Summary
Sleep homeostasis, which refers to the maintenance of sleep amount or depth following sleep deprivation, indicates that sleep and sleep-like states serve fundamental functions that cannot be bypassed [1]. Homeostasis of sleep-like behavior is observed during C. elegans lethargus, a 2-3 hour behavioral quiescent period that occurs during larval state transitions [2]. Here, we report a role for DAF-16/FOXO, a transcription factor that is active under conditions of stress [3], in the response to deprivation of lethargus quiescence. Forced locomotion during lethargus results in nuclear translocation of DAF-16. The formation of dauer larvae, a developmental state promoted by daf-16, is increased in response to quiescence deprivation. daf-16 mutants show an impaired homeostatic response to deprivation of lethargus quiescence, and are hypersensitive to the lethal effects of forced locomotion during lethargus. DAF-16 expression in muscle cells but not in neurons is sufficient to restore a homeostatic response to deprivation of quiescence, pointing to a role for muscle in sleep homeostasis. These findings are relevant to clinical observations of altered metabolic signaling in response to sleep deprivation and suggest that these signaling pathways may act in non-neuronal tissue to regulate sleep behaviors.
Results and Discussion
Elevated daf-16 signaling in response to deprivation of lethargus quiescence
We hypothesized that given the adverse consequences of sleep deprivation in other species [4, 5], deprivation of lethargus quiescence will be a stressor in C. elegans. DAF-16, a FOXO transcription factor involved in multiple stress responses [3, 6, 7], is partially activated by nuclear translocation [3, 8, 9]. The absence of food for greater than one hour results in nuclear translocation of DAF-16 [3]. While during lethargus the animal is surrounded by food, it cannot eat because there is a plug of extracellular material that occludes the buccal opening to the pharynx [10, 11], and because pharyngeal pumping, which is essential for ingestion [12], ceases [10, 11]. Since worms do not feed during lethargus, which can last up to three hours, we were surprised to observe DAF-16 distributed in the cytoplasm during the fourth larval (L4) lethargus period (Figure 1A-B). This observation extends to the first larval (L1) lethargus period. DAF-16 nuclear localization was low at hatching, but increased to a maximum during the mid L1 stage. During the L1 lethargus period, DAF-16 showed less nuclear localization than during the surrounding larval stages (Figure 1C). These observations indicate that there is reduced nuclear DAF-16 during lethargus despite the absence of feeding.
Figure 1.
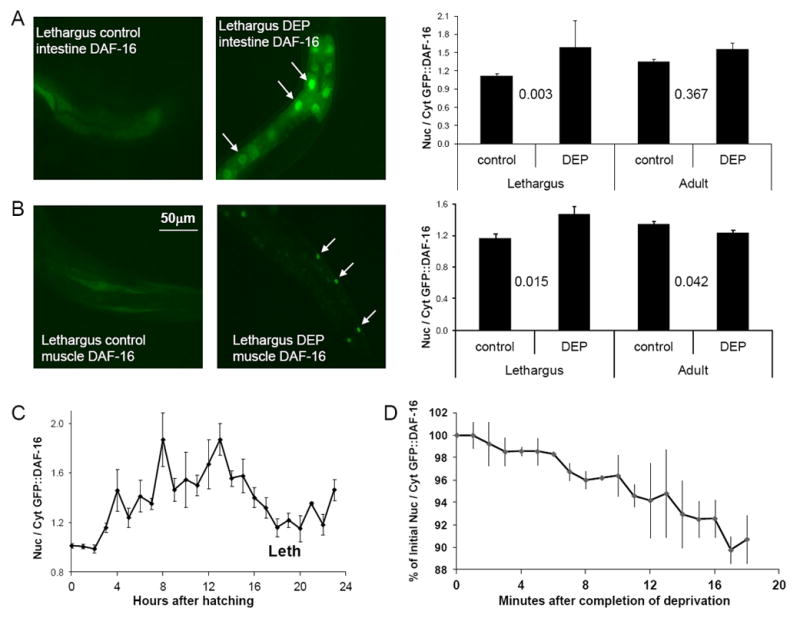
Nuclear translocation of DAF-16 as a function of developmental time (C) and in response to deprivation of lethargus quiescence in intestine (A), and body muscle (B). DEP denotes worms that had been stimulated during lethargus (Protocol 2) for 30 minutes. White arrows in the fluorescent images point to nuclei showing DAF-16∷GFP localization. The average pixel fluorescent intensity ratio between the nucleus and the cytoplasm is shown. The p value (2-tailed t test) is shown between conditions compared. N=15 for each condition. (D) The nuclear/cytoplasmic ratio decreases with time after completion of deprivation. Shown is the average of 3 worms, each mounted for imaging within 5 minutes of being deprived of L4 lethargus quiescence for 30 minutes, and then imaged for 20 minutes. Error bars denote standard deviation.
