Abstract
Background & Aims
Lipodystrophies are hypoleptinemic conditions characterized by fat loss, severe insulin resistance, hypertriglyceridemia, and ectopic fat accumulation. Nonalcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) are also features of this condition. We studied the spectrum of liver disease in lipodystrophy and the effects of leptin replacement.
Methods
This was an open-label prospective study of leptin therapy in patients with inherited and acquired lipodystrophy at the National Institutes of Health. Liver biopsies were performed at baseline (N=50) and after leptin replacement (N=27). NASH activity was assessed using the NASH Clinical Research Network (CRN) scoring system. Fasting blood glucose, triglyceride, hemoglobin A1c and liver enzymes were measured at baseline and at the time of the final liver biopsy.
Results
In leptin treated patients, 86% met criteria for NASH at baseline, while only 33% had NASH after leptin replacement for 25.8 ± 3.7 months (mean ± SE, p 0.0003). There were significant improvements in steatosis grade (reduction of mean score from 1.8 to 0.9) and ballooning injury scores (from 1.2 to 0.4), with a 44.2% reduction in mean NAFLD activity score (p < 0.0001). Patients who already had fibrosis remained stable on leptin replacement. We observed significant improvement in metabolic profile, ALT and AST. In addition to NASH, four patients with acquired generalized lipodystrophy (AGL) had autoimmune hepatitis.
Conclusions
The fundamental liver disease of lipodystrophy is NASH, although autoimmune hepatitis was observed in some patients with AGL. Leptin appears to be a highly effective therapy for NASH in hypoleptinemic lipodystrophic patients.
Keywords: Lipodystrophy, Nonalcoholic fatty liver disease (NAFLD), Nonalcoholic steatohepatitis (NASH), Leptin, Metabolic syndrome
INTRODUCTION
The lipodystrophies are a group of syndromes in which the cardinal clinical feature is partial or complete absence of adipose tissue. Lipodystrophies may have a genetic basis or be acquired as a presumed autoimmune disease. As a group they are characterized by severe insulin resistance, severe hypertriglyceridemia, low HDL cholesterol, low leptin and adiponectin, ectopic fat accumulation, non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH).
NAFLD is a chronic liver disorder frequently found in conjunction with obesity and the metabolic syndrome, and is increasingly recognized in patients with abnormal liver tests found on routine screening. Over 90% of patients with NAFLD have at least one feature of metabolic syndrome and one third of them meet the full criteria for the syndrome [1, 2]. Similarly, 80% of patients with lipodystrophy meet criteria for the metabolic syndrome [3]. Thus, lipodystrophy represents an extreme version of the common, obesity-associated “Metabolic Syndrome.” In contrast to the obesity-associated metabolic syndrome, patients with lipodystrophy have low levels of adipocyte derived hormones, including leptin [4]. Leptin deficiency therefore represents a specific therapeutic target for metabolic abnormalities in patients with lipodystrophy, including NAFLD.
We previously reported a preliminary study of the effect of recombinant human methionyl leptin (metreleptin, Amylin Pharmaceuticals, San Diego, CA) therapy in the treatment of NAFLD in lipodystrophy [5]. In the present report we had two goals: first, to further define the liver diseases of lipodystrophy, and second, to describe the longer term efficacy of metreleptin replacement in the course of NAFLD and NASH in these patients.
PATIENTS AND METHODS
Patient Population and Study Design
The protocol was approved by the Institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases. Informed consent was obtained from each patient or their legal guardian, and assent was obtained from participants under 18 years of age. All authors had access to the study data and reviewed and approved the final manuscript.
The purpose of the study was twofold: first, to determine the effects metreleptin therapy on metabolic status in patients with congenital generalized lipodystrophy (CGL), familial partial lipodystrophy (FPL), and acquired generalized lipodystrophy (AGL), and second, to define the spectrum of liver diseases in this population. Inclusion criteria for leptin replacement included hypoleptinemia (below 12 ng/mL), metabolic abnormalities such as hypertriglyceridemia and/or type 2 diabetes, and the ability to adhere to the leptin replacement protocol. Patients were recruited worldwide, and the cohort in this study included patients from North and South America, five European countries, and the Middle East.
All patients received continuous self-administered subcutaneous (SQ) metreleptin in 1–2 daily doses. Metreleptin dose was calculated based on weight and ranged between 0.06–0.24 mg/kg/day, and was adjusted to achieve metabolic control [6, 7]. Patients were seen every 4–6 months for the first year and then every 6–12 months thereafter. Laboratory data were collected during each visit. The doses of diabetes and lipid medications were adjusted as clinically indicated.
Between July 2000 and December 2011, we performed baseline liver biopsies on 50 patients with lipodystrophy. In a subset of patients, we performed one or more liver biopsies after initiation of metreleptin therapy. For patients who had more than one follow-up biopsy on therapy, only the last biopsy was included in the primary analysis. The results of paired biopsies before and 4–8 months after metreleptin initiation were previously reported in 10 of these patients [5]; however, the current report includes new biopsy data after a longer period of leptin treatment (37–61 months) in 6 of these 10 patients. In 8 patients, including the 6 just mentioned, the effects of metreleptin on 2 liver biopsies during metreleptin treatment are reported here. In these 8 patients, biopsies during metreleptin treatment were categorized as “early” (first biopsy after starting metreleptin) or “late” (second biopsy after starting metreleptin) for purposes of analysis.
