Abstract
The primary goal was to determine agonist-specific regulation of CRF2(a) receptor function. Exposure of human retinoblastoma Y79 cells to selective (UCN2, UCN3 or stresscopins) and nonselective (UCN1 or sauvagine) agonists prominently desensitized CRF2(a) receptors in a rapid, concentration-dependent manner. A considerably slower rate and smaller magnitude of desensitization developed in response to the weak agonist CRF. CRF1 receptor desensitization stimulated by CRF, cortagine or stressin1-A had no effect on CRF2(a) receptor cyclic AMP signaling. Conversely, desensitization of CRF2(a) receptors by UCN2 or UCN3 did not cross-desensitize Gs-coupled CRF1 receptor signaling. In transfected HEK293 cells, activation of CRF2(a) receptors by UCN2, UCN3 or CRF resulted in receptor phosphorylation and internalization proportional to agonist potency. Neither protein kinase A nor casein kinases mediated CRF2(a) receptor phosphorylation or desensitization. Exposure of HEK293 or U2OS cells to UCN2 or UCN3 (100 nM) produced strong βarrestin2 translocation and colocalization with membrane CRF2(a) receptors while CRF (1 µM) generated only weak βarrestin2 recruitment. βarrestin2 did not internalize with the receptor, however, indicating that transient CRF2(a) receptor-arrestin complexes dissociate at or near the cell membrane. Since deletion of the βarrestin2 gene upregulated Gs-coupled CRF2(a) receptor signaling in MEF cells, a βarrestin2 mechanism restrains Gs-coupled CRF2(a) receptor signaling activated by urocortins. We further conclude the rate and extent of homologous CRF2(a) receptor desensitization are governed by agonist-specific mechanisms affecting GRK phosphorylation, βarrestin2 recruitment, and internalization thereby producing unique signal transduction profiles that differentially affect the stress response.
1. Introduction
Hypothalamic-pituitary-adrenal (HPA) axis, defensive behavior, autonomic, metabolic, immune, and cardiovascular responses during stress and trauma are coordinated by the interplay of neuronal corticotropin releasing factor (CRF) and urocortin peptides (UCN1, UCN2, UCN3) differentially binding to and activating CRF receptors type 1 (CRF1) and type 2 (CRF2), which are members of the class B1 group of the G protein-coupled receptor (GPCR) superfamily [1–7]. Both CRF receptors are capable of signaling via the protein kinase A (PKA), protein kinase C (PKC), extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase, protein kinase B (Akt), and other pathways, although the dominant mode of signal transduction is coupling to G protein subunit Gsα and activating adenylyl cyclase to generate adenosine 3',5'-cyclic monophosphate (cyclic AMP) [1–7]. CRF1 receptor signaling generates critical defensive behaviors, HPA hormone secretion, and physiological responses required to survive trauma and stress [1–12]. Behavioral actions mediated by the CRF2 receptor are complex and contingent upon the brain site and activating agonist unlike the CRF1 receptor [1,6–9]. Emerging evidence indicates, however, forebrain CRF2 receptor signaling can be anxiogenic depending on the intensity and duration of the stress state [1,9–11]. These CRF receptor-mediated processes must be rapidly initiated in order to meet physiological demands essential for survival. Counter-regulation to restore homeostasis is equally important, however, to prevent stress-induced psychiatric and medical illnesses developing from the detrimental effects of abnormal CRF receptor signaling.
Transduction of cellular signals by G protein-coupled receptors (GPCRs) is stringently regulated to prevent the deleterious effects of unrestrained GPCR signaling. The rapid termination of signaling mediated by agonist-occupied GPCRs is referred to as homologous desensitization and involves the following: (i) Agonist-activated receptors selectively recruit a G protein-coupled receptor kinase (GRK) that phosphorylates particular serines and/or threonines in the receptor’s intracellular loops or carboxyl-terminus (C-terminus). (ii) Phosphorylated receptors attract cytoplasmic βarrestins to the cell membrane where they bind to receptors, uncoupling them from their cognate Gα via competition and steric hindrance. (iii) βarrestins subsequently target the receptors to clathrin-coated pits for endocytosis by interacting with clathrin and clathrin adapter protein AP-2. Internalized receptors are either sorted for dephosphorylation and recycling back to the plasma membrane or trafficked into lysosomes for degradation. [13–15]. The stability of the receptor-βarrestin interaction distinguishes two classes of GPCRs, termed class A and class B [15]. Class A GPCRs form transient complexes with βarrestin that dissociate at or near the plasma membrane after the receptor are directed to clathrin coated pits and consequently internalize without βarrestin. Class B receptors form stable complexes with βarrestins that internalize as a unit into endocytic vesicles and persist inside the cell. The stability of the receptor-βarrestin interaction appears to regulate the rate receptors resensitize as well as the spatial-temporal pattern of βarrestin-dependent signaling pathways [14,15].
Recently, βarrestins have been shown to contribute to desensitization and internalization of the CRF1 and CRF2(b) receptors, the peripherally expressed splice variant of the CRF2 receptor [16]. There is no information, however, about the homologous regulation of the centrally expressed CRF2(a) receptor signaling by phosphorylation, βarrestin recruitment, and internalization mechanisms. Since abnormal signaling by both CRF receptors in the extended amygdala and forebrain may contribute to the pathophysiology of human stress, anxiety, and depressive disorders, understanding control of CRF2 receptor function could provide important insight into the pathogenesis of posttraumatic stress disorder and affective illnesses.
Thus, the primary goal of this study was to investigate the desensitization profile of CRF2(a) receptors following exposure to a variety of physiological ligands. We looked not only at the desensitization of cyclic AMP signaling by the CRF2(a) receptor but also at three primary components of the desensitization process: (i) agonist-stimulated phosphorylation, (ii) βarrestin recruitment, and (iii) receptor internalization. In addition, because we have recently shown that Y79 cells co-express both CRF1 and CRF2(a) receptors [17], we were able to study potential regulatory interactions between CRF1 and CRF2(a) receptors in an endogenous setting. Results from our study reveal that desensitization of Gs-coupled CRF2(a) receptor signaling is unaffected by CRF1 receptor activity and the serine-threonine kinases, protein kinase A and casein kinases, but strongly dependent on agonist potency and βarrestin2 recruitment.
2. Methods and Materials
2.1. Reagents
Reagents for cell biology experiments were obtained from the following sources: (1) bovine serum albumin (BSA, fraction V), isobutylmethylxanthine (IBMX), and other highly purified chemicals from Sigma (St. Louis, MO); (2) aprotinin (Trasylol) from Calbiochem (San Diego, CA); (3) forskolin, PKA activators (dbcAMP, SpcAMP-S), PKA inhibitors (H89, RpcAMP-S), and casein kinase inhibitors (TBB, quinalizarin, IC261) from Calbiochem (San Diego, CA); (4) defined fetal bovine serum (#SH30070.03) from Hyclone (Logan, UT). The UCSD Cell Culture Core Facility supplied all other cell culture reagents (Mediatech-CELLGRO). The following CRF receptor agonists were purchased from Bachem (Torrance, CA; purity > 98%) or Phoenix (Belmont, CA; purity >98%) to stimulate cyclic AMP accumulation, and/or to desensitize CRF1 or CRF2 receptors: ovine CRF (oCRF); sauvagine (SVG); human/rat CRF (h/rCRF); human urocortin 1 (UCN1), urocortin 2 (UCN2), and urocortin 3 (UCN3); and human stresscopin (SCP) and stresscopin-related peptide (SRP). Drs. Aaron Hsueh and Teddy Hsu (Stanford University) kindly provided stresscopin peptides for the initial experiments. All SDS-PAGE reagents were purchased from Invitrogen-NOVEX (Carlsbad, CA). For CRF1 receptor phosphorylation experiments, the following reagents were used: (1) protein A sepharose (PrA-Seph) from Oncogene Research Products (Cambridge, MA); (2) the mouse monoclonal anti-HA (HA.11) antibody for immunoprecipitation, the FITC-labeled HA. 11 antibody (#FITC-101L) for flow cytometry in transfected HEK293 cells from BAbCO/Covance (Emeryville, CA); (3) the Alexa Fluor 488-labeled HA.11 antibody (#A488-101L) for flow cytometry in transfected MEF cells from BAbCO/Covance (Emeryville, CA).
2.2. Plasmid cDNAs
The human CRF2(a) receptor was previously amplified from a human retinoblastoma Y79 cell complementary DNA (cDNA) library by PCR and subcloned into pcDNA3 (Invitrogen, San Diego, CA) using KpnI and XbaI sites [18]. The influenza hemagglutinin (HA) epitope tag (YPYDVPDYA) was inserted between residues Ala17 and Glu18, which is an inert region of the amino-terminus (N-terminus) [19], using oligo-directed mutagenesis (Quick Change™ kit, Stratagene, La Jolla, CA). Cyclic AMP signaling by HA-tagged and wild-type CRF2(a) receptor were similar (data not shown). Construction of the βarrestin2-green fluorescent protein (GFP) expression vector has been described previously [15]. Sequences of cDNA constructs were confirmed using single-stranded DNA sequencing.
2.3. Cell culture and transfection
Suspension human retinoblastoma Y79 cultures were grown at a density of 2×107 cells/flask in RPMI-1640 and used between passages 4–25 as previously described [17,20]. Human embryonic kidney HEK293 cells were transfected with the cDNA encoding the HA-tagged human CRF2(a) receptor as previously reported [18]. Cyclic AMP signaling experiments confirmed that the sensitivity (i.e., half-maximal effective concentration, EC50) and maximum for agonist-stimulated cyclic AMP accumulation were not altered by insertion of the HA epitope tag in the CRF2(a) receptor’s N-terminus (data not shown). Likewise, the binding affinity of the CRF2(a) receptor for its agonists was also not altered by tagging the CRF2(a) receptor with the HA epitope (data not shown). For phosphorylation experiments, transiently transfected HEK293 cells were seeded at 6 × 105 cells/10-cm dish in DMEM containing 10% (v/v) FBS, 100 µg/ml streptomycin, 100 IU/ml penicillin. For confocal microscopy, HEK293 cells were cultured and transiently transfected as previously described [21]. For quantification of βarrestin-GFP translocation, human osteosarcoma U2OS cells (American Type Culture Collection) were cultured in MEM supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 10 mM HEPES, 2mM L-glutamine, and 10 µg/ml gentamicin [21]. For other experiments, wild-type (expressing both βarrestins) and βarrestin2 knockout mouse embryonic fibroblast (MEF) cell lines derived from wild-type and βarrestin2 knockout mice were cultured as previously described [22], and transiently transfected with human CRF2(a) receptor pcDNA3 using a Amaxa Biosystems Nucleofector II and following established Amaxa protocols (Amaxa-Lonza Cologne, Walkersville, MD). Transfection efficiency was assessed by co-transfecting pmaxGFP and measuring % fluorescence of cellular GFP protein expression. GFP fluorescence was similar in transfected HEK293 cells (55.4±2.2%) and transfected MEF cells (55.9±1.8%).
2.4. Agonist-stimulated cyclic AMP accumulation experiments and cyclic AMP radioimmunoassay method
Following extensive cell washing, intracellular cyclic AMP levels were measured in non- acetylated cell lysates using a double-antibody radioimmunoassay kit (cyclic AMP[125I] assay system, RPA 509; Amersham International, Little Chalfont, UK), as previously described [17,20]. In addition to UCN2 and UCN3 [2], stresscopin (SCP) and stresscopin-related peptide (SRP) are also selective CRF2 receptor agonists [23]. In Y79 cells, concentration-response curves (0–100 nM) comparing intrinsic agonist activities showed that the sensitivity (EC50) and maximum for cyclic AMP accumulation stimulated by urocortin 2 (Figure 1) and SRP were similar as were the EC50 and maximum for cyclic AMP accumulation stimulated by UCN3 and SCP (Hauger & Dautzenberg, unpublished data). Although the maximum values for cyclic AMP accumulation stimulated by UCN2 and UCN3 were comparable in recombinant HEK293 and SK-N-MC cells stably expressing CRF2a receptors, the EC50 for UCN2 was lower (i.e., higher sensitivity) than that for UCN3 in stably transfected HEK293 and human neuroblastoma SK-NMC cells [18], and in Y79 cells [17]. Thus, human UCN2 was used to stimulate cyclic AMP accumulation in Y79 cells in the majority of experiments.
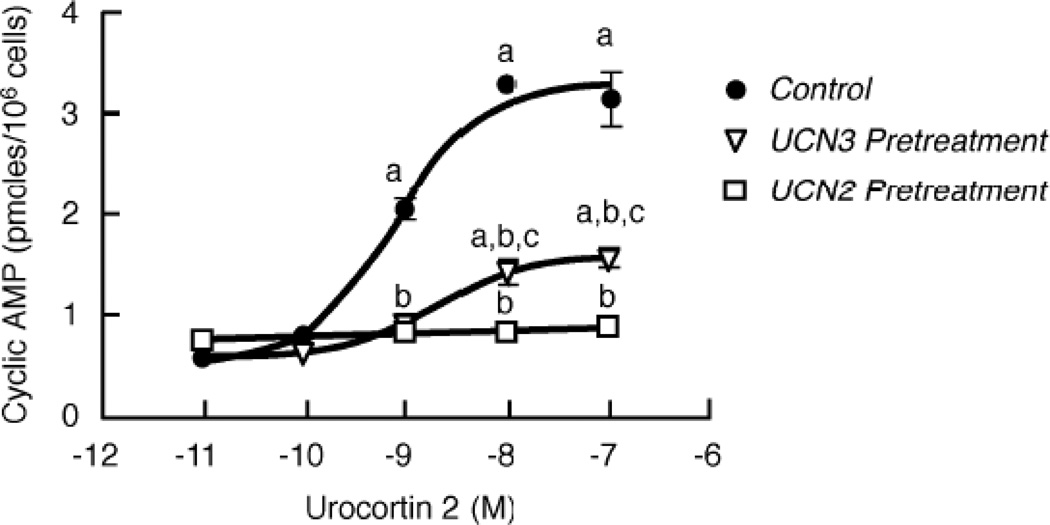
Figure 1. Effect of homologous CRF2(a) receptor desensitization on sensitivity (EC50) and maximum for the concentration-response stimulation of intracellular cyclic AMP accumulation by urocortin 2.