Does depriving worms of lethargus quiescence affect the sub-cellular distribution of DAF-16? We stimulated L4 lethargus animals to move in a liquid bacterial suspension for 30 minutes. L4 lethargus worms that had been stimulated for 30 minutes showed greater nuclear-localized DAF-16 than agematched control animals (Figure 1A-B). Adult worms, stimulated at the same frequency, did not show an increase in nuclear DAF-16 in comparison to age-matched controls (Figure 1A-B). Stimulation of animals without liquid immersion, by touching them with a wire every 20 seconds for 30 minutes, also led to DAF-16 nuclear translocation during lethargus (Figure S1B). We observed nuclear translocation of DAF-16 in both body muscle and intestinal cells (Figures 1A-B and S1B). In the nervous system, we did not see a difference in DAF-16 sub-cellular distribution between deprived and control animals (data not shown), though due to the high nucleus to cytoplasm ratio of neuron cell bodies, an effect would be difficult to detect.
DAF-16 nuclear translocation is positively regulated by the stress-activated c-Jun N-terminal kinase JNK-1 [13], and by the dafachronic acid nuclear receptor DAF-12 [14]. While the overall ratio of nuclear to cytoplasmic DAF-16 was reduced in jnk-1(gk7) mutants, as reported [13], DAF-16 was more nuclear in worms deprived of lethargus quiescence than in non-deprived animals (Figure S1C). By contrast, in daf-12(rh61rh411) mutants, the nuclear to cytoplasmic ratio of DAF-16 was not different between deprived and control animals (Figure S1C), implicating DAF-12 in this response.
Does the molecular response to sleep deprivation dissipate as the animals are allowed to sleep? Over a 20-minute recovery period following the 30-minute swimming deprivation of quiescence, we observed a re-distribution of DAF-16 from the nucleus to the cytoplasm (Figure 1D). Therefore, the C. elegans molecular response to deprivation of sleep-like behavior is transient, as has been observed in mammals and Drosophila [15-17].
To test if DAF-16 signaling is increased by deprivation of lethargus quiescence, we used a dauer formation assay as a read out of DAF-16 signaling. The dauer is a 3rd larval stage that forms under unfavorable conditions [18]. The decision to enter the dauer stage is made partially during L1 lethargus [19]. Since DAF-16 promotes dauer formation [20], we asked if deprivation of L1 lethargus quiescence increases dauer formation. To increase the propensity to form dauers, we used animals mutant for daf-8 [21]. In daf-8 mutants, increased DAF-16 signaling further increases the formation of dauers [22]. We therefore used a change in dauer formation propensity of daf-8 mutants to infer effects of DAF-16 signaling.
We deprived daf-8 mutants of quiescence by forcing them to swim in a bacterial suspension for one hour beginning at the start of L1 lethargus. Three control groups of animals were treated identically except they were (1) not forced to swim, (2) forced to swim before L1 lethargus, or (3) forced to swim after L1 lethargus. We observed a higher percentage of dauers among animals that had been forced to swim during L1 lethargus than among control animals (Figure 2). A similar result was observed with deprivation of mutants for daf-7, which encodes a TGF-β acting upstream of daf-8 [23] (Figure 2). These results are not due to the buffer in which the worms swam because we also observed an enrichment in dauer formation when daf-8 mutants were kept moving in a lawn of bacteria by touching them with a wire every 20 seconds for one hour (23/63 dauers in deprived animals; 6/100 dauers in control animals, p<0.001, Fisher 2-tailed test). Consistent with the notion that increased dauer formation is partially explained by increased DAF-16 activity in response to deprivation of L1 lethargus quiescence, the effect of deprivation on daf-7 dauer formation was attenuated by introducing the daf-16 loss-of-function mutation mu86 into the strain (Figure 2).
