding factors in the efficacy of various treatment modalities (Brown and D'Amico, 2001). Hence, to find age-appropriate medication, we tested both adolescent and adult F1 and B6 mice for binge ethanol consumption.
Sex/gender differences in AUDs is an active research area with recent studies having shown that females that drink have a higher risk of developing alcohol-associated medical problems (Medina et al., 2008, Squeglia et al., 2012, Key et al., 2006, Urbano-Marquez et al., 1995). To determine important gender-related differences in alcohol consumption, both males and females were tested using the DID paradigm.
The need for better therapies led us to test three sequential hypotheses: 1) Age and sex/gender influence alcohol consumption. 2) Alcohol-mediated brain gene expression shows age-specificity. 3) Age-divergent, neuroimmune function modulates commensurate binge drinking.
Based on a convergence of literature suggesting that age and gender are important factors to consider when developing a translational approach (Greenfield et al., 2010, Dawes and Johnson, 2004), we tested the first general hypothesis that both influence binge alcohol consumption.
After detecting a developmental difference in drinking only in male animals, we generated our second hypothesis that brain gene expression would show age and alcohol specific changes. Microarray hybridization, followed by in silico functional analyses of the transcriptome revealed age-divergent over-represented pathways related to neuroimmune function.
Numerous studies have shown that ethanol mediates its effects, in part, through mis-regulation of the neuroimmune system, leading to neuroinflammation and neurodegeneration (Davis and Syapin, 2005, Sullivan and Zahr, 2008, Cippitelli et al., 2010, Crews and Nixon, 2009). The role of the neuroimmune system had recently been implicated in regulating ethanol consumption through its interaction with the neurotransmitter system regulating the reward pathways of the brain (Crews, 2011), yet it is not completely understood. Activation of neuroimmune pathways leads to the release of cytokines, chemokines and other mediators, affecting both neuronal and non-neuronal cells. Moreover, we have previously shown that alteration in neuroimmune networks by deletion (knock out) of components caused a diminution in ethanol consumption in mice (Blednov et al., 2005a, Blednov et al., 2012).
Our third broad hypothesis was generated based on our bioinformatics pathway analyses and is thus: that neuroimmune physiology plays a role in regulating ethanol consumption in an age-divergent manner. To test our hypothesis, we used minocycline, a pharmacological modulator of neuroimmune pathways identified in our bioinformatics analyses (Kobayashi et al., 2013, Yang et al., 2007).
We measured ethanol intake in adolescent and adult F1 mice following minocycline or saline pretreatment using the DID paradigm. A parallel study was completed in C57BL/6J mice to further validate the effects of minocycline. To investigate any action of minocycline on ethanol elimination, we tested the pharmacokinetic profile of ethanol by measuring blood ethanol concentrations over time after a single dose of ethanol following minocycline pretreatment.
Results from our studies indicate an age-dependent modulation of ethanol drinking behavior by minocycline for both males and females. Our testing of sequential hypotheses, including the terminal one generated based on the output of in silico bioinformatics analyses has led to an important proof of concept: that bioinformatics analysis of brain gene expression can, and has, led to age-specific translational outcomes for the pharmacotherapeutic treatment of high alcohol consumption.
MATERIALS AND METHODS
Animal Husbandry
Adult male and female C57Bl/6J (B6) and female FVB/NJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). FVB/NJ × C57BL/6J (FVB.B6) F1 mice were bred in-house from adult female FVB/NJ and male C57BL/6J mice to produce age-matched adolescent (P30) and adult (P70) mice. All B6 mice used directly in the study were purchased. The F1 mice were weaned at postnatal day 28. All mice when assigned to the study were individually housed under standard humidity and temperature conditions, with a reverse 12-h light/dark cycle with ad libitum access to lab chow. All experimental procedures followed protocols approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee.
Alcohol Administration: Drinking-in-Dark (DID) Paradigm
Mice were housed in standard clear mouse cages with special tops built in-house to accommodate 50 ml centrifugation tubes (Falcon brand, BD Biosciences, Franklin Lakes, NJ). The Falcon tubes were fitted with dual ball-bearing sippers, and placed in an inverted position on the mouse cage tops. The DID procedure was carried out as previously described (Rhodes et al., 2005). The mice were weighed at the start of the experiment and then given access to a 20% ethanol solution, three hours into their dark cycle for four hours followed by access to water for the remaining 20 hr. This procedure was carried out for four days. The ethanol and water tubes were weighed and fluid consumption was calculated. Immediately following the last alcohol exposure, the mice were sacrificed, blood was collected for Blood Ethanol Concentration (BEC) determination, and brain tissue was harvested for further analyses.
Blood Ethanol Concentration Determination
BEC was determined from blood samples collected immediately following the DID procedure (hour 4 on Day 4) using Gas Chromatography (GC) essentially as previously described (Blednov et al., 2005a). Briefly, 20 μl of blood was collected from the head region of the mice in a tube containing 50 μl of ice cold ZnCl2. The samples were kept on ice throughout the procedure. Then, 50 μl of Ba(OH)2 and 300 μl of dH20 was added. The tubes were briefly vortexed and microfuged at max speed for 5 minutes at room temperature. The supernatant (~350 μl) was collected in crimp-top glass GC vials and sealed to prevent any evaporation of alcohol. Some samples were collected in 180 μl of water and analyzed using a headspace method (Finn et al., 2007). The BEC was quantified using four known concentrations of ethanol (0.5, 1, 2 and 4 mg/ml) in an Agilent 7683 automatic liquid sampler GC (Agilent Technologies, Palo Alto, CA).
