Key Points
STAT5 is constitutively phosphorylated in malignant B cells obtained from patients with Waldenström's macroglobulinemia.
Inhibition of STAT5 signaling significantly decreases IgM production and may be useful therapeutically for patients with high IgM levels.
Abstract
Activation of the Janus kinase family/signal transducer and activator of transcription (JAK/STAT) signaling pathway has been associated with the pathogenesis and progression of both solid and hematologic malignancies. We have detected constitutive activation of STAT5 in malignant B cells derived from patients with Waldenström's macroglobulinemia (WM). Although short hairpin RNA–mediated knockdown of the STAT5A and STAT5B isoforms did not affect cellular proliferation, loss of STAT5 significantly decreased immunoglobulin M (IgM) secretion. A similar dose-dependent inhibition of IgM secretion was observed when WM cell lines were treated with a small molecule inhibitor of STAT5. These data suggest that STAT5 is involved in regulating IgM production in WM and that inhibition of STAT5 may represent a novel therapeutic strategy for lowering IgM levels in WM patients.
Introduction
Signaling events initiated by cytokines within the bone marrow microenvironment are necessary for the viability and development of normal B cells. Such events are equally important for the maintenance of many B-cell malignancies as well, including Waldenström's macroglobulinemia (WM), a lymphoplasmacytic lymphoma characterized by high levels of serum immunoglobulin M (IgM).1,2 One of the most prominent signaling cascades activated by cytokines is the Janus kinase family/signal transducers and activators of transcription (JAK/STAT) pathway composed of 4 JAK proteins (JAK1-JAK3, Tyk2) and 7 STAT proteins (STAT1-STAT6, including STAT5A and STAT5B).3 Hyperactivation of these proteins, either in response to heightened cytokine signaling or secondary to activating mutations within the JAK/STAT pathway, leads to carcinogenesis, with numerous reports suggesting an association between constitutive STAT5 activation and uncontrolled cellular proliferation in hematologic malignancies.4-8
Despite a high degree of amino acid sequence homology, STAT5A and STAT5B are distinct transcription factors, with evidence indicating both overlapping and nonredundant functional activities for these isoforms in solid tumors.9-12 However, the activation and downstream function of the STAT5 isoforms in lymphoma have not been well characterized. Here, we have explored the relationship between STAT5 phosphorylation and the biologic activity of WM tumor cells. Our data indicate that STAT5 is hyperactive in malignant WM B cells and promotes IgM secretion, and that investigations into the targeting of both STAT5A and STAT5B clinically are warranted.
Methods
Cell lines and reagents
The WM cell lines, MWCL-1, BCWM.1, and RPCI-WM1, were cultured as described.2 CD19+CD138+ WM cells were isolated from the bone marrows of consenting patients, using positive selection beads. The STAT5 inhibitors, N′-[(4-Oxo-4H-chromen-3-yl)methylene]nicotinohydrazide and N1-(11H-indolo[3,2-c]quinolin-6-yl)-N2,N2-dimethylethane-1,2-diamine (IQDMA), were purchased from Calbiochem.
Immunohistochemistry
Paraffin-embedded bone marrow specimens were stained with anti-human pSTAT5 and visualized as outlined previously.2 All slides were observed with light microscopy (Olympus AX70, ×200 aperture 0.46, ×400 aperture 0.75, ×600 aperture 0.80; Olympus America) with images being captured with a SPOT RT camera and software (Diagnostic Instruments). A novel methodology termed SIMPLE (sequential immunoperoxidase labeling and erasing) was used to stain the sections for pSTAT5, together with either CD20 or CD138, as previously described.2 Images were prepared with Photoshop (Adobe Systems Inc.). Institutional review board approval was obtained to collect bone marrow cells from patients with WM for use in translational research projects. Informed consent was obtained in accordance with the Declaration of Helsinki.
