Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver injury across the world. It is also strongly related to other pathological conditions, including obesity, diabetes, cardiovascular diseases, and symptoms of metabolic syndrome. Pathogenesis of NAFLD remains not fully characterized but is generally attributed to the occurrence of insulin resistance, lipid metabolism dysfunction,0 oxidative stress, inflammation, and necro-apoptosis. Every potential therapeutic strategy should target one or some of these pathological events in the liver. Over the past decades, application of herbal treatment for NAFLD has received increasing attention due to its wide availability, low side effects, and proven therapeutic mechanisms and benefits. In recent years, some monomers and certain functional mixtures of herbs have been extensively examined for their potential uses in NAFLD treatment. In the present review, we selected several herbal derivatives under intense basic and/or clinical investigations by carrying out a PubMed search of English language articles relevant to herbal derivatives and NAFLD, such as polysaccharide portion of wolfberry, garlic-derived monomers, red grape–derived resveratrol, and milk thistle–derived substances. They have been shown to target the pathological events during NAFLD initiation and progression both in pre-clinical studies and clinical trials. Although more detailed mechanistic researches and long-term clinical evaluations are needed for their future applications, they offer unanticipated and great health benefits without obvious adverse effects in NAFLD therapy.
Keywords: Garlic-derived monomers, Herbal treatment, Milk thistle–derived substances, Non-alcoholic fatty liver disease, Pathogenesis, Resveratrol, Wolfberry
INTRODUCTION
Definition
As one of the metabolic disorders that induce chronic liver diseases, non-alcoholic fatty liver disease (NAFLD) currently attracts more attention than in the past decades. Although it is considered as a relatively benign form of chronic liver injury, about 20% of NAFLD patients could progress to cirrhosis and liver cancer, if not successfully retarded or reversed. Unlike alcoholic fatty liver disease (AFLD), NAFLD is not induced by the abuse or over-consumption of alcohol (below 30 g alcohol/day for men and 20 g alcohol/day for women).[1] NAFLD is classified into four types according to the severity: Type 1 NAFLD is defined by steatosis with no inflammation or fibrosis; Type 2 NAFLD is steatosis with non-specific lobular inflammation but absence of fibrosis or hepatocyte ballooning; Type 3 NAFLD is steatosis with inflammation and fibrosis of variable levels [non-alcoholic steatohepatitis (NASH)]; Type 4 NAFLD is steatosis with inflammation, hepatocyte ballooning, and fibrosis or Mallory-Denk bodies (NASH).[2,3,4]
Epidemiology
NAFLD is one of the leading causes of chronic liver disease in both developed and developing countries. In a study of adult NAFLD prevalence based on histological aspects, steatosis was present in ~70% of obese individuals and ~ 35% of lean individuals. NASH was found in ~18.5% of obese people and ~2.7% of lean people. The differences in these two sets of population are significant.[5] Several population-based studies found that the prevalence of fatty liver is 13-22% in lean non-alcoholic subjects by using ultrasound imaging as the diagnostic method.[6,7,8] In the Western world, NAFLD affects 20-35% of adult people and 5-17% of children.[9,10] Due to the content of fat in modern diet and change in the people's lifestyle, NAFLD has become one of the leading causes of chronic liver diseases in China (~15%)[11] and Hong Kong (~27.3%).[12]
It is well known that NAFLD shows gender and age differences in normal population. According to a recent survey in Japan, concerning cirrhotic NASH patients, the prevalence in women (~57%) was higher than in men (~43%).[13] However, another survey that investigated the prevalence of hepatocellular carcinoma in already established NASH patients found that men exhibited higher prevalence (~62%) when compared with women (~38%).[14] The causes of gender differentiation of NAFLD may be attributed to estrogenic endocrinology, fat distribution, and lifestyle differences (e.g., smoking population ratio is higher in men than in women). However, the details of the reason and mechanism of gender differentiation remain one of the unresolved questions in the field of hepatology.
Prevalence of NAFLD is also influenced by ethnicity. African-Americans show significantly less hepatic steatosis, although the prevalence of obesity and diabetes is relatively high.[3] Hispanic-Americans exhibit high prevalence of steatosis, while Asian-Americans only show an intermediate level of prevalence of steatosis.[9,15] Another study found that liver dysfunction in Japanese with severe obesity [body mass index >35 kg/m2] tends to be more severe than that in non-Japanese patients.[16] Differences of NAFLD prevalence are associated with different visceral adiposity and metabolic responses.
