Abstract
Osteoporosis is an aging problem. The declining bone mineral density (BMD) enhances the chances of fractures during minor falls. Effective pharmaceuticals are available for a rapid improvement of BMD. However, hormonal treatment gives serious complications, and bisphosphonates may lead to odd fractures of long bones, resulting from excessive rigidity of the cortical components. Many medicinal herbs used in Traditional Chinese Medicine, known as kidney tonics, have been tested for their effects on bone metabolism in the laboratory and clinically. Three of these, viz. Herba epimedii (淫羊藿, Yín Yáng Huò), Fructus ligustri lucidi (女貞子, Nǚ Zhēn Zi), and Fructus psoraleae (補骨脂, Bǔ Gǔ Zhī) were chosen to form a herbal formula, ELP. ELP was tested on in vitro platforms and was shown to have both osteoblastic and anti- osteoclastic action. ELP tested on ovariectomized rats also showed BMD protection. ELP was then put on a placebo- controlled randomized clinical trial. BMD protection was obvious among those women with the onset of menopause beyond 10 years (P < 0.05). A general protective trend was observed among all women under trial (P > 0.05). Although a thorough literature review on the herbal treatment effects did not give convincing answers to the use of Chinese herbs in osteoporosis, our study supports more research and trials in this area, while we are looking for safe and effective agents to keep the bone metabolism in a balanced state.
Keywords: Bone mineral density, Herbal treatment, Osteoporosis
INTRODUCTION
Bone is the most rigid tissue in the human body. Yet, it is the most dynamic and ever-changing tissue apart from blood. From birth until around age 20 years, the bone grows and stores up its calcium reserve. At the same time and under all circumstances, every bone unit is undergoing continuous changes of breakdown and restoration. These rapid, continuous changes are required for the control of calcium metabolism and to maintain the healthy state of the bone. Special cells called osteoblasts are responsible for the build-up of the bone unit, while other special cells called osteoclasts are responsible for the breakdown. Build-up and breakdown are kept at a harmonized speed, so that there is good balance between the two mechanisms that are kept at a dynamic state. The balance is affected most commonly when the breakdown mechanism supersedes the restoration process and the causes behind could be aging, nutritional, disuse, vitamin D deficiency (no sunlight), and disease conditions. Given good health, good nutrition, and freedom from major diseases, aging becomes the most unquestionable cause of bone loss.[1]
There are many ways to assess the physiological state of bone in the living individual, including clinical tests, imaging, and biochemical tests, of which imaging to detect the bone mineral density (BMD) has become the most accepted simple and reliable investigation.[2]
There have been many studies on the BMD of patients of different ages, and it has been confirmed that BMD declines with aging, and for women, menopause is accompanied with a rapid drop of BMD. When the sudden drop of BMD associated with menopause became solidly known, explorations on the prevention of the drop started. Exercises and nutritional considerations could well be effective means to prevent the decline in BMD and to maintain a balance. Nevertheless, clinicians and their clients are not satisfied with a slow maintenance. They want an immediate rise of BMD. Starting with hormonal replacement which appears logical because menopause is associated with a decline in female hormones, other pharmaceutical agents have come to market with increasing efficacy on BMD promotion.[3]
There are incidents of bone fractures at the vulnerable weight-bearing sites, particularly during accidents, when the bones have become weak because of low BMD (osteopenia and osteoporosis). The increased risks of fracture when the bone becomes osteoporotic appear logical since the weak bone is certainly less resistant to stress and bending forces. All aging people have great fear against fractures. The fear thus becomes transferred to a fear about osteoporosis. The transfer of fear could have been logical but might not be fair because there must be many other risk factors leading to fractures, apart from a low BMD. Other risk factors are the accident itself and the factors leading to the accident such as dementia, frailty, neurological symptoms, visual problems, medications, etc., led pharmaceuticals companies on an active pursuit for BMD raising drugs agents to artificially raise the BMD.
