Abstract
Human immunodeficiency virus (HIV) induces a neurological disease culminating in frank dementia referred to as HIV-associated dementia (HAD). Neurotoxins from HIV-1-infected and activated mononuclear phagocytes contribute to the neuropathogenesis of HAD. Glutamate is the predominant excitatory neurotransmitter in the mammalian central nervous system (CNS) and functions through activation of multiple receptors. Excessive glutamate production by HIV-infected macrophages in HAD may contribute to neuronal injury. Our previous studies have suggested that mitochondrial glutaminase is responsible for the excessive production of glutamate. However, how HIV-1 infection regulates glutamate over-production remains unclear. In this study, we propose that HIV infection-induced oxidative stress contributes to mitochondrial glutaminase release, which results in the excessive production of glutamate and subsequent neuronal injury. We collected conditioned media from HIV-1 infected macrophages and analyzed glutamate concentration in the media by RP-HPLC, and found that the cyclosporine A (CsA), an inhibitor of HIV-1 replication and mitochondrial permeability transition pore, and N-acetylcysteine (NAC), a remover of reactive oxygen species (ROS), not only blocked the excessive glutamate production, but also decreased the glutamate-mediated neurotoxicity. In addition, HIV-infection-induced ROS generation was accompanied with the excessive glutamate production, suggesting that oxidative stress was involved in glutamate regulation. Using the isolated rat brain mitochondria as an ex vivo model and over-expressing GFP-glutaminase fusion protein in mammalian cells as a cell model, we confirm oxidative stress-mediated mitochondrial glutaminase release during HIV-1 infection contributes to glutamate over-production and the subsequent neurotoxicity. These results may provide insight into HAD pathogenesis and a therapeutic strategy for HAD treatment.
Keywords: HIV-1 infection, Oxidative stress, Mitochondrial glutaminase, Glutamate and neurotoxicity
Introduction
HIV-1 associated neurocognitive disorders (HAND), include asymptomatic neurocognitive impairment, mild neurocognitive disorder (MND) and HIV-1-associated dementia (HAD) (Kaul et al. 2005; Kaul 2009). HAD is characterized by a constellation of cognitive, behavioral and/or motor abnormalities that affect a significant proportion of infected children and adults. The pathological correlation of HAD and HIV-1 encephalitis (HIVE), is characterized by the presence of HIV-1-infected and immune-activated brain mononuclear phagocytes (MP) (Kaul et al. 2005; Zheng and Gendelman 1997).
A great deal of research has been conducted on HAD pathogenesis, and a multitude of factors released during inflammation have been identified with the potential to cause neuronal damage. Although multiple factors play important roles in MP-mediated neuronal injury, accumulated evidence in various neurodegenerative disorders seems to indicate glutamate excitotoxicity as a prominent mechanism of neuronal damage in HAD (Erdmann et al. 2007; Tian et al. 2008a; Zhao et al. 2004). Glutamate is an important transmitter in the CNS which mediates numerous vital physiological functions through activation of multiple receptors and is important for normal neural signaling. However, too much extracellular glutamate induce excitotoxicity. Improper management of glutamate levels may impair not only its signaling properties, but can also lead to neuronal cell death via excitotoxicity, making glutamate regulation critical.
Multiple potential sources contribute to extracellular glutamate over-production during HIV-1 infection, including glutamate release from dying cells, alterations in homeostasis, and improper regulation of enzymatic glutaminase activity or expression. Our previous studies suggest mitochondrial glutaminase plays important roles in extracellular glutamate over-production in HIV-1-infected macrophages and microglia (Erdmann et al. 2007; Jiang et al. 2001; Huang et al. 2011). Glutaminase, as the predominant glutamine-utilizing enzyme in the brain, catalyzes the enzymatic conversion of glutamine (Gln) to glutamate (Glu) plus ammonia, and is generally localized to the inner membrane of mitochondria (Curthoys and Watford 1995; Holcomb et al. 2000). In the brain, glutamine is present in the extracellular fluid in high concentrations and provides an abundant substrate. Recently, we reported that the improper regulation of mitochondrial glutaminase expression and potential release from mitochondria represent the potential mechanisms of extracellular glutamate over-production during HIV-1 infection (Erdmann et al. 2009). However, how HIV-1-infection regulates glutaminase release from mitochondria needs to be further addressed.
Mitochondria participate in intracellular calcium homeostasis, reactive oxygen species generation, cell death and energy metabolism (Robertson et al. 2006). Mitochondria are a major source of intracellular reactive oxygen species (ROS) and are also particularly vulnerable to oxidative stress (Chan 2006). Monocytes are precursors of tissue macrophages, major targets of HIV-1 infection. HIV-1 infection could elevate the level of oxidative stress in macrophages which might play an important role in disease progression (Kimura et al. 1993). In addition, although few blood monocytes are infected, their resulting activation could play a key role in the pathogenesis of HIV disease by modulating their trans-endothelial migration and inducing the production of ROS. ROS participate in chronic inflammation, HIV replication, and the apoptosis of immune system cells seen in HIV-infected subjects (Elbim et al. 1999). Thus we hypothesize that the release of mitochondrial glutaminase induced by oxidative stress following HIV-1 infection in Monocyte-Derived Macrophages (MDM) plays an important role in over-production of glutamate. Confirmation of this hypothesis will help us better understand the pathogenesis of HAD.