Homologous CRF2(a) receptor desensitization was induced by pretreating Y79 cells with UCN2 (100 nM) or UCN3 (100 nM) for 30 min. Cyclic AMP levels (pmoles/106 cells) were then measured in triplicate in desensitized and control cells stimulated with 0–100 nM UCN2 for 15 min. By ANOVA, there was a significant interaction [F=68.0; p<0.0001] for group (Control or Urocortin Pretreatment) and stimulation conditions (10−11 to 10−7 M UCN2). A significant within group effect [F=114.5; p<0.0001] was found with planned comparisons indicating the following: ap<0.001 vs Basal (no UCN2). A significant across pretreatment group effect [F=208.8; p<0.0001] was detected with planned comparisons indicating the following differences: bp<0.001 vs Control (no pretreatment); cp<0.05 vs UCN2 pretreatment. These findings were confirmed in four independent experiments.
2.5. Confocal microscopy
HEK293 cells expressing the non-epitope-tagged CRF2 receptor and βarrestin2-GFP were plated on 35-mm glass bottom culture dishes (MatTek, Ashland, MA) and cultured overnight using a previously published protocol [15,24]. One hour before the experiment, the medium was removed and replaced with serum- and phenol red-free medium supplemented with 10 mM HEPES. Confocal microscopy was performed on a Zeiss laser scanning microscope. Images were acquired from live cells in real time before and after agonist addition using single excitation (488 nm). For colocalization experiments, live HEK293 cells expressing HA-tagged CRF2(a) receptors and βarrestin2-GFP were incubated with an anti-HA mouse monoclonal antibody conjugated to Alexa Fluor 594 (Invitrogen, San Diego, CA) at 37°C for 45 min in serum- and phenol red-free medium supplemented with 10 mM HEPES. Cells were then washed with this medium and evaluated by confocal microscopy. Images were collected sequentially using dual excitation (488 nm from argon laser, 543 nm from HeNe laser) and emission filter sets (band pass, 500–530 nm; long pass 560 nm) from live cells in real time before and after agonist addition.
2.6. Quantification of βarrestin2-GFP translocation
Translocation of βarrestin2-GFP was quantified as described previously [21]. In brief, U2OS cells transiently expressing CRF2(a) receptor and βarrestin2-GFP were seeded 24 h post-transfection at 15,000 cells per well in 96-well black ViewPlates (#6005182; Packard-PerkinElmer, Waltham, MA). U2OS cells were employed over HEK293 cells for their superior adherence properties and image quality [25]. After an overnight incubation at 37°C, the media was removed and replaced with 100 µL of phenol-red-free MEM with 10 mM HEPES and 2mM L-glutamine for a 45-minute incubation at 37°C. Cells were stimulated for 30 min at 37°C with various concentrations of UCN2, UCN3, or vehicle. The cells were then fixed and nuclei stained by addition of 120 µL of 4% v/v formaldehyde in PBS and 5 µg/ml Hoechst nuclear stain. After a 45-min incubation, the fixative and nuclear stain were removed and replaced with 200 µL PBS. The INCell Analyzer 3000 (GE Healthcare Biosciences, Piscataway, NJ), a laser-based, confocal imaging system, was employed to quantitate the βarrestin2-GFP translocation response, as described previously [25,26]. In brief, images were quantified using the granularity analysis GRN1 algorithm that identifies fluorescent spots or grains of βarrestin2-GFP localization based on size and fluorescence intensity. A grain size setting of 5 and an intensity gradient setting of 1.25 were employed for this analysis. Only GFP-positive cells, selected using a cell intensity signal threshold of 100, were quantitated. The reported parameter “Fgrains” represents the average fluorescence intensity of the spots or grains of βarrestin2-GFP localization.
2.7. Receptor phosphorylation assay
Phosphorylation of the CRF2 receptor was measured using a previously published method for determining CRF1 receptor phosphorylation [21,27]. Briefly, transfected HEK293 cells in 10-cm dishes were metabolically labeled for 4 h at 37°C in 5 ml of Pi-free DMEM containing 0.1% (w/v) BSA and 100 µCi/ml 32Pi. Cells were then treated with vehicle or 100 nM urocortin 2 for 10 min. In protein kinase A and casein kinase experiments, cells were pretreated for 30 min with PKA inhibitors (H89, RpcAMP) or a CK inhibitor (TBB) prior to agonist stimulation. After cells were lysed in lysis buffer (LB: 50 mM Tris, pH 8.0, 100 mM NaCl, 20 mM NaF, 10 mM Na pyrophosphate, 5 mM EDTA, 10 mg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml soybean trypsin inhibitor, 10 µg/ml pepstatin, 10 µg/ml benzamidine, 1 mM PMSF, 1 µM okadaic acid), they were pre-extracted by addition of LB containing 2 M NaCl and 8 M urea followed by overnight tumbling at 4°C. Membranes were then solubilized in LB supplemented with 1% (v/v) NP 40, 1% (w/v) Na deoxycholate and 0.1% (w/v) SDS. After clarification at 14,000 – g, solubilized membranes were pre-cleared by being incubated with 2% (v/v) protein A sepharose for 1 h at 4°C. Immunoprecipitation of HA-tagged CRF2(a) receptors was performed by adding a high affinity HA antibody (1 µl) and 2% (v/v) protein A sepharose followed by incubation overnight at 4°C. After washing of the sepharose-bound immune complexes in LB+ lacking protease inhibitors, 32P-labeled phospho-CRF2 receptors were eluted in Laemmli sample buffer for 1 h at 48°C and resolved by SDS-PAGE (10% resolving gel) after loading equal amounts of protein in each lane.
2.8. Flow cytometry receptor internalization assay
HEK293 transiently expressing the HA-tagged CRF2(a) receptor were plated in 6-well plates and incubated overnight. Afterward, the cells were incubated with UCN2, UCN3, or h/rCRF (100 nM) or media (control) for 60 min and receptor sequestration was assessed by flow cytometry using a FACS Canto as described previously [21]. Specificity of the receptor internalization signal detected by the FITC-labeled HA.11 monoclonal antibody (Covance Research Products: Denver, PA; #FITC-101L) was verified by demonstrating that cells transfected with empty vector exhibited very low immunostaining. In addition, no appreciable immunostaining of HA-tagged CRF2(a) receptor-expressing cells was measured using a FITC-labeled IgG to detect nonspecific binding. Gating was performed only on single live cell populations.
2.9. Data reduction and statistical analyses
Data reduction and statistical analyses were performed with PRISM™, Version 6.0c program for Mac OS X (GraphPad Software, San Diego, CA). Cyclic AMP levels (pmoles/10 cells) measured by RIA were calculated using a log-logit program. Cyclic AMP data are presented as mean ± SEM for basal (buffer) and agonist-stimulated levels, except for Figure 5B, Figure 8C, and supplementary Table 2 where %Control was calculated after subtracting the basal level for the pretreatment condition. EC50 (sensitivity) and maximum values were calculated from the full concentration-response curves for agonist stimulation (0–1 µM) of cyclic AMP accumulation (Figure 1) and βarrestin2 GFP translocation (Figure 12). Statistical analyses were done using one- or two-way analyses of variance (ANOVAs), depending on the experimental design, to determine significance within and across experimental groups. When statistically appropriate and indicated, planned analyses were performed using Bonferroni’s multiple comparison tests to determine significant differences between individual groups. CRF2(a) receptor phosphorylation bands were imaged, quantitated and analyzed using our previously published method [21]. Flow cytometry data was analyzed by the DIVA software (version 6.1.2).
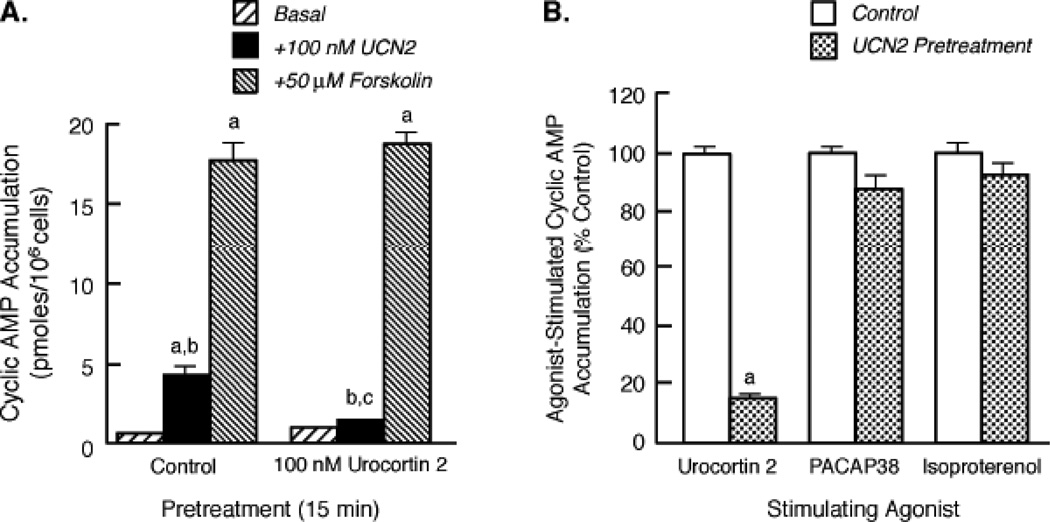
Figure 5. Effect of homologous CRF2 receptor desensitization on the ability of forskolin, PACAP38, and isoproterenol to stimulate intracellular cyclic AMP accumulation.
(A) After homologous CRF2 receptor desensitization was induced by exposing Y79 cells to UCN2, cyclic AMP levels (pmoles/106 cells) were then measured in desensitized and control (no UCN2 pretreatment) cells incubated for 15 min with buffer (Basal), UCN2, or forskolin (n=10/condition). By ANOVA, there was a significant interaction [F=5.88; p<0.005] for pretreatment group (Control, UCN2) and stimulation condition (Basal, +UCN2, or +Forskolin). A significant within group effect [F=709.7; p<0.0001] was found with planned comparisons indicating the following differences: ap<0.001 vs Basal; bp<0.001 vs Forskolin. A significant effect [F=3.59; p<0.05] was detected across the two pretreatment groups with planned comparisons indicating the following difference for +100 nM UCN2 stimulation: cp<0.001 vs Control. These findings were replicated in two independent experiments. (B) After Y79 cells were pretreated with buffer (Control) or 100 nM UCN2 for 30 min, cyclic AMP responses (%Control) to a 15-min stimulation with 100 nM UCN2, 100 nM PACAP38, or 10 µM isoproterenol (n = 12/group) were then measured in control and desensitized. By ANOVA, there were significant differences across the Control and UCN2 Pretreatment groups [F= 108.2, p < 0.0001] with planned comparisons indicating the following: ap<0.001 vs Control. These findings were replicated in two independent experiments.
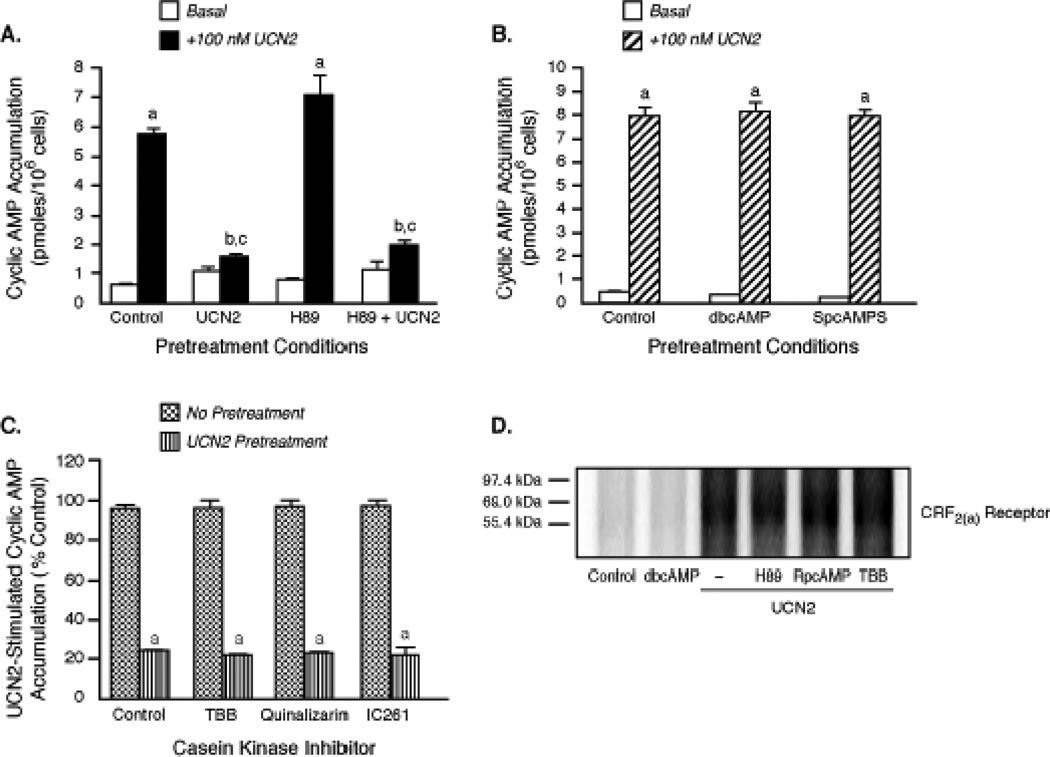
Figure 8. The role of protein kinase A and casein kinases in the regulation of Gs-coupled CRF2(a) receptor signaling.