Laboratory Analysis
Immunoassays were used to measure serum leptin levels with the use of a commercial kit (Linco Research, St. Charles, MO). Ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA) was used for measurement of Hemoglobin A1c values. Standard methods were used to determine serum chemistry values with the use of automated equipment (Beckman, Fullerton, CA).
Histological Analysis
Liver biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 4- µm intervals and stained with hematoxylin-eosin, Masson's trichrome, and Gomori iron stain. Anti-ubiquitin staining (Dako Z0458, 1:1000, heat-activated antigen retrieval in citrate buffer) was used to visualize Mallory bodies. Biopsy specimens were scored prospectively by a single hepatopathologist (DEK) blinded to the clinical data and sequence of the biopsies using the NASH CRN method [8]. Autoimmune hepatitis was diagnosed using standard biochemical and histologic criteria [9].
Statistical Methods
Values are expressed as means ± standard error (SE). The paired t test was used where applicable to compare baseline means with means after metreleptin therapy. When normality was questioned, Wilcoxon signed-rank test was used. Spearman rank correlation was used to calculate correlation coefficients between selected variables. Repeated measures ANOVA and mixed models (PROC MIXED) were used to evaluate the effect of metreleptin over time. Data were analyzed using Statview 5.0 and SAS Enterprise Guide 5.1 (SAS Institute, Inc). A P < .05 was considered statistically significant.
RESULTS
Baseline Characteristics
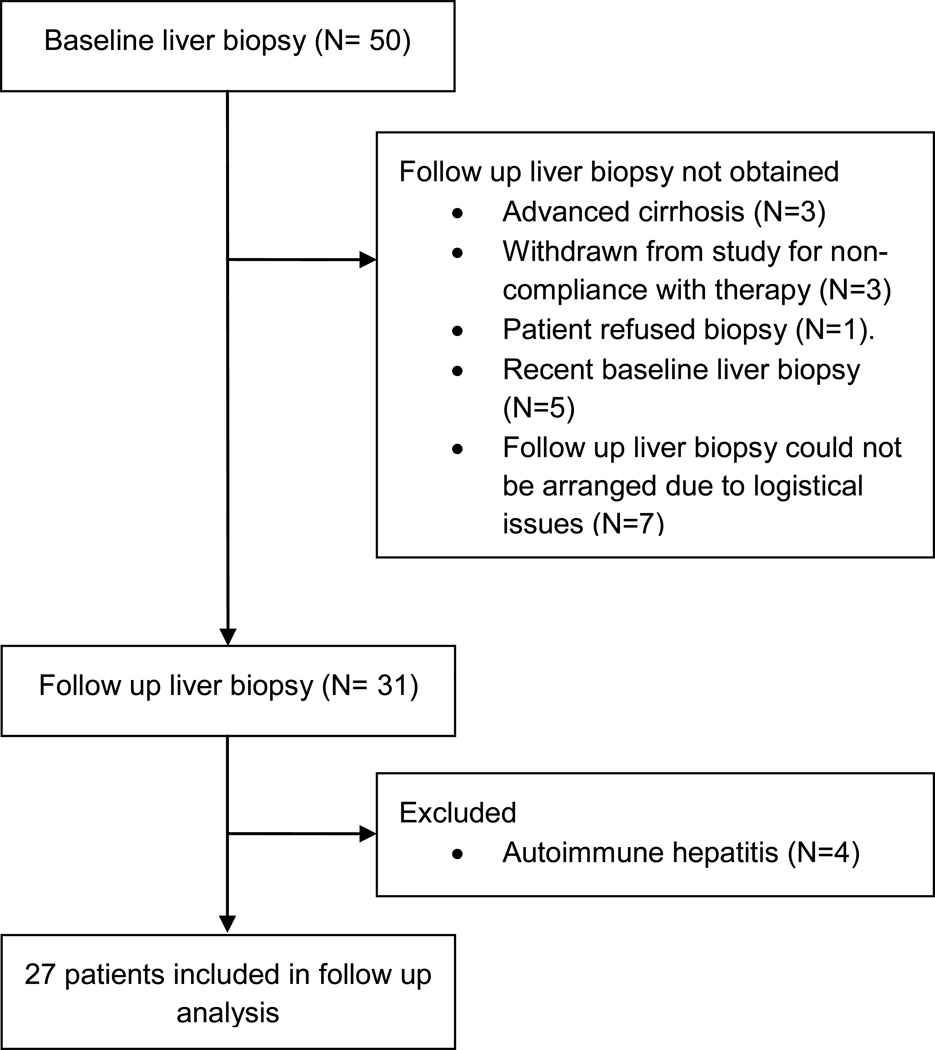
Fifty patients with different forms of lipodystrophy underwent baseline liver biopsies (Table 1). Twenty-three patients were included at baseline but were not included in the analysis of the effect of metreleptin. Four were excluded because baseline or follow up biopsies demonstrated autoimmune hepatitis. Nineteen patients did not undergo a follow up biopsy: one refused, four had advanced cirrhosis at baseline and thus there was no clinical indication for follow up biopsy, three were found to be non-compliant with metreleptin therapy, five had had a recent baseline biopsy, and there were logistical issues for planning the follow-up liver biopsies for the other seven patients (Figure 1). Twenty-seven of the patients who had at least one follow up liver biopsy after initiation of metreleptin were therefore included in the evaluation of the effect of metreleptin therapy on liver disease. The general characteristics of the subgroup used in the analysis of metreleptin effects on liver disease were comparable to that of the overall cohort (Table 1).