Figure 2.
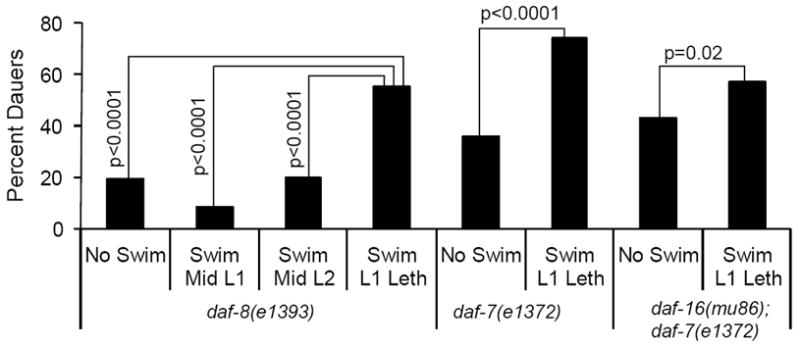
Dauer formation is increased following deprivation of lethargus quiescence. Forced swimming of daf-8(e1393) and daf-7(e1372) mutants during L1 lethargus leads to a greater percentage of dauers. Introducing the daf-16(mu86) into the daf-7 mutant attenuates the dauer-inducing effects of lethargus quiescence deprivation. N was 86-181 in each condition. P values were calculated with a twotailed Fisher’s Exact Test.
Taken together, these experiments provide evidence that increased DAF-16/FOXO signaling is a physiological consequence of the deprivation of lethargus quiescence.
daf-16 is required for the normal behavioral response to deprivation of lethargus quiescence
Previous analysis indicated that the time at which quiescence of locomotion ends is not affected by deprivation of the early part of lethargus [2]. Consistent with this observation, we detected no difference in the timing of pharyngeal pumping resumption, defecation resumption, or of ecdysis between unperturbed L4 lethargus worms and L4 lethargus worms that were forced to swim for 30 minutes starting in the first 5 minutes of L4 lethargus (Figure 3b).
Figure 3.
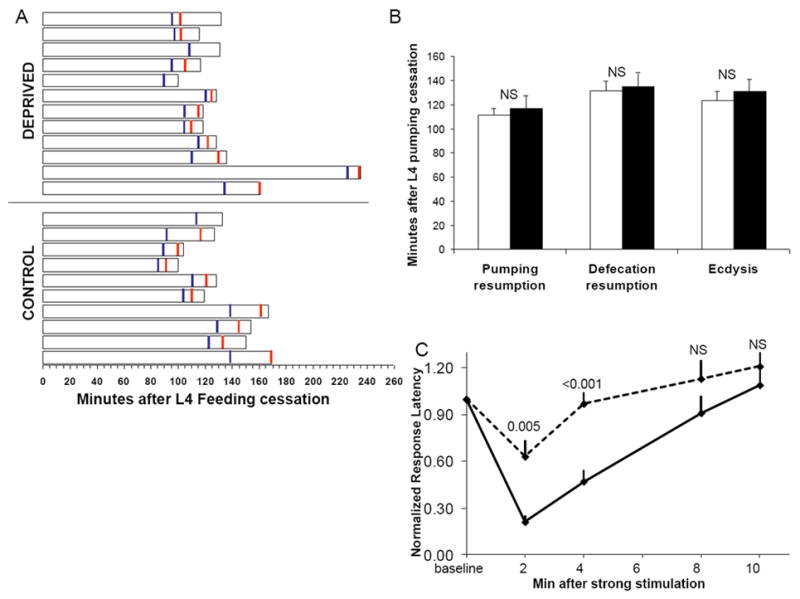
Deprivation of early L4 lethargus quiescence does not delay the timing of lethargus end points but does affect subsequent sleep quality. (A) Duration from onset of pumping cessation to ecdysis of individual worms. Blue vertical lines denote resumption of pharyngeal pumping and red vertical lines denote resumption of defecation movements. (B) Mean durations of the data presented in A. Filled bars denote deprived animals. NS denotes not significant p>0.1 (two-tailed Student’s T test). Two worms, which did not recover from the deprivation and were therefore censored (Table S2), were observed to resume body movements but not pharyngeal pumping or defecation movements and remained trapped in their prior stage cuticle. (C) Wild-type worms have shortened 1-octanol response latencies following strong stimulation during lethargus but then return to baseline elevated response latencies over 10 minutes. In contrast, worms that had been stimulated for 30 minutes during lethargus (dotted line, Protocol 2) show an accelerated return to baseline response latencies after a strong stimulus. P values shown at each time point were calculated with a two-tailed Student’s t test. N=10 worms. Error bars denote SEM. NS denotes p>0.05.
