Highlights
-
•
No study has examined neural correlates contributing to adolescent sexual riskiness.
-
•
Assessed teen contraceptive use and neural activity during impulse control task.
-
•
Sexually riskier teens recruited frontal regions less during impulse control.
Keywords: Adolescence, Brain development, Impulse control, Risky sexual behavior
Abstract
The consequences of risky sexual behavior are of public concern. Adolescents contribute disproportionately to negative consequences of risky sexual behavior. However, no research has examined the neural correlates of impulse control and real-world engagement in risky sexual behavior in this population. The aim of the present study was to examine this question. Twenty sexually active adolescents performed an impulse control task during a functional magnetic resonance imaging (fMRI) scan and risky sexual behaviors were assessed through self-report. Sexual riskiness ratings were negatively associated with activation in the prefrontal cortex during response inhibition. These results suggest that diminished engagement of impulse control circuitry may contribute to sexual riskiness in adolescents.
1. Introduction
Risky sexual behavior is a phenotypic manifestation of risky decision-making that has far-reaching consequences for individual health and public concern. In particular, unprotected sex such as a lack of condom use can place individuals at greater risk of sexually transmitted infections (STIs) as well as unintended pregnancy (Centers for Disease Control and Prevention [CDC], 2011). The vast majority of sexually active people across age groups are aware of the preventive efficacy of condom use, yet many do not use them on a consistent basis (Browne and Minichiello, 1994). Recent reports suggest that both adolescents and adults are more likely to use condoms than in the past (Reece et al., 2010), yet every year there are 19 million new cases of STIs in the United States (CDC, 2011). Adolescents contribute disproportionately to those rates–although they represent only 25% of the sexually active population, young people contribute to nearly half of all new cases of STIs and an estimated $10.9 billion annually in teen pregnancy costs each year (see Guttmacher Institute, 2013, Centers for Disease Control and Prevention, 2011). These startling statistics suggest that despite widespread knowledge of the preventive benefits of contraceptive use, many adolescents fail to translate this knowledge into action (Parsons et al., 2000). Given the prevalence of unsafe sexual behavior among adolescents, it is of concern to pinpoint key factors underlying sexual risky behaviors that may result in STIs and unintended pregnancy.
Adolescence is a unique developmental period characterized by social, motivational, affective, and cognitive changes (Crone and Dahl, 2012), all of which likely contribute to lack of contraceptive use. Previous research has focused on potential social and motivational factors that may contribute to risky sexual decision-making (Parsons et al., 2000, Aalsma et al., 2006); however, the role of cognitive processes in adolescent sexual risk-taking is less clear. There is some suggestion in the adult literature that impulse control difficulties are associated with risky sexual behavior (Eysenck, 1976, Clift et al., 1993, Pinkerton and Abramson, 1995). A recent behavioral study with adults used the go/no-go task to examine impulse control in the presence of sexual stimuli across four counterbalanced conditions (Macapagal et al., 2011). In the study, participants viewed a sexual or neutral video before performing a go/no-go task with sexual or neutral stimuli. Impulsivity was assessed with the Eysenck Personality Questionnaire (Eysenck et al., 1985). Although no relationship was found between impulsivity and task performance in neutral conditions, more impulsive individuals committed significantly more errors (i.e., failure to inhibit a response) than less impulsive individuals when attempting to inhibit a button press for sexual stimuli, specifically after viewing the sexually arousing video (Macapagal et al., 2011). In other words, poor task performance in highly impulsive individuals was specific to sexually arousing stimuli. The authors suggest that impulsivity may involve a tendency to respond to motivationally or emotionally salient stimuli (Evenden, 1999).
Given that risky sexual decisions often occur under emotionally and motivationally salient contexts, these choices may be particularly vulnerable to difficulties in impulse control (Reyna and Farley, 2006). Additionally, adolescents appear to be especially sensitive to motivational influences, perhaps leaving them more vulnerable to risky decision-making in general (Galvan et al., 2007) and risky sexual behavior in particular (Reyna and Farley, 2006). Indeed, although there is some evidence that adolescents pre-contemplate, deliberate, and prepare for sexual encounters (Reece et al., 2010), they are often unable to translate forethought into action in the heat of the moment (Reyna and Farley, 2006). An examination of the cognitive processes and traits that contribute to risky sexual decision-making in adolescents may prove useful in understanding the development of these behaviors.
