Abstract
Background
Mineralocorticoid receptor antagonists (MRA) reduce morbidity and mortality in heart failure with reduced ejection fraction (HFREF), but can cause hyperkalemia and acute kidney injury. Guidelines recommend measurement of serum potassium (K) and creatinine (Cr) before and serially after MRA initiation, but the extent to which this occurs is unknown.
Methods and Results
Using electronic data from 3 health systems 2005-2008, we performed a retrospective review of laboratory monitoring among 490 patients hospitalized for HFREF who were subsequently initiated on MRA therapy. Median age at time of MRA initiation was 73 years and 37.1% were female. Spironolactone accounted for 99.4% of MRA use. Initial ambulatory MRA dispensing occurred at hospital discharge in 70.0% of cases. In the 30 days before MRA initiation, 94.3% of patients had a K or Cr measurement. Pre-initiation K was >5.0 mmol/L in 1.4% and Cr >2.5 mg/dL in 1.7%. In the 7 days after MRA initiation among patients who remained alive and out of the hospital, 46.5% had no evidence of K measurement; by 30 days, 13.6% remained untested. Patient factors explained a small portion of post-initiation K testing (c-statistic 0.67).
Conclusions
While laboratory monitoring prior to MRA initiation for HFREF is common, laboratory monitoring following MRA initiation frequently does not meet guideline recommendations, even in patients at higher risk for complications. Quality improvement efforts that encourage the use of MRA should also include mechanisms to address recommended monitoring.
Keywords: heart failure, medication, safety, laboratory testing, mineralocorticoid receptor antagonists, aldosterone
The aldosterone / mineralocorticoid receptor antagonists (MRA)—spironolactone and eplerenone—reduce hospitalization and death among patients with heart failure and reduced left ventricular ejection fraction (HFREF). The Randomized Aldactone Evaluation Study (RALES),1 Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),2 and Eplerenone in Mild Patients Hospitalization and Survival Study (EMPHASIS)3 provide strong evidence for the use of an MRA in a broad range of patients with HFREF.
Notwithstanding their overall efficacy in selected patients, MRA carry an increased risk of hyperkalemia and acute renal injury. In RALES, the median increase in serum potassium compared to control was 0.3 mmol/L, with rates of serious hyperkalemia 2% for spironolactone versus 1% for placebo. In EPHESUS, serum potassium ≥ 6.0 mmol/L occurred in 5.5% of eplerenone-treated patients versus 3.9% of placebo. These risks are potentially exacerbated by the presence of cardiorenal syndrome or chronic kidney disease, whose prevalence was underrepresented in trial populations.4 Frequent abnormalities in serum potassium and kidney function after MRA initiation have been confirmed in observational data,5 and may translate into increased adverse events.6,7
Recognizing these potential dangers, clinical practice guidelines mirror testing protocols from MRA trials. The 2005 American College of Cardiology (ACC) / American Heart Association (AHA) Guideline for the Evaluation and Management of Heart Failure stated, “Close monitoring of serum potassium is required; potassium levels and renal function should be checked in 3 days and at 1 week after initiation of therapy and at least monthly for the first 3 months,”8 a recommendation that has remained essentially unchanged in the 20099 and 201310 guideline updates. The European Society of Cardiology guidelines have also consistently recommended “to check blood chemistry 1 week and 4 weeks after starting/increasing dose”.11,12 The extent to which these recommendations are being followed in routine practice has not been well characterized.13
Therefore, we set out to describe laboratory monitoring patterns for patients initiated on MRA in a large, multi-center, community-based cohort of adults with HFREF. Our aims were to 1) characterize patient selection for MRA therapy, 2) describe the frequency and results of pre-initiation monitoring, 3) describe the frequency and timing of post-initiation monitoring, 4) identify factors associated with failure to perform recommended laboratory monitoring after MRA initiation, and lastly 5) explore the association between early post-initiation testing and subsequent clinical outcomes.
Methods
Data Source
Participating health plans for the present study were Kaiser Permanente Colorado, Kaiser Permanente Northwest, and Fallon Community Health Plan.14,15 These health plans serve an ethnically and socioeconomically diverse population across varying clinical practice settings and geographically diverse areas. A Virtual Data Warehouse (VDW) at each site served as a distributed standardized data resource comprised of electronic datasets, populated with linked demographic, administrative, ambulatory pharmacy, outpatient laboratory test results, and health care utilization data.16,17 For the present study, laboratory data were limited to the ambulatory setting, as detailed inpatient clinical data were not consistently captured across study sites. Institutional review boards at participating sites approved the study and waiver of consent was obtained due to the nature of the study.