(A) Effect of protein kinase A (PKA) inhibition on homologous CRF2(a) receptor desensitization. In the pretreatment period, Y79 cells were first incubated with media or H89 (2 µM) for 30 min and then exposed to media (Control) or UCN2 (100 nM) in the continuing presence of H89 for an additional 15 min. Pretreated cells were then incubated with buffer (Basal, n=3) or stimulated with UCN2 (+100 nM UCN2, n=10) for an additional 15 min. By ANOVA, there was a significant interaction [F=17.09; p<0.0001] for pretreatment group (Control, UCN2, H89, H89 + UCN2) and stimulation condition (Basal or +100 nM UCN2). A significant within group effect [F=77.27; p<0.0001] was found with planned comparisons indicating the following: ap<0.001 vs Basal. A significant effect [F=12.38; p<0.0001] was found across the pretreatment groups with planned comparisons indicating the following differences in +100 nM UCN2 stimulation: bp<0.01 vs. Control; cp<0.001 vs. H89. This data was replicated in three independent experiments. (B) Effect of PKA activators on UCN 2-stimulated cyclic AMP accumulation. After Y79 cells (n=13/group) were pretreated for 1 h with dibutyryl-cyclic AMP (dbcAMP) (4 mM) or the Sp-isomer of adenosine-3’,5’-cyclic AMP monophosphorothioate (Sp-cAMPS) (500 µM) to maximally activate PKA, they were incubated with buffer or stimulated with 100 nM UCN 2 for 15 min. By ANOVA, a significant within (but not across) pretreatment group effect [F=676.5; p<0.0001] was found with planned comparisons indicating the following: ap<0.001 vs. Control. These findings were replicated in two independent experiments. (C) Effect of casein kinase (CK) inhibition on UCN2-induced CRF2(a) receptor desensitization. Y79 cells were incubated for 30 min with media (Control) or CK inhibitors TBB (20 µM), Quinalizarin (20 µM) or IC261 (100 µM). After these cells were further pretreated with media or 100 nM UCN2 for 15 min (to desensitize CRF2(a) receptors) in the continuing presence of CK inhibitors, they were incubated with buffer or stimulated with 100 nM UCN2 for 15 min. By ANOVA, there was only a significant within group (Control and CK Inhibitor) difference [F= 62.98, p < 0.0001] with planned comparisons indicating the following: ap<0.001 vs. Control. Data (% control mean ± SEM) were replicated in two independent experiments (n = 6–10/group). (D) Role of PKA or casein kinase in CRF2(a) receptor phosphorylation. After cells were pretreated for 30 min with a PKA inhibitor (2 µM H89 or 100 µM RpcAMP) or a CK inhibitor (TBB 20 µM), they stimulated with 100 nM UCN2 for 10 min. Cell were also incubated with only dbcAMP (4 mM for 10 min) to activate PKA. CRF2(a) receptor phosphorylation was then quantitated (see Methods). This data was replicated in a separate experiment.
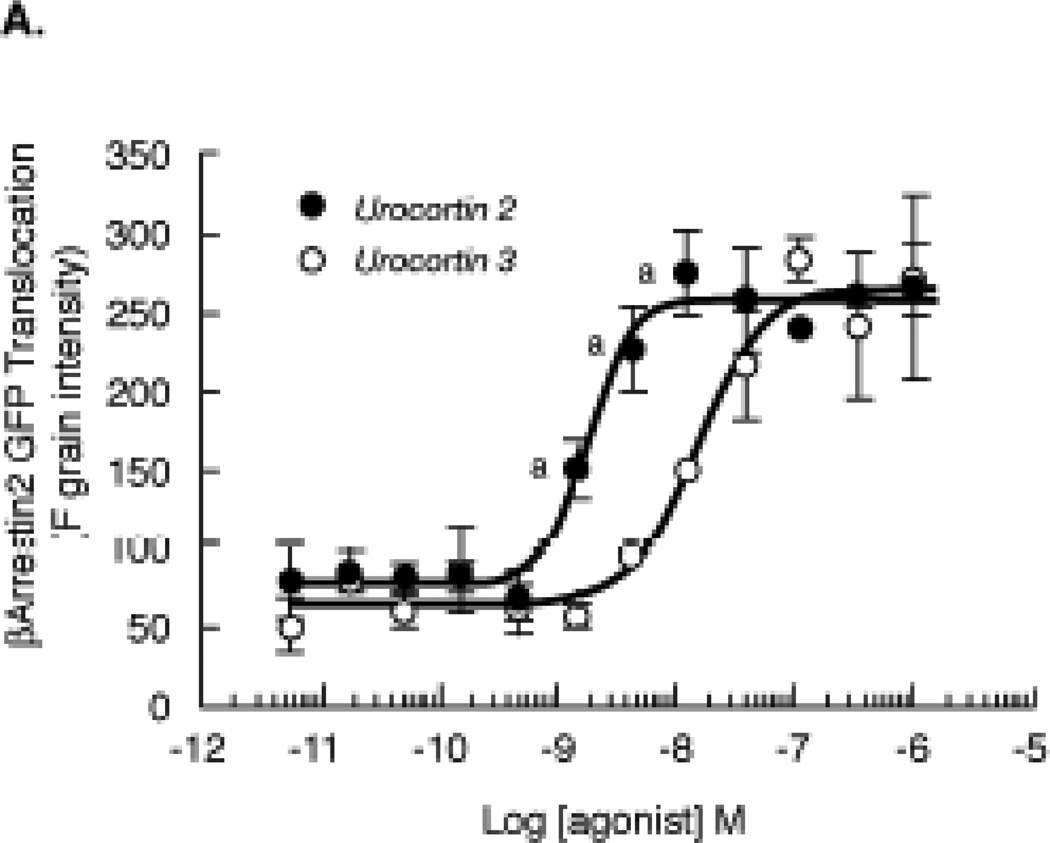
Figure 12. Quantitation of βarrestin2 translocation to the agonist-activated CRF2(a) receptors in U2OS cells.
U2OS cells transiently co-expressing βarrestin2-GFP and full-length wild-type human CRF2(a) receptors were plated in 96-well plates and cultured overnight. Cells were then stimulated with urocortin 2 or urocortin 3 (0–1000 nM) for 30 min and analyzed on the INCell Analyzer 3000. The reported parameter “Fgrains” represents the fluorescence intensity of βarrestin2-GFP localized with the receptor in spots or grains at the plasma membrane. Data represent the mean ± SEM for 3 independent experiments performed in triplicate. By ANOVA, there was a significant interaction [F=10.50; p<0.0001] for the urocortin group (UCN2, UCN3) and stimulation concentrations (10−11 to 10−6 M agonist). A significant within group effect [F=78.49; p<0.0001] was found with planned comparisons indicating significant increases in βArrrestin2 translocation over the agonist concentration range for both urocortins. A significant across pretreatment group effect [F=23.80; p<0.05] was detected with planned comparisons indicating the following: ap<0.001 vs UCN3.
3. Results
3.1. Time- and concentration-dependence of homologous desensitization of CRF2 receptors induced by urocortin 2, urocortin 3, and stresscopins
The time-dependent desensitization of CRF2(a) receptors is significantly less (p<0.001) in magnitude following exposure of human retinoblastoma Y79 cells to a saturating concentration of UCN3 (100 nM) for 5 min (34.4% decrease), 15 min (40.7% decrease), 30 min (61.2% decrease), or 1 h (66.9% decrease) compared to the ~90% desensitization of cyclic AMP signaling resulting from 100 nM UCN2 pretreatment at the same time points [17]. Since UCN2 is a more potent ligand than UCN3 at CRF2(a) receptors [2,17,18], the more rapid and greater desensitizing action of UCN2 compared to UCN3 on CRF2(a) receptors is consistent with the concept that stronger agonists can increase the rate and magnitude of homologous GPCR desensitization [28].
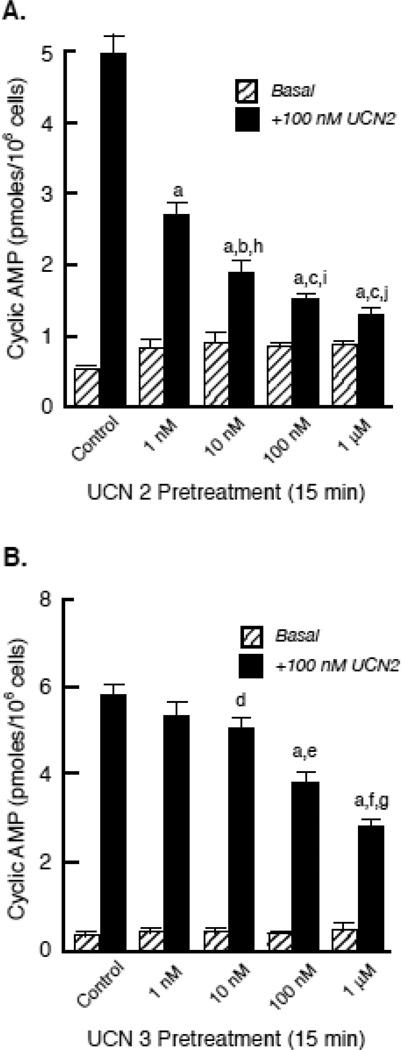
To further characterize homologous CRF2(a) receptor desensitization, we performed concentration-response curves for the stimulation of cyclic AMP accumulation following a 30-min agonist pretreatment, which is the time point of maximal CRF2(a) receptor desensitization [17]. Pretreatment of Y79 cells with a saturating concentration of UCN2 or UCN3 significantly decreased the maximum for UCN2-stimulated cyclic AMP accumulation ~65% and ~50%, respectively, compared to control cells (Figure 1, supplementary Table 1). In addition, the sensitivity (EC50) of UCN2-stimulated cyclic AMP accumulation was shifted to the right following CRF2(a) receptor desensitization induced by either agonist, however this effect was only significant for UCN2 pretreatment (supplementary Table 1). We next investigated the concentration-dependent characteristics of UCN2- or UCN3-induced CRF2(a) receptor desensitization. The maximum response for UCN2-stimulated cyclic AMP accumulation progressively decreased in cells pretreated with all UCN2 concentrations ranging from 1 nM to 1 µM (p<0.001) (Figure 2A). In contrast, UCN2-stimulated cyclic AMP accumulation was first significantly reduced by 10 nM UCN3 (p<0.01), with subsequent decreases produced by 100 nM UCN3 (p<0.001) and 1 µM UCN3 (p<0.001) (Figure 2B). At each agonist concentration, however, UCN2 pretreatment produced significantly greater homologous CRF2(a) receptor desensitization than UCN3 pretreatment (p<0.001) (Figure 2).
Figure 2. Concentration-dependence of homologous CRF2 receptor desensitization induced by urocortin 2 or 3.
After Y79 cells were pretreated with UCN2 (A), UCN3 (B), or buffer (Control), cyclic AMP levels (pmoles/106 cells) were measured in washed cells following an additional incubation with buffer (Basal) or maximal stimulation with 100 nM UCN2 for 15 min. Results were obtained in five independent experiments (n = 10 replicates per pretreatment group). By ANOVA, there was a significant interaction [F=77.41; p<0.0001] for pretreatment group (Urocortin concentration) and stimulation condition (Basal – no UCN2 or +100 nM UCN2). There were also significant within group differences for UCN2 Pretreatment [F=514.1; p<0.0001] and UCN3 Pretreatment [F=1492; p<0.0001]. Planned comparisons detected significant differences in UCN2-stimulated cyclic AMP stimulation within UCN2 Pretreatment groups (ap<0.001 vs Control; bp<0.01 vs 1 nM UCN2; cp<0.001 vs 1 nM UCN2) and within UCN3 Pretreatment groups (ap<0.001 vs Control; dp<0.01 vs Control; ep<0.01 vs 10 nM UCN3; fp<0.001 vs 10 nM UCN3; gp<0.05 vs 100 nM UCN3). When the calculated Δ cyclic AMP responses (+100 nM UCN2 stimulation - Basal cyclic AMP levels) for the UCN2 and UCN3 Pretreatment groups were compared, the ANOVA detected significant across group (UCN2 vs UCN3 Pretreatment) interactions [F=90.42; p<0.0001], with planned comparisons indicating significantly greater desensitization by UCN2 than UCN3 at the following concentrations: hp<0.001 vs 100 nM UCN3; ip<0.01 vs 1 µM UCN3; ; jp<0.01 vs 1 µM UCN3.
When Vale and colleagues cloned and sequenced UCN2 and UCN3 from mouse and human cDNA libraries [29,30], another group identified human UCN2- and UCN3-like peptides that were designated as “stresscopin” (SCP) and “stresscopin-related peptide” (SRP) [23]. In Y79 cells, the EC50 values for stimulation of cyclic AMP accumulation by the CRF2(a) receptor are similar for human UCN2 and SRP, while the EC50 values for human UCN3- and SCP-stimulated CRF2(a) receptor cyclic AMP signaling are equivalent [17]. We found that cyclic AMP responsiveness to UCN2 in Y79 cells was desensitized to the same extent by a 15-min exposure to either 100 nM UCN2 (88.6±0.8% decrease; p<0.001) or 100 nM SRP (91.5±0.9% decrease; p<0.001). Further, UCN3-stimulated cyclic AMP accumulation was reduced to the same extent by pretreating Y79 cells with UCN3 (70.4±1.5% decrease; p<0.001) or SCP (66.3±1.8%) for 30 min. Because the magnitude of homologous desensitization of CRF2(a) receptors induced by UCN2 and SRP was equivalent, as was that produced by UCN3 and SCP, subsequent experiments compared the desensitizing effects of only UCN2 and UCN3.