Osteoporosis has become a major health issue worldwide. It is defined as a condition characterized by low BMD and micro-architectural deterioration of bone tissues leading to enhanced bone fragility and a consequent increase in fracture risk.[4] Postmenopausal osteoporosis typically affects women within 10-15 years after menopause. It is complicated with fractures occurring at sites that contain relatively large amounts of cancellous bone. The prevalence of osteoporotic fracture is extremely high. In the United States, for women at 50 years of age, the lifetime risk is 17.5% for the hip fracture, 16% for vertebral fracture, and 16% for Colles’ fracture. Approximately 50% of 70-year-old women or 40% women after the menopause experience one or more fractures.[5,6]
In China, epidemiological studies have shown that the incidence of osteoporosis in the population over 40 years of age is 16.1%, over 60 years of age is 22.6%, and over 80 years of age, it is as high as 50%. Osteoporosis in the spine not only leads of fractures, but would also produce pain and back deformities, which seriously affect the patients’ quality of life.[7]
Estrogen plays an important role in coordinating the activities of the bone-forming osteoblasts and bone-resorbing osteoclasts in bone homeostasis. Ovarian hormonal deficiency is one of the most important factors leading to postmenopausal osteoporosis, which has made hormonal replacement therapy popular in the early days for the prevention of bone loss in postmenopausal women. However, treatment with estrogen has well-known side effects such as breast soreness and nausea, and in the long-term, may lead to increased risk of breast and uterine cancer development and also venous thrombosis.[8,9]
Subsequently, when other therapeutic measures are developed to correct the deteriorating BMD, the direction is one of quick correction. This concept is acceptable when the loss of BMD is sharp and severe, under which circumstance, effective restoration is preferred. However, when the BMD is only starting to decline, there is a doubtful necessity of bringing it up quickly and artificially using drug treatment. It might be more favorable just to maintain the bone mineral contents and prevent any uncontrolled decline. Food supplements, therefore, could be a valuable offer.
The intention of this review is to explore the biological effects of the herbs used to maintain bone health, and then study the reported results of clinical treatment. Our own experience in the search for effective herbal treatment for the maintenance of bone health will be finally described.
PHYTOTHERAPY FOR OSTEOPOROSIS AS REVEALED FROM CHINESE LITERATURE
Laboratory studies
Natural herbs have been widely used in clinical practice in China and other countries since ancient times. Certain natural herbs have potential effects in promoting gonadal function and fracture healing, and therefore are suitable items for current research to counteract and prevent postmenopausal osteoporosis.
In China, bone health is considered closely related to kidney function. Thus, “kidney deficiency” (腎虛, Shèn Xū) is considered the root of all pathologies related to bones and joints, which include arthritis and osteoporosis. Treatment accordingly follows the principle of strengthening the kidney function when the bones and joints are considered “weak”.[10]
Of the many herbs reported to be useful for kidney strengthening, around 30 are popular and 6-8 are most frequently used. They are: Herba epimedii (淫羊藿 Yín Yáng Huò), Fructus psoraleae (補骨脂 Bǔ Gǔ Zhī), Radix rehmanniae (生地黃 Shēng Dì Huáng), Rhizoma drynariae (骨碎補 Gǔ Suì Bǔ), Herba cistanches (肉蓯蓉 Ròu Cōng Róng), and Cortex eucommaie (杜仲 Dù Zhòng).