Table 1.
Baseline characteristics and clinical changes on metreleptin.
| Cohort with baseline liver biopsy (N=50) |
Cohort with paired liver biopsies (N=27) |
|
|---|---|---|
| Demographics | ||
|
Age (yrs) [Mean ± SE (range)] |
26 ± 2 (7.8–68.3) | 29 ±3 (7.8–68.3) |
| Female (%) | 42(84) | 21(77.7) |
| Forms of lipodystrophy [N (%)] | ||
| AGL | 13 (26) | 5(18.5) |
| APL | 5 (10) | 3 (11.1) |
| CGL (AGPAT2 mutation) | 8 (16) | 6 (22.2) |
| CGL (BSCL2 mutation) | 10 (20) | 4(14.8) |
| FPL (LMNA mutation) | 12 (24) | 7 (25.9) |
| FPL (PPARY mutation) | 2 (4) | 2 (7) |
| Baseline Serum Leptin (ng/dL) [Mean ± SE (range)] |
2.74 ± 0.39 (0.404–12.21) | 2.72 ± 0.5 (0.5–10.1) |
| Clinical data | ||||
|---|---|---|---|---|
| Baseline | After leptin therapy | |||
| Mean ± SE (range) | Mean ± SE (range) | p Value | ||
|
Fasting triglycerides (mg/dL) |
1017 ± 296 (49– 12697) |
952 ± 291 (108– 7420) |
303 ± 65 (86– 1825) |
0.0002 |
| Fasting glucose (mg/dL) | 178 ± 13 (49–478) | 178 ± 15 (49–333) | 117 ± 9 (57–267) | 0.004 |
| HbA1c (%) | 8.2 ± 0.3 (4.5–11.9) | 7.9 ± 0.4 (4.5–11.9) | 6.3 ± 0.2 (4.5–8.5) | 0.0009 |
| ALT (U/L) | 106 ± 18 (18–726) | 99 ± 19 (18–386) | 53 ± 12 (9–282) | 0.002 |
| AST (U/L) | 71 ± 11 (15–380) | 66 ± 11 (15–258) | 35 ± 5 (11–102) | 0.002 |
AGL, Acquired Generalized Lipodystrophy; CGL, Congenital Generalized Lipodystrophy; FPL, Familial Partial Lipodystrophy; APL, Acquired Partial Lipodystrophy; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase.
Figure 1.
Cohort of 50 patients with baseline liver biopsy and the subset of 27 patients included in the analysis of the effects of metreleptin on liver histology.
Mean ALT and AST prior to metreleptin treatment were 106 U/L and 71 U/L, respectively; 66% of the patients had elevated transaminases (AST > 34 U/L, ALT > 41 U/L) at baseline (Table 1).
Baseline Liver Histology
At baseline, 90% of the biopsies showed evidence of fatty liver disease and 82% met diagnostic criteria for either borderline (20%) or definite (62%) steatohepatitis (Table 2). One pediatric patient showed a pattern of portal fibrosis and zone 1 steatosis that has been previously reported in children with fatty liver disease due to obesity and diabetes [10]. Common pathologic findings included typical zone 3 injury with ballooning in 74% and Mallory-Denk bodies evident on routine stains in 12%. Using the more sensitive technique of ubiquitin immunostain, Mallory-Denk bodies were seen in 37% of cases.
Table 2.
Liver histology from 50 patients with baseline biopsies
| Portal Areas (mean, range) | 14.3 (5–46) |
|---|---|
| Steatosis Grade (mean) | 1.7 |
| 0 (<5% of hepatocytes) | 5 (10)1 |
| 1 (5–33% of hepatocytes) | 19 (38) |
| 2 (33–67% of hepatocytes) | 13 (26) |
| 3 (>67% of hepatocytes) | 13 (26) |
| Microvesicular Steatosis | 5 (10) |
| Lobular Inflammation Grade (mean) | 1.3 |
| 0 (no foci) | 2 (4) |
| 1 (<2 foci/20x hpf) | 34 (68) |
| 2 (2–4 foci/20x hpf) | 9 (18) |
| 3 (>4 foci/20x hpf) | 5 (10) |
| Portal Inflammation (mean score) | 1 |
| 0 (None) | 7 (14) |
| 1 (Mild) | 34 (68) |
| 2 (More than mild) | 9 (18) |
| Ballooning Injury (mean score) | 1.1 |
| 0 (None) | 13 (26) |
| 1 (Few) | 17 (34) |
| 2 (Many) | 20 (40) |
| Acidophil Bodies | 18 (37) |
| Megamitochondria | 7 (14) |
| Mallory Bodies | 6 (12) |
| Fibrosis (mean stage) | 2.1 |
| 0 (None) | 7 (14) |
| 1A (Mild perisinusoidal only) | 6 (12) |
| 1B (Moderate perisinuosoidal) | 3 (6) |
| 1C (Periportal only) | 3 (6) |
| 2 (Periportal and perisinusoidal) | 10 (20) |
| 3 (Bridging fibrosis) | 13 (26) |
| 4 (Cirrhosis) | 8 (16) |
| NAFLD Activity Score (mean) | 4.2 |
| Diagnostic Classification | |
| Not Steatohepatitis | 9 (18) |
| Borderline Steatohepatitis | 10 (20)2 |
| Definite Steatohepatitis | 31 (62) |
hpf, High Power Field.