Patient Population
All persons aged ≥21 years with diagnosed HF based on a hospitalization with a primary discharge diagnosis of HF between January 1, 2005 through December 31, 2008 using International Classification of Diseases, 9th Edition (ICD-9) codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.x. Prior studies have shown a positive predictive value of >95% for HF when compared with Framingham clinical criteria.18-20
Assessments of left ventricular ejection fraction (LVEF) were ascertained for each HF patient from echocardiograms, nuclear imaging modalities, and left ventriculography test results available from site-specific databases complemented by manual chart review. The measure obtained closest to the index date of study entry was used. We restricted the cohort to HFREF by requiring the summary LVEF to be quantitatively ≤40% or qualitatively described as “moderately” or “severely” reduced.21 Patients without a documented LVEF measurement were excluded (24.6%).
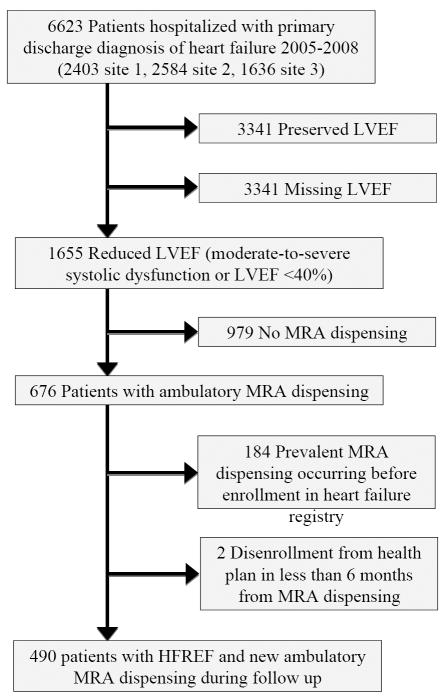
Patients were required to have a new pharmacy dispensing of either spironolactone or eplerenone at any time after HF hospitalization, with no prior dispensing of these agents (Figure 1). MRA use was determined using filled outpatient prescriptions from health plan databases.
Figure 1.
Cohort selection.
Covariates
Baseline covariates used to describe the cohort and perform multivariate modeling were chosen a priori based on presumed interactions with MRA therapy, previously published HF prognostic models, and availability within the VDW. We determined the presence of coexisting illnesses based on diagnoses or procedures using relevant ICD-9 codes, CPT procedure codes, as well as site-specific diabetes mellitus and cancer registries.17
Outcomes
Reflecting clinical guideline recommendations, we assessed serum potassium and creatinine measurement in the 30-days preceding MRA dispensing, the 7 days following MRA dispensing, and the 30 days following MRA dispensing. We also used a Kaplan-Meier estimate for time to the first serum potassium measure following MRA dispensing during available follow up. Subjects were censored at the time they were hospitalized, died, disenrolled from the health plan, or reached the end of study follow-up (December 31, 2008). Hospitalizations were identified from each site’s VDW. Deaths were identified from hospital and billing claims databases, administrative health plan databases, state death certificate registries, and Social Security Administration files as available at each site.15,19
Statistical Analysis
We described baseline patient characteristics overall and stratified by serum potassium measure, no measure, or death/hospitalization in the 7 days after initial MRA dispensing. Continuous variables were ordinalized using cut points chosen based on clinically meaningful values. Missing covariate data were treated as a separate category. Statistical significance was evaluated using Wilcoxon rank sum tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables.
Step-wise multivariable logistic regression was employed to examine the independent relationship between baseline characteristics and failure to perform laboratory monitoring in the week after MRA initiation, with model performance characterized using c-statistics and Nagelkerek pseudo-R2. Variable selection included predetermined key variables of clinical interest (age, gender, baseline serum potassium and creatinine), as well as additional variables with significant univariate associations. Missing heart rate and blood pressure measures were imputed to the median.
Cox proportional hazards models were used to assess the relationship between testing 1-7 days after MRA initiation and the outcome of all-cause hospitalization or death 8-90 days after MRA initiation; variables included in the model were taken from the Yale readmission risk calculator.22
All analyses were conducted using SAS statistical software, version 9.1 (Cary, NC).
Results
In total, 490 patients with HFREF were initiated on MRA in the ambulatory setting. Median age was 73.6 years and 37.1% were female (Table 1). Spironolactone accounted for 99.4% of MRA use and eplerenone 0.6% of use. The starting dose of MRA was 12.5 mg per day in 33.9%, 25 mg per day in 60.0%, and 50 mg per day in 7.1%. MRA was initially dispensed in 57.8% of patients at the time of a hospital discharge and within 1-7 days of a hospital discharge in an additional 12.2% of the cohort. Concomitant use of angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) was 73.5%; concomitant dispensing of both ACEI and ARB in the setting of MRA initiation was 0.6%. Use of an MRA in the absence of a loop diuretic was 23.7%. Use of an MRA with concomitant use of oral potassium supplementation was 40.8%.
Table 1.