Expression and Bioinformatics Analyses
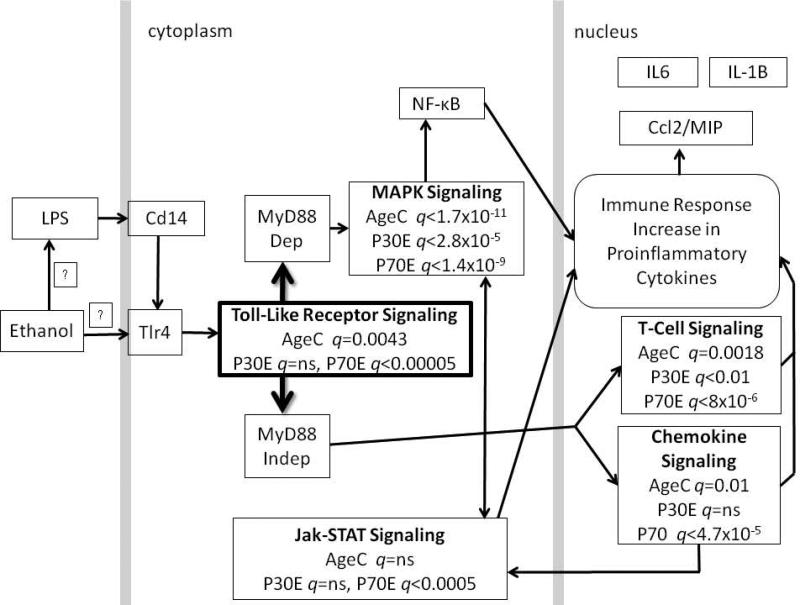
Immediately following the DID procedure (hour 4 on day 4), brains were harvested for gene expression (transcriptome) analysis from the adolescent (P30) and adult (P70) male F1 mice (n = 5 / for P30C, P30E, P70C, P70E). Total RNA was isolated from the brain tissue using RNA-STAT 60 (catalog no. Cs502, Tel-Test Inc., TX, USA) as per manufacturer's protocol. The RNA was reverse transcribed, with the resulting cDNA flurolabeled and then hybridized to whole genome custom cDNA arrays. Microarray assays and statistical analyses were performed as described previously (Mulligan et al., 2008, Mulligan et al., 2006). ANOVA, Student's t-tests and PLS (partial least squares) pattern analysis were completed for the expression data, using the Alcohol Research Integrator, an in-house database (http://aridb.ttuhsc.edu/cgi-bin/genedb.pl login=guest, password=guest). Posthoc t-tests for an age effect (P30C v P70C), overall ethanol dose response, and an ethanol-response for each age group (P30C v P30E and P70C v P70E) were completed. To uncover any alcohol dose-response effects, statistical analyses for correlation of BEC and expression was completed for each gene [36,562 unflagged spots, including 24,566 known genes and Riken clones]. Expression of known genes, with duplicates removed [11,682], for the twenty samples (n = 5/group for P30C, P30E, P70C, P70E) was then sorted based on a stringency of p < 0.05 to generate several gene lists as follows: To determine whether a priori age differences contribute to any potential ethanol responses, an AgeC (differences between control P30 and P70) gene list was generated (P30C vs P70C: 1405 genes, p < 0.05 = q < 0.13). After genes that showed significant response by dose (396 genes, t-statistic < 2.4, n-2=8, df=2) were removed, separate gene lists for a simple age / ethanol response in both the adolescent (P30C vs. P30E: 763 genes, p < 0.05 = q < 0.42) and adult (P70C vs. P70E: 1132 genes, p < 0.05 = q < 0.19) mice were generated. The dataset is included as Supplemental Table 1. Bioinformatics analyses were completed using the web-based database tool, WebGestalt (Zhang et al., 2005). Statistically significant over-representation of biological pathways was identified by comparing input gene expression data sets to the default mouse genome. Statistical significance for the pathway analyses was set with a Benjamini and Hochberg FDR (false discovery rate) (Benjamini and Hochberg, 1995) of q < 0.05. The pathway information was then manually compared for similarities and differences between the age groups. When several pathways related to neuroimmune function were identified as age/ethanol-divergent, the WebGestalt results were used to generate Figure 2.
Figure 2.
Pathway analysis of alcohol-induced gene expression revealed significant over-representation of immune pathways predominantly in adult mice, with little or no significance in the adolescents. Various biological pathways that directly or indirectly have a role in immune response signaling are shown, with the false discovery rate (FDR) protected significance (q < 0.05) value for both age groups stated for each pathway. Three unbiased WebGestalt (Zhang et al., 2005) analyses were used to determine pathway differences in age- and alcohol-related brain transcriptome changes (AgeC = comparison between adolescent and adult controls, P30E = adolescent control compared to adolescent ethanol drinking, P70E = adult control compared to adult ethanol drinking). The cartoon was generated based on potential mechanisms, as determined in silico, that may influence binge alcohol consumption. Arrows indicate direction of response with initial Tlr4 action leading to either MyD88 dependent or independent downstream changes. It is unknown how alcohol directly acts on neuroimmune function, yet genetic and functional studies have shown a role for LPS, Cd14 and Tlr4. Therefore, a boxed “?” is used to depict possible action. Mechanisms known to occur in the cytoplasm and nucleus are shown in their respective compartments divided by gray bars. However, although action is likely to predominantly occur via microglia, cell specificity remains to be determined in future studies. Abbreviations are: Ccl2/MIP, chemokine (C-C motif) ligand 2 / macrophage inflammatory protein; Cd14, monocyte differentiation antigen Cd14; IL-1B, interleukin 1 beta; IL6, interleukin 6; Jak-STAT, Janus kinase - signal transducer and activator of transcription; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor of kappa light polypeptide gene enhancer in B cells; Tlr4, Toll-like receptor 4.
Drug Administration
Alcohol was administered to the mice as noted under the DID paradigm. To test the effect of minocycline on ethanol drinking, drug was administered to the mice as a solution of its salt form, Minocycline HCl (Sigma Aldrich, St. Louis, MO, catalog no. 13614-98-7), dissolved in Phosphate Buffered Saline (PBS, pH 7.4), at a dose of 50 mg/kg via an intraperitoneal (i.p.) injection (Agrawal et al., 2011). Similarly, a separate group of mice received saline injections at 10 ml/kg i.p., and served as a control group. The mice were injected with saline for three days prior to treatment and then minocycline or saline was administered daily for four days (spanning the entire DID procedure), 20 hr before the start of the DID procedure. [F1 mice: P30♀, n = 8 saline, n = 8 minocycline; P30♂, n = 7 saline, n = 7 minocycline; P70♀, n = 10 saline, n = 11 minocycline; P70♂, n = 9 saline, n = 9 minocycline. B6 mice: P30♀, n = 8 saline, n = 8 minocycline; P30♂, n = 7 saline, n = 7 minocycline; P70♀, n = 11 saline, n = 11 minocycline; P70♂, n = 21 saline, n = 21 minocycline.] Blood samples were collected and processed at the end of DID for BEC measurements.