Data are presented as N (%) unless otherwise indicated.
One pediatric patient had a borderline zone 1 pattern.
One of the patients who had NASH at baseline had concomitant chronic hepatitis B. One patient with AGL had autoimmune hepatitis at baseline; this patient did not have histologic evidence of steatosis or NASH.
Baseline Subgroup Observations
We studied a broad spectrum of both congenital and acquired forms of lipodystrophy (Table 1). In this cohort we had10 patients with mutations in the BSCL2 gene, encoding the protein seipin. This subgroup was characterized by the most extreme fibrosis at an early age; (mean age 12.5± 1.4 years; range 4.6–17.8 at the time of baseline liver biopsy) (Table-3). As we have previously reported, these patients had the lowest triglyceride levels (289 ± 84, range: 49–879 mg/dl; compared to 1200 ± 364, range: 100–12697 mg/dl in the remaining 40 patients) [6]. Similarly, in the entire baseline cohort, there was no correlation between the severity of liver fibrosis and triglyceride level (P=0.24), fasting glucose (P=0.45), hemoglobin A1c (P=0.78), or fasting insulin (P=0.29). It is of note that four of the twelve patients with other causes of lipodystrophy (not BSCL2 mutations) who had bridging fibrosis or cirrhosis had another concomitant liver disease: either autoimmune hepatitis or viral hepatitis (Table 3).
Table 3.
Baseline fibrosis score in patients with different types of lipodystrophy
| Fibrosis Score | AGL (N=13) (Age 9–68 years) |
APL (N=5) (Age 10– 30 years) |
CGL; AGPAT2 (N=8) (Age 14 47 years) |
CGL; BSCL2 (N=10) (Age 8–18 years) |
FPL; LMNA (N=12) (Age 18–64 years) |
FPL; PPARY (N=2) (Age 32 and 35 years) |
|---|---|---|---|---|---|---|
| 0 (None) | 2 | 0 | 0 | 0 | 4 | 1 |
|
1A (Mild perisinusoidal only) |
0 | 0 | 3 | 0 | 3 | 0 |
|
1B (Moderate perisinuosoidal) |
1 | 1 | 1 | 0 | 0 | 0 |
| 1C (Periportal only) | 1 | 0 | 1 | 0 | 1 | 0 |
|
2 (Periportal and perisinusoidal) |
2 | 2 | 2 | 1 | 2 | 1 |
| 3 (Bridging fibrosis) | 41 | 2 | 12 | 4 | 2 | 0 |
| 4 (Cirrhosis) | 3 | 0 | 0 | 5 | 0 | 0 |
AGL, Acquired Generalized Lipodystrophy; CGL, Congenital Generalized Lipodystrophy; FPL, Familial Partial Lipodystrophy; APL, Acquired Partial Lipodystrophy.
Three out 4 patients in the AGL group with bridging fibrosis also had autoimmune hepatitis
The patient with AGPAT2 mutation in the CGL group who had bridging fibrosis also had concomitant hepatitis B.
Effects of Metreleptin
Eighty-one percent (22 out of 27) of the patients included in the analysis of the effect of metreleptin on their liver disease had a follow up liver biopsy one year or more after initiation of the therapy; the remaining 5 patients had follow up liver biopsies less than one year after initiation of metreleptin. All patients continued to receive metreleptin at the time of the follow up liver biopsies. The effects of metreleptin on laboratory parameters in the 27 patients included in this analysis are shown in Table 1. There were significant reductions in fasting triglycerides, fasting glucose, and hemoglobin A1c. ALT decreased from 99 ± 19 to 53 ± 12 (P 0.002), and AST decreased from 66 ± 11 to 35 ± 5 (P 0.002).
The average time on metreleptin therapy at the time of the last follow up biopsy was 25.8 months (median 15 months, range 4–68 months), and the average time between the first and last biopsy was 27.8 months (median 15 months, range 4–84 months). In 3 patients, the initial pretreatment biopsy was obtained more than 6 months prior to metreleptin initiation (10, 10, and 23 months).