Flow cytometry
WM cells were fixed and permeabilized, before staining, with STAT antibodies or respective isotype controls (Phosflow, BD Biosciences). Cells were analyzed on a FACSCalibur, and data were processed using FlowJo software (TreeStar Inc.).
shRNA-mediated knockdown of STAT5A and STAT5B
STAT5A- and STAT5B-targeting short hairpin RNAs (shRNAs) in the doxycycline-inducible pTRIPZ vector system were purchased from Open Biosystems. Recombinant lentiviral particles were expressed after transient transfection of HEK293T cells using the TransLenti Viral Packaging System (Open Biosystems). Viral supernatant was then used to transduce MWCL-1 cells, and after 48 hours, cells were selected with puromycin. Specificity and efficiency of doxycycline-induced knockdown was determined after 72 hours by western blotting.
Enzyme-linked immunosorbent assay
Cell-free supernatants were collected, and the concentration of IgM was determined according to the manufacturer’s protocol (Human IgM Enzyme-Linked Immunosorbent Assay Kit; Bethyl Laboratories, Inc.).
Proliferation assay
Cells were cultured with either a STAT5 inhibitor or doxycycline for 48 or 72 hours, respectively. Tritiated thymidine (3H-TdR; 5.0 Ci/mmol [185 GBq/mmol]; Amersham) was added 18 hours before scintillation counting.
Immunoprecipitation and immunoblotting
Cell lysates from BCWM.1 and MWCL-1 cells were incubated overnight with either anti-STAT5A or anti-STAT5B antibodies, followed by the addition of Protein G Dynabeads (Invitrogen) for an additional 24 hours. Beads were collected using a magnet and heated for 10 minutes in 4X Laemmli Loading Buffer (Bio-Rad). Protein was separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. After transfer onto nitrocellulose membranes, blots were probed using antibodies targeted toward pSTAT5 (Y694/Y699) (Abcam), STAT5A, or STAT5B (both Santa Cruz Biotechnology, Inc.). Western blotting for STAT1, STAT3, and actin was performed using antibodies from Santa Cruz Biotechnology, Inc.
Statistical analysis
Statistical analysis was performed with GraphPad Prism v5.0d (GraphPad Software), using the Student t test. Significance was set at P < .05.
Results and discussion
Constitutive activation of STAT5 in WM
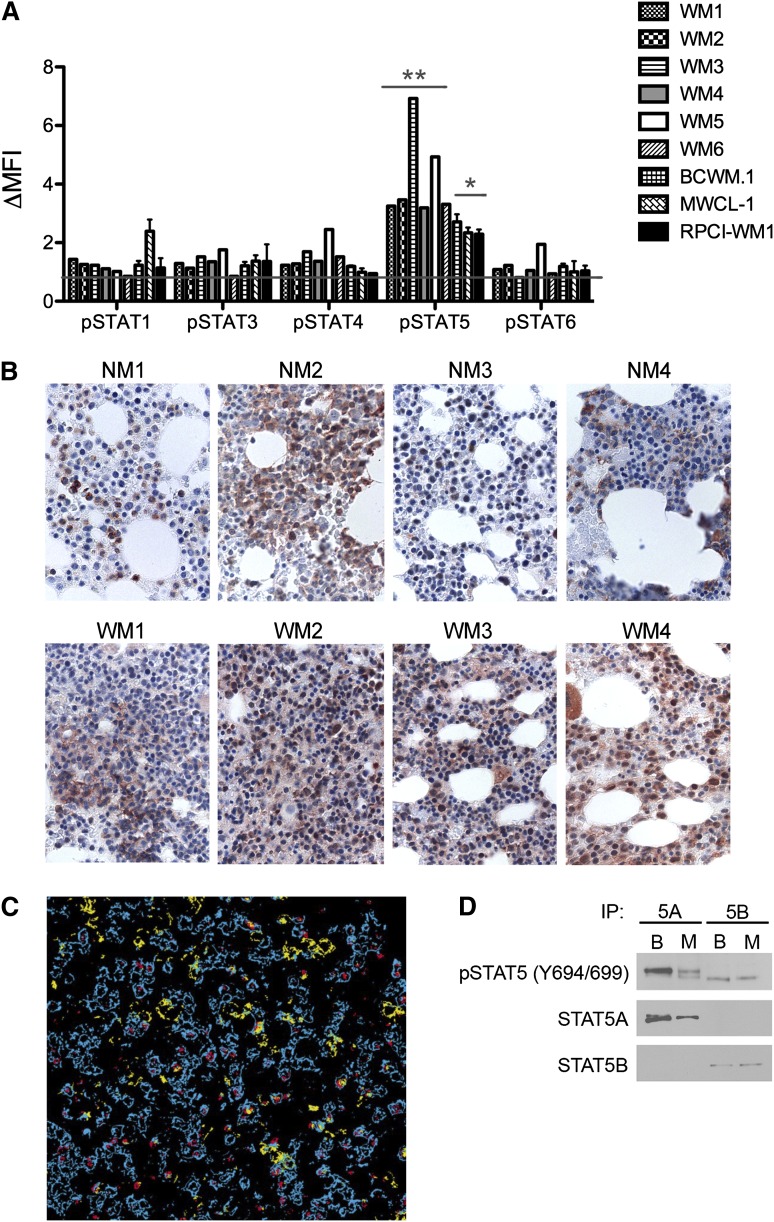