No matter how many biologically active chemical components are contained in the herbs and how complex their mechanisms of action could be, the phytoestrogens in the herbal preparations, viz. certain flavones, isoflavones, flavanones, flavonols, coumestans, and lignans, could be playing an important role in the amelioration of postmenopausal bone loss.[11,12,13] The effects of phytoestrogens have been reported in both laboratory studies and clinical trials. Studies in ovariectomized rats have suggested that the known phytoestrogens such as coumestrol, genistein, and daidzein can reduce bone loss.[14,15]
Phytochemical analyses have revealed that the six most commonly used herbs just quoted contain more specific components such as icariin in H. epimedii (淫羊藿 Yín Yáng Huò) and isopsoralen in F. psoraleae (補骨脂 Bǔ Gǔ Zhī). These effective components have estrogen-like effects and may become new phytoestrogens for future research.[15]
Clinical studies
Xu, et al. reported in 2008 that 63 randomized control trials (RCTs) were conducted in China in which postmenopausal bone loss was treated with oral administration of herbs. Only 14 of the trials met the criteria of RCT. Treatment duration of the included trials ranged from 1 to 6 months. In 11 out of the 14 trials, the treatment groups were treated with patent herbal formulae. There were obvious defects in the design of the clinical trials. Firstly, the use of blinding and stratified randomization according to age and length of menopause were not employed. Secondly, the small sample sizes in some of the trials were a matter of concern. Thirdly, prior drug history was not stated explicitly. Fourthly, reporting BMD change as mean differences with associated standard deviations was not sufficient for data analysis. The authors compared the results of herbal treatment with those of hormonal treatment and reported that the prevention of bone loss at the classical regions of lumbar and femoral regions was about the same, and that adverse effects like uterine bleeding and breast pain were not objectively described. These studies could be further criticized because of short treatment durations.[16]
STUDIES DONE IN HONG KONG
Our laboratory is aware of the importance of osteoporosis related to aging. We are of the view that osteoporosis, though contributing toward the risks of bone fractures, might not be the major culprit. Hence, clinicians’ concern might not be a rapid boosting of the BMD, but a long-term maintenance of the bone quality. In this regard, highly effective therapeutic agents that artificially suppress osteoclastic activities might not be the best choice for bone health, unless the BMD has reached a dangerously low level. This consideration might appear particularly logical when potent osteoclast antagonists, viz. the bisphosphonates, are showing incidents of adverse effects like odd fractures and cardiac complications when taken for a long duration.[17,18]
We selected three herbs classically used for “kidney insufficiency,” for our laboratory and clinical studies.
Laboratory studies
The herbs used were H. epimedii (淫羊藿 Yín Yáng Huò), Fructus ligustri lucidi (女貞子 Nǚ Zhēn Zi), and F. psoraleae (補骨脂 Bǔ Gǔ Zhī) – ELP. They were mixed in the ratio 10:8:2, and were boiled, extracted, and prepared in a powder form.
ELP were first tested in the laboratory to establish the scientific evidence of their bone supportive activities. The known active compounds of the three herbs, ELP, are listed in Table 1. The anabolic effects of ELP on bone formation were evaluated using cultured rat osteoblast-like osteosarcoma cell line UMR-106 and rat mesenchymal stem cells (MSCs). To test for bone resorption, RAW 264.7 cell line was used to investigate the inhibition of osteoclast formations. A dosing effect with several concentrations (1 g/day, 0.5 g/day, and 0.175 g/day) was tested in ovariectomized, and calcium deficiency-induced osteoporotic rats.[19]
Table 1.
Known active compounds of ELP

RESULTS
In the in vitro studies, ELP significantly increased the proliferation of UMR-106 cells in a dose-dependent manner from 50 to 400 μg/ml [Figure 1]. For MSCs, ELP stimulated the osteogenic differentiation as indicated by the increase of bone markers such as alkaline phosphatase (ALP) activity and matrix calcium mineralization. ELP elevated the ALP activity significantly at concentrations of 50 and 100 μg/ml [Figure 2a]. The amount of mineralization also increased significantly with ELP [Figure 2b]. In regards to bone resorption, ELP significantly inhibited osteoclast formation from RAW 264.7 cells upon receptor activation of nuclear factor-kappa B ligand induction on the 4th day of treatment, at a concentration of 80 and 160 μg/ml. The difference between treated and untreated cultures of osteoclasts reached statistical significance (P ≤ 0.05). The experimental data, therefore, illustrated that the formula ELP had positive effects on bone metabolism through a double process of osteoblastic promotion and osteoclastic inhibition.
Figure 1.