In contrast to the timing of the events marking the completion of lethargus, which were unaffected by deprivation of quiescence, the arousal threshold of animals was strongly affected by deprivation (Figure 3c). Wild-type animals forced to swim for 30 minutes during lethargus showed an accelerated return to sleep-like behavior, as demonstrated by an increased response latency to the noxious chemical 1-octanol [2]. The increased response latency after deprivation is not explained by irreversible damage to the animal, because the 1-octanol response latency of 10 animals four hours after the completion of deprivation during L4 lethargus, during their early adult stage, (3.3±3.0 seconds, mean±SD) was not different from that of 10 age-matched control animals (2.3±1.1 seconds, p=0.7, Mann-Whitney U Test). Therefore, the quality but not the duration of quiescent behavior is affected by deprivation of quiescence.
In contrast to wild-type worms, daf-16(mu86) and daf-16(mgDf50) mutants [22, 24], did not demonstrate elevated 1-octanol response latencies following deprivation of lethargus quiescence (Figure 4). This defect in the homeostatic response is not explained by reduced baseline quiescence since total quiescence during L4 lethargus (107.1±17.9 min) and L4 lethargus duration (3.08±0.24 hrs) of 16 daf-16(mu86) mutant animals were not different from these measurements in 104 wild-type animals (97.9 ±15.3 min, 3.04±0.22 hrs, respectively. Shown are mean±SD). Arousal threshold was also not defective in these mutants, as baseline 1-octanol response latencies of unperturbed worms in L4 lethargus were not reduced in daf-16(mgDf50) (11.5±4.7, N=80, p=0.19) and were slightly increased in daf-16(mu86) mutants (15.8±4.4, N=60, p=0.001) in comparison to wild-type worms (12.8±5.6, N=70). Therefore, daf-16 is required for the normal homeostatic response to deprivation of lethargus quiescence without impairing the baseline sleep-like behavior. This suggests that baseline sleep behavior and the behavioral response to sleep deprivation can be genetically separated, as has been shown for sleep in other animals [25, 26].
Figure 4.