Pharmacokinetics of Ethanol
To test the effect of minocycline on the metabolism of ethanol in vivo, pharmacokinetic profiling of ethanol was done post-minocycline administration. Adult male and female F1 or B6 mice were injected with a single dose of minocycline (50 mg/kg i.p.) or saline as done in the DID procedure [F1 mice: P70♀, n = 10 saline, n = 11 minocycline; P70♂, n = 9 saline, n = 9 minocycline. B6 mice: P70♀, n = 6 saline, n = 7 minocycline; P70♂, n = 6 saline, n = 7 minocycline]. Twenty-one hours post-injection, mice were gavaged with a 4 g/kg dose of ethanol. For the F1 mice, food was removed from the mice cages 2 hr before gavage and replaced 1 hr after gavage to prevent any alteration in the metabolism of ethanol caused by differential presence of food in the stomach. Blood samples (20 μl) were collected from the mice at 90, 120, 150 min post-gavage by the cheek puncture blood draw method and at 180 min by sacrificing the mice. Minor changes in the procedure were made for the B6 mice based on elimination data obtained from the F1 mice. For the B6 mice, food was taken off 2 hr before and replaced 2 hr after gavage. The blood samples were collected at 165, 210, 265 and 310 min time intervals post-gavage in a similar manner as was done for the F1 mice. The blood samples were processed and analyzed as described above for BEC analysis.
Statistics
For all the DID experiments, ethanol intake was calculated in g/kg body weight of the mice over 4 hr/ day or was averaged over the four days of the DID procedure. In general, outliers were removed from the analysis if they were out of a mean ± 2σ range of the group (See http://www.danielsoper.com/statcalc3/default.aspx for more details). Ethanol intake differences between adolescent and adult F1 mice were compared using a 2-way ANOVA (Age × Days) separately for males and females. A Tukey's post hoc test was used to determine differences between adolescent and adult mice for individual days. Differences in ethanol intake or BEC, between saline or minocycline treated, adolescent and adult, male and female mice were compared using a 3-way multiple design ANOVA (Sex × Age × Treatment), followed by Tukey's or Student's t-test post hoc analysis. For ethanol kinetic studies, the slope of the linear regression line during elimination phase was calculated for each mouse and then treatment groups were compared using the Student's t-test. In general, any mouse that failed to follow the zero order kinetics of ethanol metabolism was considered an outlier for the analysis. Differences in kinetic BEC between treatment groups were tested using a 2-way ANOVA (Sex × Treatment) followed by Bonferroni's post hoc analysis. GraphPad Prism 5.0 (GraphPad Prism Software Inc., San Diego, CA, USA) and R v2.13.2 (R Development Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org, 2006) was used for statistical analysis with significance set as p < 0.05. Statistics used for expression and pathway analyses were described above in the Expression and Bioinformatics Analyses sections.
RESULTS
DID Consumption Differs by Age and Sex
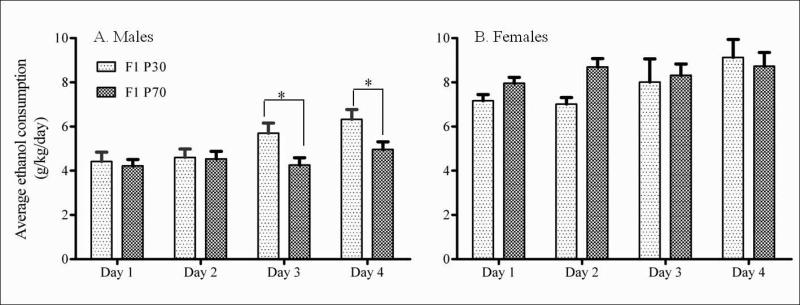
The two-point hypothesis that age and sex influences ethanol drinking was confirmed: Under the four day limited access DID paradigm of voluntary ethanol consumption, adolescent (P30) male mice showed both an increase over time, and higher overall alcohol drinking than adult (P70) male mice (F (1, 34) = 8.139, p < 0.05, Figure 1A). Initial ethanol consumption (Days 1 and 2) in both age groups was similar (P30, x̄ = 4.50 ± 0.29(σx̄); P70, x̄ = 4.38 ± 0.22(σx̄); p = ns) and importantly, as the days of ethanol exposure continued, adolescent mice consumed significantly higher amounts of alcohol than adults (Days 3 and 4) (P30, x̄ = 6.01 ± 0.33(σx̄); P70, x̄ = 4.61 ± 0.25(σx̄); p < 0.05, Figure 1A), signifying a development of increased drinking over time in the adolescent mice. Pharmacologically relevant blood ethanol levels were obtained for both the adolescent (n = 5, BEC = 1.2-2.4 mg/ml) and adult F1 mice (n = 5, BEC = 0.7-1.8 mg/ml) with no significant difference (P30, x̄ = 1.8 ± 0.3(σx̄); P70, x̄ = 1.3 ± 0.3(σx̄); p = ns) between the two age groups. Although the adolescent animals drank more on day 4, the animals were intoxicated to similar physiological levels at the time of tissue collection, likely due to differences in food consumption or timing of drinking within the Day 4 DID exposure. Female F1 mice were also tested; however, they showed no age differences in consumption over time (F (1, 13) = 1.854, p = ns, Figure 1B). 2-way ANOVA confirmed a significant difference between genders for adult (F (1,24) = 104.3, p <0.0001) and adolescent (F (1, 23) = 17.16, p = 0.0004) F1 mice. Post-hoc analyses revealed females drank significantly more than their male counterparts every day over the 4 days of DID (p <0.0001 for adults and p <0.05 for adolescents).