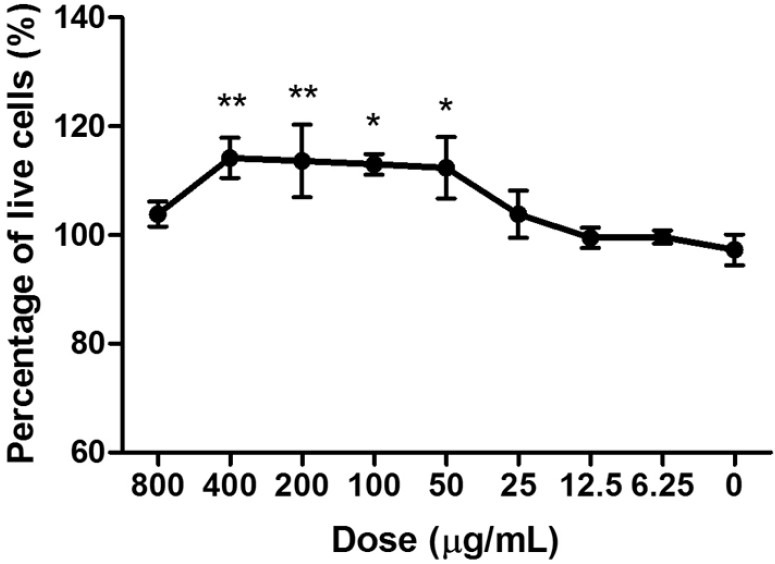
Effect of ELP water extract on the cell viability of osteoblast-like UMR-106 cells for 3 days at different concentrations. Data are the means (SEM; error bars) from three independent experiments in triplicate. *P < 0.05; **P < 0.01 for difference in percentage of live cells from respective baseline culture without treatment
Figure 2a.
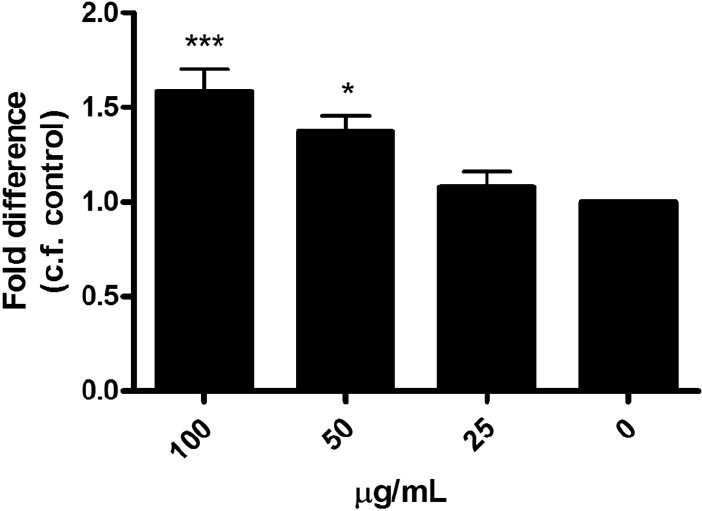
Dose effect of ELP on the ALP activity in rat MSCs on day 7
Figure 2b.
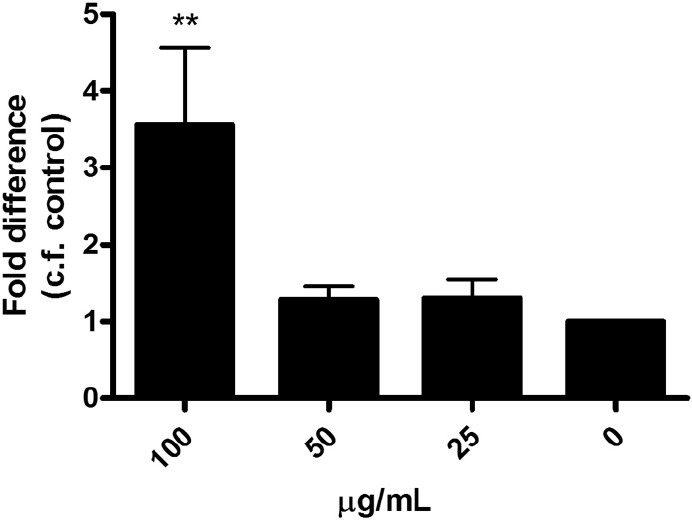
Dose effect of ELP on the matrix mineralization in rat MSCs on day 7 (***P < 0.001)
In the ovariectomized, and calcium deficiency-induced osteoporotic model, after 11 weeks of ELP treatment, it was demonstrated that there was preservation of BMD at the proximal femur in a dose-dependent manner with a preference for higher dosages. No significant increase in uterine weight was observed in the ELP treated rats. In addition, the microarray data of kidney tissues revealed that ELP was able to down-regulate the expression of phase II drug metabolizing enzymes, similar to the effects of estrogens.[19]
Clinical studies
The design of the clinical trial was of randomized, double-blind, and placebo-controlled nature. Proper ethical approval was obtained before starting the clinical trial and proper consent was given by the clients.