Effects of Metreleptin on Steatohepatitis: Analysis of final biopsy during metreleptin treatment
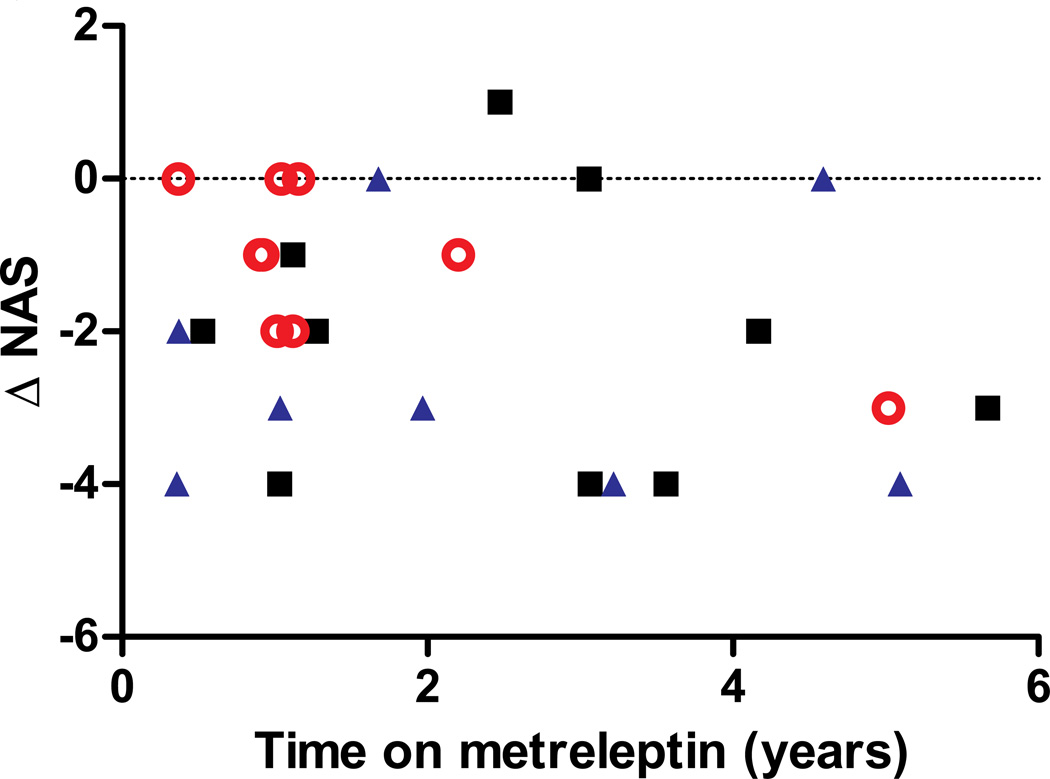
Aggregate changes in NAFLD Activity Scores (NAS) are shown in Table 4, and data for individual patients is in the Supplemental Appendix. Figure 3 shows the changes in the NAFLD activity score for each individual, color coded by lipodystrophy subtype. Twenty patients had improvement in their NAS, six patients showed no difference, and one patient’s NAS slightly increased. There was not a significant correlation between duration of metreleptin treatment and change in NAS for the overall cohort, although this relationship was statistically significant for the subgroup with FPL (P=0.04, n=8).
Table 4.
Comparison of liver histology at baseline and on metreleptin.
| Baseline N=27 |
On Leptin N=27 |
p value1 | |
|---|---|---|---|
| Steatosis Grade (mean) | 1.8 | 0.9 | 0.0004 |
| 0 (<5% of hepatocytes) | 2 (7) | 12 (44) | |
| 1 (5–33% of hepatocytes) | 10 (37) | 9 (33) | |
| 2 (33–67% of hepatocytes) | 7 (26) | 4 (15) | |
| 3 (>67% of hepatocytes) | 8 (30) | 2 (7) | |
| Lobular Inflammation Grade (mean) | 1.3 | 1.1 | 0.097 |
| 0 (no foci) | 1 (4) | 3 (11) | |
| 1 (<2 foci/20x hpf) | 19 (70) | 18 (67) | |
| 2 (2–4 foci/20x hpf) | 4 (15) | 5 (19) | |
| 3 (>4 foci/20x hpf) | 3 (11) | 1 (4) | |
| Portal Inflammation (mean score) | 1.1 | 0.9 | 0.13 |
| 0 (None) | 3 (11) | 7 (26) | |
| 1 (Mild) | 18 (67) | 15 (56) | |
| 2 (More than mild) | 6 (22) | 5 (19) | |
| Ballooning Injury (mean score) | 1.2 | 0.4 | 0.0002 |
| 0 (None) | 5 (19) | 18 (67) | |
| 1 (Few) | 11 (41) | 6 (22) | |
| 2 (Many) | 11 (41) | 3 (11) | |
| Fibrosis (mean stage) | 1.9 | 1.9 | 1 |
| NAFLD Activity Score (mean) | 4.3 | 2.4 | <0.0001 |
| Diagnostic Classification | |||
| Not Steatohepatitis | 4 (15) | 18 (67) | 0.0003 |
| Borderline Steatohepatitis | 5 (19)1 | 3 (11)2 | |
| Definite Steatohepatitis | 18 (67) | 6 (22) | |
hpf, High Power Field.
Paired t-test for continuous variables; Wilcoxon for ordered nominal variables.
One pediatric patient had a borderline zone 1 pattern.
Figure 3.
Nonalcoholic fatty liver disease activity score difference (ΔNAS) before and after metreleptin therapy versus time on metreleptin replacement. Black squares represent patients with congenital generalized lipodystrophy, red circles are patients with familial partial lipodystrophy, and blue triangles are patients with acquired lipodystrophy (generalized and partial). There was not a statistically significant correlation between ΔNAS and the duration of metreleptin treatment.