Figure 1.
Age influenced male, but not female, DID alcohol consumption. Ethanol consumption is shown for F1 mice over four days using the DID paradigm for A. males and B. females. The bars represent average ethanol intake in g/kg/day over the four days for adolescent (P30, light textured; n = 18) and adult (P70, dark textured; n = 18) mice as mean ± S.E.M. Two-way ANOVA revealed a significant overall difference in drinking between the adolescent and adult male mice (F (1,34) = 8.139, p < 0.05) but not in the female mice (F (1, 13) = 1.854, p = ns; n = 7 (P30), n = 8 (P70)). Tukey's post-hoc analysis revealed a significant day effect between adolescent and adult male mice for days 3 and 4 (*p < 0.05).
Bioinformatics Analyses Revealed Age Specificity for Alcohol-Mediated Neuroimmune Responses
Microarray hybridization and analyses were carried out only in the ethanol-exposed male mice due to the fact that the females showed no age difference in consumption. Three separate bioinformatics analyses were completed using WebGestalt (Zhang et al., 2005) for: 1) age differences in control mice, and ethanol-mediated responses in 2) P30 adolescent or 3) P70 adult male mouse brain. The collective results revealed over-representation of several immune regulatory pathways, in age-control and predominantly in the P70, and not P30, ethanol-exposed mice as shown in Figure 2. These results led to the generation of our current hypothesis that neuroimmune physiology plays a role in ethanol drinking in an age-specific manner.
The neuroimmune pathways found to be differentially changed by bioinformatics analyses, Toll-like Receptor Signaling, MAPK Signaling, Jak-STAT Signaling, T-Cell Signaling, and Chemokine Signaling, are all related to microglia action. When searching for a potential pharmacologic agent to modulate neuroimmune pathway activity in microglia, there were few glia-selective options that were known to cross the blood brain barrier and could be given intraperitoneally (Agrawal et al., 2011). The best option appeared to be minocycline (Yoon et al., 2012), which is known to reduce TLR4 (Kobayashi et al., 2013, Yang et al., 2007) and other forms of microglial activation (Soliman et al., 2010, Orio et al., 2010, Guasti et al., 2009). Therefore, the bioinformatics analyses strongly suggested that minocycline would be effective in reducing drinking in adult, but not adolescent mice.
Minocycline Decreased Adult, But Not Adolescent, DID Drinking in F1 Mice
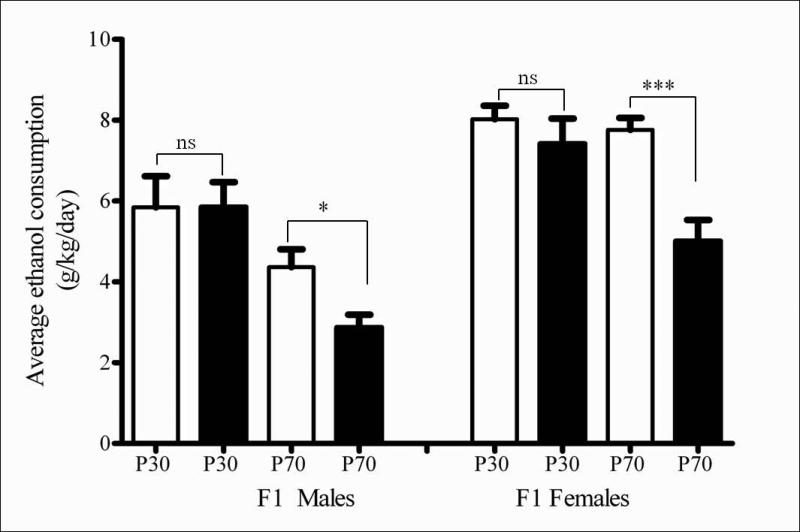
Minocycline pre-treatment had a significant overall reduction effect on ethanol consumption in the adult male (F (1, 16) = 15.06, p < 0.05) and female (F (1, 19) = 31.30, p < 0.001), but not in adolescent male (F (1, 13) = 1.810, p = ns) or female (F (1, 15) = 2.458, p = ns) mice (Figure 3). A 3-way ANOVA overall revealed significant main effects of sex (F (1, 69) = 46.629, p < 0.0001), age (F (1, 69) = 32.302, p < 0.0001) and treatment (F (1, 69) = 12.333, p < 0.001) on ethanol intake. The degree of reduction seen in adults was evident after the first day of treatment and did not change on subsequent days (data not shown), similar to our previous findings (Agrawal et al, 2011).
Figure 3.
Minocycline administration reduced DID drinking in adult (P70), but not adolescent (P30) F1 mice. The bars represent average ethanol consumption in g/kg over the 4 days of measurement as mean ± S.E.M. for adolescent (P30) and adult (P70), saline (open bars) or minocycline (filled bars) treated male or female mice. Minocycline administration did not affect ethanol consumption in adolescent male (F (1,13) = 1.810, p = ns) or female (F (1,15) = 2.458, p = ns) mice, but caused a significant reduction in adult DID drinking in male (F (1,16) = 15.06, p < 0.05) and female (F (1, 19) = 31.30, p < 0.001) mice, compared to saline treated mice as determined using 3-way ANOVA. (*p < 0.05, ***p < 0.001, ns = not significant).