The inclusion criteria were: Women who experienced menopause for at least 1 year, with their lumbar spine BMD lower than 0.891 g/cm2, which indicated osteopenia. Those suffering from serious concomitant diseases and were maintained on complicated medications, and those suffering from psychiatric or addiction disorders were excluded.
Two hundred and twenty-eight patients from volunteers in community centers in Hong Kong were screened and 78 were found unsuitable. A total of 150 subjects were considered adequate for this study basing on the assumption that at least 1% difference in BMD could be observed between the treatment and placebo groups with 90% power, if the standard deviation of BMD measurements was 3.6%.
The subjects enrolled were randomized to either ELP (75 cases) or placebo (75 cases) group. In the ELP group, the mean age of subjects was 58.4 ± 3.6 years, the mean time since last menses was 8.1 ± 4.4 years, and the mean age of menopause onset was 50.4 ± 3.8 years, whereas in the control group, they were 58.4 ± 3.8 years, 8.4 ± 4.7 years, and 50.0 ± 4.2 years, respectively. There were no significant differences between the ELP and placebo groups in demographic and baseline BMD characteristics. The intention-to-treat (ITT) approach was adopted [Figure 3].
Figure 3.
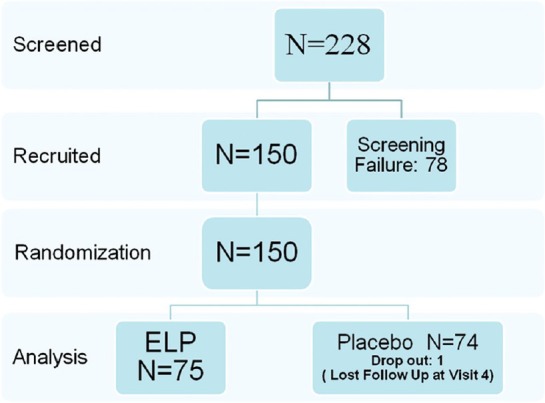
Flowchart for inclusion of patients
The primary outcome was the BMD of the lumbar spine assessed at baseline and 6 and 12 months after treatment, using a Hologic type 4500 DEXA machine. Secondary end points included peripheral quantitative computed tomography (pQCT) measurements of the distal tibia and an analysis of the physical components of the quality of life (SF-36 questionnaire). Only the four physical domains relevant to bone and joint problems were evaluated. Among the 150 subjects, 79 subjects (41 in the ELP group and 38 in the placebo group) received pQCT examination.
Adverse events (AEs) were carefully monitored and checking for biochemical functions of the liver and kidney was done for all clients before and after treatment. The resulting data were analyzed using to the ITT principles.
RESULTS
In the ELP group, 75 subjects completed the 12-month clinical study; 74 subjects in the placebo group completed the 12-month study: The mean drug compliance rates were 73.7% and 81.2% in the ELP and placebo groups, respectively. There were no statistical differences between the two groups.
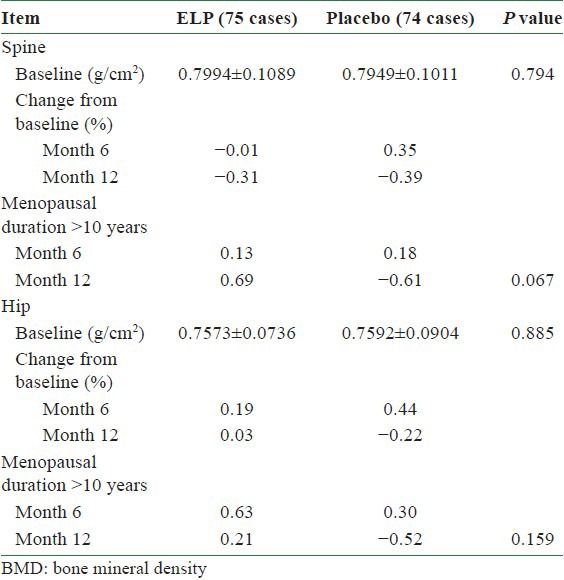
Both ELP and placebo groups showed a decrease in spine BMD over the 12-months treatment period (−0.31% in the ELP group and −0.39% in the placebo group). However, the spine BMD of the ELP group was increased by 0.69% in subjects with more than 10 years duration of menopause. In contrast, the placebo group of the same stratum decreased by 0.61% (P = 0.067).