Advances in neuroimaging have enabled researchers to establish a neural basis for risky decision-making during adolescence, a developmental period of significant brain maturation (Somerville and Casey, 2010). There is evidence that frontal regions implicated in regulatory processes undergo a protracted development, while subcortical limbic regions display heightened sensitivity to emotional stimuli and reward, potentially leaving adolescents vulnerable to risky decision-making (Casey et al., 2008). This developmental imbalance between neural systems likely grants adolescents greater cognitive flexibility (Crone and Dahl, 2012), though may leave adolescents less able to inhibit impulses, especially in emotionally arousing contexts. This may be represented behaviorally through a failure to inhibit the impulse to engage in sexual intercourse despite a lack of contraceptives. The remodeling of fronto-striatal regions implicated in regulatory and motivational processes has been tied to forms of risk-taking during adolescence, such as substance use (Clark et al., 2008) and gambling (Chambers and Potenza, 2003). Yet the connection between risky sexual behavior and the regulatory mechanisms in the brain remains unexamined in adolescents, despite the prevalence and gravity of the consequences of these behaviors.
The goal of the present study was to examine the association between naturalistic levels of contraceptive use and neural correlates of impulse control during a basic go/no-go task performed during functional magnetic resonance imaging (fMRI) in adolescents. Neural correlates of impulse control have previously been assessed using the go/no-go task and linked to real-world behaviors in a sample of smokers attempting to quit (Berkman et al., 2011). Using the adolescents’ self-report of protection against unwanted pregnancy grants the ability to examine how individual differences in neural correlates of impulse control relate to this behavior. We hypothesized that adolescents reporting greater levels of risky sexual behavior (i.e., less contraceptive use) would exhibit less activation in frontal regions involved in regulation, as shown previously in adults (Aron and Poldrack, 2006) and adolescents (Cohen et al., 2010).
2. Methods
2.1. Participants
Forty eight adolescents participated in an fMRI scan. Only sexually experienced adolescents (n = 20) were included in the current analyses, as we wished to examine individual variability in self-reported sexual risk taking, as measured by contraceptive use, and its relationship to neurocognitive indicators of inhibition. The rate of sexually active adolescents in our sample (42%) is similar to national trends (CDC, 2011). Participants ranged in age from 15–17 years (Mage = 16.36; 7 males, 13 females). All subjects were right-handed, free of metal, and reported no current medication except birth control. Participants completed written consent and assent in accordance with UCLA's Institutional Review Board and were compensated for their participation.
2.2. Questionnaire measures
2.2.1. Risky sexual behavior
Although multiple variables were collected to assess risky sexual behavior (e.g., number of partners, age of first sexual intercourse), riskiness level of contraceptive method used was selected to assess risky sexual decision-making. The reason for focusing on this variable is because lack of contraceptive use, more so than other behaviors, most directly relates to contraction of STIs or unintended pregnancy (CDC, 2011) and in-the-moment impulsive decisions (Donohew et al., 2000). Behaviors were assessed through self-report and questions were phrased with respect to “sexual intercourse,” the definition of which was left to the adolescent (e.g., “The last time you had sexual intercourse, what contraceptive method did you or your partner use? (Choose all that apply).”) Contraceptive use options included: no method was used, condoms, birth control pills patch or shot (Depo-Provera), withdrawal, some other method. A composite score, or “sexual riskiness rating,” was then created from the participants’ response. A higher rating indicated greater riskiness of protection method used at last intercourse (1 = condom and birth control, 2 = only condom, 3 = only birth control, 4 = withdrawal, 5 = none). As no participant selected “some other method” in their response, this item was not included in the composite score.