Minocycline Does Not Affect Blood Ethanol Concentration
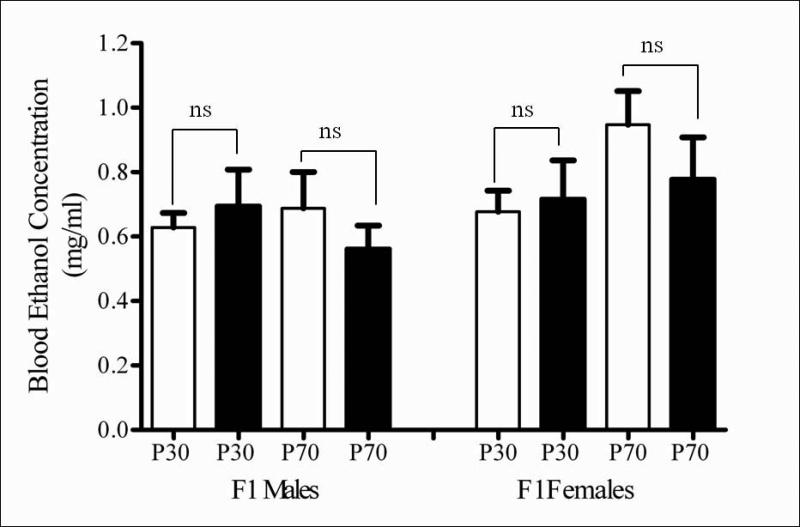
Analysis of blood for ethanol content at the end of the DID paradigm with minocycline administration revealed no effect of the drug on the blood ethanol levels compared to saline treatment (Figure 4). A 3-way ANOVA revealed no significant overall effect of sex (F (1, 48) = 0.0271, p = ns), age (F (1, 48) = 0.3622, p = ns) or treatment (F (1, 48) = 2.0131, p = ns) on the BEC. Hence, no further post-hoc analyses were done.
Figure 4.
Minocycline administration did not affect blood ethanol concentrations (BEC) in adolescent and adult male and female DID treated F1 mice. The bars represent average BEC in mg/ml measured at the end of DID paradigm as mean ± S.E.M. for saline (open bars) or minocycline (filled bars) treated male and female mice. A 3-way ANOVA revealed no significant effect of minocycline on BEC in either the adolescent or adult F1 mice (F (1, 48) = 0.3622, p = ns), compared to saline treated mice. (ns = not significant).
Minocycline Does Not Alter the Pharmacokinetics of Ethanol
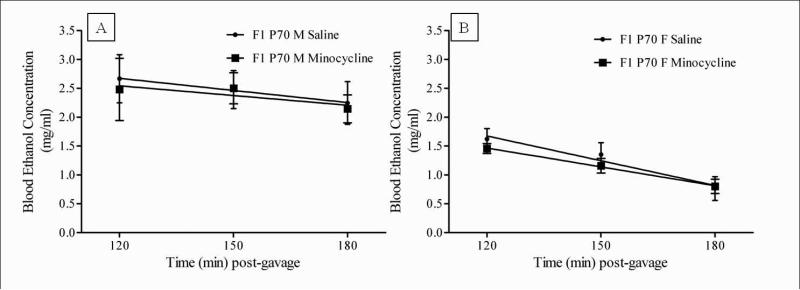
To examine a possible effect of minocycline on alteration in the pharmacokinetic elimination of ethanol, blood profiling for ethanol content was done in the adult F1 mice following drug treatment. The adolescent mice were not tested because they showed no effect of the drug. A 2-way (Sex × Treatment) ANOVA was completed on the slope of the elimination curve for both saline and minocycline treated adult mice. No effect of sex (F (1, 24 ) = 1.8521, p = ns, Figure 5) or treatment (F (1, 24) = 0.4929, p = ns, Figure 5) was revealed, suggesting there was no significant difference in ethanol elimination of the saline or minocycline treated mice.
Figure 5.
Minocycline administration did not affect ethanol elimination in either A. male and B. female adult (P70) F1 mice. Average BEC at 120, 150 and 180 min post-gavage are represented as mean ± S.E.M. for the saline (triangles) and minocycline (squares) treated mice. Lines represent linear regression analysis for estimation of elimination rates. Minocycline treatment did not alter the elimination in either the male or female mice (F (1, 24) = 0.4929, p = ns), compared to saline treated mice. (ns = not significant).
Minocycline Has an Age-Divergent Effect on Ethanol Intake in C57BL/6J Mice
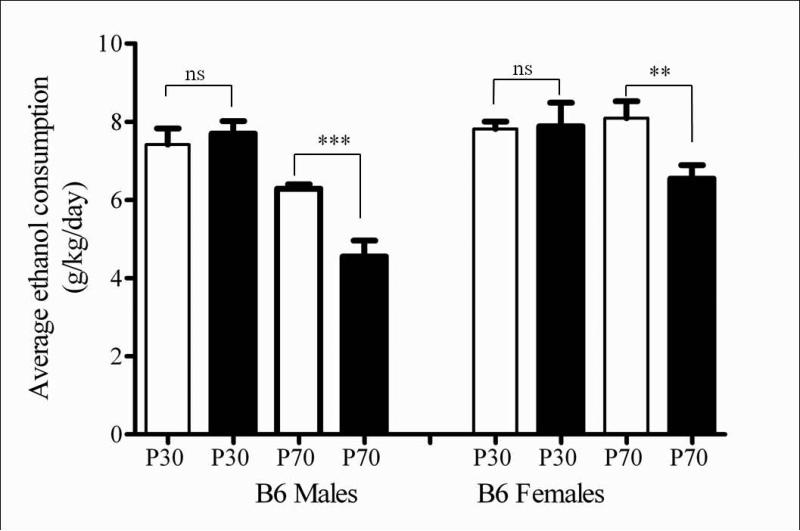
Consistent with the results observed in the F1 mice, B6 mice also showed an age-divergent minocycline response of decreased ethanol intake using the DID paradigm (Figure 6). A 3-way ANOVA revealed an overall significant main effect of sex (F (1, 76) = 17.6185, p < 0.0001), age (F (1, 76) = 28.678, p < 0.0001) and treatment (F (1, 76) = 20.307, p < 0.0001), as well as a significant interaction of age and treatment (F (1, 76) = 6.366, p < 0.05). Minocycline treatment did not affect ethanol consumption in the adolescent male (F (1, 12) = 1.639, p = ns) and female (F (1, 14) = 1.426, p = ns) mice. Further, minocycline treatment caused a significant reduction in ethanol intake in the adult male (F (1, 40) = 12.93, p < 0.001, Figure 6) and female (F (1, 20) = 1.580, p < 0.05, see Figure 6) mice, compared to saline treated controls. The degree of reduction seen was evident after the first day of treatment and did not change on subsequent days (data not shown).