Characteristics of patients with prior hospitalization for heart failure with reduced left ventricular ejection fraction at the time of initiation of a mineralocorticoid receptor antagonist, stratified by death/hospitalization, serum potassium measure, or no measure in the 7 days after initial MRA dispensing.
|
|
EXCLUDED due to death or hospitalization within 7 days | ELIGIBLE for 7 day testing comparisons N=443 (90.4% of 490)
|
||
|---|---|---|---|---|
| Characteristic | TESTING ≤7 days from MRA dispensing | NO testing ≤7 days of MRA dispensing | P value* | |
| N=47 (9.6% of 490) | N=237 (53.5% of 443) | N=206 (46.5% of 443) | ||
|
| ||||
| Age in years, median (IQR) | 76 (66, 84) | 73 (62, 81) | 74 (61, 80) | 0.97 |
|
| ||||
| Age categories, n (%) | ||||
|
| ||||
| Age ≤65 | 10 (21.3%) | 76 (32.1%) | 64 (31.1%) | 0.58 |
|
| ||||
| Age 65-74 | 10 (21.3%) | 60 (25.3%) | 45 (21.8%) | |
|
| ||||
| Age ≥75 | 27 (57.5%) | 101 (42.6%) | 97 (47.1%) | |
|
| ||||
| Female gender, n (%) | 22 (46.8%) | 87 (36.7%) | 73 (35.4%) | 0.78 |
|
| ||||
| Cardiac History, n (%) | ||||
|
| ||||
| LVEF, median (IQR) | 0.25 (0.20, 0.33) | 0.25 (0.20-0.33) | 0.25 (0.20-0.30) | 0.65 |
|
| ||||
| Missing LVEF | 8 (17.0%) | 82 (34.6%) | 56 (27.2%) | 0.12 |
|
| ||||
| Acute myocardial infarction | 7 (14.9%) | 39 (16.5%) | 23 (11.2%) | 0.11 |
|
| ||||
| Coronary artery bypass surgery | 2 (4.3%) | 17 (7.2%) | 8 (3.9%) | 0.14 |
|
| ||||
| Coronary stent or angioplasty | 5 (10.6%) | 30 (12.7%) | 24 (11.7%) | 0.75 |
|
| ||||
| Atrial fibrillation or flutter | 9 (19.1%) | 48 (20.3%) | 33 (16.0%) | 0.25 |
|
| ||||
| Ventricular tachycardia | 2 (4.3%) | 16 (6.8%) | 4 (1.9%) | 0.02 |
|
| ||||
| Rheumatic valvular disease | 7 (14.9%) | 11 (4.6%) | 5 (2.4%) | 0.21 |
|
| ||||
| ICD | 4 (8.5%) | 19 (8.0%) | 18 (8.7%) | 0.78 |
|
| ||||
| Pacemaker | 5 (10.6%) | 23 (9.7%) | 18 (8.7%) | 0.73 |
|
| ||||
| Medical History, n (%) | ||||
|
| ||||
| Ischemic stroke or TIA | 4 (8.5%) | 16 (6.8%) | 9 (4.4%) | 0.28 |
|
| ||||
| Peripheral arterial disease | 16 (34.0%) | 27 (11.4%) | 26 (12.6%) | 0.69 |
|
| ||||
| Dyslipidemia | 27 (57.4%) | 126 (53.2%) | 97 (47.1%) | 0.20 |
|
| ||||
| Hypertension | 22 (46.8%) | 117 (49.4%) | 100 (48.5%) | 0.86 |
|
| ||||
| Diabetes mellitus | 9 (19.1%) | 42 (17.7%) | 38 (18.4%) | 0.42 |
|
| ||||
| Diagnosed dementia | 8 (17.0%) | 25 (10.5%) | 22 (10.7%) | 0.96 |
|
| ||||
| Diagnosed depression | 8 (17.0%) | 51 (21.5%) | 38 (18.4%) | 0.42 |
|
| ||||
| Chronic lung disease | 20 (42.6%) | 85 (35.9%) | 80 (38.8%) | 0.52 |
|
| ||||
| Chronic liver disease | 2 (4.3%) | 9 (3.8%) | 13 (6.3%) | 0.23 |
|
| ||||
| Systemic cancer | 12 (25.5%) | 16 (6.8%) | 18 (8.7%) | 0.43 |
|
| ||||
| Medications at initial dispensing ambulatory MRA | ||||
|
| ||||
| Spironolactone | 47 (100%) | 235 (99.2%) | 205 (99.5%) | 0.99 |
|
| ||||
| Eplerenone | 0 (0%) | 2 (0.8%) | 1 (0.5%) | |
|
| ||||
| Starting MRA dose, mg/24hr | ||||
| ≤12.5 mg | 11 (23.4%) | 92 (38.8%) | 63 (30.6%) | 0.08 |
| 25 mg | 20 (63.8%) | 127 (53.6%) | 132 (64.1%) | |
| ≥50 mg | 6 (12.8%) | 18 (7.6%) | 11 (5.3%) | |
|
| ||||