2.3. fMRI paradigm
2.3.1. Impulse control task
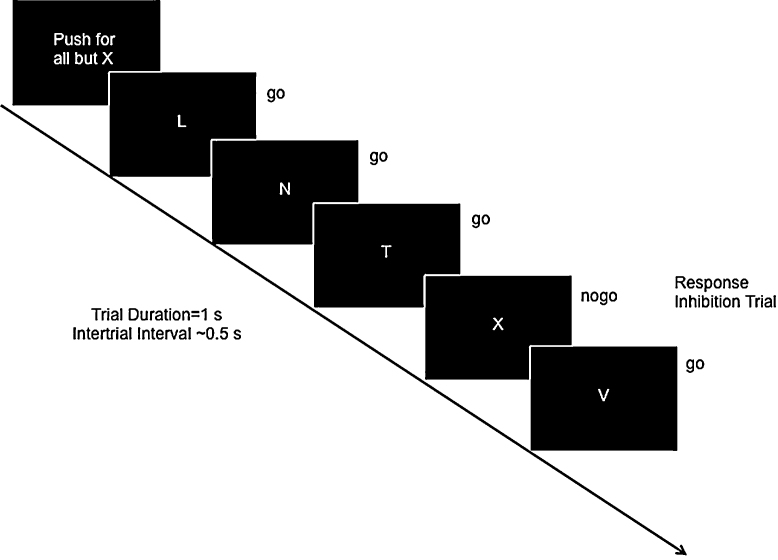
Participants completed a standard go/no-go (GNG) task to examine neural correlates of cognitive control (Fig. 1). Participants were presented with a series of rapid trials (1 s each), each displaying a single letter, and were instructed to respond with a button press as quickly as possible to all letters except for X. The X occurred on 25% of trials. Thus, participants developed a pre-potent response to press (go) upon stimulus onset, and must inhibit the go response on X trials (no-go). Response inhibition was operationalized as successful no-go compared to go trials (overriding the pre-potent response). Participants completed 5 blocks during one functional run. Each block contained 10 no-go trials and 30 go trials presented in random order. The inter-trial interval (ITI) was jittered according to a random gamma distribution (M = 0.75 s). Each block (40 trials and ITIs) lasted 70 s, and each block was separated by a 12-s rest period.
Fig. 1.
The go/no-go task. Participants were instructed to press the button as quickly as possible for all letters (“go” trials) except the letter X (“no-go” trials). “No-go” trials occurred randomly, 25% of the time.
2.4. fMRI data acquisition and analysis
2.4.1. fMRI data acquisition
Imaging data were collected using a 3 T Siemens TrioMRI scanner. The task was presented on a computer screen through scanner-compatible goggles. The GNG task consisted of 200 images [slice thickness, 4 mm; 34 slices; TR = 2 s; TE = 30 ms; flip angle = 90°; matrix = 64 × 64; FOV = 200 mm; voxel size 3 mm × 3 mm × 4 mm]. A T2*weighted, matched bandwidth (MBW), high-resolution, anatomical scan and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan were acquired for registration purposes (TR: 2.3; TE: 2.1; FOV: 256; matrix: 192 × 192; sagittal plane; slice thickness: 1 mm; 160 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
2.4.2. fMRI data preprocessing and analysis
Analyses were performed using FSL 4.1.6 (www.fmrib.ox.ac.uk/fsl). All images were skull-stripped using FSL BET. The images were realigned to compensate for small head movements (Jenkinson et al., 2002). No participants exceeded > 2 mm in translational movement. Data were smoothed using a 5-mm FWHM Gaussian kernel to increase the signal-to-noise ratio, and filtered in the temporal domain using a nonlinear high-pass filter (100-s cutoff). EPI images were registered to the MBW, then to the MPRAGE, and finally into standard MNI space (MNI152, T1 2 mm) using linear registration with FSL FLIRT.
One general linear model (GLM) was defined for the GNG task, which included multiple regressors for each event type: successful go trials, successful no-go trials, and false alarms. Events were modeled with a 1 s duration. The rest periods and jittered inter-trial intervals were not explicitly modeled and therefore served as an implicit baseline. Temporal derivatives and motion parameters were included as covariates of no interest for all regressors.