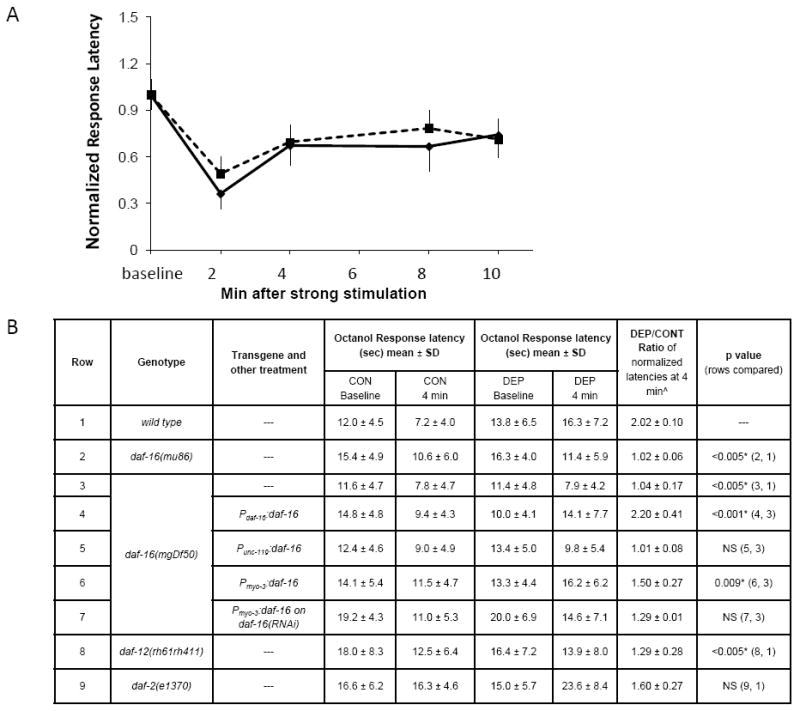
daf-16 mutants are defective in the homeostatic behavioral response to deprivation of lethargus quiescence. A. daf-16(mgDf50) mutants have 1-octanol response latencies following 30-minutes of stimulation (Protocol 2) during lethargus (dotted line) similar to those of non-deprived animals (solid line). At all times points, p>0.2, Student’s t test. N=10 worms. Error bars denote SEM. B. 1-octanol response latencies at baseline and at 4 minutes after strong stimulation without (CON) and with (DEP) a 30-minute deprivation of lethargus quiescence (Protocol 2). ˆAverage of 3-5 trials, with 10 worms per trial. *Significant after Bonferroni correction for multiple testing.
To ask whether constitutively nuclear DAF-16 affects the homeostatic response to deprivation of lethargus quiescence, we tested animals with a reduction of function mutation in daf-2. The daf-2 mutant e1370 had an elevated baseline 1-octanol response latency both during L4 lethargus (Figure 4B) as well as during the adult stage (5.9±2.4 in daf-2(e1370) versus 3.9±2.0 in wild-type adults, p=0.01, Mann-Whitney U Test). The response latency was further elevated after deprivation of lethargus quiescence (Figure 4B), indicating that nuclear DAF-16 does not occlude a homeostatic response.
Finally, we tested the homeostatic response to deprivation of lethargus quiescence in daf-12(rh61rh411) mutants. Like daf-16 mutants, daf-12 mutants had a defective homeostatic response to deprivation of lethargus quiescence (Figure 4B). A recent report showed that daf-12 positively regulates DAF-16 via its negative regulation of two DAF-16 negative regulators, AKT-1 and LIN-14 [27]. AKT-1 is a particularly attractive candidate to mediate a sleep homeostatic response since it is a target of the metabolically sensitive TOR kinase [28], an enzyme that is negatively regulated by sleep deprivation in mice [29].
Role for muscle cells in the response to deprivation of lethargus quiescence
Mechanical stimulation during lethargus has two effects: the animal is awake at a time when it normally sleeps, and muscle contractions occur at a time when the animal is normally still. These effects occur in every experiment where a laboratory animal is forced to move during its quiescent sleep time.
To test the role of muscle in the homeostatic response to deprivation of lethargus quiescence, we restored DAF-16 function in muscle and other tissues. As expected, expression of daf-16 under the control of its own promoter rescued the defective homeostatic response to deprivation of lethargus quiescence (Figure 4B). In addition, daf-16 expression in body muscle cells partially restored the homeostatic response of daf-16 mutants to deprivation of lethargus quiescence (Figure 4B). This rescue was abrogated when these transgenic animals were treated with daf-16(RNAi), indicating that it was the muscle expression of daf-16 that was restoring the homeostatic response (Figure 4B). In contrast to its effect in muscle, daf-16 expression in neurons did not restore the homeostatic response of daf-16 mutants (Figure 4B).
The homeostatic behavioral response may be explained by a physiological change in sensory neurons, interneurons, motor neurons, or muscle, or by a change in a combination of these sites. To study the integrity of neuromuscular function in response to deprivation of lethargus quiescence, we stimulated cholinergic motor neurons optogenetically. This stimulation, which causes a muscle contraction and therefore shortening of the worm’s body [30], caused the same magnitude of muscle contraction in lethargus worms that had not been perturbed (4.6±1.8% of initial body length, N=8) as in lethargus worms that had been forced to move for 30 minutes (5.0±1.8%, N=8, p=0.60, Mann-Whitney U test). Therefore, although body muscle cells are involved in the homeostatic response to deprivation of lethargus quiescence, the reduced responsiveness to 1-octanol is likely to be explained by effects outside the muscle or the neuromuscular junction.