Figure 6.
Minocycline administration reduced DID drinking in adult (P70), but not adolescent (P30) C57BL/6J mice. The bars represent average ethanol consumption in g/kg over the 4 days of measurement as mean ± S.E.M. for saline (open bars) or minocycline treated (filled bars) male and female mice. Minocycline administration did not affect ethanol consumption in adolescent male (F (1, 12) = 1.639, p = ns) and female (F (1, 14) = 1.426, p = ns) mice, but caused a significant reduction in adult male (F (1, 40) = 12.93, p = ns) and female (F (1, 20) = 1.580, p < 0.05), compared to saline treated mice. (*p < 0.05, **p < 0.01, ***p < 0.001, ns = not significant).
Similar results using a 2-bottle choice alcohol consumption paradigm and adult male and female mice have been previously reported by our lab (Agrawal et al., 2011).
Minocycline Does Not Alter the Pharmacokinetics of Ethanol in B6 Mice
The B6 pharmacokinetic profile for ethanol elimation following minocycline or saline pre-treatment were simliar to the F1 results in that there was no effect of drug on ethanol metabolism. A 2-way (Sex × Treatment) ANOVA on the slopes of the elimination curve for saline and minocycline treated adult mice revealed an overall effect of Sex (F (1, 24) = 8.5105, p < 0.01, Figure 7) and no signficant effect of treatment (F (1, 24) = 0.0615, p = ns, Figure 7), suggesting no significant difference in ethanol elimination of the saline or minocycline treated mice.
Figure 7.
Minocycline administration did not affect ethanol elimination in either A. male or B. female adult (P70) B6 mice. Average BEC at 165, 210, 255, and 300 min post-gavage are represented as mean ± SEM for the saline (triangles) and minocycline (squares) treated mice. Lines represent linear regression analysis for estimation of elimination rates. Minocycline treatment did not alter the rate of ethanol elimination in either the male or female mice (F (1, 24) = 0.0615, p = ns), compared to saline treated mice. (ns = not significant).
DISCUSSION
The age and gender of individuals who drink have a significant impact on the severity and progression of alcohol use problems. Alcoholism subtypes also break along these lines with Early Onset or Type II Alcoholism (EOA) and Late Onset or Type I Alcoholism (LOA) showing distinct phenotypic differences as well as some therapeutic distinctions (Babor et al., 1992). Gender differences in alcohol responses are still not well understood, but some significant differences have been reported. For instance, women, even when they drink the same per weight, show more tissue damage than men (Squeglia et al., 2012). It was with this in mind that we conducted our study using male and female animals at both adolescent and adult ages.
The complexity of AUD makes development of new pharmacotherapies challenging. Currently, treatment for alcohol problems is limited and includes behavioral modification programs and a small number of medications, including some that were first used many decades ago (Volpicelli et al., 1992, Kranzler and Van Kirk, 2001, Johnson et al., 2003). None are highly effective, as would be expected for a complex disorder with underlying subtypes (Anton et al., 2006). As a consequence, new drugs for treatment are much needed; especially those targeting AUD subtypes or as individualized medications. The overarching purpose of our initial experiment was to identify new targets and to focus our effort on those with specific translational promise.
Our goal was to use the DID/F1 mouse model to find age and sex differences. Results from the DID experiments showed that females drank more per body weight than males, which is a common observation in rodent studies (Melon et al., 2013), although variations are observed, most likely dependent upon differences in the drinking model and strains of mice used. The male mice began drinking equivalently on day one, and by day three, the adolescent group showed elevated consumption, suggesting that the act of drinking altered their consumption. In humans, young drinkers tend to drink more per binge episode (Bennett et al., 1999, Raivich et al., 1999), making our animal model consistent with epidemiological reports. Adolescence is a known ‘critical period’ for AUD risk but the mechanisms underlying either EOA or LOA development are not understood.
Although the ethanol intake, measured in g/kg body weight, was higher in the females compared to the males, no age difference in drinking was seen in the female mice. The reason for the lack of age difference in drinking in the F1 females is not known at this time. A follow-up power analysis showed that no difference would be expected for even very large sample sizes (Cohen's d = 0.02). Since only the male F1 mice showed an age-developmental difference in consumption, we limited our microarray hybridization to RNA extracted from male mouse brain. Blood ethanol concentration (BEC) at the end of the DID period on day 4 did not differ by age despite the fact that the adolescent mice drank more alcohol. The most likely explanation is that consumption of both food and alcohol solution varied over time within the DID window. It is important to note that all animals of both age groups showed pharmacologically relevant and over-lapping BEC, with a similar physiological range, that of motor incoordination, but not CNS-mediated sedation. In order to focus on age-differences rather than a potential dose-response, we first determined expression that was significantly influenced by dose, and these 396 genes were removed from the age × alcohol-response analyses. WebGestalt pathway analyses then uncovered several significantly over-represented pathways related to neuroimmune function predominantly in the adults as shown in Figure 2. The data revealed important control and alcohol-responsive age-specific components.
Bioinformatic pathway analysis uncovered transcriptomic changes in genes reported to be linked to common processes (ie, a pathway). The central role of the “Toll-like Receptor Signaling” pathway and the concurrent significant changes in “MAPK Signaling” and “Jak-STAT Signaling”, coupled with changes in “Chemokine Signaling” pathways immediately pointed to brain immune (neuroimmune) cells as potential targets for pharmacologic intervention. The most obvious brain immune cell known to utilize all of these pathways is the brain microglia. They are the primary brain immune cell (Gehrmann et al., 1995, Aloisi, 2001) and respond to several signals via TLR4 activation (Zhang et al., 2012, Scheffel et al., 2012). Furthermore, NF-κB and STAT-1 pathways are activated downstream of TLR4 activation in microglia (Qin et al., 2005), the chemokine receptor C×C3R1 expressed on microglia is important for the CNS effects of LPS (Corona et al., 2010), MAPK signaling is involved in microglia activation (Zhao et al., 2007), and ethanol has been reported to activate TLR4 on microglia (Fernandez-Lizarbe et al., 2009).