Results
The inhibition of HIV-1 replication and the reduction of oxidative stress decrease glutamate production during HIV-1 infection
Monocytes/Macrophages (M/M) play a pivotal role as a source of virus during the whole course of HIV-1 infection. In addition, enhanced oxidative stress is involved in the pathogenesis of HIV-1 infection (Aquaro et al. 2007). Our previous data demonstrate that HIV-1-infection increases the extracellular glutamate production in HIV-1-infected MDM conditioned medium (Erdmann et al. 2007; Zhao et al. 2004). To validate the relationship between HIV-1 replication, oxidative stress and excessive glutamate production, human monocytes were differentiated into MDM, and then pre-treated with 5 μM CsA or 10 mM NAC, respectively before HIV-1ADA infection. After 3 days of infection with or without CsA or NAC, old culture medium was removed and replaced with fresh neurobasal medium containing 5 mM glutamine with or without CsA or NAC for overnight incubation. Supernatants were collected and the total concentration of glutamate was measured by RP-HPLC (Fig. 1a). Our results demonstrate that HIV-1-infected MDM show a dramatic increase in glutamate concentration, however, CsA, an immune suppressor, or NAC, a ROS scavenger, blocked the increase of glutamate. Testing of supernatants (10 μl) for RTase activity (Fig. 1b) show that CsA completely prevents HIV-1 from replication, whereas, NAC had no effect, suggesting HIV-1-mediated glutamate production is related to HIV-1-induced oxidative stress in HIV-1-infected MDM.
Fig. 1.
The inhibition of HIV-1 application and the reduction of oxidative stress decrease glutamate production. a Human MDM were pre-treated with 5 μM CsA or 10 mM NAC for 30 min, and then infected with HIV-1ADA overnight. After 3 days infection, cells were washed three times with PBS and incubated for 24 h in serum-free neurobasal media containing 5 mM glutamine with or without CsA and NAC. The concentrations of glutamate were determined by RP-HPLC. All data are generated using the absolute concentration of glutamate (μmol/L) in MCM, and results are shown as average±SD of triplicate samples and are representative of three independent experiments (n=3) with MDM from at least 3 different donors, # denotes p<0.001 in comparison to control; ## denotes p<0.001 in comparison with HIV-MCM; ** denotes p<0.001 in comparison with HIV-MCM. b Samples were tested for RTase activity. # denotes p<0.001 in comparison to control; ## denotes p<0.001 in comparison with control
HIV-1 infection induces ROS generation in MDM contributing to mitochondrial oxidative stress
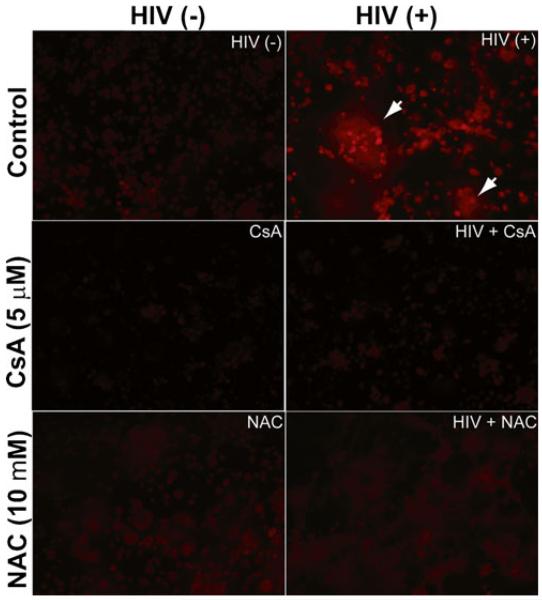
Free radical production and, consequently, oxidative stress play an important role in the pathogenesis of acquired immune deficiency syndrome (AIDS) and cause damage to lipids, proteins, and DNA (Price et al. 2006). Macrophages are major targets of HIV-1 infection, and HIV-1 infection could elevate the level of oxidative stress in macrophages which may play an important role in disease progression (Kimura et al. 1993). We examined ROS levels after HIV-1 infection to investigate the effects of oxidative stress on glutamate production in HIV-1-infected MDM. We cultured macrophages and infected cells with HIV-1. After 3 days infection, cells were stained with dihydroethidium (DHE) which has been used extensively in tissue culture experiments to evaluate ROS production (Peshavariya et al. 2007), and then fixed with 4 % PFA. Cellular ROS generation was determined by the conversion of DHE to an ethidium derivative that exhibits peak fluorescence in the red spectrum (excitation 480 nm; emission 586 nm) (Fig. 2). The results demonstrate that HIV-1 infection dramatically increases ROS generation in MDM, especially in giant cells caused by HIV-1 infection. However, inhibition of HIV replication by CsA blocks the ROS generation. Although NAC has no effect on HIV-1 replication, it also completely removed the ROS induced by HIV-1 infection. These results suggest ROS generation is correlated to excessive glutamate production.
Fig. 2.