| Potassium supplement | 18 (38.3%) | 107 (45.1%) | 75 (36.4%) | 0.06 |
|
| ||||
| Loop diuretic | 32 (68.1%) | 187 (78.9%) | 155 (75.2%) | 0.36 |
|
| ||||
| Thiazide-type diuretic | 4 (8.5%) | 33 (13.9%) | 19 (9.2%) | 0.13 |
|
| ||||
| ACEI | 22 (46.8%) | 156 (65.8%) | 135 (65.6%) | 0.95 |
|
| ||||
| ARB | 2 (4.3%) | 24 (10.1%) | 24 (11.7%) | 0.61 |
|
| ||||
| Beta-blocker | 26 (55.3%) | 190 (80.2%) | 164 (79.6%) | 0.88 |
|
| ||||
| Digoxin | 17 (36.2%) | 104 (43.9%) | 89 (43.2%) | 0.89 |
|
| ||||
| Vitals | ||||
|
| ||||
| Systolic blood pressure, mmHg, median (IQR) | 121 (108, 132) | 122 (110, 140) | 120 (110, 140) | 0.90 |
|
| ||||
| Missing Systolic BP | 5 (10.6%) | 11 (4.6%) | 17 (8.3%) | 0.12 |
|
| ||||
| <=90 | 5 (10.6%) | 13 (5.5%) | 9 (4.4%) | 0.30 |
|
| ||||
| 91-110 | 3 (6.4%) | 58 (24.5%) | 52 (25.2%) | |
|
| ||||
| 111-140 | 13 (27.7%) | 103 (43.5%) | 89 (43.2%) | |
|
| ||||
| 141-160 | 21 (44.7%) | 40 (16.9%) | 22 (10.7%) | |
|
| ||||
| >160 | 3 (6.4%) | 12 (5.1%) | 17 (8.3%) | |
|
| ||||
| Heart rate, bpm, median (IQR) | 80 (64, 96) | 83.5 (72, 99) | 78 (66, 89) | <0.001 |
|
| ||||
| Missing heart rate | 5 (10.6%) | 11 (4.6%) | 18 (8.7%) | 0.08 |
|
| ||||
| Discharged to a facility location (nursing home, skilled nursing, rehab unit, or another hospital) | 9 (3.8%) | 12 (5.8%) | ||
p values are from Fisher’s exact test and chi-square test for categorical variables and Wilcoxon Rank Sum for continuous variables, and compare testing within 7 days to no testing within 7 days.
MRA=mineralocorticoid receptor antagonist; IQR=interquartile range; LVEF=left ventricular ejection fraction; ICD=implantable cardioverter-defibrillator; TIA=transient ischemia attack; ACEI=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker.
Pre-initiation laboratory testing
In the 30 days before initial MRA dispensing, ambulatory serum potassium measurement was noted for 69.0%; an additional 25.3% of patients had no ambulatory monitoring but were hospitalized during this pre-initiation period, presumably with laboratory monitoring during the hospitalization. Therefore, 5.7% of patients had no direct or indirect evidence of a serum potassium measurement in the 30 days before MRA initiation. Serum creatinine measurements closely paralleled serum potassium measures (absolute differences <1%). Among patients with available measurements, median pre-initiation serum potassium was 4.1 mmol/L (IQR 3.9-4.5); 1.2% [n=6] of patients had a potassium level above the recommended cutoff of 5.0 mmol/L on the measurement immediately preceding MRA initiation. Median pre-initiation serum creatinine was 1.4 mg/dL (IQR 1.1-1.6); 1.6% [n=8] had a creatinine level above the recommended cutoff of 2.5 mg/dL on the measurement immediately preceding MRA initiation (Table 2).
Table 2.
Pre-initiation laboratory testing of patients with prior hospitalization for heart failure with reduced left ventricular ejection fraction (HFREF) at the time of initiation of a mineralocorticoid receptor antagonist (MRA), stratified by serum potassium measure, or no measure in the 7 days after initial MRA dispensing.