We, and others, have previously shown a role for neuroimmune modulation in high alcohol consumption (Agrawal et al., 2011, Blednov et al., 2011, Blednov et al., 2005a, Blednov et al., 2012, Liu et al., 2011). Therefore, based on our bioinformatics analyses, we tested the newly generated hypothesis that suppression of neuroimmune function would alter drinking in adult, but not, adolescent mice. As mentioned previously, our search for a potential pharmacologic agent to modulate neuroimmune pathway activity in microglia revealed there were few glia-selective options (Agrawal et al., 2011). The best appeared to be minocycline (Yoon et al., 2012), which crosses the blood brain barrier and is known to reduce TLR4 (Kobayashi et al., 2013, Yang et al., 2007) and other forms of microglial activation (Soliman et al., 2010, Orio et al., 2010, Guasti et al., 2009). Therefore, the bioinformatics results strongly suggest minocycline would be effective in reducing drinking in adult, but not adolescent mice. Minocycline pretreatment effects shown in Figures 3 and 6 indicated a rejection of the null hypothesis, that no age-related difference in DID consumption would be detected. These results are important as a proof of concept; that bioinformatics analyses can, and do, provide a useful tool for the identification of potential pharmacotherapeutic targets (Ai et al., 2013).
In order for our results to have a practical application, we needed to show that minocycline did not reduce drinking by changing ethanol metabolism. Pharmacokinetic elimination studies in both genders and genotypes provided evidence that the observed reduction in drinking was due to a pharmacodynamic mechanism rather than metabolic changes. Future studies are planned to elucidate the mechanism(s) involved.
Collectively, the data suggests that neuroimmune responses to binge alcohol consumption differ in adolescent and adult mice. Several general mechanisms could explain the differences, including, but not limited to known age differences in microRNA expression (Kapsimali et al., 2007) and/or immune development changes as seen in the age-control comparison in Fig 2. Suppression of an alcohol-mediated immune response decreased drinking in adult mice, and may also do so in LOA, but not EOA individuals. Minocycline is an FDA approved, and commonly used drug and thus, translational studies would allow facile efficacy testing. A recent review suggests consideration of minocycline use for other diseases/disorders with a neuroimmune component (Garrido-Mesa et al., 2013). The neuroimmune suppressive effect of minocycline is not fully understood. Minocycline is a tetracycline antibiotic, but its anti-inflammatory and neuroimmune actions are independent of it bactericidal effects (e. g., Kielian et al., 2007, Sewell et al., 1996). Minocycline is known to down-regulate expression of pro-inflammatory genes and mediators in both the peripheral and central immune systems (Homsi et al., 2009, Kloppenburg et al., 1996, Nikodemova et al., 2010, Henry et al., 2008, Huang et al., 2009), through such mechanisms as divalent ion chelation, inhibition of matrix metalloproteases (MMPs), and scavenging of reactive oxygen species, that likely contributes to its neuroimmune and neuroprotective effects (Plane et al., 2010, Bahrami et al., 2012). As mentioned previously, NF-κB and STAT-1 pathways are activated downstream of TLR4 activation in microglia (Qin et al., 2005) and MAPK signaling also is involved in microglia activation (Zhao et al., 2007). This in turn leads to morphological changes in microglia and production of cytokines, chemokines, and their receptors (for a review see Raivich et al., 1999). Given that ethanol has been reported to stimulate NF-κB and MAPKs, and activate TLR4 on microglia (Fernandez-Lizarbe et al., 2009) it is tempting to assume that the anti-drinking properties of minocycline are due to interactions with brain microglia. However, further study is needed to support this assumption.
Our study, for the first time, showed an age-divergent role of neuroimmune pathways in regulating an ethanol-drinking phenotype. Importantly, we also showed the age-specific effectiveness of the neuroimmune modulator, minocycline, in reducing ethanol consumption in adult mice under the DID limited access paradigm. Generation of new hypotheses from our bioinformatics analytical results serves as an important proof of concept and suggests that drugs acting on neuroimmune pathway targets may represent potential new treatments for modulating ethanol consumption in specific AUD subtypes.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by NIAAA INIA grants U01AA13475 (SEB), U01AA13520 (YAB) and the South Plains Alcohol and Addiction Research Center.
Footnotes
Conflict of Interest Statement
All authors declare that there are no conflicts of interest.