The hip BMD of the ELP group remained unchanged over 12-month treatment, whereas the placebo group decreased by 0.22%. In the subjects with more than 10 years duration of menopause, the hip BMD increased by 0.21%, compared with a decrease of 0.52% in the placebo group [Table 2].
Table 2.
Comparison of BMD changes
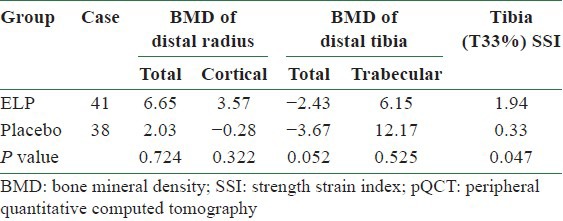
After 12 months of treatment with the herbal preparation, the distal tibia pQCT decreased by 2.43% in the ELP group compared with 3.67% in the placebo group (P = 0.052). The tibia (T33%) strength–strain index (SSI) was increased by 1.94% in the ELP group compared with 0.33% in the placebo group (P = 0.047) [Table 3].[20]
Table 3.
Annual changes of BMD at different anatomic sites measured by pQCT (%)
Comparison of QOL
SF-36 questionnaire showed remarkable improvement in physical function and physical role in the ELP group compared with the baseline, but failed to reach dominance over the placebo group [Figure 4]. AEs were insignificant.
Figure 4.
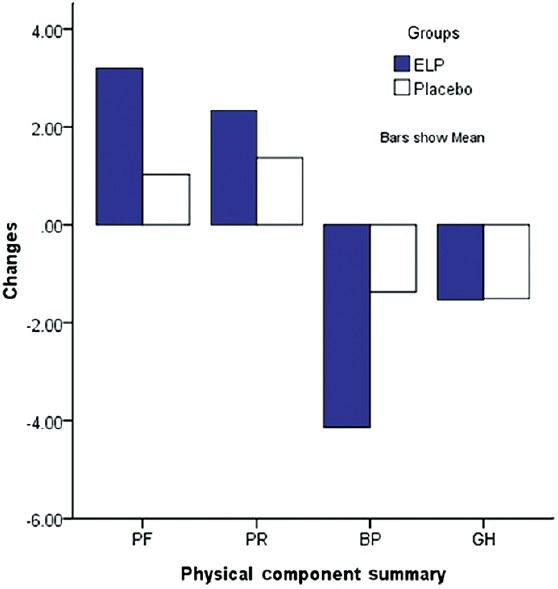
Changes in the four subscales of the SF-36 after 12 months of treatment
DISCUSSION
Traditional Chinese Medicine is built on the principle of maintaining physiological harmony. Since specific pathological changes are unknown to the ancient experts, Chinese medicine aims, not at specific targets, but at the maintenance of normal “internal balance.” The well-known theory of “yin and yang” is a philosophical expression of excess and deficiency which need to be balanced. In the case of osteoporosis prevention, when Chinese herbs are used, the rationale would be one of balanced bone formation and bone resorption. On a cellular level, it would be a balance between osteoblastic construction and osteoclastic destruction. The expected responses would be a steady BMD, and not a rapidly increasing one. However, when we design a protocol for an osteoporosis prevention trial using Chinese herbs, the end point would best follow the current trend which relies on the BMD levels.
In Traditional Chinese Medicine, osteoporosis has not been described. However, syndromes with low back pain, back deformities, loosening teeth, etc., which appear in classical records, could be the manifestations of osteoporosis. Chinese herbs have been widely used in orthopedic practice and many of their therapeutic effects on skeletal problems and fractures have been reported.[21,22,23] In our study, we have selected three common herbs from the pool of Chinese medicine, which are known to be good for bone health, to form a cocktail. The three herbs have been used both as a traditional drug and as food items for “kidney strengthening” in soup making for normal meals. The safety of these herbs has been proven in our clinical trial, as no significant adverse effects are demonstrated.