The FSL FEAT package was used for statistical analysis. Regressors of interest were created using a stick function of the event duration at the onset time of each trial with a canonical (double-gamma) HRF. A group-level analysis was performed using the FMRIB Local Analysis of Mixed Effects module in FSL (Beckmann et al., 2003). The sexual riskiness rating was demeaned and entered as a regressor in whole brain regression analyses. Thresholded Z statistic images were prepared to show clusters determined by a corrected, cluster-forming threshold of Z > 2.3 and an extent threshold of p < .05 familywise error corrected using the Theory of Gaussian Random Fields (Poline et al., 1997). Outliers were de-weighted in the multi-subject statistics using mixture modeling (Woolrich, 2008). For visualization, statistical maps of all analyses were projected onto a study-specific average brain of the participants.
3. Results
3.1. Behavioral results
Participants’ behavior was normally distributed on the 1–5 scale of sexual riskiness rating for method of protection used (MRiskinessRating = 2.9, SD = 1.6). A breakdown of participant distribution by category is provided in Table 1. No significant associations were observed for sexual riskiness rating and participants’ gender or age.
Table 1.
Participant distribution by “Riskiness Rating” of protection method used at last intercourse.
| Riskiness Rating | Description | N |
|---|---|---|
| 1 | Condom and birth control | 4 |
| 2 | Condom only | 7 |
| 3 | Birth control only | 1 |
| 4 | Withdrawal | 3 |
| 5 | No method used | 5 |
On the GNG task, participants successfully inhibited 81.48% (SD = 7.17) of the no-go trials (i.e., withheld the button press to the no-go trials), ranging from 40 to 100%. The mean response time on go trials was 410.1 ms (SD = 39.3). No significant associations were found between the sexual riskiness rating and task performance or reaction time.
3.2. fMRI results
3.2.1. Main effects
First, main effects of go responses and inhibition (no-go) trials on neural activation were examined. The omnibus GLM analysis of the imaging data identified activation in the occipital cortex during go responses compared to baseline and in the insula, superior parietal, lateral occipital, and superior frontal cortex during no-go trials compared to baseline (Table 2). Second, the omnibus GLM analysis for the no-go > go contrast revealed a main effect of activation in the insula, precuneus, middle frontal gyrus, posterior cingulate gyrus, parietal cortex, and occipital cortex (Table 2). Finally, the omnibus GLM analysis for the go > no-go contrast identified activation in the cerebellum, lateral temporal cortex, medial frontal gyrus, and postcentral gyrus during go trials compared to no-go (Table 2).
Table 2.
Neural activation during go and no-go trials.
| Trial type | Region | x | y | z | Z-max | Voxels |
|---|---|---|---|---|---|---|
| Main effects | ||||||
| Go | R occipital cortex | 50 | −70 | −8 | 5.17 | 1157 |
| No-go | R superior parietal cortex | 42 | −48 | 48 | 4.97 | 2365 |
| L superior parietal cortex | −48 | −48 | 48 | 4.12 | 1133 | |
| R insula | 32 | 18 | 8 | 4.27 | 1750 | |
| L insula | −36 | 16 | 10 | 4.03 | 542 | |
| R lateral occipital cortex | 52 | −66 | 4 | 4.85 | 956 | |
| R superior frontal gyrus | 16 | 8 | 62 | 4.09 | 638 | |
| No-go > go | R superior parietal cortex | 42 | −48 | 46 | 5.05 | 5993 |
| L inferior parietal cortex | −62 | −44 | 28 | 4.44 | 2706 | |
| R occipital cortex | 28 | −92 | −6 | 4.24 | 422 | |
| L occipital cortex | −22 | −100 | −14 | 3.85 | 371 | |
| R posterior cingulate gyrus | 4 | −22 | 36 | 3.68 | 402 | |