Of the 27 patients, 86% had borderline or definite NASH at baseline and only 33% had NASH after leptin replacement for 25.8 ± 3.7 months (p = 0.0002). This improvement was reflected specifically by improvement in steatosis (reduction from a mean score of 1.8 to 0.9) and ballooning injury (reduction from a mean score of 1.2 to 0.4) (Figure 2).Ten patients with non-zero scores for steatosis and thirteen with non-zero scores for ballooning had scores of zero on follow-up. No patients showed worsened ballooning and only one patient had a higher steatosis score on follow-up. Inflammation was generally mild both at baseline and follow-up. The reduction in observed steatosis and ballooning injury resulted in a drop in the mean NAS from 4.3 to 2.4 (p<0.0001).
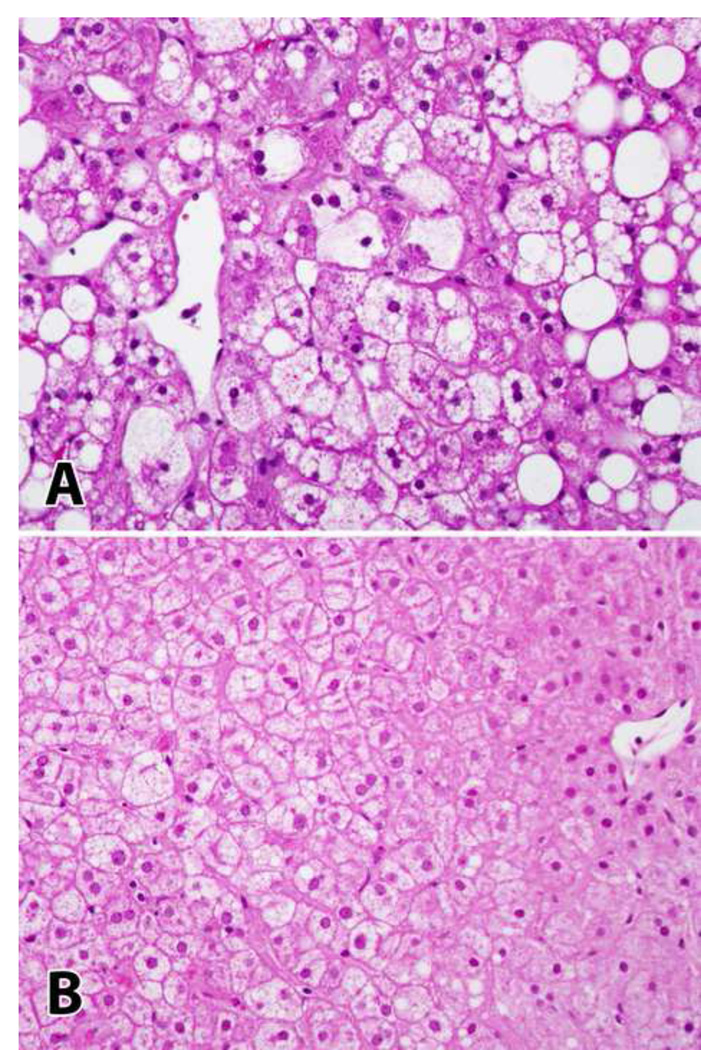
Figure 2.
Improvement of histology on leptin therapy. Patient NIH-56. Panel A shows characteristic changes of steatohepatitis prior to leptin therapy. Panel B shows resolution of steatosis and ballooning injury on follow-up biopsy. (H&E, 400x magnification).
Improvements in NAS and steatosis were not associated with changes in body weight or BMI. Body weight decreased by 2.5 kg from baseline to the time of final biopsy (P=0.6), and BMI decreased by 0.6 kg/m2 (P=0.5). Neither body weight nor BMI were significant predictors of NAS or steatosis, and changes in NAS and steatosis with metreleptin remained statistically significant (P<0.001) after adjustment for weight or BMI. Sensitivity analyses excluding growing children showed the same results.
There was a general clinical and histopathological stability in patients on leptin replacement who already had fibrosis at baseline. Importantly there was no worsening of fibrosis while on leptin therapy.
Effects of Metreleptin on Steatohepatitis: Analysis of repeated biopsies during metreleptin treatment
Eight patients with NASH as the primary liver disease present (i.e. without autoimmune hepatitis) had two biopsies after the initiation of metreleptin. The effects of metreleptin on liver pathology (steatosis, fibrosis, and overall NAFLD activity score) over time are shown in Supplemental Figure 1. The two biopsies on metreleptin treatment were categorized as “early” (9.3 ± 1.8 months on metreleptin, range 5–17 months), or “late” (41.7 ± 5.4 months on metreleptin, range 13–60 months). NAFLD activity scores were significantly lower on metreleptin versus at baseline (P=0.0067), both at the early (P=0.009) and late (p=0.0033) time points. Although mean NAFLD activity scores were lower at the late (1.9 ± 0.6) than the early (2.2 ± 0.5) time point, this difference was not statistically significant (P=0.62). Steatosis scores were significantly lower on metreleptin versus at baseline (P=0.0015), both at the early (P=0.0043) and late (p=0.0006) time points. Although mean steatosis scores were lower at the late (0.37 ± 0.2) than the early (0.75 ± 0.2) time point, this difference was not statistically significant (P=0.32). Fibrosis scores were identical (mean 2.25) at baseline and at the early and late time points on metreleptin (P=1.0).