The best results were shown among the group of clients who had experienced menopause for over 10 years. This group of patients when treated with ELP for 12 months showed better results than the placebo group. Other women with shorter periods of menopause also showed a better trend of bone preservation which, however, was statistically insignificant. If BMD represents the standard changes in the bone density, pQCT which assesses the distal tibia is more sensitive to early influences and less potent effects. In our clinical trial, the supportive effects of ELP on the distal tibia were shown and statistical significance was reached.
Although there are many reports in China endorsing the therapeutic value of various herbs originally used as “kidney tonics” against “kidney deficiencies,” the claims have not been based on reliable clinical trials. Since ELP is not aiming at any specific target of bone metabolism, its efficacy would be inferior to target-oriented pharmaceuticals. A longer period of observation and a larger sample size might be mandatory for a proper biostatistical revelation of the results of treatment. ELP might be considered capable of providing additional support on top of the essential needs against osteoporosis, like vitamin D and calcium. Herbal treatments in Chinese medicine are well known to be working slowly and non-specifically. Although the mechanism of action of the three herbs in EPL has been shown in our laboratory to be both pro-osteogenic and anti-resorptive, more explorations on the mode of action of the herbs need to be done. We also need to know whether the support on the BMD is through a cellular level, or indirectly via an increased absorption or decreased excretion of calcium, and whether vitamin D is involved.
An evidence-based methodology has been followed in the development of ELP as an effective agent for the prevention of osteoporosis, not for active treatment. Long-term effects and safety are of more importance than rapid rises in BMD and in preventive therapy. In prevention, evidences of steady maintenance could be more desirable than specific responses, and multiple foci of attention could be better alternatives to specific, single target therapy.
Some chemicals that the ELP formula contains are already known.[24] Chemists and pharmacologists could further fractionate the extracts to work out the groups of chemicals that are mainly responsible for the bioactivities and are responsible for the anti-osteoporotic effects.
CONCLUSION
Traditional practitioners emphasize on the clinical responses of individual patients. They are not totally supportive of RCTs which insist on group analysis and uniform treatment. Indeed, traditional practitioners prefer treating their patients according to their syndrome presentation, i.e. the “clinical pattern” apparent to the attending clinician. They are looking forward to a balancing effect rather than an abrupt effective control of the problem. In the situation of osteoporosis, current clinical practice has chosen BMD measurement as a convenient tool of assessment. The time taken to achieve an observable improvement in BMD while using herbal supplement is expected to be longer and the intensity of improvement is also expected to be milder since the nature of treatment is not specific.
Modern pharmaceuticals aim at quick and specific responses. The popular pharmaceutical agents elevate BMD within a short period for the need of those people at high risk. However, a proportion might respond too well to give rise to overhardening of cortical components of the long bone, which might lead to odd fractures at unusual sites.[17,18] On the other hand, herbal treatment is supportive and slow. It offers prevention and maintenance rather than over-active treatment.
ACKNOWLEDGMENTS
The author is indebted to the Ming Lai Foundation and Hong Kong and Macau Tam Wah Ching Chinese Medicine Resource Centre for the support given to the team responsible for the study. The author discloses no conflicts of interest in this work.