REFERENCES
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain Behav Immun. 2011;25(Suppl 1):S165–S169. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai Z, Wang J, Xu Y, Teng Y. Bioinformatics analysis reveals potential candidate drugs for cervical cancer. J Obstet Gynaecol Res. 2013;39:1052–8. doi: 10.1111/jog.12022. [DOI] [PubMed] [Google Scholar]
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, Locastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, Delboca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Bahrami F, Morris DL, Pourgholami MH. Tetracyclines: drugs with huge therapeutic potential. Mini Rev Med Chem. 2012;12:44–52. doi: 10.2174/138955712798868977. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statis Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bennett ME, Mccrady BS, Johnson V, Pandina RJ. Problem drinking from young adulthood to adulthood: patterns, predictors and outcomes. J Stud Alcohol. 1999;60:605–14. doi: 10.15288/jsa.1999.60.605. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VMM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005a;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005b;29:1949–58. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–20. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, D'amico EJ. Outcomes of alcohol treatment for adolescents. Recent Dev Alcohol. 2001;15:307–27. doi: 10.1007/978-0-306-47193-3_18. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann N Y Acad Sci. 2004;1021:234–44. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, Eskay RL, Heilig M. Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biol Psychiatry. 2010;67:823–30. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O'connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT. Ethanol induction of innate immune genes creates the neurobiology of addiction. Alcohol. 2011;45:269. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of Neurodegeneration and Regeneration in Alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels GM, Buck KJ. Expression profiling identifies strain-specific changes associated with ethanol withdrawal in mice. Genes Brain Behav. 2002;1:35–45. doi: 10.1046/j.1601-1848.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Interactions of alcohol and nitric-oxide synthase in the brain. Brain Res Brain Res Rev. 2005;49:494–504. doi: 10.1016/j.brainresrev.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Johnson BA. Pharmacotherapeutic trials in adolescent alcohol use disorders: opportunities and challenges. Alcohol Alcohol. 2004;39:166–77. doi: 10.1093/alcalc/agh045. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Strother WN, Mcclintick JN, Tian H, Stephens M, Jerome RE, Lumeng L, Li TK, Mcbride WJ. Gene expression in the hippocampus of inbred alcohol-preferring and -nonpreferring rats. Genes Brain Behav. 2005;4:20–30. doi: 10.1111/j.1601-183X.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–44. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41). Alcohol Clin Exp Res. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res. 2013;67:18–30. doi: 10.1016/j.phrs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Pettinati HM, O'malley S, Randall PK, Randall CL. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin Exp Res. 2010;34:1803–12. doi: 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasti L, Richardson D, Jhaveri M, Eldeeb K, Barrett D, Elphick MR, Alexander SP, Kendall D, Michael GJ, Chapman V. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol Pain. 2009;5:35. doi: 10.1186/1744-8069-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflamm. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi S, Federico F, Croci N, Palmier B, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 2009;1291:122–32. doi: 10.1016/j.brainres.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Huang TY, Chu HC, Lin YL, Lin CK, Hsieh TY, Chang WK, Chao YC, Liao CL. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol. 2009;237:69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, Diclemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Kapsimali M, Kloosterman WP, De Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns RT, Miles MF. Microarray analysis of ethanol-induced changes in gene expression. Methods Mol Biol. 2008;447:395–410. doi: 10.1007/978-1-59745-242-7_26. [DOI] [PubMed] [Google Scholar]
- Key J, Hodgson S, Omar RZ, Jensen TK, Thompson SG, Boobis AR, Davies DS, Elliott P. Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issues. Cancer Causes Control. 2006;17:759–70. doi: 10.1007/s10552-006-0011-0. [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Ruhe JJ. Minocycline modulates neuroinflammation independently of its antimicrobial activity in staphylococcus aureus-induced brain abscess. Am J Pathol. 2007;171:1199–214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg M, Brinkman BM, De Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, Dijkmans BA, Verweij C. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996;40:934–40. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–41. [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–82. [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr., Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr., Aurelian L. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108:4465–70. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Mcqueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Wray KN, Moore EM, Boehm SL., 2nd Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacol Biochem Behav. 2013;104:177–87. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Boehm SL, 2nd, Owen JA, Levin PS, Berman AE, Blednov YA, Crabbe JC, Williams RW, Miles MF, Bergeson SE. Alcohol Trait and Transcriptional Genomic Analysis of C57BL/6 Substrains. Genes Brain Behav. 2008;7:677–89. doi: 10.1111/j.1601-183X.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–73. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Adron Harris R, Ponomarev I. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:659–70. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Lee J, Fabry Z, Duncan ID. Minocycline attenuates experimental autoimmune encephalomyelitis in rats by reducing T cell infiltration into the spinal cord. J Neuroimmunol. 2010;219:33–7. doi: 10.1016/j.jneuroim.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Orio L, Llopis N, Torres E, Izco M, O'shea E, Colado MI. A study on the mechanisms by which minocycline protects against MDMA (‘ecstasy’)-induced neurotoxicity of 5-HT cortical neurons. Neurotox Res. 2010;18:187–99. doi: 10.1007/s12640-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for Minocycline Neuroprotection. Arch Neurol. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin HW, Wilson CA, Lee SJ, Zhao XY, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-kappa B and STAT-1 alpha in macrophages and microglia. Blood. 2005;106:3114–3122. doi: 10.1182/blood-2005-02-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Scheffel J, Regen T, Van Rossum D, Seifert S, Ribes S, Nau R, Parsa R, Harris RA, Boddeke HW, Chuang HN, Pukrop T, Wessels JT, Jurgens T, Merkler D, Bruck W, Schnaars M, Simons M, Kettenmann H, Hanisch UK. Toll-like receptor activation reveals developmental reorganization and unmasks responder subsets of microglia. Glia. 2012;60:1930–43. doi: 10.1002/glia.22409. [DOI] [PubMed] [Google Scholar]
- Sewell KL, Breedveld F, Furrie E, O'brien J, Brinckerhoff C, Dynesius-Trentham R, Nosaka Y, Trentham DE. The effect of minocycline in rat models of inflammatory arthritis: correlation of arthritis suppression with enhanced T cell calcium flux. Cell Immunol. 1996;167:195–204. doi: 10.1006/cimm.1996.0027. [DOI] [PubMed] [Google Scholar]
- Soliman GM, Choi AO, Maysinger D, Winnik FM. Minocycline block copolymer micelles and their anti-inflammatory effects on microglia. Macromol Biosci. 2010;10:278–88. doi: 10.1002/mabi.200900259. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220:529–39. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM. Neuroinflammation as a neurotoxic mechanism in alcoholism: Commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Exp Neurol. 2008;213:10–17. doi: 10.1016/j.expneurol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Bhave SV, Hoffman PL. Selective breeding, quantitative trait locus analysis, and gene arrays identify candidate genes for complex drug-related behaviors. J Neurosci. 2003;23:4491–8. doi: 10.1523/JNEUROSCI.23-11-04491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano-Marquez A, Estruch R, Fernandez-Sola J, Nicolas JM, Pare JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. Jama. 1995;274:149–54. doi: 10.1001/jama.1995.03530020067034. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO. Minocycline and sulforaphane inhibited lipopolysaccharide-mediated retinal microglial activation. Mol Vis. 2007;13:1083–93. [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Patel D, Dougherty PM. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience. 2012;221:214–24. doi: 10.1016/j.neuroscience.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–8. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Immunolocalization of Toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. Neuroimmunomodulation. 2012;19:10–9. doi: 10.1159/000326771. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.