Clinical Course of Patients with Advanced Cirrhosis at Baseline
Four patients (8%) in the larger cohort of 50 had advanced cirrhosis associated with clinical features of portal hypertension at baseline, and were not included in the analysis of effects of metreleptin on liver histology. One patient died of liver failure after 17 months of metreleptin treatment. The second patient has been treated with metreleptin for 6.5 years, with stable liver function and thrombocytopenia (platelet range 30K-75K). The third patient has had stable hepato-pulmonary syndrome during 3.5 years of metreleptin replacement. The fourth patient had esophageal varices, splenomegaly, thrombocytopenia, and prior episodes of hepatic encephalopathy baseline. During 2 years on metreleptin therapy, she has one additional episode of hepatic encephalopathy and has otherwise been stable.
Recognition of Autoimmune Hepatitis During Metreleptin Therapy
In addition to the one patient known to have autoimmune hepatitis at baseline, another three patients were observed to have autoimmune hepatitis over the course of follow up, while on metreleptin. Two out of the four patients with autoimmune hepatitis also had fatty liver disease at baseline. Metreleptin therapy was continued in two patients with autoimmune hepatitis, one of whom had fatty liver disease at baseline. One patient with autoimmune hepatitis died of renal failure. One patient with autoimmune hepatitis was withdrawn from the study due to inability to adhere to the metreleptin replacement protocol for psychiatric reasons. The three surviving patients have been followed for varying periods of time (4–12 years) and have been on corticosteroids and/or other immunosuppressive therapy for treatment of autoimmune hepatitis.
DISCUSSION
In the present study we describe the liver diseases associated with lipodystrophy and the long term effect of metreleptin therapy on NAFLD of lipodystrophy. The major liver disease of lipodystrophy is NAFLD, and at baseline 82% of our patients with lipodystrophy have NASH. The extent of liver fibrosis was more severe in patients with mutations of BSCL2, a subtype of CGL. In the subgroup of patients with AGL, autoimmune hepatitis may coexist with NAFLD. We also demonstrated that metreleptin replacement reverses NAFLD and NASH to a significant degree, and that these changes were not attributable to weight loss. Although we were unable to show an improvement in fibrosis, our cohort showed no progression of fibrosis on metreleptin therapy. The significant reduction in NAFLD activity scores was paralleled by significant reductions in metabolic parameters, including triglycerides, fasting glucose, hemoglobin A1c and transaminases. The majority of the benefit of metreleptin on steatosis and NAFLD activity scores appears to occur early after the initiation of metreleptin. There was a significant correlation between duration of metreleptin treatment and change in NAFLD activity score in the subgroup with FPL; however, this subgroup analysis should be interpreted with caution due to small patient numbers.
This data confirms our initial small preliminary study, adding follow up biopsy data on 17 previously unreported patients, new biopsy data on 6 of the 10 previously reported patients, and extending the follow up duration from a mean of 6 months to a mean of 2 years. It is clear that a larger sample size and randomized control trial would have been preferable. However, given the rarity of lipodystrophy, this is the largest sample size existing in this patient population, and the only study of the effects of metreleptin therapy on liver disease. In the absence of a control group, it is possible that the improvement in NASH scores was spontaneous, and therefore we cannot definitively establish a causative relationship between this improvement and metreleptin therapy. However data from placebo groups in clinical trials of obesity-associated NAFLD have shown that improvements in NAFLD are generally linked to weight loss. In contrast, lipodystrophic patients cannot substantially alter the course of their disease by lifestyle modification without metreleptin therapy.
NAFLD is a characteristic feature in both lipodystrophy and the common metabolic syndrome. The pathologic features of both conditions are similar and include inflammation and ballooning, which can progress to fibrosis [11]. Although the pathogenesis of NASH is not well-understood, insulin resistance and oxidative stress are likely to be major culprits in both obesity and lipodystrophy associated NASH [1, 12, 13]. The spectrum of NAFLD starts with the asymptomatic accumulation of fat in hepatocytes (steatosis) and can progress to NASH and even cirrhosis and end stage liver disease in patients with no history of excessive alcohol intake [12, 14]. As the disease progresses to cirrhosis, fat is less prominent. Given the fact that some individuals with steatosis will never progress to steatohepatitis, other, as yet unknown events, are important in the pathogenesis of NASH.
While the presumed sequence of events that goes from accumulation of fat to oxidative stress to inflammation and fibrosis is not precisely defined, insulin resistance appears to be an important underlying factor. However, insulin resistance is not a sufficient stimulus for the development of NASH in the absence of fat accumulation in the liver. This has been demonstrated in patients with insulin receptor mutations [15] or autoantibodies against insulin receptor [16] who have extreme insulin resistance, but do not have ectopic fat deposition in the liver and do not develop metabolic liver disease. This observation reinforces the essential role of ectopic fat in the pathogenesis of NASH. In addition, we have observed that while ectopic fat appears to be important in the pathogenesis of NASH, there is no good correlation between circulating triglyceride levels and the severity of NASH, as observed in our lipodystrophic patients with BSCL2 mutations, who tend to have lower triglyceride levels.