Constitutive STAT signaling is associated with the pathogenesis and progression of many malignancies.4,6,13 A flow cytometry–based characterization of the baseline activation status of several STAT proteins in malignant CD19+CD138+ cells obtained from the bone marrows of 6 patients with WM, as well as in 3 WM cell lines, revealed significant phosphorylation of STAT5 (Figure 1A). Phosphorylation was greatest in the patient samples, possibly as a result of in vivo exposure to STAT5-activating cytokines before harvesting. No other STAT protein was consistently phosphorylated. To confirm the hyperactivation of STAT5 in WM, immunohistochemical staining for pSTAT5 was performed on bone marrows obtained from both healthy controls and WM patients. Although pSTAT5 staining was detected in all marrows, staining was more intense and uniform in the specimens obtained from patients with WM (Figure 1B). Sequential staining of bone marrow specimens revealed STAT5 phosphorylation in both CD20+- and CD138+-expressing cell populations (Figure 1C). Furthermore, immunoprecipitation experiments revealed baseline phosphorylation of both the STAT5A and STAT5B isoforms in the BCWM.1 and MWCL-1 cell lines (Figure 1D). Together, these data indicate that STAT5 is constitutively phosphorylated in malignant WM B cells.
Figure 1.
Activation of STAT5 in malignant WM B cells. (A) Baseline STAT phosphorylation was measured in freshly sorted CD19+CD138+ cells obtained from the bone marrows of patients with WM (n = 6) and MWCL-1, BCWM.1, and RPCI-WM1 cells. After fixation and permeabilization, tyrosine phosphorylation of STATs 1, 3, 4, 5, and 6 was determined via fluorescence-activated cell sorter analysis. Normalization for nonspecific antibody binding was performed by dividing the mean fluorescence intensity associated with the specific phospho-antibody signal by the mean fluorescence intensity (MFI) of the isotype control. Data are presented as the normalized MFI (ΔMFI). Significance of STAT5 phosphorylation relative to other STAT proteins was determined by the Student t test for both primary cells and WM cell lines. *P < .05; **P < .01. (B) Immunohistochemical staining of pSTAT5 (brown) in bone marrow sections obtained from 4 consenting patients with WM (WM1-WM4) and 4 normal bone marrows (NM1-NM4) was performed using a polyclonal anti-pSTAT5 antibody, as described in “Methods.” Slides were visualized on an Olympus Provus AX70 light microscope, and images shown are original magnification ×400. (C) Costaining of pSTAT5 (red) with CD20+-expressing (blue) and CD138+-expressing (yellow) B cells in WM bone marrow. (D) Immunoprecipitation for STAT5A (5A) and STAT5B (5B) was performed on unstimulated BCWM.1 (column B) and MWCL-1 (column M) cells followed by western blotting and detection of STAT5A, STAT5B, and pSTAT5.