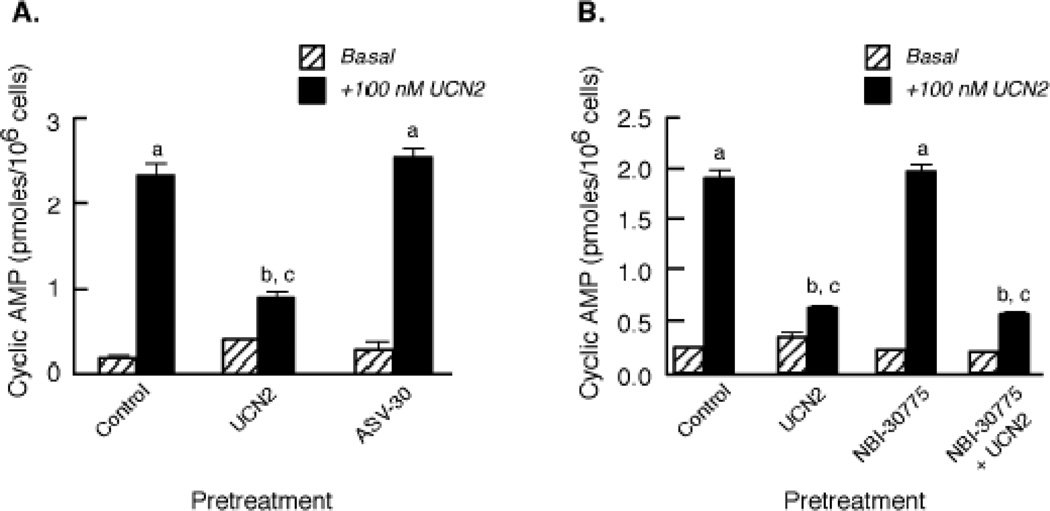
To further confirm that the observed desensitization response resulted from the specific homologous actions of UCN2 and UCN3 on the CRF2(a) receptor protein, we investigated the effect of the CRF2(a) receptor-selective antagonist, antisauvagine-30 (ASV-30) in Y79 cells. ASV-30 pretreatment failed to desensitize UCN2-stimulated cyclic AMP accumulation compared to the large CRF2(a) receptor desensitization (77.9±2.3%; p<0.001) caused by UCN2 pretreatment (Figure 3A). Because CRF1 receptor cyclic AMP signaling has been reported to be activated by high concentrations of UCN2 [29], co-activation of CRF1 receptors could contribute to CRF2(a) receptor desensitization. When Y79 cells were pretreated with a saturating concentration of the small molecule antagonist NBI-30775 that selectively blocks CRF1 receptor activation [5], the magnitudes of CRF2(a) receptor desensitization produced by exposure to 100 nM UCN2 for 30 min were found to be similar in control Y79 cells (82.9±1.3%) and NBI-30775-pretreated cells (79.7±1.0%) (Figure 3B).
Figure 3. Effect of CRF1 and CRF2 receptor antagonists on UCN2-stimulated cyclic AMP accumulation.
(A) The effects of 15-min pretreatment with the selective CRF2 receptor antagonist antisauvagine-30 (ASV-30 100 nM), UCN2 (100 nM), or buffer (Control) on UCN2-stimulated cyclic AMP accumulation were compared. Data (mean ± SEM) are cyclic AMP levels (pmoles/106 cells; n = 9) measured in cells incubated with buffer (Basal) or maximally stimulated with 100 nM UCN2 for 15 min (+100 nM UCN2) after the pretreatment period. By ANOVA, there was a significant interaction [F=94.43; p<0.0001] for pretreatment group (Control, UCN2, ASV-30) and stimulation conditions (Basal – no UCN2 or +100 nM UCN2). A significant within group effect [F=750.7; p<0.0001] was observed with planned comparisons indicating the following: ap<0.001 vs Basal. There was also a significant effect [F=63.88; p<0.0001] across the pretreatment groups with planned comparisons indicating the following differences in +100 nM UCN2 stimulation: bp<0.001 vs Control; cp<0.001 vs ASV-30. These results were replicated in two independent experiments. (B) After Y79 cells were pretreated with 100 nM UCN2 or buffer (Control) for 30 min in the presence or absence of the selective CRF1 receptor antagonist NBI-30775, cyclic AMP levels (pmoles/106 cells; n = 9) were measured in washed cells incubated with buffer (Basal) or maximally stimulated with 100 nM UCN2 for 15 min (+100 nM UCN2). By ANOVA, there was a significant interaction [F=265.4; p<0.0001] for pretreatment group (Control, UCN2, NBI-30775, NBI-30775 + UCN2) and stimulation condition (Basal or +100 nM UCN2). A significant within group effect was observed with planned comparisons indicating the following: ap<0.001 vs Basal. There was also a significant effect [F=239.2; p<0.0001] across the pretreatment groups with planned comparisons indicating the following differences in +100 nM UCN2 stimulation: bp<0.001 vs Control; cp<0.001 vs NBI-30775. These results were replicated in two independent experiments.
3.2. Homologous CRF2 receptor desensitization induced by nonselective agonists urocortin 1, sauvagine, or corticotropin releasing factor
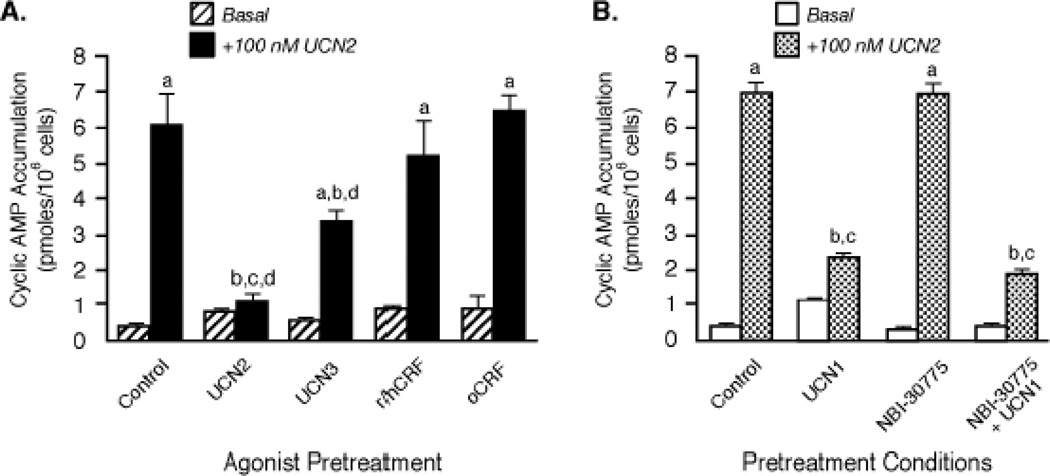
Affinities and potencies of nonselective agonists for CRF receptors vary considerably. Urocortin 1 (UCN1) has an equivalent affinity and potency at both CRF receptors while human/rat CRF (h/rCRF) and ovine CRF (oCRF) bind to CRF2 receptors with ~45-fold and ~185-fold lower affinities, respectively [1–5,18]. Pretreatment with h/rCRF for 30 min resulted in a markedly lower magnitude of CRF2(a) receptor desensitization than that induced by the three urocortin peptides (Figure 4A,B). Furthermore, UCN2-stimulated cyclic AMP accumulation by retinoblastoma CRF2(a) receptors failed to desensitize during a 30-min (Figure 4A) or 1-h exposure to oCRF (Control: 4.02±0.53 pmoles/106 cells; 100 nM oCRF pretreatment: 4.00±0.30 pmoles/106 cells). Because UCN1 is the most non-selective CRF receptor agonist with equal potency at CRF1 and CRF2 receptors [1–5,18], we determined if any of the observed desensitization was attributable to UCN1 activation of CRF1 receptors. In these experiments, Y79 cells were incubated with a saturating concentration of UCN1 (100 nM) for the longer agonist pretreatment period (1 h) required to desensitize CRF1 receptors [20,31], with and without the selective CRF1 receptor antagonist NBI-30775 (1 µM) [5]. There was no statistically significant difference in the prominent homologous desensitization of UCN2-stimulated cyclic AMP accumulation resulting from UCN1 exposure in NBI-30775-pretreated cells (79.3±1.4% decrease; p<0.001) and cells exposed to UCN1 alone (Figure 4B). Similar to UCN1, the frog neuropeptide sauvagine also binds to both CRF1 and CRF2 receptors with equivalent subnanomolar affinities and equipotently activates Gs-coupled cyclic AMP signaling by both CRF receptors [1,2,18]. We have previously reported that sauvagine can desensitize retinoblastoma CRF1 receptors to a similar extent when Y79 cells are exposed to oCRF or UCN1 [31]. Equivalent degrees of homologous CRF2(a) receptor desensitization were observed when Y79 cells were pretreated for 15 min or 1 h with 100 nM sauvagine in the presence of the CRF1-selective antagonist NBI-30775 (38.0% and 69.0% desensitization, respectively) or without CRF1 receptor antagonism (39.8% and 62.0% desensitization, respectively), commensurate with the affinity of sauvagine for the CRF2 receptor. Collectively, these data show not only that the nonselective CRF receptor ligands desensitize endogenous CRF2(a) receptors independent of their activation of CRF1 receptors but also that the nonselective ligands with high affinity for CRF2(a) receptors promote strong desensitization, a finding consistent with the results observed for the selective CRF2(a) agonists.
Figure 4. Comparison of the magnitude of homologous CRF2 receptor desensitization induced by urocortins and CRF.
(A) After Y79 cells were pretreated with 100 nM UCN2, UCN3, r/hCRF, or oCRF for 30 min, cyclic AMP levels (pmoles/106 cells; n = 6) were measured in washed cells incubated with buffer (Basal) or maximally stimulated for 15 min (+100 nM UCN2). By ANOVA, there was a significant interaction [F=64.04; p<0.0001] for pretreatment group (Control, UCN2, UCN3, CRF) and stimulation condition (Basal or +100 nM UCN2). A significant within group effect was found with planned comparisons indicating the following: ap<0.001 vs Basal. There was also a significant effect [F=239.2; p<0.0001] across pretreatment groups with planned comparisons indicating the following differences for +100 nM UCN2 stimulation: bp<0.001 vs Control; cp<0.001 vs r/hCRF; dp<0.001 vs oCRF. These results were replicated in five independent experiments. (B) After Y79 cells were pretreated for 1 h with buffer (Control) or 100 nM urocortin 1 (UCN1) in the presence or absence of the selective CRF1 receptor antagonist NBI-30775, they were then incubated with buffer (Basal) or maximally stimulated with 100 nM UCN2 for 15 min and cyclic AMP levels (pmoles/106 cells, n=9) measured. By ANOVA, there was a significant interaction [F=197.1; p<0.0001] for pretreatment group (Control, UCN1, NBI-30775, NBI-30775 + UCN1) and stimulation condition (Basal or +100 nM UCN2). A significant within group effect was found with planned comparisons indicating the following: ap<0.001 vs Basal. There was also a significant effect [F=138.9; p<0.0001] across the pretreatment groups with planned comparisons indicating the following differences for +100 nM UCN2 stimulation: bp<0.001 vs Control; cp<0.001 vs NBI-30775. These results were replicated in three independent experiments.
3.3. Effect of CRF2(a) receptor desensitization on direct stimulation of cyclic AMP accumulation by forskolin and on Gs-coupled PAC1 and adrenergic receptor signaling in Y79 cells
In contrast to UCN2 exposure inducing a 72% desensitization of CRF2(a) receptors, the level of cyclic AMP responses stimulated by 50 µM forskolin in Y79 cells pretreated with 100 nM UCN2 for the same time period did not differ from forskolin-stimulated cyclic AMP accumulation in control cells (Figure 5A). This data indicates that the intrinsic activity of adenylyl cyclase was not altered by homologous desensitization of CRF2(a) receptors induced by UCN2 exposure. Functional β-adrenergic receptors have been identified in Y79 cells [20]. Previously we demonstrated that Y79 cells also endogenously express pituitary adenylyl cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1), another regulator of the stress response, and that PAC1 receptors undergo rapid homologous desensitization via a GRK3 mechanism [32]. To test for heterologous regulation of CRF2(a) receptors, UCN2-stimulated cyclic AMP accumulation was measured in Y79 cells in which a large degree of PAC1 receptor desensitization (56%) was induced by PACAP38 pretreatment (10 nM for 1 h) [32]. UCN2-stimulated cyclic AMP accumulation was not desensitized by PACAP38 pretreatment. Conversely, pretreatment of Y79 cells with 100 nM UCN2 for 30 min failed to alter PACAP38- and isoproterenol-stimulated cyclic AMP accumulation while causing a prominent desensitization (85%; p<0.001) of CRF2(a) receptors (Figure 5B). We conclude that PAC1 receptor signaling does not induce heterologous desensitization of the retinoblastoma CRF2(a) receptor, and CRF2(a) receptor signaling does not result in heterologous desensitization of the PAC1 or β-adrenergic receptors.