REFERENCES
- 1.Riggs BL, Melton LJ., 3rd The worldwide problem of osteoporosis: Insights efforded by epidemiology. Bone. 1995;17(5 Suppl):S505–11. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 2.Dambacher MA, Schmitt S, Schacht E, Ito M, Neff M, Muller R, et al. Bone structures in vitro and in vivo in animals and men: A view into the future. J Miner Stoffwechsel. 2004;2:22–8. [Google Scholar]
- 3.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: Scientific review. JAMA. 2002;88:872–81. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health, US. NIH Consensus Statement of Osteoporosis prevention, diagnosis and therapy. [Last accessed on 2000 Mar]. Available from: http://Consensus.nih.gov . [PubMed]
- 5.Lau EM. The epidemiology of osteoporosis in Asia. In: Lau EM, Ho SC, Leung S, Woo J, editors. In Osteoporosis in Asia: Crossing the frontiers. Singapore: World Scientific; 1997. pp. 1–20. [Google Scholar]
- 6.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of osteoporotic fractures research group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 7.Li NH, Ou PZ, Zhu HM, Yang DZ, Zheng PR, Liao EY. Epidemiological study on osteoporosis in middle-aged and elderly population in China. Chin J Clin Rehabil. 2002;6:758–9. [Google Scholar]
- 8.Christiansen C. Prevention and treatment of osteoporosis with hormone replacement therapy. Int J Fertil Menopausal Stud. 1993;38(Suppl 1):45–54. [PubMed] [Google Scholar]
- 9.Lindsay R, Hart DM, Aitken JM, MacDonald EB, Anderson JB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delay onset of oestrogen treatment. Lancet. 1976;1:1038–41. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 10.Cai L, Li H, Wei Y. Effect of Yang-Xue Bu-Shen tablet on ovarian function in animal model of Yang-deficiency. Chin J Integr Tradit West Med. 1998;18:620–2. [PubMed] [Google Scholar]
- 11.Fanti P, Monier-Faugere MC, Geng Z, Schmidt J, Morris PE, Cohen D, et al. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos Int. 1998;8:274–81. doi: 10.1007/s001980050065. [DOI] [PubMed] [Google Scholar]
- 12.Xu M, Zhang XH, Liu SX. Phytoestrogens in herbal medicine. Chin Drug Res Clin Pharmacol. 2002;13:261–3. [Google Scholar]
- 13.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: Evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78(3 Suppl):593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 14.Ishida H, Uesugi T, Hirai K, Toda T, Nukaya H, Yokotsuka K, et al. Preventive effects of the plant isoflavones, daidzin and genistin, on bone loss in ovariectomized rats fed a calcium-deficient diet. Biol Pharm Bull. 1998;21:62–6. doi: 10.1248/bpb.21.62. [DOI] [PubMed] [Google Scholar]
- 15.Huang HF, You JS. The use of Chinese herbal medicine on experimental fracture healing. Am J Chin Med. 1997;25:351–6. doi: 10.1142/S0192415X97000391. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Qi C, Deng B, Deng PX, Mo CW. Phytotherapy versus hormonal therapy for postmenopausal bone loss: A meta-analysis. Osteoporos Int. 2009;20:519–26. doi: 10.1007/s00198-008-0724-x. [DOI] [PubMed] [Google Scholar]
- 17.Neviaser AS, Lane JM, Lorich DG. Bisphosphonates and odd fractures. J Orth Trauma. 2008;22:346–8. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 18.Kwek EB, Koh JS, Howe TS. More on atypical fractures of the femoral diaphysis. N Engl J Med. 2008;359:316–7. doi: 10.1056/NEJMc080861. [DOI] [PubMed] [Google Scholar]
- 19.Leung PC, Ko EC, Siu WS. Developing an effective food supplement for the prevention of osteoporosis functional foods. Health Dis. 2011;9:379–88. [Google Scholar]
- 20.Leung PC, Ko EC, Siu WS. Developing an effective health supplement for the prevention of Osteoporosis. Int J Osteoporosis Metab Disord. 2012;5:1–12. [Google Scholar]
- 21.Cai L, Li H, Wei Y. Effect of Yang Xue Bu Shen tablets on ovarian function in animal model of Yang deficiency. Chin J Integr Tradit West Med. 1998;18:620–2. [PubMed] [Google Scholar]
- 22.Qin L, Zhang G, Hung WY, Shi Y, Leung K, Yeung HY, et al. Phytoestrogen rich herb formula “XLGB” prevent ovx-induced deterioration of musculoskeletal tissues. J Bone Miner Metab. 2005;23(Suppl):55–61. doi: 10.1007/BF03026324. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Lai WP, Wong MS. Effects of Fracutus Ligustri lucidi extract on bone turn over and calcium balsnce in ovariectomized rats. Biot Pharm Bull. 2006;29:291–6. doi: 10.1248/bpb.29.291. [DOI] [PubMed] [Google Scholar]
- 24.Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY, Yao XS, et al. The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid Based Complement Alternat Med. 2005;2:353–61. doi: 10.1093/ecam/neh101. [DOI] [PMC free article] [PubMed] [Google Scholar]