HIV-1 infection induces ROS generation in human MDM. Human MDM were pre-treated with 5 μM CsA or 10 mM NAC for 30 min, and then infected with HIV-1ADA overnight. After 3 days infection, cells were stained by loading with 5 μM DHE for 15 min at 37 °C. Cells were washed with PBS and fixed with 4 % PFA for 7 min at room temperature and then subjected to fluorescence microscope. DHE was oxidized to red fluorescent ethidium by ROS indicated in the left upper panel. Staining results are representative of three independent experiments (n=3). Giant cells were indicated with arrows
Oxidative stress induces the release of glutaminase from mitochondria ex vivo and in vitro
Mitochondria not only facilitate the production of ATP through the electron transport chain (ETC), but also participate in intracellular calcium homeostasis, ROS generation and cell death (Robertson et al. 2006). Importantly, mitochondria as the main intracellular sources of ROS are also the targets of ROS. Our previous data demonstrate that the process of HIV-1 infection leads to a release of mitochondrial glutaminase from mitochondria into the cytoplasm in HIV-1-infected MDM (Erdmann et al. 2009). Thus, we examined whether glutaminase localized in the mitochondria was released from injured mitochondria following oxidative stress. First, we isolated intact mitochondria from rat brain and treated the isolate with H2O2 followed by analysis of the supernatants with cytochrome c and glutaminase specific antibodies (Fig. 3a). Our results show that H2O2 induces the release of glutaminase and cytochrome c into supernatant parts of isolated mitochondria in a dose-dependent manner. However, the amounts of glutaminase and cytochrome c in supernatants of mitochondria were decreased when the isolated mitochondria were pre-treated with CsA, a specific inhibitor of PTPC (permeability transition pore complex) before H2O2 stimulation. The presence of glutaminase in the supernatant of isolated mitochondria suggests the possibility of mitochondrial glutaminase release. This is coincident with the release of cytochrome c upon H2O2 stimulation (Fig. 3b and c).
Fig. 3.
Mitochondrial permeability transition pore complex (PTPC) inhibition blocks H2O2-induced glutaminase release from mitochondria. Rat brain mitochondria were isolated and stimulated ex vivo with H2O2 (0.1, 0.5, or 1 mM) with or without CsA (5 μM) treatment. Mitochondrial supernatants were collected by centrifuge at 10,000 g and then subjected to Western blotting analysis using anti-glutaminase and anti-cytochrome c antibodies, and VDAC1 as a loading control (a). Levels of glutaminase (b) and Cytochrome c (c) were normalized as a ratio to VDAC1 after densimetrical quantification and shown as fold change over control. Quantification results were shown as average±SEM in three independent experiments (n=3), # denotes p<0.05 in comparison to control; ## denotes p<0.05 in comparison with 1.0 mM H2O2-treated group; * denotes p<0.05 in comparison with control group; ** denotes p <0.05 in comparison to 1.0 mM H2O2-treated group
Astrocytes provide more fine mitochondria structure as compared to macrophages. To better observe the morphology of mitochondria and the translocation of glutaminase from mitochondria to cytoplasm in vitro, we co-transfected human astrocytes with pEGFP-N1 (empty vector), pEGFP-GA125 (truncated glutaminase fused with GFP) with the mitochondrion-targeted DsRed (mtDsRed) plasmid, and then treated cells with 100 μM H2O2. The distributions of glutaminase (EGFP fusion protein) and mitochondria (red) in cells were investigated (Fig. 4). The results demonstrate that GFP protein is evenly distributed in the whole cell including cytoplasm, nucleus and mitochondria. Additionally, H2O2 treatment has no effect on the distribution of GFP, but mitochondria undergo fragmentation (Fig. 4 iii, iii-1). In contrast, the distribution of GFP-GA125 (glutaminase) fusion protein overlaps well with mitochondrial structure, whereas glutaminase is redistributed following mitochondrial fragmentation after H2O2 stimulation. Most of the GFP-GA125 proteins are still co-localized with mitochondria and distributed around nucleus, however, some GFP-GA125 proteins are present in the cytoplasm without co-localization with mitochondria (Fig. 4 vi, vi-1), suggesting that some of the mitochondrial glutaminase is redistributed from mitochondria to cytoplasm. Furthermore, we transfected Hela cells with pEGFP-GA125 (glutaminase) plasmid, pre-treated transfected cells with NAC and CsA, separately, and then treated cells with H2O2. Cells were subjected to subcellular fractionation and western blotting analysis. Our results show that H2O2 stimulation increases the amount of glutaminase-GFP in the cytoplasmic fraction (Fig. 5), consistent with the fluorescence imaging results (Fig. 4 vi-1). However, inhibiting PTPC opening with its inhibitor, CsA and scavenging ROS with NAC, prevented the translocation of GFP fusion protein from mitochondria to cytoplasm. All these data suggest that glutaminase originally localized in mitochondria translocates from the mitochondrial matrix into cytoplasm after oxidative stress, which may contribute to the excessive production of glutamate.
Fig. 4.
Oxidative stress induces the translocation of mitochondrial glutaminase. Human fetal astrocytes were co-transfected with pEGFP-N1 or pEGFP-GA1-125 together with mito-Ds-Red (specific labeling mitochondria). Post-transfection 24 h, cells were treated with 100 μM H2O2 and fixed with 4 % paraformaldehyde (PFA), and then subjected to nuclear staining with DAPI. After washing, cells were mounted with SlowFade light anti-fade reagent (Molecular Probes) and analyzed by Zeiss Axiovert microscope (arrows indicate the area with no the co-localization between mitochondria and glutaminase in cytoplasm)
Fig. 5.

The inhibition of PTPC opening and a ROS scavenger blocks oxidative stress-mediated translocation of mitochondrial glutaminase. Hela Cells were transfected with pEGFP-GA215 alone. Post-transfection 24 h, cells were pre-treated with 5 μM CsA or 10 mM NAC for 30 min, and then treated with 200 μM H2O2 for another 24 h. Cells were subjected to subcellular fractionation and western blotting analysis with anti-GFP antibody, and β-actin as a loading control
Inhibition of excessive glutamate production by regulating glutaminase release from mitochondria prevents glutamate-mediated neurotoxicity
To examine whether the inhibition of HIV replication by CsA and the removing of ROS by NAC will decrease the glutamate production by MDM following HIV-1 infection and subsequent glutamate-mediated neurotoxicity, we collected the conditioned media and measured the glutamate concentrations in MCM (Fig. 1a), and then we treated rat cortical neurons (RCN) with 30 % MCM and examined the neurotoxicity of MCM by MAP-2 ELISA analysis (Fig. 6). In addition, we also compared the neurotoxicity induced by control MDM-conditioned media to that by serum-free neurobasal medium. Although the neurotoxicity induced by control MDM-conditioned media was slightly higher than that by serum-free neurobasal medium, no significant difference was observed. The results demonstrate that HIV-1-infected MCM significantly induces neurotoxicity due to high concentration of glutamate in MCM, however, removing ROS by NAC attenuates the neurotoxicity, similar to the inhibition of HIV-1 replication by CsA. These results further suggest that oxidative stress plays an important role in excessive glutamate production from HIV-1-infected MDM and subsequent neurotoxicity.