3.4. Lack of cross-desensitization of CRF1 and CRF2 receptors by selective agonists
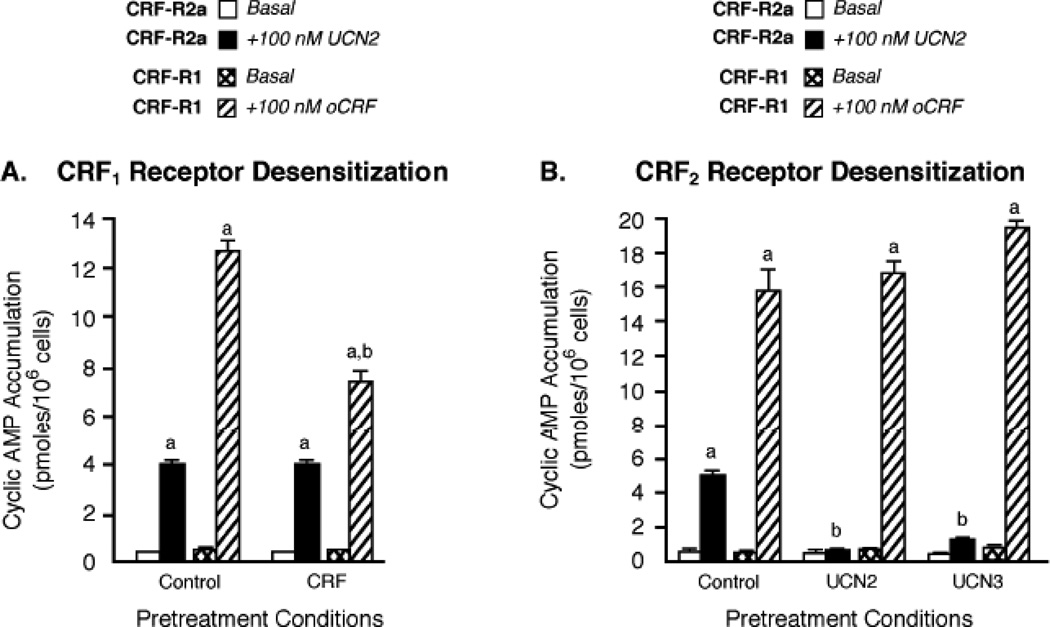
One receptor can regulate a related receptor subtype within the same GPCR subfamily. For example, H1 and H2 histamine receptors become cross-desensitized via a GRK2 mechanism and both histamine receptors undergo co-internalization upon agonist pretreatment of either H1 or H2 receptors [33]. Since Y79 cells express both CRF1 and CRF2(a) receptors [17], we were able to determine if a cross-talk mechanism co-regulated both CRF receptors. We first employed CRF1-preferring agonists stressin-1A [34] and cortagine [35] to investigate whether or not CRF1 receptor activation regulated CRF2(a) receptors in Y79 cells. While 1-h pretreatment of Y79 cells with 100 nM stressin-1A or cortagine significantly desensitized CRF-stimulated cyclic AMP accumulation by CRF1 receptors, neither CRF1 receptor-preferring agonist desensitized UCN2-simulated cyclic AMP accumulation by CRF2(a) receptors (supplementary Table 2). As shown earlier, pretreating Y79 cells with the selective CRF1 receptor antagonist NBI-30775 to block UCN1-stimulated CRF1 receptor activation had no effect on homologous CRF2(a) receptor desensitization induced by UCN1 (Figure 4B), an agonist that equipotently activates both CRF receptor subtypes with high affinity. To further confirm that CRF1 receptor signaling does not regulate CRF2(a) receptors, Y79 cells were pretreated with 100 nM oCRF for 1 h to maximally desensitize CRF1 receptors and then stimulated with 100 nM UCN2. CRF2(a) receptors were not desensitized to any extent in Y79 cells in which the 1-h oCRF exposure desensitized CRF1 receptors by decreasing oCRF-stimulated cyclic AMP accumulation 43.6±6.7 % (Figure 6A).
Figure 6. Independence of homologous desensitization processes controlling signal transduction by CRF1 and CRF2 receptors co-expressed in Y79 cells.
(A) The effect of prolonged CRF exposure on Gs-coupled CRF2 receptor signaling was determined by pretreating Y79 cells with buffer (Control) or 100 nM oCRF for 1 h (to induce maximal CRF1 receptor desensitization [20]). Cyclic AMP accumulation (pmoles/106 cells; n = 9) was then measured after CRF2(a) receptors (CRF-R2a) were maximally stimulated by UCN2 (+100 nM UCN2) or CRF1 receptors (CRF-R1) were maximally stimulated by CRF (+100 nM oCRF) for 15 min. By ANOVA, there was a significant interaction [F=27.92; p<0.0001] for pretreatment conditions (Control, CRF) and stimulation group (Basal, +100 nM UCN2 or +100 nM oCRF). A significant within group effect was found with planned comparisons indicating the following: ap<0.001 vs Basal. There was also a significant effect across the pretreatment groups with planned comparisons indicating the following differences for +100 nM CRF stimulation: bp<0.001 vs Control. These results were replicated in three independent experiments. (B) The effect of prolonged urocortin exposure on Gs-coupled CRF1 receptor signaling was assessed in Y79 cells by incubation with buffer (Control), or pretreatment with 100 nM UCN2 or UCN3 for 1 h (to induce maximal CRF2 receptor desensitization). Cyclic AMP accumulation (pmoles/106 cells; n = 13) was then measured after CRF1 receptors (CRF-R1) were maximally stimulated by CRF (+100 nM oCRF) or CRF2(a) receptors (CRF-R2a) were maximally stimulated by UCN2 (+100 nM UCN2) for 15 min. By ANOVA, there was a significant interaction [F=11.20; p<0.0001] for pretreatment conditions (Control, UCN2, UCN3) and stimulation group (Basal, +100 nM UCN2 or +100 nM oCRF). A significant within group effect was found with planned comparisons indicating the following: ap<0.001 vs Basal. There was also a significant effect across the pretreatment groups with planned comparisons indicating the following differences for +100 nM UCN2 stimulation: bp<0.001 vs Control. These results were replicated in three independent experiments. These results were replicated in four independent experiments.
We next investigated whether UCN2- or UCN3-induced CRF2(a) receptor activation heterologously desensitized retinoblastoma CRF1 receptors. The levels of CRF1 receptor cyclic AMP signaling in Y79 cells exhibiting strong CRF2(a) receptor desensitization induced by UCN2 or UCN3, however, were similar to the level of CRF1 receptor cyclic AMP signaling in control cells without UCN2 or UCN3 pretreatment (Figure 6B). Therefore, after having tested a large repertoire of both selective and non-selective CRF receptor agonists, we conclude that there is no significant cross-desensitization of CRF1 and CRF2(a) receptors endogenously expressed in Y79 cells.
3.5. Agonist-induced phosphorylation of CRF2(a) receptors
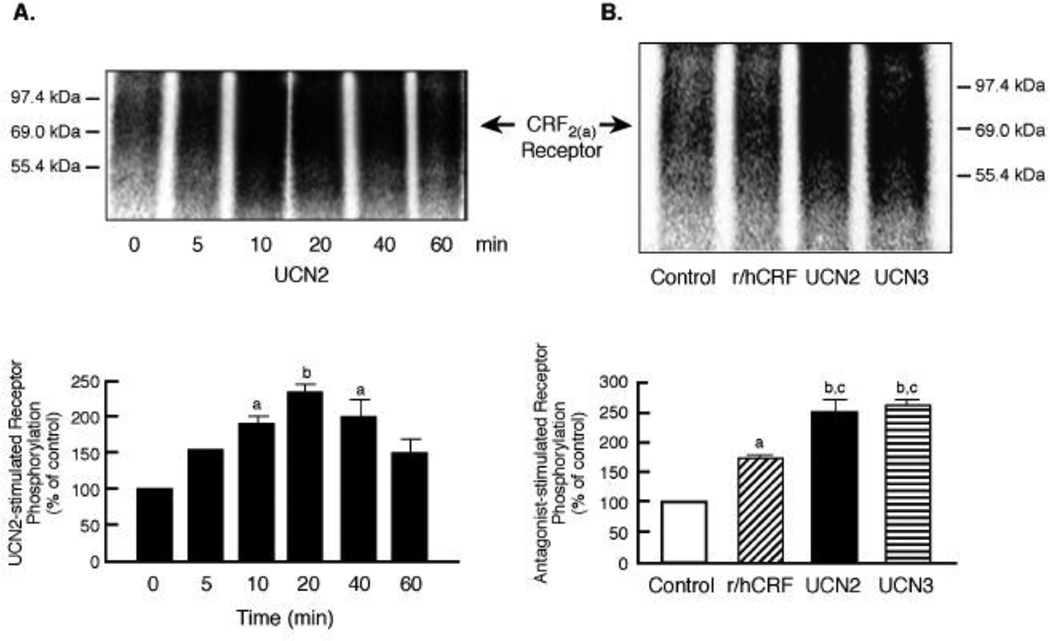
According to the classic model of homologous desensitization, agonist-activated receptors are phosphorylated by a family of G protein-coupled receptor kinases (GRKs) on specific serine/threonine residues in the receptor’s third intracellular loop and/or carboxyl terminus [13,14]. Since there are no commercially available antibodies with the necessary high specificity and sensitivity to detect endogenous CRF receptors after phosphorylation and internalization, we used our well-characterized assays for measuring phosphorylation and subsequent trafficking of membrane CRF receptors with an HA epitope inserted at an inert region of the N-terminus and expressed in HEK293 cells [21,27]. Stimulation of HA-CRF2(a) receptor-expressing cells with UCN2 resulted in a time-dependent increase in a dense phospho-CRF2(a) band with a Mr of 60–70 kDa in membrane fractions (Figure 7A), consistent with the known molecular size of the CRF2(a) receptor protein [1–4]. CRF2(a) receptor phosphorylation band density after 10-, 20-, and 40-min incubation with UCN2 was significantly increased 2.3- to 2.5-fold greater than the basal phosphorylation in control cells treated with media. The magnitudes of CRF2(a) receptor phosphorylation stimulated by UCN2 and UCN3 (100 nM for 10 min) were similar (Figure 7B). In contrast, a low level of CRF2(a) receptor phosphorylation was measured in HEK293 cells incubated with 100 nM h/rCRF for 10 min (Figure 7B). Thus, CRF, an agonist deficient in its ability to induce CRF2(a) receptor desensitization, also exhibited a very weak capacity to stimulate CRF2(a) receptor phosphorylation.
Figure 7. Agonist-stimulated phosphorylation of membrane CRF2(a) receptors.
(A) 72 h after transfection with HA-tagged CRF2(a) receptor cDNA, HEK293 cells were metabolically labeled with 32Pi and then stimulated with 100 nM UCN2 for 0–60 min. By ANOVA with planned comparisons, the levels of phosphorylated CRF2(a) receptor (Mr 60–70 kDa) measured by densitometry were significantly increased: ap<0.05 vs 0 min UCN2 (Control); bp<0.01 vs 0 min (Control). This data was replicated in three independent experiments. (B) The magnitude of CRF2(a) receptor phosphorylation was compared following a 10 min stimulation with buffer (Control) or 100 nM agonist (r/hCRF, UCN2, UCN3). ANOVA with planned comparisons detected the following significant differences in CRF2(a) phosphorylation bands: ap<0.01 vs Control; bp<0.001 vs Control; cp<0.01 vs r/hCRF. This data was replicated in three independent experiments.
3.6. Effect of serine-threonine kinases on Gs-coupled CRF2(a) receptor signaling
Protein kinase A (PKA) can homologously desensitize certain Gs-coupled GPCRs by phosphorylating serines and/or threonines within PKA consensus sites on the receptor’s intracellular loops and/or C-terminus [13]. The CRF2(a) receptor possesses a potential PKA phosphorylation site (RAS) at the beginning of the serine-threonine-rich sequence in the IC3 loop [1–3]. Thus, we determined if a PKA mechanism mediated homologous or heterologous CRF2(a) receptor desensitization. Preincubating Y79 cells with the cell-permeable PKA inhibitor H89 prior to UCN2 pretreatment failed to alter homologous CRF2(a) receptor desensitization (Figure 8A). Furthermore, the magnitude of UCN2-stimulated cyclic AMP accumulation was similar in Y79 cells in which PKA was maximally activated by phosphodiesterase-resistant dibutyryl cyclic AMP or SpcAMP-S and in control cells without PKA activation (Figure 8B).
Casein kinases CK1 and CK2 can also desensitize GPCR signaling by phosphorylating intracellular serines and/threonines [36]. Since there are potential CK phosphorylation sites located within the IC3 loop (TTSE) and C-terminus (SIPT) of the CRF2(a) receptor [1–3], we examined the effect of CK1 and CK2 inhibitors on the homologous CRF2(a) receptor desensitization process in Y79 cells. UCN2-induced CRF2(a) receptor desensitization was not inhibited in cells pretreated with the non-selective CK inhibitor TBB (75.6±1.0%), the CK2-specific inhibitor quinalizarin (74.1±1.0%), or the CK1-specific inhibitor IC261 (74.0±2.4%), compared to control cells without CK inhibitor pretreatment (74.4±1.1%) (Figure 8C).
In other experiments, we determined if PKA or casein kinase phosphorylated the HA-tagged CRF2(a) receptor. CRF2(a) receptor phosphorylation was not detected following maximal activation of PKA with dbcAMP (Figure 8D). In addition, pretreatment of HEK293 cells with PKA inhibitors H89 or RpcAMP did not reduce the 2-fold increase in CRF2(a) receptor phosphorylation stimulated by a saturating concentration of UCN2 (100 nM) (Figure 8D). Furthermore, UCN2 was still able to induce a 2-fold phosphorylation of the CRF2(a) receptor in HEK293 cells in which CK1 and CK2 had been inhibited by TBB pretreatment (Figure 8D). Hence, neither PKA nor casein kinase mechanisms are involved in the phosphorylation and desensitization of CRF2(a) receptors.