Deprivation of lethargus quiescence is lethal
We noted that 11% (N=265) of wild-type worms did not recover after forced movement for 30 minutes during L4 lethargus. Microscopic inspection of these arrested animals showed that, while they had secreted and assembled an adult cuticle as evidenced by the presence of a new cuticle lining the buccal cavity (Figure S3A) and adult-specific alae (Figure S3E), they were unsuccessful in escaping from the L4 cuticle (Figure S3A-D). That is, these worms had a molting-defective (Mlt) phenotype [31].
We used daf-16 mutants to sensitize the animals to the lethal effects of deprivation of lethargus quiescence. 18% of daf-16(mu86) (N=55), 57% of daf-16(mgDf50) (N=44), 53% daf-16(m26) (N=55) and 38% of daf-16(m27) (N=63) mutants died after 30 minutes of continuous forced swimming movement during lethargus. To address the possibility that the lethality was caused by mechanical injury, we mechanically stimulated first larval stage (L1) daf-16 mutants. Beginning 14 hours after first exposure to food (bacteria) and repeating once every hour for a different cohort of worms, daf-16 L1 worms were transferred from an agar surface into buffer where they were stimulated for an hour using a vortex to agitate the buffer. To insure that all worms were receiving identical stimulation, worms of various ages were agitated simultaneously on the vortex. We observed a peak in lethality for L1 worms that were agitated at a time that corresponded to L1 lethargus in daf-16 mutants (Figure S3F).
It is possible that worms in lethargus are more sensitive to mechanical stimulation due to fragile properties of their exoskeleton at that stage. To control for the stage of stimulation, we mechanically stimulated pairs of daf-16 worms, which were at the identical stage of L4 lethargus. We stimulated one worm, which we term “experimental”, to move continuously by mechanically stimulating it each time it stopped moving. We stimulated the second animal, which we term “yoked control”, each time the experimental worm was stimulated but irrespective of whether or not this yoked control animal was quiescent. The experiment was continued until the experimental animal no longer responded to stimulation. Thus, the experimental worm was totally deprived of quiescence whereas the yoked control animal was only partially deprived of quiescence. Six of eight experimental animals died as a consequence of this stimulation, which had a duration of 59±5 minutes (mean±SEM), whereas zero of eight yoked control animals died (p=0.02, 1-tailed Fisher’s Exact Test). These results suggest that it is the prevention of lethargus quiescence and not solely the mechanical stimulation during lethargus that results in the lethal phenotype.
We consider four explanations for the lethality induced by deprivation of lethargus quiescence. First, we may be injuring the worm by frequent mechanical stimulation during lethargus. However, the lethality observed in animals stimulated identically to yoked controls during lethargus suggests a more specific mechanism. It is also possible that locomotion during lethargus prevents the animal from engaging in movements required for ecdysis. Flipping movements, where the animal rotates along its longitudinal axis, have been observed during the final 10-15 minutes of lethargus and have been proposed to be required for ecdysis [11]. However, this explanation does not account for the increased incidence of ecdysis defects in daf-16 mutants, which do not show enhanced flipping movements. Third, it is possible that the ecdysis defect is caused by poor maturation of the new cuticle when the animal is forced to move continuously. Maturation of the cuticle may require immobility, much like wet mortar requires immobility in order to harden appropriately to a concrete. While adult cuticle forms in sleepdeprived molting defective animals, we cannot be certain that this cuticle forms entirely properly. Finally, the defect in ecdysis may be caused by a defect in regulation of metabolism; that is, the sustained locomotion during the normally quiescent lethargus period consumes metabolic resources normally reserved for the molting process. This explanation is supported by our observation that mutants for daf-16, a key integrator of stress and metabolism [32], show enhanced sensitivity to the effects of deprivation of lethargus quiescence.