|
|
EXCLUDED due to death or hospitalization within 7 days | ELIGIBLE for 7 day testing comparisons N=443 (90.4% of 490)
|
||
|---|---|---|---|---|
| Characteristic | TESTING ≤7 days from MRA dispensing | NO testing ≤7 days of MRA dispensing | P value* | |
| N=47 (9.6% of 490) | N=237 (53.5% of 443) | N=206 (46.5% of 443) | ||
|
| ||||
| Potassium, serum, prior to initiation of MRA (mmol/L), median, (IQR) (N=299) | 4.1 (3.6, 4.4) | 4.2 (3.9-4.5) | 4.1 (3.9-4.5) | 0.61 |
|
| ||||
| Potassium, serum, prior to initiation of MRA (mmol/L), n (%) | ||||
|
| ||||
| >5.51 | 0 | 0 | 0 | 0.31 |
|
| ||||
| 5.01-5.50 | 0 | 5 (2.1%) | 1 (0.5%) | |
|
| ||||
| 4.51-5.00 | 5 (13.5%) | 30 (12.7%) | 29 (14.1%) | |
|
| ||||
| 4.01-4.50 | 24 (37.8%) | 65 (27.4%) | 43 (20.9%) | |
|
| ||||
| 3.51-4.00 | 9 (24.3%) | 61 (25.7%) | 42 (20.4%) | |
|
| ||||
| <=3.50 | 9 (24.3%) | 10 (4.2%) | 13 (6.3%) | |
|
| ||||
| Labs not available: Hospitalized within prior 30 days† | 7 (14.9%) | 51 (21.5%) | 66 (32.0%) | 0.012 |
|
| ||||
| No labs, no hospitalization within prior 30 days | 3 (6.4%) | 15 (6.3%) | 12 (5.8%) | 0.83 |
|
| ||||
| Creatinine, serum, prior to initiation of MRA (mg/dL), median, (IQR) (N=307) | 1.1 (0.9,1.5) (N=47) | 1.2 (1.0-1.4) (N=176) | 1.1 (0.9-1.4) (N=131) | 0.10 |
|
| ||||
| Creatinine, serum, prior to initiation of MRA (mg/dL), n (%) | ||||
|
| ||||
| >3.00 | 1 (2.8%) | 2 (0.8%) | 0 (0%) | 0.62 |
|
| ||||
| 2.51-3.00 | 0 | 3 (1.3%) | 2 (1.0%) | |
|
| ||||
| 2.01-2.50 | 4 (11.1%) | 6 (2.5%) | 3 (1.5%) | |
|
| ||||
| 1.51-2.00 | 3 (8.3%) | 15 (6.3%) | 13 (6.3%) | |
|
| ||||
| 1.01-1.50 | 15 (41.7%) | 98 (41.4%) | 63 (30.6%) | |
|
| ||||
| <=1.00 | 13 (36.1%) | 52 (21.9%) | 50 (24.3%) | |
|
| ||||
| Labs not available, hospitalized within prior 30 days† | 8 (17.0%) | 48 (20.3%) | 63 (30.6%) | 0.012 |
|
| ||||
| No labs, no hospitalization within prior 30 days | 3 (6.4%) | 13 (5.5%) | 12 (5.8%) | 0.88 |
p values are from Fisher’s exact test and chi-square test for categorical variables and Wilcoxon Rank Sum for continuous variables, and compare testing within 7 days to no testing within 7 days.
Missing labs prior to MRA initiation are primarily due to hospitalization in prior 30 days from hospital facilities where laboratory data was unavailable; 81% of subjects missing an outpatient serum potassium measure prior to MRA start were hospitalized in prior 30 days. Percentages were not significantly different for subjects with and without potassium monitoring within 7 days of MRA start: 76.9% and 84.6%, respectively; p=0.24.
MRA=mineralocorticoid receptor antagonist; IQR=interquartile range.
Post-initiation laboratory testing
Among the 443 patients who remained alive and out of the hospital (non-hospitalized and no urgent or emergency care visits) in the 7 days after MRA initiation, 46.5% (n=206) of patients did not have evidence of serum potassium measured 1-7 days following MRA dispensing. For the subset of patients with first ambulatory MRA dispensing occurring at or within 7 days of a hospital discharge, 43.6% (n=136/312) had no evidence of 7-day post-initiation serum potassium measurement; for those with first ambulatory MRA dispensing greater than a week after hospital discharge, 53.4% (n=70/131) had no evidence of 7-day post-initiation serum potassium measurement. After excluding patients (n=75) who were hospitalized, died, or had health plan enrollment termination in the 8-30 days after MRA initiation,13.6% (n=50/368) of patients had no evidence of serum potassium measurement in the entire 30 days from MRA dispensing. For those patients who were monitored within 7 days and did not suffer death or hospitalization in the 30 days after MRA dispensing, the rate of a second potassium measurement in between days 8-30 was 67.5% (n=127/203). Figure 2 depicts a Kaplan-Meier analysis of time to potassium measurement, with censoring for hospitalization, death, or health plan disenrollment.
Figure 2.