Fig. 6.
The inhibition of glutamate over-production by inhibiting HIV-1 replication and ROS generation decreases glutamate-mediated neurotoxicity. RCN were treated for 3 days with serum-free neurobasal medium or 30 % MCM collected from HIV-1-infected MDM with or without CsA or NAC. Cells were fixed and then subjected to ELISA with anti-MAP-2 antibody, and the absorbance was read at 450 nm. Data values represent SEM from three independent experiments (n=3). ## denotes p<0.05 in comparison to control; ns denotes no significance between Con-MCM and serum-free neurobasal medium
Discussion
The findings about elevated glutamate levels in AIDS patients’ cerebrospinal fluid (CSF) have been correlated to HIV-induced neurodegeneration (Ferrarese et al. 2001) and in turn raise hopes to develop potential neuroprotective drugs for delaying and restricting HAD. Clinical applications for these findings also require identification of the source for the elevated CSF glutamate levels. In this study, our results demonstrate that excessive glutamate production by HIV-1 infected macrophages and subsequent neurotoxicity by HIV-1 infected MCM are attenuated by inhibiting HIV-1 replication with CsA and scavenging ROS with NAC. Moreover, we found that HIV-1 infection-mediated ROS generation in MDM plays an important role in the regulation of extracellular glutamate over-production in MDM during HIV-1 infection, which may be attributed to mitochondrial glutaminase release from mitochondrial matrix to cytosol. And this translocation of glutaminase may contribute to the excessive conversion of glutamate from glutamine leading to subsequent neurotoxicity. These findings provide insights into the pathogenesis of HIV-associated dementia and the role of infected macrophages during HIV-1 infection.
HIV-infected mononuclear phagocytes were recognized as primary mediators of inflammation and excitotoxicity (Kaul et al. 2005; Kaul et al. 2001; Zheng et al. 2001a; Zheng et al. 2001b). HIV-infected macrophages release various neurotoxins including glutamate, quinolinic acid, platelet activating factor, reactive oxygen species (ROS), NTox, Tat and gp120, which directly or indirectly influence neuronal functions. Our studies confirm that HIV-1 infection of human macrophages results in a pathogenic increase of glutamate concentration (Fig. 1a) and the subsequent neurotoxicity (Fig. 6). Cyclosporine (CsA) is a very potent immunosuppressive agent that has an apparent selective action on T lymphocyte-dependent immune responses and has been used extensively in clinical transplantation to prevent solid organ allograft rejection and graft-versus-host disease (GVHD) (Borel 1976; Borel et al. 1976; Hess et al. 1986). In addition, hyperactivation of the immune system has emerged as an important clinical marker of HIV disease progression to AIDS, and CsA, an immune response suppressor, also blocks HIV-1 infectivity via two independent mechanisms, the first involving HIV-1 capsid (CA) interaction with target cell cyclophilin A (CypA) and the second involving HIV-1 envelope glycoprotein (Env) in producer cells (Argyropoulos and Mouzaki 2006; Sokolskaja et al. 2010). Thus the treatment with CsA in macrophages during HIV-1 infection attenuates the HIV-1 replication (Fig. 1b) followed by the inhibition of excessive glutamate production in HIV-infected MCM (Fig. 1a). Moreover, NAC (N-Acetyl L-Cysteine) acts as a free-radical scavenger and inhibits the production of reactive oxygen species which can damage cellular structures (Yang et al. 2007). Our results demonstrate that NAC has no effect on HIV-1 replication, but decreases the glutamate production in HIV-infected MCM and the HIV-infected MCM-mediated neurotoxicity (Fig. 1a and b, Fig. 6), suggesting ROS plays an important role in glutamate production during HIV-1 infection. Our fluorescence imaging results further confirm the ROS generation during HIV-1 infection (Fig. 2).