Biological effects of STAT5 knockdown in WM
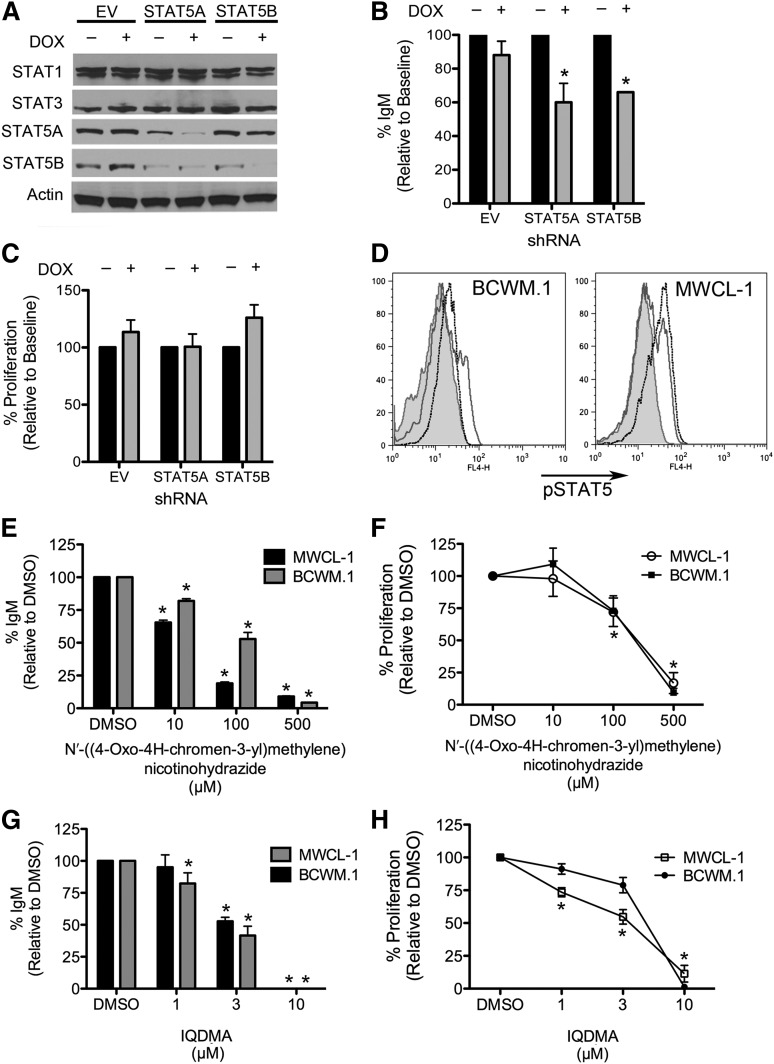
Hyperactivation of STAT5 has been reported in other hematological malignancies, including Hodgkin lymphoma, acute myeloid leukemia, and acute lymphocytic leukemia, and inhibition of STAT5 phosphorylation, either chemically or using shRNA-mediated approaches, has been observed to significantly enhance apoptosis and limit cellular proliferation.4,14 We have employed both methodologies here to assess the biological relevance of constitutive STAT5 phosphorylation in WM. Decreasing the expression of either STAT5A or STAT5B in MWCL-1 cells, using isoform-specific, doxycycline-inducible shRNA, significantly decreased IgM levels as compared with cells transduced with an empty vector shRNA (Figure 2A-B). These results were subsequently confirmed in BCWM.1 cells (data not shown). Interestingly, the STAT5A- and STAT5B-mediated decreases in IgM secretion do not appear to occur secondary to decreases in overall cell number, as inhibition of neither STAT5A nor STAT5B had an effect on the level of cellular proliferation (Figure 2C). STAT5 has been previously reported to drive cellular proliferation through activation of cyclin D1.15 However, it is possible that both isoforms need to be inhibited to see effects on proliferation, as knocking down only STAT5A or STAT5B may lead to compensation by the other isoform, an effect that has been observed after inhibition of other STAT proteins.16,17
Figure 2.