| L middle frontal gyrus | −42 | 36 | 40 | 3.37 | 447 | |
| R insula | 30 | 18 | −10 | 4.69 | 6739 | |
| L insula | −40 | 12 | −6 | 3.9 | 840 | |
| R precuneus | 6 | −70 | 44 | 3.59 | 361 | |
| Go > no-go | R cerebellum | 14 | −52 | −16 | 4.09 | 1529 |
| R lateral temporal cortex | 36 | −44 | −2 | 3.74 | 451 | |
| L medial frontal gyrus | −6 | 50 | −4 | 3.79 | 858 | |
| L postcentral gyrus | −48 | −20 | 54 | 3.59 | 642 | |
| Positive correlation with sexual riskiness rating | ||||||
| Go > no-go | R superior frontal gyrus | 32 | 50 | 26 | 3.64 | 1035 |
| L superior frontal gyrus | −4 | 20 | 48 | 3.77 | 429 | |
| R inferior parietal lobule | 42 | −46 | 50 | 3.53 | 538 | |
| L inferior parietal lobule | −46 | −52 | 52 | 3.42 | 887 | |
| L insula | −46 | −14 | 6 | 3.16 | 498 | |
| L middle frontal gyrus | −32 | 36 | 18 | 3.58 | 400 | |
| Negative correlation with sexual riskiness rating | ||||||
| No-go | L lateral occipital cortex | −24 | −72 | 38 | 4.07 | 1066 |
| L superior parietal cortex | −42 | −50 | 40 | 3.67 | 971 | |
| R parietal cortex | 50 | −28 | 54 | 4.15 | 497 | |
| L superior temporal cortex | −60 | −10 | 2 | 3.78 | 673 | |
| R insula | 34 | 22 | 6 | 3.56 | 652 | |
| R inferior frontal gyrus | 46 | 24 | 4 | 2.53 | ||
| No-go > go | R superior parietal cortex | 42 | −46 | 50 | 3.53 | 538 |
| L superior parietal cortex | −46 | −52 | 52 | 3.42 | 887 | |
| L superior frontal gyrus | −4 | 20 | 48 | 3.77 | 429 | |
| L temporal cortex | −46 | −14 | 6 | 3.16 | 498 | |
| L middle frontal gyrus | −32 | 36 | 18 | 3.58 | 400 | |
| L insula | −32 | 18 | 8 | 2.9 | ||
| R superior frontal gyrus | 32 | 50 | 26 | 3.64 | 1035 | |
| R inferior frontal gyrus | 34 | 26 | 8 | 2.89 | ||
| R insula | 40 | 10 | 8 | 2.62 | ||
Note: x, y, and z refer to MNI coordinates; Z-max refers to the peak level of activation intensity; Voxels refers to the number of voxels in each significant cluster; L and R refer to left and right hemispheres.
3.2.2. Correlations between neural activation and sexual riskiness
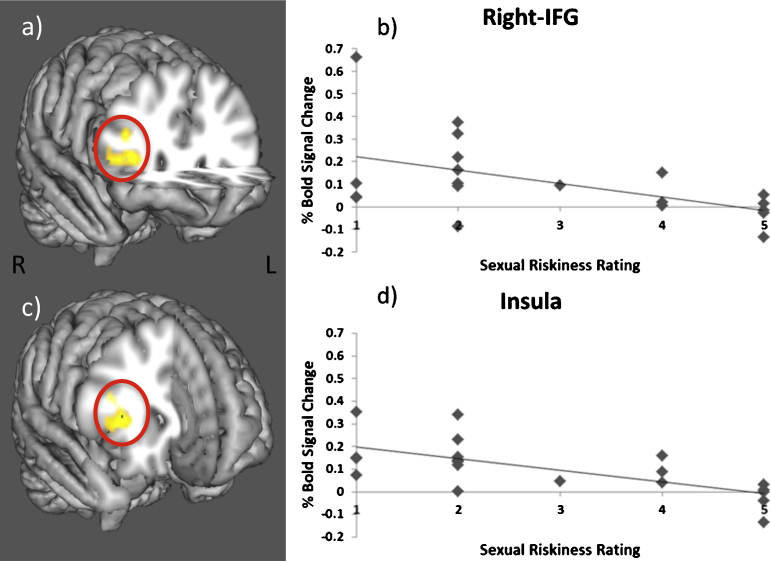
To test whether sexual riskiness was associated with brain activation during task performance, correlation analyses were conducted between sexual riskiness rating and neural activation during go trials and inhibition (no-go) trials. The contrasts examined were go > baseline, go > no-go, no-go > baseline, and no-go > go. There were no significant positive or negative correlations between sexual riskiness and neural activation for go > baseline. There was a significant positive correlation between sexual riskiness and neural activation during go > no-go in the superior frontal gyrus, inferior parietal lobule, insula, and middle frontal gyrus. There were no significant negative correlations for this contrast. There were no significant positive correlations between sexual riskiness and neural activation during inhibition in either the no-go > baseline or no-go > go contrasts. There was a significant negative correlation between sexual riskiness and neural response for no-go > baseline in the superior parietal, lateral occipital, superior temporal cortex, insula, and right inferior frontal gyrus (rIFG) (Table 2). There was also a significant negative association between sexual riskiness and neural response for the no-go > go contrast in the parietal and temporal cortex, superior, middle, and inferior frontal gyri, and the insula (Table 2). The negative correlations for the no-go > go contrast in the rIFG and insula are illustrated in Fig. 2. In other words, sexually riskier teens showed less recruitment of frontal regions during impulse control.