Mitochondria as a metabolic and apoptotic center, participate in intracellular calcium homeostasis, reactive oxygen species generation and cell death in addition to energy metabolism (Robertson et al. 2006). Meanwhile, mitochondria are also the targets of ROS (Chan 2006), which results in the mitochondrial oxidative stresses. It was well-known that the conversion of glutamine to glutamate is attributed to glutaminase, which is a mitochondrial enzyme. Thus, how mitochondrial glutaminase is regulated by HIV-1-mediated oxidative stress and its contribution to extracellular glutamate production becomes an important question for understanding the mechanism of HIV-infection mediated neurotoxicity. The relationship between excessive glutamate production, mitochondrial glutaminase and oxidative stress was further investigated by analyzing the localization of mitochondrial glutaminase after oxidative stress in vitro and ex vivo. Our results demonstrated that oxidative stress mimicked by H2O2 treatment induced the translocation of glutaminase from mitochondrial matrix to cytosol in either isolated mitochondria model or cell model (Figs. 3, 4 and 5). It is generally acknowledged that opening of the permeability transition pore (PTP) located at the contact site of the inner and outer membrane of a mitochondrion could be involved in cytochrome c release during apoptotic stimulus (Desagher and Martinou 2000). Moreover, PTP was considered a mediator of cell death and has been hypothesized to minimally consist of the voltage-dependent anion channel (Vdac) in the outer membrane, the adenine-nucleotide translocase (Ant) in the inner membrane and cyclophilin-D in the matrix (Baines et al. 2007; De Pinto and Palmieri 1992; Marzo et al. 1998). Both Ant and Vdac are easily oxidized by ROS, which leads to PTP opening and cyto c release (Kanno et al. 2004; Le Bras et al. 2005; Qin et al. 2009). Our ex vivo results demonstrate that H2O2 treatment induces the translocation of both glutaminase and cyto c from mitochondria to cytosol. Cyclosporin A (CsA) (a ligand of the mitochondrial cyclophilin D, CypD) blunts the release of cyto c and mitochondrial glutaminase, further supporting this possibility. These findings suggest that either specific inhibitors of mitochondrial glutaminase or anti-oxidants will prevent excessive glutamate production and subsequent glutamate -mediated neurotoxicity.
Taken together, along with the facts that mitochondrial glutaminase expression in HIV-1-infected/activated macrophages can be regulated by glutaminase promoter regulation (Zhao et al. 2012) and miRNA regulation (Gao et al. 2009), HIV-1-infection mediated ROS generation may regulate mitochondrial glutaminase release. All these potential mechanisms act to increase the conversion of glutamine to glutamate, which contributes to subsequent neurotoxicity. Not only do these findings provide insight into the intricate mechanisms underlying the pathogenesis of HIV-1-associated dementia, but they also present novel opportunities for the design and development of more effective therapeutic strategies.
Materials and methods
Isolation and culture of primary monocyte-derived macrophages, rat cortical neurons (RCN)
Human monocytes were isolated from peripheral blood mononuclear cells (PBMCs). Primary monocytes were cultured as adherent monolayers at a density of 1.1×106 cells/well in 24-well plates and differentiated for 7 days in Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO Invitrogen Corp., Carlsbad, CA) supplemented with 10 % heat-inactivated pooled human serum (Cambrex Bio Science, Walkersville, MD), 50 μg/ml gentamicin (GIBCO Invitrogen Corp.), 10 μg/ml ciprofloxacin (Fisher Scientific, Dubuque, IA), and 1000 U/ml highly purified recombinant human macrophage-colony stimulating factor (M-CSF, a generous gift from Wyeth Pharmaceutical, Cambridge, MA). Primary human astrocytes were isolated from fetal brain tissue cortexes, as previously described (Ghorpade et al. 2003). Cells were cultured at a density of 2×107 cells/150 cm2 in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, GIBCO Invitrogen Corp) with 10 % heat-inactivated fetal bovine serum (FBS, GIBCO Invitrogen Corp), and an antibiotic mixture containing penicillin and streptomycin (Invitrogen Corp). Primary cortical neurons were prepared from cortices of embryonic day 17 to 18 (E17-18) Sasco Sprague–Dawley rat fetuses. Briefly, the cortex was dissected and individual cells were mechanically dissociated in Neurobasal™ medium (Invitrogen Corp.) and differentiated in Neurobasal™ medium containing B27 supplement (GIBCO, Invitrogen Corp.), 0.5 mM glutamine (Sigma-Aldrich, Milwaukee, WI, USA) and 25 μg/ml penicillin-streptomycin (GIBCO, Invitrogen, Corp.). For neurotoxicity assays, neurons were plated onto poly-D-lysine-coated 96-well plates at a density of 5×104 cells/well. Cultured neurons were assumed to be mature at day 7–12 after plating. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Infection of monocyte-derived macrophages (MDM) and collection of MDM-conditioned medium (MCM)
HIV-1ADA was isolated from the peripheral blood mononuclear cells (PBMCs) of an infected patient with Kaposi’s sarcoma (Gendelman et al. 1988). Primary viruses were cultured in phytohemagglutinin (PHA, 5 μg/ml, Sigma-Aldrich) stimulated PBMCs for 14 days before titration. Stock viruses were screened for mycoplasma and endotoxin using hybridization and Limulus amebocyte lysate assays, respectively. The 50 % tissue culture infective dose (TCID50) of each viral stock was determined by monitoring infection of TZM-bl cells obtained through NIH AIDS Research & Reference Reagent Program as described previously (Marozsan et al. 2004; Wei et al. 2002; Derdeyn et al. 2000). Seven days after plating, MDM were infected with HIV-1ADA (TCID50/ml=6.31 × 104) or pre-treated with 10 mM NAC (Sigma-Aldrich) or 5 μM CsA (Sigma-Aldrich) and then infected with HIV-1ADA. On the second day after infection, medium was removed and substituted with MDM culture medium (DMEM with 10 % heat-inactivated pooled human serum, 50 μg/ml gentamicin, and 10 μg/ml ciprofloxacin) with or without NAC or CsA. Three days after infection, cells were changed to 0.5 ml/well fresh serum-free Neurobasal medium (supplemented with 25 ug/ml penicillin-streptomycin and 5 mM glutamine) with or without NAC or CsA overnight. Culture supernatants were collected as MCM and subsequently stored at −80 °C, which were subjected to HPLC analysis and neurotoxicity assay.