Animal models of NAFLD have only limited utility in improving the understanding of human physiology, as mice can develop fatty liver but not the complete picture of NASH and cirrhosis. There are mouse models expressing different features, but none which shows all the features of NASH [17–20]. The link between leptin and NAFLD and NASH is yet to be elucidated. Leptin deficient ob/ob mice develop significant steatosis, which can be reversed by leptin replacement, but do not progress to NASH spontaneously [21]. Similarly, in our patient population with lipodystrophy, leptin deficiency is the target for treatment of hepatic steatosis and NASH. In contrast, in rodent models of NASH, leptin has been demonstrated to be involved in innate and humoral immunity and to have a proinflammatory and profibrogenic role [22, 23]. To answer further questions about these conflicting data from rodents and humans, we need more in depth information on the role of leptin in obesity-related NASH. Further complicating this picture is the fact that, in contrast to lipodystrophy, leptin levels are elevated in obesity related steatosis and NASH, and thus leptin deficiency is not a treatment target.
An important observation in this study is that patients with AGL may have autoimmune hepatitis in addition to NASH. This was recognized prior to metreleptin treatment in one patient, and identified only after initiation of metreleptin in three others. A critical point that arises from this question is whether metreleptin might have contributed to the development of autoimmune hepatitis in these three patients. AGL is a form of lipodystrophy which is often associated with organ specific autoimmune diseases such as type 1 diabetes, dysregulated humoral immunity [24], or complement abnormalities [25]. Autoimmune hepatitis has been observed only in patients with AGL, and not in other forms of lipodystrophy that are not associated with immune dysfunction. For these reasons, we believe it is likely that autoimmune hepatitis is another example of organ specific autoimmunity resulting from the underlying immunologic abnormality in AGL, rather than a consequence of metreleptin treatment. Supporting this notion, we are aware of two patients with AGL who were diagnosed with autoimmune hepatitis at a young age (4 and 11 years) and have never been on metreleptin therapy.
In conclusion, these studies demonstrate the varied liver diseases seen in lipodystrophy. Further, they document the centrality of leptin deficiency in producing the metabolic and hepatic abnormalities, and, most importantly, the effect of leptin replacement therapy in ameliorating both the metabolic and hepatic abnormalities of lipodystrophy. Improvements in NASH score presumably represents stabilization or improvement of the course of the disease. Since treatment of the metabolic abnormalities in lipodystrophy and metabolic syndrome require prolonged therapy, it is likely that a prolonged course of treatment will be needed for liver disease as well. In our clinical trials, metabolic abnormalities have been the primary indicators for metreleptin therapy. However, the new observation that features of NASH, including fibrosis, maybe seen at a very early age in lipodystrophy suggests that liver disease, particularly in young children, should be considered as a primary indication for metreleptin therapy.
Supplementary Material
Acknowledgements
We would like to acknowledge many fellows of the NIH Inter-institute Endocrine Training Program, the nursing staff of the NIH Clinical Center, other professionals, and patients, who have participated in this study. We would like to thank Amylin Pharmaceuticals for metreleptin used in this study.
Financial Support: This work was supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute.
Abbreviations
- (NAFLD)
Nonalcoholic Fatty liver disease
- (NASH)
Nonalcoholic steatohepatitis
- (CRN)
Clinical Research Network
- (ALT)
Alanine aminotransferase
- (AST)
Aspartate aminotransferase
- (AGL)
Acquired Generalized Lipodystrophy
- (CGL)
congenital generalized lipodystrophy
- (FPL)
familial partial lipodystrophy
- (SE)
standard error
- (NAS)
NAFLD activity score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Authors have nothing to disclose.
REFERENCES
- 1.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Gorden P, Lupsa BC, Chong AY, Lungu AO. Is there a human model for the 'metabolic syndrome' with a defined aetiology? Diabetologia. 2010;53:1534–1536. doi: 10.1007/s00125-010-1719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. The Journal of clinical endocrinology and metabolism. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 5.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 6.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 7.Chan JL, Lutz K, Cochran E, Huang W, Peters Y, Weyer C, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 9.Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 10.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. doi: 10.1053/j.gastro.2008.08.050. e1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255–262. doi: 10.1097/00004836-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 13.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 14.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 15.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87–100. doi: 10.1097/00005792-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Nagarajan P, Mahesh Kumar MJ, Venkatesan R, Majundar SS, Juyal RC. Genetically modified mouse models for the study of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:1141–1153. doi: 10.3748/wjg.v18.i11.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 19.Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, et al. Hyperresponsivity to Low-Dose Endotoxin during Progression to Nonalcoholic Steatohepatitis Is Regulated by Leptin-Mediated Signaling. Cell Metab. 2012;16:44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Reitman ML. Leptin in the liver: a toxic or beneficial mix? Cell Metab. 2012;16:1–2. doi: 10.1016/j.cmet.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138–144. doi: 10.1016/j.hepres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Ikejima K, Honda H, Yoshikawa M, Hirose M, Kitamura T, Takei Y, et al. Leptin augments inflammatory and profibrogenic responses in the murine liver induced by hepatotoxic chemicals. Hepatology. 2001;34:288–297. doi: 10.1053/jhep.2001.26518. [DOI] [PubMed] [Google Scholar]
- 23.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 24.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003;82:129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Savage DB, Semple RK, Clatworthy MR, Lyons PA, Morgan BP, Cochran EK, et al. Complement abnormalities in acquired lipodystrophy revisited. The Journal of clinical endocrinology and metabolism. 2009;94:10–16. doi: 10.1210/jc.2008-1703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.