3.7. Agonist-induced CRF2(a) receptor internalization
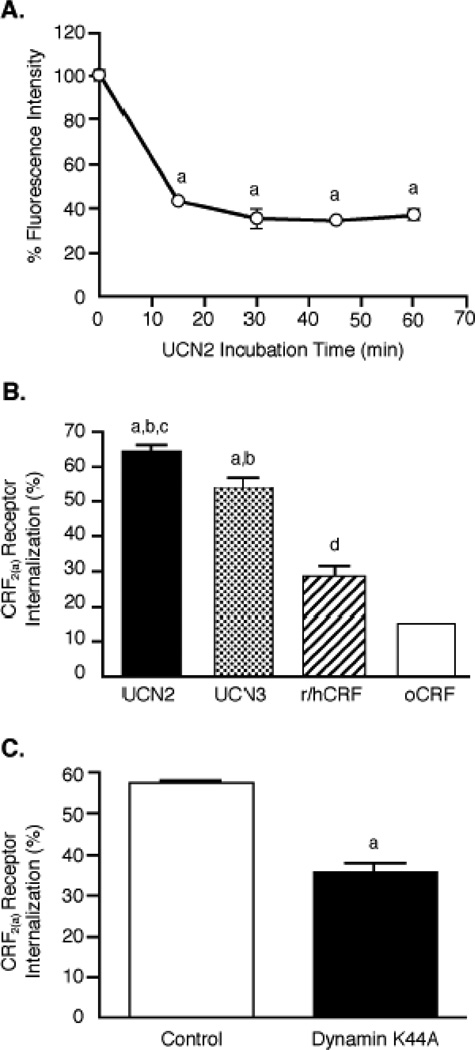
We next used flow cytometry to study agonist-induced differences in internalization of CRF2(a) receptors in transfected HEK293 cells that were used for the phosphorylation assay. In time course experiments (Figure 9A), CRF2(a) receptors rapidly internalized in cells stimulated with 100 nM UCN2 for 15 min (56.9±0.2%), 30 min (64.4±2.0%), 45 min (65.8±0.2%), and 60 min (63.1±1.3%). Because we observed differential desensitization of CRF2(a) receptors by urocortins and CRF in Y79 cells, we compared the magnitude of CRF2(a) receptor internalization in transfected HEK293 cells stimulated with 100 nM agonist for 30 min. CRF2(a) receptors internalized to a significantly (p<0.001) greater extent in response to UCN2 (64.4±2.0%) or UCN3 (53.7±3.7%) in comparison to the weak internalization resulting from h/rCRF (27.9±3.5%) or oCRF (14.9±0.1%) (Figure 9B). CRF2(a) receptor internalization was significantly greater following UCN2 than UCN3 (p<0.05) and following r/hCRF than oCRF (p<0.05). Finally, we determined the effect of the dynamin dominant negative mutant K44A on the CRF2(a) receptor sequestration process. Internalization of CRF2(a) receptors stimulated by a 30-min UCN2 exposure was inhibited ~50% (p<0.001) in HEK293 cells co-transfected with K44A compared to cells co-transfected with an empty vector (Figure 9C), suggesting that CRF2(a) receptors internalize via a clathrin-mediated pathway.
Figure 9. Agonist-stimulated CRF2(a) receptor internalization in HEK293 cells.
(A) Flow cytometry was used to measure the time course for internalization of HA-tagged CRF2(a) receptors in HEK293 cells transfected 72 h earlier and then incubated with 100 nM UCN2 for 0-60 min (n=2–4/timepoint). By ANOVA, there were significant differences across the timepoint groups [F= 429.2; p < 0.0001] with planned comparisons indicating the following: ap<0.001 vs 0 min. This data was confirmed in five independent experiments. (B) CRF2(a) receptor internalization was measured in transfected HEK293 stimulated for 30 min with 100 nM UCN2, UCN3, r/hCRF, or oCRF (n=2–3/group). By ANOVA, there were significant differences across the agonist stimulation groups [F= 56.65; p < 0.0001] with planned indicating the following: ap<0.001 vs oCRF; bp<0.001 vs r/hCRF; cp<0.05 vs UCN3; dp<0.05 vs oCRF. This data was confirmed in three independent experiments. (C) After CRF2(a)-expressing HEK293 cells were transfected 72 h earlier with the dynamin dominant negative mutant K44A or empty vector, CRF2(a) receptor internalization was measured by flow cytometry following a 30-min stimulation with 100 nM UCN2 (n=2/group). By t-test, the groups were statistically different: ap<0.0001 vs Control. These results were replicated in four independent experiments. Vasopressin V2 receptor internalization in V2-expressing HEK293 cells incubated with 100 nM arginine vasopressin for 30 min was also significantly decreased (p=0.02) by dynamin K44A (29.8±3.7%) compared to control vector (50.0±0.2%).
3.8. Characteristics of βarrestin2 recruitment by CRF2(a) receptors
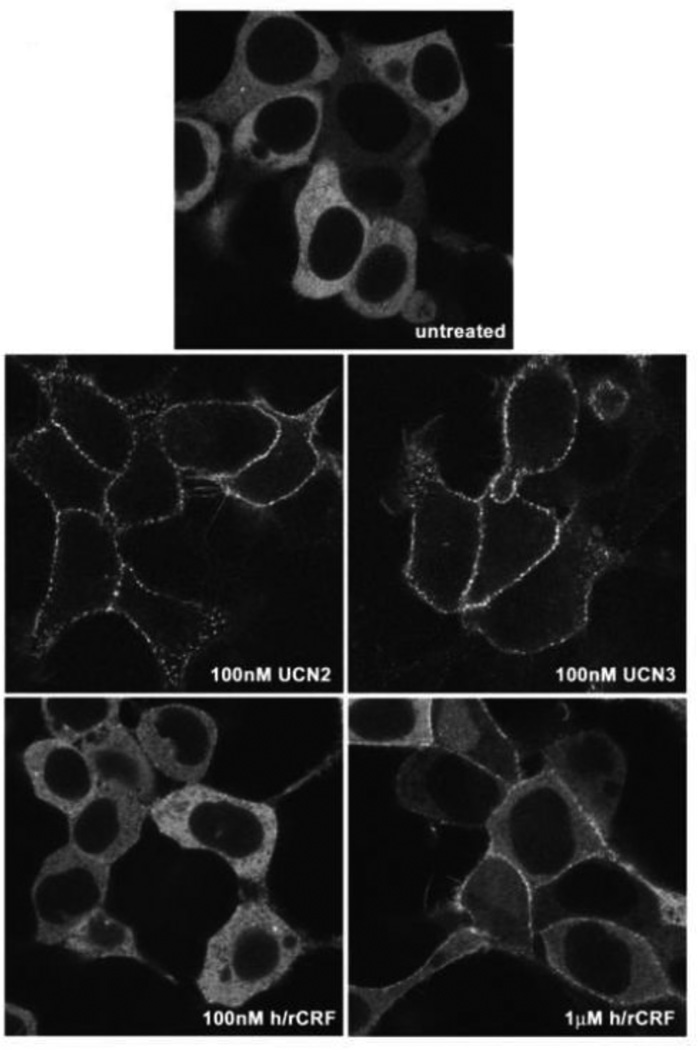
GRK-mediated phosphorylation of receptor proteins can increase the affinity of many GPCRs for βarrestins up to 30-fold thereby promoting recruitment of βarrestin proteins from the cytoplasm to the cell surface [13]. Binding of recruited βarrestins to GRK-phosphorylated GPCRs then sterically uncouples the receptor from its cognate G protein, resulting in the “arrest” or termination of agonist-mediated signal transduction [13–15]. βArrestins further contribute to the desensitization of many GPCRs by targeting them to clathrin-coated pits for endocytosis, resulting in the spatial uncoupling of the GPCR from the plasma membrane. To determine in real time and in living cells if activated CRF2(a) receptors associate with βarrestin proteins, and whether the pattern of βarrestin recruitment varies in an agonist-specific manner, we employed a βarrestin2-GFP fusion protein. HEK293 cells were co-transfected with CRF2(a) receptor and βarrestin2-GFP. The transfected cells were scanned using a confocal microscope prior to and after treatment with UCN2, UCN3, or h/rCRF. In the absence of agonist, βarrestin2-GFP remained evenly distributed throughout the cytoplasm and was excluded from the nucleus (Figure 10). In cells exposed to 100 nM UCN2 or 100 nM UCN3 for 10 min, βarrestin2 was strongly recruited from the cytoplasm to membrane CRF2(a) receptors, depleting the observable cytoplasmic pool of βarrestin2-GFP (Figure 10). In contrast, stimulation of CRF2(a) receptor-expressing HEK293 cells with 100 nM h/rCRF for 10 min resulted in minimal recruitment of βarrestin2-GFP to the cell membrane with the majority of βarrestin2 remaining in the cytoplasm (Figure 10). Treatment with 1µM h/rCRF for 10 min elicited a greater level of βarrestin2 recruitment that was still, however, substantially weaker than that induced by 100 nM UCN2 and UCN3 (Figure 10). For all three ligands, a punctuate pattern of fluorescence was observed at the plasma membrane that likely reflects the localization of βarrestin2-GFP with CRF2(a) receptors in clathrin coated pits (Figure 10). Longer agonist incubations of up to 1 hour did not alter the magnitude or pattern of βarrestin2 recruitment to the cell surface.
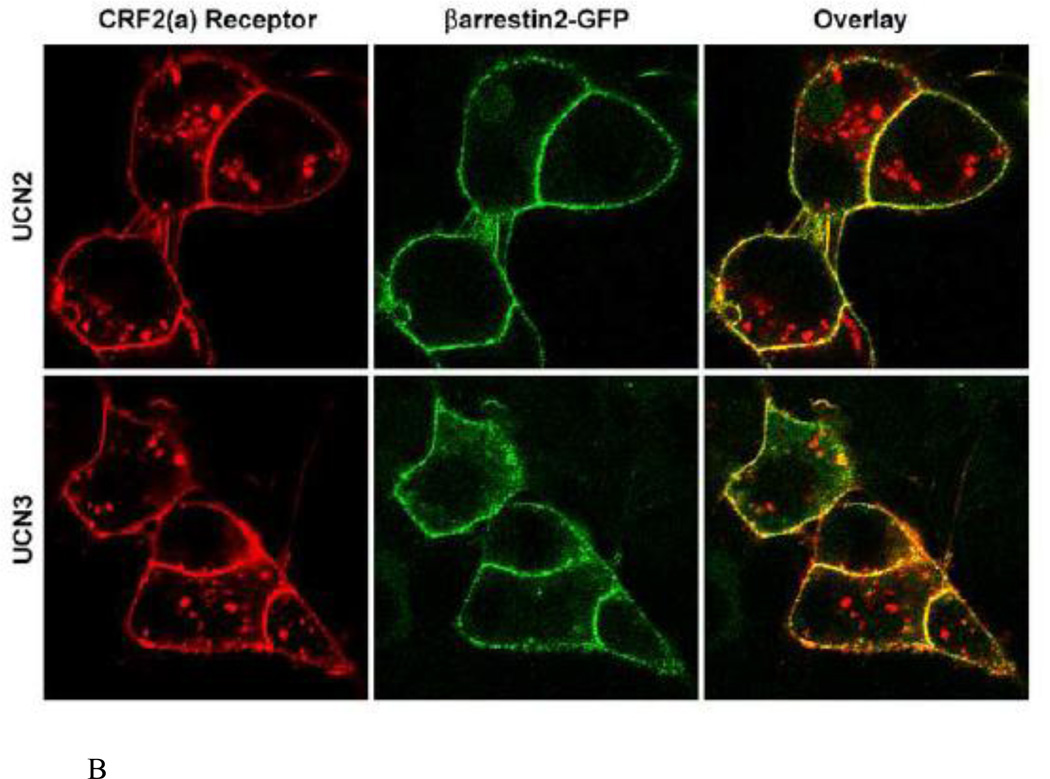
Figure 10. Recruitment of βarrestin2 by agonist-activated human CRF2(a) receptors in HEK293 cells.
Confocal microscopy was used to evaluate the interaction of βarrestin2-GFP with full-length wild-type CRF2(a) receptors transiently expressed in HEK293 cells. This representative experiment shows the distribution of βarrestin2-GFP in untreated cells and cells incubated with UCN2 (100 nM), UCN3 (100 nM), or r/hCRF (100 nM or 1 µM) for approximately 10 min.
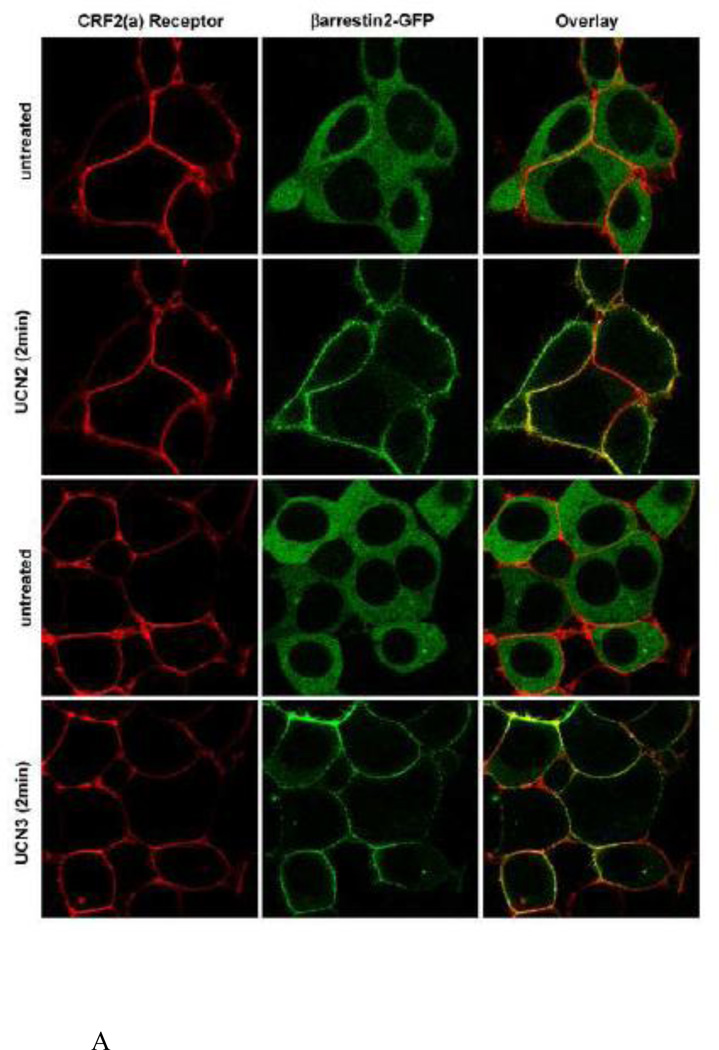
The interaction of CRF2(a) receptors with βarrestin2 was further characterized by performing colocalization experiments in live HEK293 cells. Prior to agonist treatment, HA tagged-CRF2(a) receptors (red) localized to the plasma membrane and βarrestin2-GFP (green) resided in the cytoplasm (Figure 11 A). When these same HEK293 cells were stimulated with 100 nM UCN2 or UCN3, βarrestin2 rapidly translocated within 2 minutes to the cell surface and prominently colocalized (yellow) with membrane CRF2(a) receptors (Figure 11A). Following a 30-min treatment with UCN2 or UCN3, CRF2(a) receptors were strongly internalized into endocytic vesicles (Figure 11B). βarrestin2 proteins, however, did not traffic with these receptors inside the cell but continued to localize with CRF2(a) receptors at the plasma membrane (Figure 11B). Taken together, these data suggest that the CRF2(a) receptor, like the CRF1 receptor, is a “class A” GPCR in terms of its interaction with βarrestin2, and this property is unaffected by binding UCN2 or UCN3 as the ligand. However, the magnitude of βarrestin2 recruitment by the CRF2(a) receptor depends dramatically upon the activating ligand with the more potent desensitizers UCN2 and UCN3 inducing a much stronger response.