Analyses of glutamate in conditioned media by RP-HPLC and reverse transcriptase (RTase) activity
RP-HPLC analysis was performed using an Agilent Technologies 1200 series liquid chromatograph (Agilent Technologies, Germany) as described previously (Zhao et al. 2004). In brief, 250 μl of sample was mixed with equal volumes of 3 % perchloric acid (Sigma-Aldrich), and then immediately neutralized with 11.5 μl saturated potassium carbonate (Sigma-Aldrich). Samples were centrifuged at 12,000 g for 15 min at 4 °C, and then injected into an RP-HPLC system. Glutamate detection was monitored using a fluorescence detector with wavelengths of excitation at 340 nm and emission at 450 nm. HIV-1 reverse transcriptase (RTase) activity was determined from triplicate samples of cell culture supernatants as previously described (Koenig et al. 1986). Briefly, 10 μl of supernatant were incubated in a reaction mixture of 0.05 % Nonidet P-40, 10 μg of poly (A)/ml, 0.25 μg of oligo (dT)/ml, 5 mM dithiothreitol, 150 mM KCl, 15 mM MgCl2, and [3H]TTP in Tris–HCl buffer (pH 7.9) for 24 h at 37 °C. Radiolabeled nucleotides were precipitated with cold 10 % trichloroacetic acid on filter paper plates in an automatic cell harvester and washed with 95 % ethanol. Radioactivity was estimated by liquid scintillation spectroscopy.
Determination of ROS production
ROS were detected by staining the HIV-1-infected macrophages with dihydroethidium (DHE) (Molecular Probes, Carlsbad, CA) as previously described (Pan et al. 2010) with some modifications. Briefly, cells were loaded with 5 μM DHE for 15 min at 37 °C, and 5 % CO2 and then were washed with PBS and returned to media for a 30 min recovery period. The cells were fixed with 4 % PFA for 7 min at room temperature and then subjected to fluorescence microscope.
Colorimetric MAP-2 ELISA for neurotoxicity analysis
Quantitative ELISA for rat cortical neurons was performed as previously described (Zheng et al. 2001a) with minor modifications. In brief, rat cortical neurons cultured in poly-D-lysine-coated 96-well plate and at 7 days were exposed to different macrophage-derived conditioned medium (MCM). After 3 days of treatment, cultures were fixed with 4 % paraformaldehyde (PFA) in PBS. The cells were treated with 1 % hydrogen peroxide (H2O2) in methanol for 20 min after being washed three times with PBS. The cells were then incubated with MAP-2 monoclonal antibody (Millipore, MAB3418, Billerica, MA) after being blocked with 3 % normal goat serum. Cells were washed with PBS and then incubated with anti-mouse biotinylated antibody (Vector Laboratories, Inc., BA-1400, Burlingame, CA) for 30 min at room temperature. After additional washes with PBS, avidin/biotin complex (ABC) solution (Vector Laboratories, Inc., SP-2001, Burlingame, CA) was added for 30 min at room temperature. Cells were washed with PBS and then rinsed with ddH2O. Color was developed using 3,3′,5,5′-tetramethyl-benzidine (TMB, Vector Laboratories, Inc.) at room temperature for 2–4 min, and terminated with 2 M H2SO4 (Sigma-Aldrich). The absorbance was read at 450 nm on a plate reader (Bio-Rad Laboratories).
Analysis of glutaminase release by isolated mitochondria
Rats (Sprague–Dawley) were killed by decapitation. Brain mitochondria isolation was performed according to previously described methods (Tian et al. 2008b). Isolated mitochondria were equally treated with different concentrations of hydrogen peroxide (H2O2) at 25 °C for 60 min. In addition, isolated mitochondria were pre-incubated with 5 μM CsA for 10 min, and then treated with H2O2 for another 60 min. The samples were then centrifuged at 12,000 g for 15 min at 4 °C. The supernatants were subjected to immunoblotting analysis using mitochondrial glutaminase and cytochrome c specific antibodies, mitochondrial conversed VDAC1 protein as a loading control.
Cell transfection and cell fractionation
Human fetal astrocytes were isolated from human fetal brain tissue (gestational age 13–16 weeks). Cells cultured on cover-slips were co-transfected with pEGFP-N1, pEGFP-GA1-125 (a fusion protein of truncated glutaminase with EGFP), separately, together with mito-Ds-Red (mitochondrial localization sequence fused to Ds-Red). Post-transfection 24 h, cells were treated with 200 μM H2O2 for another 24 h, then fixed with 4 % paraformaldehyde (PFA), permeabilized with 0.1 % Triton X-100 in PBS and subjected to nuclear staining with DAPI. After washing, cells were mounted with SlowFade light antifade reagent (Molecular Probes) and analyzed by Zeiss Axiovert microscopey. Hela cells cultured on 100 mm culture dishes were transfected with pEGFP-GA1-125 alone. Post-transfection 24 h, cells were treated with 200 μM H2O2 for another 24 h together with or without 5 μM CsA and 10 mM NAC, and then subjected to subcellular fractionation by differential centrifugation as described previously (Tian et al. 2008b). Briefly, cells were harvested through trypsin digestion, and then centrifuged and resuspended in three volumes of hypotonic buffer [210 mM sucrose, 70 mM mannitol, 10 mM HEPES (pH 7.4), 1 mM EDTA] containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). After gentle homogenization with a Dounce homogenizer, cell lysates were centrifuged at 1,000 g for 5 min to remove unbroken cells and nuclei, and the cytosolic fractions were obtained by further centrifugation at 10,000 g for 30 min at 4 °C and subjected to western blotting analysis with GFP antibody (Sigma), β-actin as a loading control.