Biological relevance of STAT5 activation in WM. (A) Expression of STAT5A and STAT5B was individually inhibited through the use of a doxycycline-inducible shRNA system transduced into MWCL-1 cells. After a 72-hour treatment with doxycycline (500 ng/mL), cells were lysed and immunoblotted for STAT1, STAT3, STAT5A, STAT5B, and actin, and protein expression was compared with that observed in cells transduced with an empty vector shRNA (EV). Western blotting was performed on 3 separate experiments. Representative blots are shown. (B) After 72-hour induction of STAT5A or STAT5B shRNA with 500 ng/mL doxycycline, IgM was measured by enzyme-linked immunosorbent assay (B), cellular proliferation was measured by 3H-TdR incorporation (C), and levels were compared with those detected in the empty vector-transduced cells (EV). (D) Effect of a small molecule inhibitor of STAT5 on STAT5 activation status in BCWM.1 and MWCL-1 cells. Shaded histograms represent isotype control, dotted lines represent baseline STAT5 phosphorylation, and solid lines represent STAT5 phosphorylation after 24-hour treatment with 10 μM N′-[(4-Oxo-4H-chromen-3-yl)methylene] nicotinohydrazide. Representative plots of 3 separate experiments are shown. (E-H) BCWM.1 and MWCL-1 cells were treated with increasing concentrations of N′-[(4-Oxo-4H-chromen-3-yl)methylene] nicotinohydrazide or IQDMA for 48 hours. IgM was measured by enzyme-linked immunosorbent assay (E,G), and cellular proliferation was measured by 3H-TdR incorporation (F,H). All experiments were performed in triplicate. *P < .05 as determined by the Student t test.
These experiments were then repeated using known small molecule inhibitors of both isoforms of STAT5, N′-[(4-Oxo-4H-chromen-3-yl)methylene]nicotinohydrazide and IQDMA.18,19 A dose-dependent decrease in IgM secretion was observed after incubation of WM cell lines with both small molecule inhibitors, providing the first data to indicate that STAT5 regulates IgM secretion by malignant B cells (Figure 2E,G). The small molecule inhibitors also significantly decreased cellular proliferation, albeit at slightly higher concentrations than those needed to affect IgM levels (Figure 2F,H). These results support the existence of a compensatory mechanism between STAT5A and STAT5B, such that both isoforms need to be inhibited before cellular proliferation is affected.
In conclusion, we have identified high levels of STAT5 activation in malignant WM B cells. As inhibition of both STAT5 isoforms either alone or together led to significant reductions in IgM levels, addition of a STAT5 inhibitor to current treatment protocols could provide a useful clinical strategy for lowering IgM levels in patients with WM.
Acknowledgments
This work was supported in part by grants from the International Waldenström Macroglobulinemia Foundation and the Leukemia and Lymphoma Society Translational Research Program.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.S.H., A.J.N., and S.M.A. designed experiments; L.S.H. and S.C.Z. performed experiments; L.S.H., A.J.N., Z.-Z.Y., F.J.S., A.J.N., and S.M.A. analyzed data; and L.S.H. and S.M.A. wrote the manuscript with input from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ansell.stephen@mayo.edu.