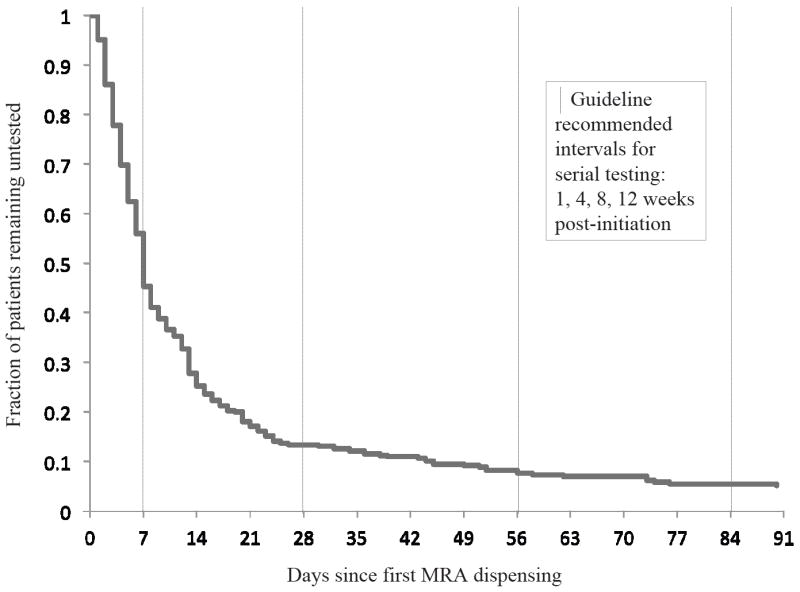
Kaplan-Meier curve of time to serum potassium measurement from first mineralocorticoid receptor antagonist (MRA) dispensing, with censoring for death, hospitalization, or plan disenrollment.
Factors associated with failure to monitor
A combination of patient demographics, comorbidities, laboratory testing, vital signs, and medication use explained a relatively small portion of post-initiation K testing patterns (cstatistic 0.67). See Table 3 for details.
Table 3.
Performance of baseline factors in predicting serum potassium testing in the 7 days after mineralocorticoid receptor antagonist (MRA) initiation.
| Model Covariates | c-statistic | Pseudo R2 |
|---|---|---|
| Age, gender | 0.50 | 0.0007 |
| Age, gender, pre-initiation serum potassium and creatinine* | 0.56 | 0.0192 |
| Age, gender, pre-initiation serum potassium and creatinine, MI, CABG, PCI, V-tach, dyslipidemia, starting dose of MRA, prescription for potassium chloride, prescription for thiazide diuretic, heart rate, systolic blood pressure† | 0.67 | 0.1129 |
| Age, gender, K levels, CR levels, MI, CABG, PCI, V-tach, starting dose of MRA, drug indicators for potassium chloride and thiazide diuretic, dyslipidemia, heart rate, systolic BP, site, year of MRA start | 0.70 | 0.1620 |
Pre-initiation serum potassium and creatinine were categorized: potassium missing, ≤4.50 mmol, and > 4.50 mmol; creatinine missing, ≤1.50 mg/dL, and >1.50 mg/dL.
Median used for missing heart rate and systolic blood pressure. Addition of LVEF as continuous covariate for subjects with a quantitative measure did not significantly influence c-statistic or R.
Association with post-initiation monitoring and subsequent outcomes
In the 8-90 days after MRA initiation among patients that remained alive and out of the hospital in the first 7 days, death occurred in 6.2% (n=14/225) of those with 7-day serum potassium testing compared to 6.1% (n=12/196) of those without 7-day potassium testing (0.31 versus 0.29 deaths per person years, respectively; p=0.97). All cause hospitalization was also similar, with 1.70 hospitalizations per person years for patients with 7-day post-initiation serum potassium testing compared to 1.61 hospitalizations per person years for patients without 7-day testing (p=0.41). Cox proportional hazards modeling was not statistically significant for the association between testing 1-7 days post MRA initiation and mortality or hospitalization 8-90 days post-initiation, although was underpowered to assess small but clinically meaningful differences (adjusted hazards ratio 1.18, 95% confidence interval 0.83-1.62).
Discussion
In this study of patients with HFREF in managed care plans, laboratory monitoring following initiation of MRA frequently did not meet guideline recommendations. While almost all patients had a baseline serum potassium and creatinine test (or a hospitalization with assumed testing) in the month before initiation of MRA, nearly half of patients had no evidence of a repeat serum potassium and creatinine measurement in the 7 days following initial MRA dispensing. Due to concerns about MRA-mediated hyperkalemia and renal dysfunction, particularly among patients outside of the narrow eligibility criteria and close supervision inherent in randomized controlled trials, such testing has been recommended since 2005, with all major heart failure clinical practice guidelines currently endorsing testing within at least a week of MRA initiation and again at 4 weeks.8-11,23. Not only were there gaps between observed and recommended post-initiation testing patterns, testing had little association with risk (i.e., lack of testing was not confined to the lowest risk patients). Finally, MRA initiation appeared to occur at a dose higher than recommended or with concomitant potassium supplementation in a significant minority of patients. These results highlight a need for education and systems of care that enhance appropriate safety monitoring, particularly if quality improvement initiatives24 and performance measures25 are implemented to increase the use of MRA in patients with HFREF.
The laboratory testing patterns seen here are concordant with older studies documenting suboptimal monitoring following initiation of MRA13 and other high-risk medications in general populations.26 In a study looking at laboratory evaluation among all ambulatory patients dispensed spironolactone in 1999-2000 within 10 health maintenance organizations (regardless of indication), 27.7% of patients had not had a follow-up test for potassium and creatinine over the next 13 months.13 Contemporary patterns of laboratory monitoring have not been described in detail for HFREF populations, and speak to the novelty of our findings.