Figure 11. Colocalization of βarrestin2 with cell-surface but not internalized agonist-activated CRF2(a) receptors.
HEK293 cells transiently expressing βarrestin2-GFP and HA-CRF2(a) receptors were prelabeled with Alexa Fluor 594 conjugated anti-HA monoclonal antibody and evaluated by confocal microscopy. (A) Distribution of CRF2(a) receptor immunofluorescence (red) and βarrestin2-GFP fluorescence (green) in the same cells before and after a 2 min treatment with 100 nM UCN2 or UCN3. (B) Distribution of CRF2(a) receptor immunofluorescence (red) and βarrestin2-GFP fluorescence (green) after an ~30 min treatment with 100 nM UCN2 or UCN3. Colocalization of βarrestin2-GFP with the receptor is indicated by the yellow color in the overlay.
At the saturating concentration of 100 nM, UCN2 and UCN3 appear visually to stimulate the same magnitude of βarrestin2 recruitment to the CRF2(a) receptor expressed in HEK293 cells (Figure 10). To determine if differences exist in the level of βarrestin2 recruitment induced by these ligands and to investigate the concentration dependence of the response, we transiently expressed CRF2(a) receptors in U2OS cells and analyzed the redistribution of βarrestin2-GFP using the INCell Analyzer 3000 [26]. U2OS cells were utilized for quantitation because of their superior adherent and morphological properties that allow for more sensitive measurements [25,37]. UCN2 and UCN3 concentration-response curves for βarrestin2-GFP translocation to CRF2(a) receptors are depicted in Figure 12. Consistent with our findings in HEK293 cells, no significant differences were observed in the magnitude of βarrestin2 recruitment to the cell membrane in response to saturating concentrations of UCN2 (259 ± 6 Fgrains) or UCN3 (266 ± 10 Fgrains). However, an almost 10-fold difference (p<0.0001) was observed for the EC50 values of βarrestin2 recruitment by the CRF2(a) receptor activated by UCN2 (1.76 ± 0.06 nM) and UCN3 (15.13 ± 0.08 nM), and these values correspond closely with the reported Kd values for CRF2(a) receptor binding of these two selective ligands [17,18,29,30].
3.9. βarrestin2 regulation of Gs-coupled CRF2(a) receptor signaling
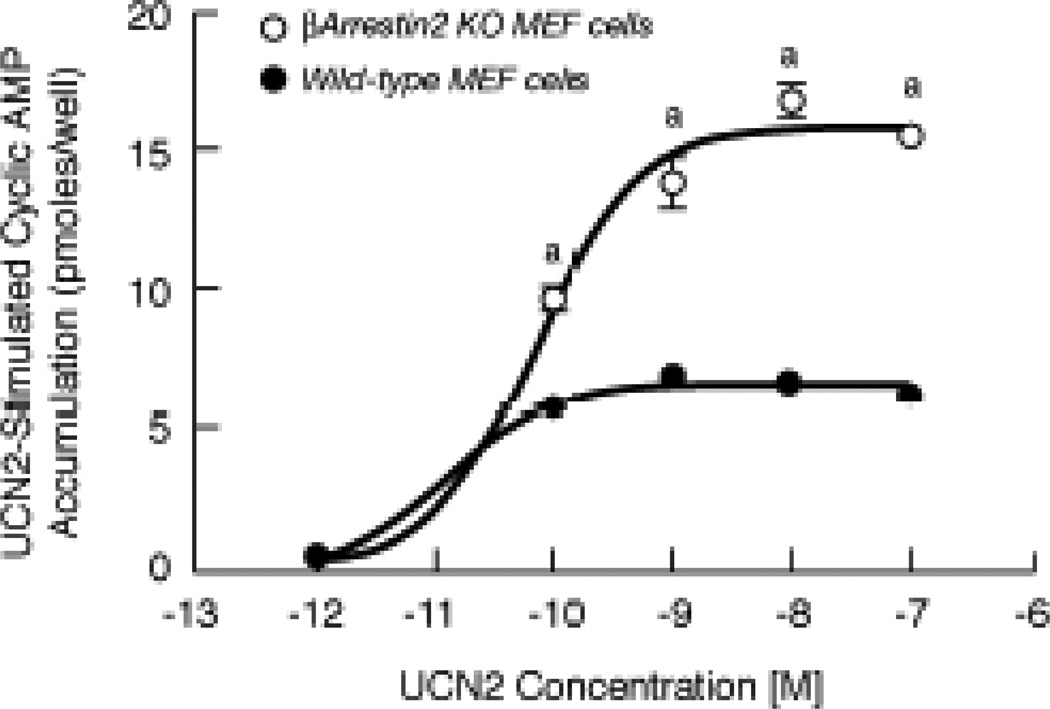
To investigate further βarrestin2 control of CRF2(a) receptor signal transduction, we assessed Gs-coupled CRF2(a) receptor signaling in mouse embryonic fibroblast (MEF) cell lines derived from wild-type and βarrestin knockout (KO) mice, which serve as an ideal cell system for studying βarrestin-dependent mechanisms [22]. Cell surface expression of CRF2(a) receptors was not significantly different in the βarrestin2 KO MEF cells (44.5 ± 3.4%) and wild-type MEF cells (37.5 ± 3.4%) 48 h following transfection. When UCN2 concentration-response curves for the stimulation of cyclic AMP accumulation were measured, we found that the maximum for UCN2-stimulated cyclic AMP accumulation was significantly increased more than 2-fold in βarrestin2 KO MEF cells (15.82 ± 0.40 pmoles/well; p<0.001) compared to wild-type MEF cells with endogenous βarrestin expression (6.59 ± 0.15 pmoles/well) (Figure 13).
Figure 13. Dose-response stimulation of cyclic AMP accumulation by urocortin 2 in wild-type and βarrestin2 knockout mouse embryonic fibroblast (MEF) cells.
In two experiments, cyclic AMP levels (pmoles/well) were measured in quadruplicate in CRF2(a) receptor-expressing MEF cells stimulated with 0–100 nM UCN2 for 15 min. By ANOVA, there was a significant interaction [F=50.13; p<0.0001] for MEF cell group and stimulation conditions (10−12 to 10−7 M UCN2). A significant within group effect [F=251.9; p<0.0001] was observed with planned comparisons indicating significant increases in cyclic AMP accumulation over the UCN2 concentration range. A significant across MEF cell group effect [F=534.7; p<0.0001] was detected with planned comparisons indicating the following difference: ap<0.001 vs Wild-type MEF cells.
4. Discussion
The data reported herein establishes that the human CRF2(a) receptor is stringently regulated by a rapid and strong desensitization mechanism following activation by the urocortins or related stresscopin peptides but not CRF. Furthermore, UCN2 and UCN3 at saturating concentrations stimulated similar high levels of CRF2(a) receptor phosphorylation and maximal βarrestin2 recruitment while CRF2(a) receptors homologously desensitized and internalized via the dynamin/clathrin-mediated pathway to a greater degree in response to UCN2 compared to UCN3. In contrast, CRF induced very low levels of CRF2(a) receptor phosphorylation, βarrestin2 recruitment, and internalization. These findings indicate the CRF2(a) receptor adopts distinct conformations depending on the bound agonist that can modulate its βarrestin2 recruitment, subcellular trafficking and functional regulation, perhaps through differences in picketing and fencing of the cell membrane by the actin cytoskeleton known to be involved in synaptic plasticity [38]. We also discovered that deletion of the βarrestin2 gene in MEF cells significantly upregulated Gs-coupled CRF2(a) receptor signaling activated by UCN2 and unrestrained by rapid homologous desensitization. Likewise, isoproterenol-stimulated cyclic AMP accumulation is abnormally high and prolonged in βarrestin2 knockout MEF cells expressing β2-adrenergic adrenergic receptors [22], and UCN2-induced CRF2(b) receptor desensitization is greatly impaired when βarrestin2 protein expression is knocked down by siRNA in HEK293 cells [16]. Neither protein kinase A nor casein kinase mechanisms, however, contributed to CRF2(a) receptor phosphorylation and desensitization. Overall, our study suggests that the rate and magnitude of homologous desensitization of Gs-coupled signaling by the CRF2(a) receptor is governed by the affinity and selectivity of agonist-induced conformations for triggering GRK-dependent phosphorylation, βarrestin2 translocation, and dynamin/clathrin-dependent internalization.
Unlike the CRF1 receptor, the CRF2(a) receptor binds and is activated by agonists with a broad range of potencies. Therefore, we assessed the ability of strong and weak ligands to desensitize retinoblastoma CRF2(a) receptors. Although stresscopin’s N-terminus is two amino acids longer than the N-terminus of human UCN3, while the N-terminus of stresscopin-related peptide is five amino acids longer than the N-terminus of human UCN2 [23], the robust levels of CRF2(a) receptor desensitization were equivalent when comparing the effects of SCP to UCN3 and SRP to UCN2. Importantly, desensitization of cyclic AMP signaling by CRF2(a) receptors generated by exposure to high-affinity, selective (UCN2, UCN3) or non-selective (UCN1, sauvagine) ligands was strikingly greater than that induced by the low-affinity, non-selective ligand CRF. Moreover, CRF2(a) receptors were only minimally phosphorylated and internalized weakly in response to high CRF concentrations known to saturate the receptor protein in contrast to the high magnitude and rapid rate of CRF2(a) receptor phosphorylation and internalization elicited by the urocortins. Similarly, a recent report has shown that the CRF2(b) receptor, the peripheral CRF2 splice variant primarily expressed in cardiovascular, gastrointestinal, and muscle cells, homologously desensitized and internalized to a substantially greater extent in response to UCN2 compared to CRF in HEK293 cells [16]. Interestingly, activation of the β2-adrenergic receptor by different agonists not only influenced the velocity and extent of GRK-mediated phosphorylation and desensitization but also resulted in different serine and threonine residues being phosphorylated in this receptor’s C-terminus [39,40]. Furthermore, a recent fluorescence energy transfer (FRET) study concluded that weaker βarrestin2 recruitment and internalization of the β2-adrenergic receptor activated by norepinephrine compared to stronger regulatory responses induced by epinephrine was a consequence of GRK5-mediated phosphorylation being considerably less for norepinephrine compared to epinephrine potentially resulting in different receptor conformations [41]. Similarly, different serine-threonine phosphorylation “bar codes” on the CRF2(a) receptor activated by urocortins or CRF may generate different conformations that govern the rate and magnitude of βarrestin2 recruitment to cell surface CRF2(a) receptors and their subsequent internalization.
Our study using real-time confocal microscopy in live cells provides the first evidence that the human CRF2(a) receptor activated by UCN2 or UCN3 rapidly and strongly recruits GFP-labeled βarrestin2 to the plasma membrane where βarrestin2 proteins colocalize with CRF2(a) receptor proteins. Similar to the CRF1 receptor [21,42], βarrestin2 dissociates from the activated CRF2(a) receptor at or near the plasma membrane and fails to internalize with the CRF2(a) receptor as a unit into endocytic vesicles. Therefore, both CRF receptor isoforms exhibit a “class A” GPCR-arrestin interaction. A recent study using immunohistochemistry in fixed HEK293 cells found that the peripherally expressed CRF2(b) receptor also exhibits a “class A” interaction with βarrestins [16]. To quantify differences in βarrestin2-GFP recruitment stimulated by the urocortins, we transiently expressed the CRF2(a) receptors in U2OS cells, which allow for accurate and sensitive quantitation due to their superior adherent and morphological properties [21]. Although saturating concentrations of UCN2 and UCN3 stimulated similar high levels of CRF2(a) receptor phosphorylation and maximal βarrestin2 recruitment, we found the βarrestin2 recruitment process to be more sensitive when CRF2(a) receptors were activated by UCN2 compared to UCN3, in accordance with the more rapid desensitizing action of UCN2 compared to UCN3. Since UCN3 induces less CRF2(a) receptor desensitization and internalization than that resulting from UCN2, the higher residual level of phosphorylated membrane CRF2(a) receptors during UCN3 exposure may interact with recruited βarrestin2 remaining near the membrane to promote UCN3-specific βarrestin2-mediated signaling and other cellular events.