References
- 1.Elsawa SF, Novak AJ, Ziesmer SC, et al. Comprehensive analysis of tumor microenvironment cytokines in Waldenstrom macroglobulinemia identifies CCL5 as a novel modulator of IL-6 activity. Blood. 2011;118(20):5540–5549. doi: 10.1182/blood-2011-04-351742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge LS, Ziesmer SC, Yang ZZ, et al. IL-21 in the bone marrow microenvironment contributes to IgM secretion and proliferation of malignant cells in Waldenstrom macroglobulinemia. Blood. 2012;120(18):3774-3782. [DOI] [PubMed]
- 3.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 4.Joliot V, Cormier F, Medyouf H, Alcalde H, Ghysdael J. Constitutive STAT5 activation specifically cooperates with the loss of p53 function in B-cell lymphomagenesis. Oncogene. 2006;25(33):4573–4584. doi: 10.1038/sj.onc.1209480. [DOI] [PubMed] [Google Scholar]
- 5.Liu RY, Fan C, Garcia R, Jove R, Zuckerman KS. Constitutive activation of the JAK2/STAT5 signal transduction pathway correlates with growth factor independence of megakaryocytic leukemic cell lines. Blood. 1999;93(7):2369–2379. [PubMed] [Google Scholar]
- 6.Harir N, Pecquet C, Kerenyi M, et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood. 2007;109(4):1678–1686. doi: 10.1182/blood-2006-01-029918. [DOI] [PubMed] [Google Scholar]
- 7.Martini M, Hohaus S, Petrucci G, et al. Phosphorylated STAT5 represents a new possible prognostic marker in Hodgkin lymphoma. Am J Clin Pathol. 2008;129(3):472–477. doi: 10.1309/63H1A83HRTBQ07DB. [DOI] [PubMed] [Google Scholar]
- 8.Heltemes-Harris LM, Willette MJL, Ramsey LB, et al. Ebf1 or Pax5 haploinsufficiency synergizes with STAT5 activation to initiate acute lymphoblastic leukemia. J Exp Med. 2011;208(6):1135-1149. [DOI] [PMC free article] [PubMed]
- 9.Yao Z, Cui Y, Watford WT, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA. 2006;103(4):1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93(5):841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 11.Leong PL, Xi S, Drenning SD, et al. Differential function of STAT5 isoforms in head and neck cancer growth control. Oncogene. 2002;21(18):2846–2853. doi: 10.1038/sj.onc.1205385. [DOI] [PubMed] [Google Scholar]
- 12.Xi S, Zhang Q, Gooding WE, Smithgall TE, Grandis JR. Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res. 2003;63(20):6763–6771. [PubMed] [Google Scholar]
- 13.Fox EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells. Mol Endocrinol. 2008;22(8):1781–1796. doi: 10.1210/me.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui ALF, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18(17):4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magné S, Caron S, Charon M, Rouyez MC, Dusanter-Fourt I. STAT5 and Oct-1 form a stable complex that modulates cyclin D1 expression. Mol Cell Biol. 2003;23(24):8934–8945. doi: 10.1128/MCB.23.24.8934-8945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Pereira AP, Tininini S, Strobl B, et al. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci USA. 2002;99(12):8043-8047. [DOI] [PMC free article] [PubMed]
- 17.Cui Y, Hosui A, Sun R, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46(2):504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- 18.Müller J, Sperl B, Reindl W, Kiessling A, Berg T. Discovery of chromone-based inhibitors of the transcription factor STAT5. ChemBioChem. 2008;9(5):723–727. doi: 10.1002/cbic.200700701. [DOI] [PubMed] [Google Scholar]
- 19.Yang SH, Chien CM, Su JC, Chen YL, Chang LS, Lin SR. Novel indoloquinoline derivative, IQDMA, inhibits STAT5 signaling associated with apoptosis in K562 cells. J Biochem Mol Toxicol. 2008;22(6):396–404. doi: 10.1002/jbt.20254. [DOI] [PubMed] [Google Scholar]