Fig. 2.
Neural response during no-go > go trials. Neural responses in the rIFG (x = 34, y = 26, z = 8) (a) and insula (x = 40, y = 10, z = 8) (c) that negatively correlate with sexual riskiness rating during successful response inhibition. For illustration only, the scatterplots depict the correlations between risky sexual behavior and activation during response inhibition trials in the rIFG (r = −.57, p < .05) (b) and insula (r = −.51, p < .05) (d).
4. Discussion
This study examined behavioral and neural responses to an impulse control task in a sample of sexually active adolescents reporting varying levels of riskiness in contraceptive use. Findings revealed that sexual riskiness was correlated with neural response such that there was less activation in the insula and rIFG during response inhibition trials for those reporting less contraceptive use. In other words, sexually riskier teens showed less recruitment of frontal regions implicated in impulse control and cognitive control of emotion regulation (Cohen et al., 2010, Aron and Poldrack, 2006, Casey et al., 1997). No behavioral differences on inhibitory task performance were found, which may be due to limited sample size. Neuroimaging findings support our hypothesis that neural correlates of impulse control are related to sexual riskiness in adolescents.
Impulsive personality traits have been consistently recognized as a factor contributing to sexual risk-taking (see Hoyle et al., 2000 for a review), and behavioral studies have assessed the role of impulsiveness as a cognitive process that may underlie risky sexual decision-making in adults (Macapagal et al., 2011). Engaging in impulsive sexual behavior is likely a function of deficits in impulse control as well as heightened susceptibility to emotional arousal. Indeed, recent neuroimaging studies have associated heightened neural responses to sexual cues in reward circuitry with subsequent impulsive sexual behavior in adults (Demos et al., 2012). However, no study has yet employed neuroimaging techniques to examine neural underpinnings of impulsivity as they relate to risky sexual behavior. Furthermore, no study has investigated the connection between neural response and engagement in risky sexual behavior during adolescence, a period when social and developmental factors contribute to an increased vulnerability to risky sexual decisions. Importantly, adolescence is a period when motivational and inhibitory regions of the brain undergo extensive remodeling. Our findings provide the first empirical evidence of impaired recruitment of frontal regions during response inhibition in sexually riskier teens, suggesting that diminished impulse control may underlie adolescent sexual riskiness. As cognitive and affective processes undergo drastic changes during adolescence, it is possible that distinct cognitive mechanisms contribute to lack of contraceptive use during adolescence and adulthood. Further work will be needed to evaluate the potentially separate cognitive processes and neurobiology that may be associated with the same behavior in different developmental populations.
As this was the first study to assess neurocognitive correlates of adolescent risky sexual behavior, we employed a basic GNG task without the use of sexual stimuli to isolate neural correlates of cognitive control and provide a simple measure of response inhibition. It should be mentioned that no-go trials were modeled separately from error trials, so that reported brain activation represents successful response inhibition. Prior work using the GNG task has demonstrated engagement of the IFG for trials in which suppression was correctly invoked (Luna and Sweeney, 2004, Durston et al., 2002), suggesting that the rIFG is necessary for behavioral inhibition (Aron et al., 2003, Aron and Poldrack, 2006, Chambers et al., 2006). Specifically, individuals less able to inhibit impulses display less activation in the rIFG (Aron and Poldrack, 2006). From a developmental perspective, adolescents display less activation than adults in the rIFG during impulse control (Cohen et al., 2010). We interpret our finding that sexually riskier adolescents showed less recruitment of the rIFG during no-go compared to go trials to suggest impaired recruitment of impulse control circuitry when inhibiting a response. Additionally, results demonstrate that sexually riskier adolescents displayed greater activation in frontal regions during go compared to no-go trials, which may reflect a heightened motor response and orientation toward impulsive action (Goya-Maldonado et al., 2010).