In contrast, exposure of CRF2(a) receptors to h/rCRF in living cells produced a strikingly weak translocation of βarrestin2 to the membrane. Specifically, we found a markedly lower magnitude and slower rate of βarrestin2 recruitment to CRF2(a) receptors in response to h/rCRF concentrations as high as 1 µM compared to the remarkably more rapid and greater βarrestin2 translocation response induced by 100 nM UCN2 and UCN3. This observation agrees with our other findings that h/rCRF induced weak phosphorylation, desensitization, and internalization of the CRF2(a) receptor. A recent study reported, however, similar magnitudes of βarrestin2 translocation to cell surface CRF2(b) receptors activated by 100 nM UCN2 or h/rCRF [16]. The 406–414 amino acid CRF(2a) receptor and the 430–438 amino-acid CRF(2b) receptor splice variants have identical sequences in their intracellular loops and C-terminal regions [1–6]. Substantial differences in their distal N-terminal extracellular domains may confer, however, distinct agonist-induced conformations to the two CRF2 receptor splice variants that result in differences in the extent and/or pattern of GRK phosphorylation and βarrestin2 recruitment in response to UCN2 or h/rCRF. Alternatively, activation of CRF2(a) and CRF2(b) receptors by the same ligand may differentially expose phosphorylation-independent βarrestin2 binding motifs. While GRK-mediated GPCR phosphorylation is critical for βarrestin recruitment [13,14], recent studies have also shown that agonist-induced changes in receptor conformation independent of GRK phosphorylation can also play an important role in the interaction of βarrestin2 with GPCRs including the CRF1 receptor [21,43,44].
5. Conclusion
We have found unique profiles of CRF2(a) receptor phosphorylation, βarrestin2 recruitment, homologous desensitization, and dynamin/clathrin-mediated internalization conferred by binding of urocortins or CRF. Thus, differential desensitizing and internalizing actions of urocortins and CRF may generate agonist-specific regulation of CRF2(a) receptors in brain neurons depending on which ligand is present. Increasing evidence indicates that “biased agonists” promote and stabilize distinct receptor conformations with functional selectivity for specific signal transduction pathways [14,45]. Previous studies using transfected CHO cells have demonstrated strong ERK signaling by recombinant CRF2(b) receptors activated by UCN2 but not CRF [46]. Our preliminary experiments have detected a large stimulation of Akt phosphorylation by CRF2(a) receptors activated by UCN2 or UCN3 compared to minimal effects from binding CRF [Olivares-Reyes & Hauger, unpublished data]. Rapid and strong recruitment of βarrestin2 to the CRF2(a) receptor during sustained exposure to UCN2 or UCN3 may shift signaling from G protein- to βarrestin2-dependent pathways. CRF2(a) receptors are highly expressed in the hippocampus, the bed nucleus of the stria terminalis, and other regions of the and extended amygdala that have been implicated in posttraumatic stress disorder (PTSD) and mood pathophysiology [1,2,47]. Furthermore, prolonged severe stress and trauma upregulate expression of limbic CRF2(a) receptors [48,49]. Stress also promotes CRF2(a) receptor trafficking from the cytoplasm to the cell surface thereby rendering dorsal raphe neurons hyperexcitable (i.e., abnormally high discharge rates) due to excessive CRF2(a) signaling [50]. Importantly, G protein-coupled hypersignaling by brain CRF2(a) receptors has been found to promote startle hyperreactivity, persistent anxiety-like behavior, and contextual fear in mouse models of PTSD [10,11,48–51]. Thus, the failure to rapidly and strongly desensitize limbic CRF2(a) receptors upregulated to abnormally high levels following stress and trauma due to deficient βarrestin2 recruitment may be an important mechanism contributing to PTSD [52].
Supplementary Material
Highlights.
CRF2(a) receptors are rapidly and strongly desensitized by urocortins but not CRF.
Urocortins but not CRF strongly internalize CRF2(a) into dynamin/clathrin pits.
Urocortins but not CRF induce rapid, strong class A CRF2(a)-βarrestin2 interactions.
Gs-coupled CRF2(a) signaling is upregulated in cells with a βarrestin2 gene deletion.
Agonist-specific receptor conformations govern Gs-coupled CRF2 signaling regulation.
Acknowledgements
Dr. Hauger was supported by a BLR&D Merit Review grant from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. Dr. Hauger also received support from the VA Center of Excellence for Stress and Mental Health (CESAMH), and NIH/NIA (AG018386) and NIH/NIMH (MH074697) RO1 grants. Dr. Oakley received support from the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, and completed a portion of the confocal microscopy experiments while he was employed at Xsira Pharmaceuticals (Morrisville, NC). Dr. Olivares-Reyes was supported by CINVESTAV-IPN, a UC MEXUS-CONACYT grant for collaborative projects, and a Grant for Research on Health from Fundacion Miguel Aleman 2010. Receptor internalization and cell surface receptor expression measurements were performed at the Flow Cytometry Research Core of the San Diego VA Research Service that is supported by DVA and a NIH/NIAID RO1 (AI36214). We also grateful acknowledge Dr. Dimitri Grigoriadis and Neurocrine Biotechnology (La Jolla, CA) for generously providing NBI-30775 under an approved material transfer agreement. Dr. Robert Lefkowitz (HHMI, Duke University) kindly provided the βarrestin KO and wild-type MEF cells under a material transfer agreement.
Keywords/Abbreviations
- CK1
casein kinase 1
- CK2
casein kinase 2
- CRF
corticotropin releasing factor receptor
- CRF1 receptor
CRF receptor type 1
- CRF2(a) receptor
CRF receptor type 2(a)
- K44A
dynamin dominant negative mutant
- GPCR
k G protein-coupled receptor
- GRK
GPCR kinase; βarrestin2
- PKA
protein kinase A
- PKC
protein kinase C
- UCN1
urocortin 1
- UCN2
urocortin 2
- UCN3
urocortin 3
- SCP
stresscopin
- SRP
stresscopin-related peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Conflict of Interest
The authors have no conflict of interest to report.
References
- 1.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS & Neurological Disorders – Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrin MH, Vale W. Chapter 25: Corticotropin-releasing factor receptors. In: Pangalos MN, Davies CH, editors. Understanding G protein-coupled receptors and their role in the CNS. New York: Oxford University Press; 2002. pp. 505–526. [Google Scholar]
- 3.Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Brit J Pharmacol. 2012;166:85–97. doi: 10.1111/j.1476-5381.2011.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 5.Grigoriadis DE. The corticotropin-releasing factor receptor: a novel target for the treatment of depression and anxiety-related disorders. Expert Opin Ther Targets. 2005;9:651–684. doi: 10.1517/14728222.9.4.651. [DOI] [PubMed] [Google Scholar]
- 6.Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann NY Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 8.Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–889. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Waselus M, Valentino RJ, Van Bockstaele E. Collaterized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebow M, Neufeld-Cohen A, Kuperman Y, Tsoory M, Gil S, Chen A. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J Neurosci. 2012;32:6906–6916. doi: 10.1523/JNEUROSCI.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 14.Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, β-arrestin1, and β-arrestin2 with G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 16.Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2b endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol Endocrinol. 2008;22:689–706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutknecht E, Hauger RL, Van der Linden I, Georges Vauquelin G, Dautzenberg FM. Expression, binding, and signaling properties of CRF2(a) receptors endogenously expressed in human retinoblastoma Y79 cells: passage-dependent regulation of functional receptors. J Neurochem. 2008;104:926–936. doi: 10.1111/j.1471-4159.2007.05052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dautzenberg FM, Gutknecht E, Van der Linden I, Olivares-Reyes JA, Dürrenberger F, Hauger RL. Cell type specific calcium signaling by corticotropin-releasing factor type 1 (CRF1) and 2a (CRF2(a)) receptors: Gq coupling in human embryonic kidney 293 but not SK-N-MC neuroblastoma cells. Biochem Pharmacol. 2004;68:1833–1844. doi: 10.1016/j.bcp.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Palchaudhuri MR, Hauger RL, Wille S, Fuchs E, Dautzenberg FM. Isolation and pharmacological characterization of two functional splice variants of corticotropin releasing factor type 2 receptor from Tupaia belangeri. J Neuroendocrinol. 1999;11:419–428. doi: 10.1046/j.1365-2826.1999.00348.x. [DOI] [PubMed] [Google Scholar]
- 20.Dautzenberg FM, Braun S, Hauger RL. GRK3 mediates desensitization of CRF1 receptors: a potential mechanism regulating stress adaptation. Am J Physiol. 2001;280:R935–R946. doi: 10.1152/ajpregu.2001.280.4.R935. [DOI] [PubMed] [Google Scholar]
- 21.Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl terminal and intracellular loop sites for CRF1 receptor phosphorylation and βarrestin2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol. 2007;293:R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohout TA, Lin F-T, Perry SJ, Conner DA, Lefkowitz RJ. β-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 24.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled-β-arrestin complexes after receptor endocytosis. J Biol Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 25.Oakley RH, Cowan CL, Hudson CC, Loomis CR. Transfluor provides a universal cell-based assay for screening G protein coupled receptors. In: Minor L, editor. Handbook of Assay Development in Drug Discovery. New York: Marcel Dekker; 2006. pp. 435–457. [Google Scholar]
- 26.Oakley RH, Hudson CC, Cruickshank RD, Meyers DM, Payne RE, Rehm SM, Loomis CR. The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay Drug Develop Tech. 2002;1:21–30. doi: 10.1089/154065802761001275. [DOI] [PubMed] [Google Scholar]
- 27.Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Comm. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- 28.Clark RB, Knoll BJ, Barber R. Partial agonists and G protein-coupled receptor desensitization. Trends Pharmacol Sci. 1999;20:279–286. doi: 10.1016/s0165-6147(99)01351-6. [DOI] [PubMed] [Google Scholar]
- 29.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci (USA) 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dautzenberg FM, Wille S, Braun S, Hauger RL. GRK3 regulation during CRF- and urocortin-induced CRF1 receptor desensitization. Biochem Biophys Res Commun. 2002b;298:303–308. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- 32.Dautzenberg FM, Hauger RL. G protein receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor, type 1 in human Y-79 retinoblastoma cells. Neuropharmacology. 2001;40:394–407. doi: 10.1016/s0028-3908(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 33.Alonso N, Fernandez N, Notcovich C, Monczor F, Simaan M, Baldi A, Gutkind S, Davio C, Shayo C. Cross-desensitization and cointernalization of H1 and H2 histamine receptors reveal new insights into histamine signal integration. Mol Pharmacol. 2013;83:1087–1098. doi: 10.1124/mol.112.083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivier J, Gulyas J, Kunitake K, DiGruccio M, Cantle JP, Perrin MH, Donaldson C, Vaughan J, Million M, Gourceroi G, Adelson DW, Rivier C, Tache Y, Vale W. Stressin-1A, a potent corticotrophin releasing factor receptor 1 (CRF1)-selective peptide agonist. J Med Chem. 2007;50:1668–1674. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci USA. 2004;101:9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobin A. Are we beta-ARKing up the wrong tree? Casein kinase 1 alpha provides an additional pathway for GPCR phosphorylation. Trends Pharmcol Sci. 2002:337–343. doi: 10.1016/s0165-6147(02)02043-6. [DOI] [PubMed] [Google Scholar]
- 37.Oakley RH, Barak LS, Caron MG. Real time imaging of GPCR-mediated arrestin translocation as a strategy to evaluate receptor-protein interactions. In: George SR, O’Dowd BF, editors. G Protein Coupled Receptor-Protein Interactions. Hoboken, NJ: John Wiley & Sons; 2005. pp. 53–80. [Google Scholar]
- 38.Milan-Lobo L, Gsandtner I, Gaubitzer E, Runzler D, Buchmayer F, Kohler G, Bonci A, Freissmuth M, Sitte HH. Subtype-specific differences in corticotropin-releasing factor receptor complexes detected by fluorescence spectroscopy. Mol Pharmacol. 2009;76:1196–1210. doi: 10.1124/mol.109.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krasel C, Vilardaga JP, Bunemann M, Lohse MJ. Kinetics of G-protein-coupled receptor signaling and desensitization. Biochem Soc Trans. 2004;32:1029–1031. doi: 10.1042/BST0321029. [DOI] [PubMed] [Google Scholar]
- 40.Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the β2-adrenergic receptor. Biochemistry. 2005;44:6133–6143. doi: 10.1021/bi0475469. [46] Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the β2-adrenergic receptor. Biochemistry 2005;44:6133-6143. [DOI] [PubMed] [Google Scholar]
- 41.Reiner S, Ambrosio M, Hoffmann C, Lohse MJ. Differential signaling of the endogenous agonist at the beta2-adrenergic receptor. J Biol Chem. 2010;285:36188–36198. doi: 10.1074/jbc.M110.175604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- 43.Marion S, Oakley RH, Kim KM, Caron MG, Barak LS. A beta-arrestin binding determinant common to the second-intracellular loops of Rhodopsin-family G protein-coupled receptors. J Biol Chem. 2006;281:2932–2938. doi: 10.1074/jbc.M508074200. [DOI] [PubMed] [Google Scholar]
- 44.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Brit Pharmacol. 2008;153(Suppl 1):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011 doi: 10.1016/j.molmed.2010.11.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase-1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2b by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- 47.Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 48.Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura-Silva AP, Pego JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OFX, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08262.x. in press. [DOI] [PubMed] [Google Scholar]
- 50.Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neufeld-Cohen A, Kelly PAT, Paul ED, Carter RN, Skinner E, Olverman HJ, Vaughan JM, Issler O, Kuperman Y, Lowry CA, Vale WW, Seckl JR, Chen A, Jamieson PM. Chronic activation of corticotropin-releasing factor receptor type 2 receptors reveals a key role of 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol Psychiatry. 2012;72:437–447. doi: 10.1016/j.biopsych.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hauger RL, Olivares-Reyes JA, Braun S, Dautzenberg FM, Oakley RH. Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology. 2012;62:705–714. doi: 10.1016/j.neuropharm.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.