The results presented here do not address the reasons for nonadherence to monitoring recommendations for MRA use in HFREF. Prior study has shown that a computerized system of monitoring alerts managed by pharmacists increased the number of patients who received laboratory safety monitoring of drug therapy;27 in contrast, laboratory monitoring alerts within a computerized physician order entry system have not improved monitoring.28 These data suggest that physicians may not be best positioned to order follow up testing. Furthermore, our study assessed the actual performance of laboratory testing, not the intent of prescribing clinicians to obtain such testing in follow-up. The high rate of pre-initiation testing (the absence of which may allow a provider not to prescribe MRA) but subsequently low post-initiation testing (over which a provider has less control) suggest that many gaps in recommended testing may be related to system execution and patient adherence with such testing.
Whether greater monitoring may improve safety and help increase the benefit of MRA use in real-world HFREF patients remains to be determined.7,29 Theoretically, improvements in adherence to guideline recommended laboratory monitoring can lead to pre-emptive changes in MRA dosing, thereby avoiding some unnecessary adverse events. Yet, interventions that improve laboratory monitoring have not necessarily translated into cost-effective mechanisms to improve clinical outcomes, particularly if not concentrated among high-risk patients30 and targeted to the health care providers best suited to implement suggested monitoring.31
The policy implications of laboratory testing related to MRA use are important. Appropriate MRA use is the lowest of major recommended therapies for HFREF.24, 32 Yet, a joint report from the ACC/AHA Task Force on Performance Measures and the American Medical Association Physician Consortium for Performance Improvement decided not to include MRA use: “…treatment with aldosterone receptor antagonists was considered but not developed because of the large number of patients excluded from the denominator because of renal insufficiency or hyperkalemia before or during treatment with these agents. In addition, the development of serious renal failure or hyperkalemia in large numbers of patients might be an unintended consequence of the broad implementation of such a measure.”25 Development of effective systems for laboratory monitoring before and after MRA initiation may assuage these concerns, thereby allowing for responsible MRA performance measures that help larger numbers of real-world HFREF patients realize the benefits of MRA seen in randomized trials.
Potential Limitations
Insured populations in our participating health plans may not be fully representative of the general U.S. population or international populations. Nevertheless, the demographic diversity represented across 3 geographically diverse health plans, as well as the community-based nature of health care delivery, suggest that findings from our cohort are likely to be highly generalizable to patients with HFREF in “real-world” practice settings. The data used here to define MRA initiation come from the dispensing date of prescribed MRA, which may misclassify some patients who start their drug at a later date or who do not end up taking it at all. Laboratory testing may occur outside the health plan electronic data capture, either at distant sites or at contract hospitals; however, the potential for such missing data is small as patients are financially discouraged from out-of-system testing and our methods accounted for non-network hospitalizations. Most patients initiating ambulatory MRA within a day of hospital discharge presumably had been started on MRA during hospitalization with laboratory monitoring during the hospital course; regardless, such patients are relatively high risk for ongoing serum potassium and creatinine changes following discharge, and thus multiple organizations now recommend clinic follow up and laboratory testing within a week after any HF hospital discharge.
Conclusion
Laboratory monitoring following initiation of an MRA in real world practice frequently does not meet guideline recommendations. Given the known risks of MRA, quality improvement efforts that encourage the use of MRA for HFREF should also consider effective mechanisms to ensure appropriate monitoring. The extent to which poor monitoring reduces safety and explains the lack of benefit for MRA seen in observational studies should be further evaluated.
Acknowledgments
The authors wish to thank all of the project managers, data programmers, and analysts for their critical technical contributions and support that made this study possible.
Sources of Funding
This study and Dr Allen’s time were supported by 1K23HL105896 from National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health. Additional support for cohort creation was provided by the American Recovery and Reinvestment Act grant 1RC1HL099395 (PRESERVE Study) administered through the NHLBI. Dr Peterson is supported by grant K08 HS019814-01 from the Agency for Healthcare Research and Quality.
Footnotes
Disclosures
None.