The consequences of total sleep deprivation (TSD) have been extensively examined in rats, which die when subjected to TSD. The major documented consequences of TSD are skin lesions and weight loss despite increased food intake [5]; in contrast to these systemic effects, no defects have been observed in the rat brain [33]. In Drosophila, a genetic perturbation outside the nervous system affects sleep [16] and the animal’s fat stores affect its response to sleep deprivation [34]. Effects of exercise on sleep continuity have been documented in rats [35] as well as in humans [36]. It is therefore not surprising that in C. elegans too, homeostasis of lethargus sleep-like behavior involves signaling outside the nervous system, in muscle. It will be of future interest to examine the effect of genetic perturbations in muscle on mammalian sleep homeostasis.
Total sleep deprivation promotes insulin resistance in humans [37, 38] as well as in human adipocytes [39]. Our data extend these observations to one of the most primitive sleep-like states described, and suggest that C. elegans lethargus can be used as model system to gain a mechanistic understanding of this clinical phenomenon of high public health importance.
Supplementary Material
Highlights.
► DAF-16 moves to the nucleus upon deprivation of C. elegans sleep-like behavior ► DAF-16 is required for the homeostatic response to sleep deprivation ► DAF-16 functions in muscle in the homeostatic response to sleep deprivation ► Deprivation of C. elegans lethargus sleep-like behavior is lethal
Acknowledgments
We thank B. Freedman for technical assistance, A. Sehgal, T. Lamitina, G. Kao, D. Biron, and members of the Raizen lab for comments, and Cynthia Kenyon and Erik Jorgensen for reagents. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). D.M.R was supported by NS064030 from the NIH and by a NARSAD Young Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 3.Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 4.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 5.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. 1989. Sleep. 2002;25:68–87. [PubMed] [Google Scholar]
- 6.Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 7.Miyata S, Begun J, Troemel ER, Ausubel FM. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics. 2008;178:903–918. doi: 10.1534/genetics.107.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 9.Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 10.Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nature Neuroscience. 2007;10:1300–1307. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 11.Singh RN, Sulston JE. Some observations on moulting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]
- 12.Avery L, Horvitz HR. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell. 1987;51:1071–1078. doi: 10.1016/0092-8674(87)90593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 16.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson SE, Duricka DL, Campbell K, Churchill L, Krueger JM. Homer1a and 1bc levels in the rat somatosensory cortex vary with the time of day and sleep loss. Neuroscience letters. 2004;367:105–108. doi: 10.1016/j.neulet.2004.05.089. [DOI] [PubMed] [Google Scholar]
- 18.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Developmental Biology. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 19.Swanson MM, Riddle DL. Critical periods in the development of the Caenorhabditis elegans dauer larva. Developmental Biology. 1981;84:27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park D, Estevez A, Riddle DL. Antagonistic Smad transcription factors control the dauer/non-dauer switch in C. elegans. Development. 2010;137:477–485. doi: 10.1242/dev.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 23.Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 24.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 25.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Y, Wollam J, Magner D, Karalay O, Antebi A. A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science. 2012;338:1472–1476. doi: 10.1126/science.1228967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S, et al. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics. 2012;44:981–991. doi: 10.1152/physiolgenomics.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A. Optogenetic analysis of synaptic function. Nat Methods. 2008;5:895–902. doi: 10.1038/nmeth.1252. [DOI] [PubMed] [Google Scholar]
- 31.Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- 32.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 33.Cirelli C, Shaw PJ, Rechtschaffen A, Tononi G. No evidence of brain cell degeneration after long-term sleep deprivation in rats. Brain Res. 1999;840:184–193. doi: 10.1016/s0006-8993(99)01768-0. [DOI] [PubMed] [Google Scholar]
- 34.Thimgan MS, Suzuki Y, Seugnet L, Gottschalk L, Shaw PJ. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanco-Centurion CA, Shiromani PJ. Beneficial effects of regular exercise on sleep in old F344 rats. Neurobiol Aging. 2006;27:1859–1869. doi: 10.1016/j.neurobiolaging.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355–365. xi. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 38.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–557. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