Statistical analysis
Data were shown as the mean±SD. Data were analyzed by ANOVA, followed by the Student’st-test for paired observations. Significance was determined if p< 0.05, p<0.01, or p<0.001.
Acknowledgements
We kindly thank Dr. Terry D. Hexum for comments on the manuscript and Drs.Yunlong Huang, Hui Peng, Ling Ye, Lixia Zhao, Myhanh Che, Li Wu and Kristin Leland Wavrin, who provided support for this work. Julie Ditter, Lenal M Bottoms, Johna Belling, and Robin Taylor provided outstanding administrative and secretarial support. This work was supported in part by research grants by the National Institutes of Health: R01 NS 41858–01, R01 NS 061642–01, 3R01NS61642-2S1, R21 MH 083525–01, P01 NS043985, and P20 RR15635-01 (JZ) and National Natural Science Foundation of China (NSFC) # 81028007.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest
Contributor Information
Changhai Tian, Department of Pharmacology/Experimental Neuroscience, Laboratory of Neuroimmunology and Regenerative Therapy, University of Nebraska Medical Center, 985930 Nebraska Medical Center, Omaha, NE 68198-5930, USA.
Lijun Sun, Department of Pharmacology/Experimental Neuroscience, Laboratory of Neuroimmunology and Regenerative Therapy, University of Nebraska Medical Center, 985930 Nebraska Medical Center, Omaha, NE 68198-5930, USA.
Beibei Jia, Department of Pharmacology/Experimental Neuroscience, Laboratory of Neuroimmunology and Regenerative Therapy, University of Nebraska Medical Center, 985930 Nebraska Medical Center, Omaha, NE 68198-5930, USA.
Kangmu Ma, Department of Pharmacology/Experimental Neuroscience, Laboratory of Neuroimmunology and Regenerative Therapy, University of Nebraska Medical Center, 985930 Nebraska Medical Center, Omaha, NE 68198-5930, USA.
Norman Curthoys, Department of Biochemistry and Molecular Biology, Colorado State University, Fort Collins, CO 80523, USA.
Jianqing Ding, Department of Neurology & Institute of Neurology, Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
Jialin Zheng, Department of Pharmacology/Experimental Neuroscience, Laboratory of Neuroimmunology and Regenerative Therapy, University of Nebraska Medical Center, 985930 Nebraska Medical Center, Omaha, NE 68198-5930, USA; Department of Pathology/Microbiology, University of Nebraska Medical Center, Omaha, NE 68198-5930, USA.
References
- Aquaro S, Muscoli C, Ranazzi A, Pollicita M, Granato T, Masuelli L, Modesti A, Perno CF, Mollace V. The contribution of peroxynitrite generation in HIV replication in human primary macrophages. Retrovirology. 2007;4:76. doi: 10.1186/1742-4690-4-76. doi:10.1186/1742-4690-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulos C, Mouzaki A. Immunosuppressive drugs in HIV disease. Curr Top Med Chem. 2006;6(16):1769–1789. doi: 10.2174/156802606778194271. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9(5):550–555. doi: 10.1038/ncb1575. doi:10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel JF. Comparative study of in vitro and in vivo drug effects on cell-mediated cytotoxicity. Immunology. 1976;31(4):631–641. [PMC free article] [PubMed] [Google Scholar]
- Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. doi:10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- De Pinto VD, Palmieri F. Transmembrane arrangement of mitochondrial porin or voltage-dependent anion channel (VDAC) J Bioenerg Biomembr. 1992;24(1):21–26. doi: 10.1007/BF00769526. [DOI] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O’Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74(18):8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10(9):369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Elbim C, Pillet S, Prevost MH, Preira A, Girard PM, Rogine N, Matusani H, Hakim J, Israel N, Gougerot-Pocidalo MA. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: relationship with viral load. J Virol. 1999;73(6):4561–4566. doi: 10.1128/jvi.73.6.4561-4566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann N, Tian C, Huang Y, Zhao J, Herek S, Curthoys N, Zheng J. In vitro glutaminase regulation and mechanisms of glutamate generation in HIV-1-infected macrophage. J Neurochem. 2009;109(2):551–561. doi: 10.1111/j.1471-4159.2009.05989.x. doi:10.1111/j.1471-4159.2009.05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann N, Zhao J, Lopez AL, Herek S, Curthoys N, Hexum TD, Tsukamoto T, Ferraris D, Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102(2):539–549. doi: 10.1111/j.1471-4159.2007.04594.x. doi:10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57(4):671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. doi: 10.1038/nature07823. doi:10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade A, Holter S, Borgmann K, Persidsky R, Wu L. HIV-1 and IL-1 beta regulate Fas ligand expression in human astrocytes through the NF-kappa B pathway. J Neuroimmunol. 2003;141(1-2):141–149. doi: 10.1016/s0165-5728(03)00222-4. [DOI] [PubMed] [Google Scholar]
- Hess AD, Colombani PM, Esa AH. Cyclosporine and the immune response: basic aspects. Crit Rev Immunol. 1986;6(2):123–149. [PubMed] [Google Scholar]
- Holcomb T, Taylor L, Trohkimoinen J, Curthoys NP. Isolation, characterization and expression of a human brain mitochondrial glutaminase cDNA. Brain Res Mol Brain Res. 2000;76(1):56–63. doi: 10.1016/s0169-328x(99)00331-9. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao L, Jia B, Wu L, Li Y, Curthoys N, Zheng JC. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci. 2011;31(42):15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. doi:10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117(1-2):97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- Kanno T, Sato EE, Muranaka S, Fujita H, Fujiwara T, Utsumi T, Inoue M, Utsumi K. Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria. Free Radic Res. 2004;38(1):27–35. doi: 10.1080/10715760310001626266. [DOI] [PubMed] [Google Scholar]
- Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22(3):315–320. doi: 10.1097/WCO.0b013e328329cf3c. doi:10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. doi:10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kaul R, Rowland-Jones SL, Kimani J, Fowke K, Dong T, Kiama P, Rutherford J, Njagi E, Mwangi F, Rostron T, Onyango J, Oyugi J, MacDonald KS, Bwayo JJ, Plummer FA. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol Lett. 2001;79(1-2):3–13. doi: 10.1016/s0165-2478(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kameoka M, Ikuta K. Amplification of superoxide anion generation in phagocytic cells by HIV-1 infection. FEBS Lett. 1993;326(1-3):232–236. doi: 10.1016/0014-5793(93)81797-4. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Le Bras M, Clement MV, Pervaiz S, Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol. 2005;20(1):205–219. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang Y, Tian C, Taylor L, Curthoys N, Wang Y, Vernon H, Zheng J. Interferon-α regulates glutaminase 1 promoter through STAT1 phosphorylation: Relevance to HIV-1 associated neurocognitive disorders. PLoS One. 2012 doi: 10.1371/journal.pone.0032995. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marozsan AJ, Fraundorf E, Abraha A, Baird H, Moore D, Troyer R, Nankja I, Arts EJ. Relationships between infectious titer, capsid protein levels, and reverse transcriptase activities of diverse human immunodeficiency virus type 1 isolates. J Virol. 2004;78(20):11130–11141. doi: 10.1128/JVI.78.20.11130-11141.2004. doi:10.1128/JVI.78.20.11130-11141.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Kroemer G. The central role of the mitochondrial megachannel in apoptosis: evidence obtained with intact cells, isolated mitochondria, and purified protein complexes. Biomed Pharmacother. 1998;52(6):248–251. doi: 10.1016/S0753-3322(98)80009-7. doi:10.1016/S0753-3322(98)80009-7. [DOI] [PubMed] [Google Scholar]
- Pan J, Chang Q, Wang X, Son Y, Zhang Z, Chen G, Luo J, Bi Y, Chen F, Shi X. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem Res Toxicol. 2010;23(3):568–577. doi: 10.1021/tx9003193. doi:10.1021/tx9003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41(6):699–712. doi: 10.1080/10715760701297354. doi:10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- Price TO, Uras F, Banks WA, Ercal N. A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp Neurol. 2006;201(1):193–202. doi: 10.1016/j.expneurol.2006.03.030. doi:10.1016/j.expneurol.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Qin G, Meng X, Wang Q, Tian S. Oxidative damage of mitochondrial proteins contributes to fruit senescence: a redox proteomics analysis. J Proteome Res. 2009;8(5):2449–2462. doi: 10.1021/pr801046m. doi:10.1021/pr801046m. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Soane L, Siegel ZT, Fiskum G. The potential role of mitochondria in pediatric traumatic brain injury. Dev Neurosci. 2006;28(4-5):432–446. doi: 10.1159/000094169. [DOI] [PubMed] [Google Scholar]
- Sokolskaja E, Olivari S, Zufferey M, Strambio-De-Castillia C, Pizzato M, Luban J. Cyclosporine blocks incorporation of HIV-1 envelope glycoprotein into virions. J Virol. 2010;84(9):4851–4855. doi: 10.1128/JVI.01699-09. doi:10.1128/JVI.01699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Erdmann N, Zhao J, Cao Z, Peng H, Zheng J. HIV-infected macrophages mediate neuronal apoptosis through mitochondrial glutaminase. J Neurochem. 2008a;105(3):994–1005. doi: 10.1111/j.1471-4159.2007.05197.x. doi:10.1111/j.1471-4159.2007.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Gao P, Zheng Y, Yue W, Wang X, Jin H, Chen Q. Redox status of thioredoxin-1 (TRX1) determines the sensitivity of human liver carcinoma cells (HepG2) to arsenic trioxide-induced cell death. Cell Res. 2008b;18(4):458–471. doi: 10.1038/cr.2007.112. doi:10.1038/cr.2007.112. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Su Y, Richmond A. Antioxidants tiron and N-acetyl-L-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-kappaB pathway. Free Radic Biol Med. 2007;42(9):1369–1380. doi: 10.1016/j.freeradbiomed.2007.01.036. doi:10.1016/j.freeradbiomed.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Lopez AL, Erichsen D, Herek S, Cotter RL, Curthoys NP, Zheng J. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. J Neurochem. 2004;88(1):169–180. doi: 10.1046/j.1471-4159.2003.02146.x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Gendelman HE. The HIV-1 associated dementia complex: a metabolic encephalopathy fueled by viral replication in mononuclear phagocytes. Curr Opin Neurol. 1997;10(4):319–325. [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Cotter RL, Lopez AL, Ghorpade A, Persidsky Y, Xiong H, Leisman GB, Che MH, Gendelman HE. HIV-1 infected and immune competent mononuclear phagocytes induce quantitative alterations in neuronal dendritic arbor: relevance for HIV-1-associated dementia. Neurotox Res. 2001a;3(5):443–459. doi: 10.1007/BF03033203. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Persidsky Y, Williams CE, Cotter RL, Zink W, Ryan L, Ghorpade A, Lewis K, Gendelman HE. HIV-1 infected immune competent mononuclear phagocytes influence the pathways to neuronal demise. Neurotox Res. 2001b;3(5):461–484. doi: 10.1007/BF03033204. [DOI] [PubMed] [Google Scholar]