Additionally, we found that the insula showed differential activation during response inhibition with varying levels of reported sexual riskiness. The insula has been demonstrated to control attention to emotionally evocative stimuli (Ochsner and Gross, 2008) such that cognitive distraction diminishes the salience of an emotional response and reduces activity in the insula (Frankenstein et al., 2001). The reduced insula activation we observed in sexually riskier teens during response inhibition may indicate a reduced level of engagement and attention. Developmental fMRI studies using the GNG task have shown that adults exhibit increased activation during successful response inhibition in the insula (Tamm et al., 2002) and rIFG (Rubia et al., 2007) when compared to adolescents. Development of cognitive and emotional processes may contribute to immature impulse control during adolescence and consequently greater levels of risky sexual behavior at this time.
Although this study has strengths, there are a few limitations to note. The sample of adolescents used for the purposes of this paper was part of a larger dataset not recruited on the basis of engagement in sexual activity. Although the percentage of adolescents reporting prior sexual activity (42%) was comparable to national rates (46%) (Guttmacher Institute, 2013), this limited our sample size, which may account for the lack of behavioral results. Additionally, the questionnaires used were phrased with respect to sexual intercourse, limiting the scope of risky sexual behaviors that we were able to assess with this study. Questionnaires did not assess sexual orientation; youth in same gender relationships may choose to not report contraceptive use because they reason there is no biological risk of pregnancy. However, failure to use contraceptives may still be considered risky due to concerns about STIs. Finally, we recognize that impulse control is merely one aspect of several that contribute to adolescent risky sexual decision-making (e.g., perceptions of risks and benefits, peer norms, length and type of relationship, gender). As the GNG task is a measure of impulse control, we limited the scope of our analyses and interpretations to impulsiveness. Sample size did not allow for a comparison of sex differences in the relation between risky sexual behavior and brain activation during impulse control. Future research evaluating a broader range of risky sexual activities utilizing tasks with sexual stimuli may further inform the relationship between adolescent sexual decision-making and the brain. Larger sample sizes will allow for greater ability to elucidate individual and gender differences on neural and behavioral levels. These findings are meant to serve as a preliminary exploration to address a question which has not been examined in the literature previously.
In summary, results from this study provide the first evidence of differential brain activation in prefrontal regions during cognitive control for sexually riskier adolescents. Specifically, the finding that adolescents who demonstrated less recruitment of the rIFG and insula also tended to display a lack of contraceptive use converges with prior work demonstrating the role of frontal regions in guiding appropriate actions and estimating future outcomes of risky choices (Miller and Cohen, 2001). These findings suggest that deficiencies in recruitment of the rIFG and insula may put adolescents with less ability to self-regulate at greater risk of impulsive sexual decision-making. Research such as this will hopefully expand our understanding of risky sexual behavior in adolescents, particularly in those who struggle with impulse control disorders and related psychopathology.
Conflicts of interest
The authors report no conflicts of interest.
Acknowledgements
This research was supported in part by a grant from the NSF dissertation improvement grant, NICHD, UCLA Center for Culture, Brain, and Development, SRCD Dissertation Grant , and the UC MEXUS Dissertation Grant . The authors gratefully acknowledge the research participants and their families.