References
- 1.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. New Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. New Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 3.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. New Engl J Med. 2011;364:11–21. [Google Scholar]
- 4.Masoudi FA, Gross CP, Wang Y, Rathore SS, Havranek EP, Foody JM, Krumholz HM. Adoption of spironolactone therapy for older patients with heart failure and left ventricular systolic dysfunction in the united states, 1998-2001. Circulation. 2005;112:39–47. doi: 10.1161/CIRCULATIONAHA.104.527549. [DOI] [PubMed] [Google Scholar]
- 5.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Cardiac Fail. 2004;10:297–303. doi: 10.1016/j.cardfail.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. New Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Heywood JT, Liu Y, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Associations between outpatient heart failure process of care measures and mortality. Circulation. 2011;123:1601–1610. doi: 10.1161/CIRCULATIONAHA.110.989632. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 11.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Lévy S, Linde C, Lopez-Sendon JL, Nieminen MS, Piérard L, Remme WJ. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 13.Raebel MA, McClure DL, Chan KA, Simon SR, Feldstein AC, Lafata JE, Andrade SE, Gunter MJ, Nelson WW, Roblin D, Platt R. Laboratory evaluation of potassium and creatinine among ambulatory patients prescribed spironolactone: Are we monitoring for hyperkalemia? Ann Pharmacother. 2007;41:193–200. doi: 10.1345/aph.1H520. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Magid DJ, Wells B, Sung SH, Cassidy-Bushrow AE, Greenlee RT, Langer RD, Lieu TA, Margolis KL, Masoudi FA, McNeal CJ, Murata GH, Newton KM, Novotny R, Reynolds K, Roblin DW, Smith DH, Vupputuri S, White RE, Olson J, Rumsfeld JS, Gurwitz JH. The cardiovascular research network: A new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Int Med. 2008;168:2415–2421. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 16.Magid DJ, Gurwitz JH, Rumsfeld JS, Go AS. Creating a research data network for cardiovascular disease: The CVRN. Expert Rev Cardiovasc Ther. 2008;6:1043–1045. doi: 10.1586/14779072.6.8.1043. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: The anemia in chronic heart failure: Outcomes and resource utilization (ANCHOR) study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 20.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham Study. New Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 21.Hendel RC, Budoff MJ, Cardella JF, Chambers CE, Dent JM, Fitzgerald DM, Hodgson JM, Klodas E, Kramer CM, Stillman AE, Tilkemeier PL, Ward RP, Weigold WG, White RD, Woodard PK. ACC/AHA/ACR/ASE/ASNC/HRS/NASCI/RSNA/SAIP/SCAO/SCCT/SCMR/SIR 2008 key data elements and definitions for cardiac imaging a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (writing committee to develop clinical data standards for cardiac imaging) J Am Coll Cardiol. 2009;53:91–124. doi: 10.1016/j.jacc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 23.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 comprehensive heart failure practice guideline. J Cardiac Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: Primary results of the registry to improve the use of evidence-based heart failure therapies in the outpatient setting (IMPROVE HF) Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 25.Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE, Jr, Goodlin SJ, Grady KL, Hundley RF, Jessup M, Lynn TE, Masoudi FA, Nilasena D, Pina IL, Rockswold PD, Sadwin LB, Sikkema JD, Sincak CA, Spertus J, Torcson PJ, Torres E, Williams MV, Wong JB. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2012;125:2382–2401. doi: 10.1161/CIR.0b013e3182507bec. [DOI] [PubMed] [Google Scholar]
- 26.Tjia J, Field TS, Garber LD, Donovan JL, Kanaan AO, Raebel MA, Zhao Y, Fuller JC, Gagne SJ, Fischer SH, Gurwitz JH. Development and pilot testing of guidelines to monitor high-risk medications in the ambulatory setting. Am J Manag Care. 2010;16:489–496. [PubMed] [Google Scholar]
- 27.Raebel MA, Chester EA, Newsom EE, Lyons EE, Kelleher JA, Long C, Miller C, Magid DJ. Randomized trial to improve laboratory safety monitoring of ongoing drug therapy in ambulatory patients. Pharmacotherapy. 2006;26:619–626. doi: 10.1592/phco.26.5.619. [DOI] [PubMed] [Google Scholar]
- 28.Palen TE, Raebel M, Lyons E, Magid DM. Evaluation of laboratory monitoring alerts within a computerized physician order entry system for medication orders. Am J Manag Care. 2006;12:389–395. [PubMed] [Google Scholar]
- 29.Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Qualls LG, Peterson ED, Fonarow GC, Curtis LH. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. doi: 10.1001/jama.2012.14795. [DOI] [PubMed] [Google Scholar]
- 30.Smith DH, Raebel MA, Chan KA, Johnson ES, Petrik AF, Weiss JR, Yang X, Feldstein A. An economic evaluation of a laboratory monitoring program for renin-angiotensin system agents. Med Decis Making. 2011;31:315–324. doi: 10.1177/0272989X10379918. [DOI] [PubMed] [Google Scholar]
- 31.Smith DH, Feldstein AC, Perrin NA, Yang X, Rix MM, Raebel MA, Magid DJ, Simon SR, Soumerai SB. Improving laboratory monitoring of medications: An economic analysis alongside a clinical trial. Am J Manag Care. 2009;15:281–289. [PubMed] [Google Scholar]
- 32.Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Setoguchi S, Hernandez AF, Fonarow GC. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986. doi: 10.1016/j.ahj.2013.03.007. [DOI] [PubMed] [Google Scholar]