References
- Aalsma M.C., Fortenberry J.D., Sayegh M.A., Orr D.P. Family and friend closeness to adolescent sexual partners in relationship to condom use. Journal of Adolescent Health. 2006;38:173–178. doi: 10.1016/j.jadohealth.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B., Lieberman M.D. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22(4):498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne J., Minichiello V. The condom: why more people don’t put it on. Sociology of Health and Illness. 1994;16:229–251. [Google Scholar]
- Casey B.J., Castellanos F.X., Giedd J.N., Marsh W.L., Hamburger S.D., Schubert A.B., Vauss Y.C., Vaituzis A.C., Dickstein D.P., Sarfatti S.E., Rapoport J.L. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Development Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . U.S.; Atlanta, GA: 2011. National Center for Health Statistics. [Google Scholar]
- Chambers R.A., Potenza M.N. Neurodevelopment, impulsivity, and adolescent gambling. Journal of Gambling Studies. 2003;19(1):53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Bellgrove M.A., Stokes M.G., Henderson T.R., Garavan H., Robertson I.H., Morris A.P., Mattingley J.B. Executive ‘brake failure’ following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18(3):444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Clark D.B., Thatcher D.L., Tapert S.F. Alcohol, psychological dysregulation, and adolescent brain development. Alcoholism: Clinical and Experimental Research. 2008;32(3):375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Clift S.M., Wilkins J.C., Davidson E.A.F. Impulsiveness, venturesomeness and sexual risk-taking among heterosexual GUM clinic attenders. Personality and Individual Differences. 1993;15(4):403–410. [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. Decoding developmental differences and individual variability in response inhibition through predictive analyses across individuals. Frontiers in Human Neuroscience. 2010;4(47) doi: 10.3389/fnhum.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Demos K.E., Heatherton T.F., Kelley W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. Journal of Neuroscience. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohew L., Zimmerman R., Cupp P.S., Novak S., Colon S., Abell R. Sensation seeking, impulsive decision-making, and risky sex: implications for risk-taking and design of interventions. Personality and Individual Differences. 2000;28(6):1079–1091. [Google Scholar]
- Durston S., Thomas K.M., Yang Y., Ulug A.M., Zimmerman R.D., Casey B.J. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. [Google Scholar]
- Evenden J.L. Varieties of impulsivity. Psychopharmacology (Berlin) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Eysenck H.J. Open Books; London: 1976. Sex and Personality. [Google Scholar]
- Eysenck S.B.G., Pearson P.R., Easting G., Allsopp J.F. Age norms of impulsivity, venturesomeness, and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- Frankenstein U.N., Richter W., McIntyre M.C., Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14(4):827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Goya-Maldonado R., Walther S., Simon J., Stippich C., Weisbrod M., Kaiser S. Motor impulsivity and the ventrolateral prefrontal cortex. Psychiatry Research. 2010;183(1):89–91. doi: 10.1016/j.pscychresns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Guttmacher Institute . In Brief. 2013. Facts on American teens’ sexual and reproductive health. Retrieved from: http://www.guttmacher.org/pubs/FB-ATSRH.pdf. [Google Scholar]
- Hoyle R.H., Fejfar M.C., Miller J.D. Personality and sexual risk taking: a quantitative review. Journal of Personality. 2000;68(6):1203–1231. doi: 10.1111/1467-6494.00132. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Macapagal K.R., Janssen E., Fridberg D.J., Finn P.R., Heiman J.R. The effects of impulsivity, sexual arousability, and abstract intellectual ability on men's and women's go/no-go task performance. Archives of Sexual Behavior. 2011;40(5):995–1006. doi: 10.1007/s10508-010-9676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J.T., Halkitis P.N., Bimbi D., Borkowski T. Perceptions of the benefits and costs associated with condom use and unprotected sex among late adolescent college students. Journal of Adolescence. 2000;23(4):377–391. doi: 10.1006/jado.2000.0326. [DOI] [PubMed] [Google Scholar]
- Pinkerton S.D., Abramson P.R. Decision making and personality factors in sexual risk-taking for HIV/AIDS: a theoretical integration. Personality and Individual Differences. 1995;19(5):713–723. [Google Scholar]
- Poline J.B., Worsley K.J., Evans A.C., Friston K.J. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5(2):83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Reece M., Herbenick D., Schick V., Sanders S.A., Dodge B., Fortenberry J.D. Condom use rates in a national probability sample of males and females ages 14–94 in the United States. Journal of Sexual Medicine. 2010;7(5):266–276. doi: 10.1111/j.1743-6109.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- Reyna V.F., Farley F. Risk and rationality in adolescent decision-making: implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7(1):1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Taylor E., Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Casey B.J. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]