Pathological mechanisms
During the past two decades, although some cellular processes of NAFLD have been extensively studied, the exact pathogenesis of NAFLD remains largely unknown. Few years ago, a pathological model of NAFLD named “multi-hit” model described the pathological mechanisms of NAFLD initiation and progression.[17] In this model, the first hit in NAFLD is the dysfunction of fatty acid metabolism which leads to insulin resistance and altered signaling transductions, thereby making the hepatocytes vulnerable to the following multiple hits. Mitochondrial fatty acid oxidation which induces oxidative stress, expression of pro-inflammatory cytokines and chemokines that is nuclear factor kappa B (NF-κB) dependent, expression of pro-fibrogenic factors, and adipocytokines are considered as the possible causative factors that contribute to cell inflammation and necrosis/apoptosis with subsequent activation of fibrogenic cascade.[17,18] Detailed pathogenic events in the initiation and development of NAFLD are summarized in Figure 1. Very recently, the “multi-hit” model has been amended to a “multiple parallel hit” model in which steatosis and NASH are discrete entities rather than two points of the NAFLD spectrum, not only from a histological but also from a pathophysiological standpoint.[19,20]
Figure 1.
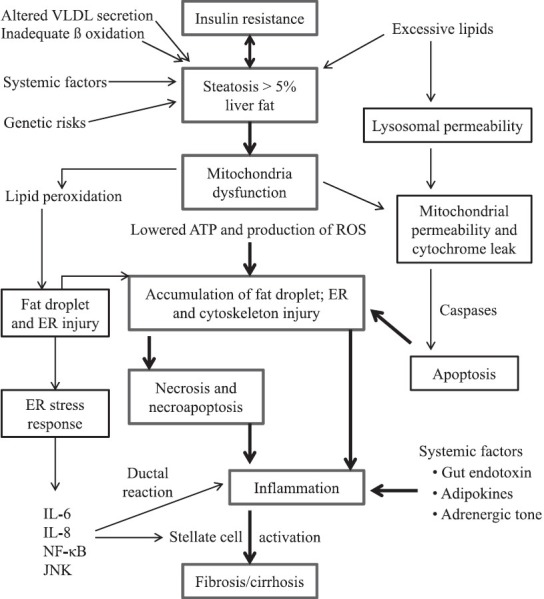
Illustration of molecular events involved in the pathogenesis of non-alcoholic fatty liver disease
Current therapies
To date, there are two major categories of NAFLD therapies: (1) lifestyle interventions (including weight reduction, dietary modification, and physical exercise) and (2) pharmaceutical therapies. Weight reduction and dietary modification are the most recognized strategies for the control of NAFLD. In recent years, emerging evidence suggests that long-term and moderate-intensity exercise is a very effective and safe way to retard the progression of not only NAFLD but also some other kinds of metabolic diseases, including obesity and hyperlipidemia. However, the underlying mechanisms of lifestyle interventions in attenuating the progression of NAFLD still remains largely unknown, although a large number of clinical studies have confirmed their therapeutic potentials. For pharmaceutical therapies, a wide range of drugs, including antioxidants, insulin sensitizers, lipid lowering agents, and renin–angiotensin system blockers, have been applied in clinical trials. However, pharmaceutical therapies for NAFLD exhibited few positive outcomes in clinical trials, although animal and cellular studies contributed several promising advancements.[21,22]
In recent years, the beneficial effects of herbal derivatives on NAFLD progression have received increasing attention since these substances hold several advantages: (1) widely available around the world, particularly in Chinese societies; (2) natural products with low or minimal side effects; and (3) some of them have been extensively studied in modern basic and clinical studies.[23] In the current review, we mainly focus on the recent advances of herbal derivatives in the amelioration of NAFLD in laboratory studies and, if applicable, clinical trials.
RECENT FINDINGS FROM HERBAL TREATMENT OF NAFLD
Lycii fructus (枸杞子 Gǒu Qǐ Zǐ, wolfberry, goji berry)
Wolfberry is the fruit of plant Lycium barbarum of the family Solanaceae. It is a famous drug or supplement in traditional Chinese medicine in which it holds beneficial properties on both liver and eyes.[24] In terms of substances, the polysaccharide portion of wolfberry (often referred as LBP) represents the most important part. Modern studies indicate that LBP possesses a wide range of biological actions, including antioxidant effect, immunoregulation, neuroprotection, control of glucose metabolism, and anti-tumor activities. Clinical trials also found that intake of LBP juice increases the number of lymphocytes and levels of interleukin-2 and immunoglobulin G in human beings. LBP also was found to increase the serum levels of antioxidants while decreasing the lipid peroxide formation.[25,26] In the liver, early reports demonstrated that treatment with LBP inhibited proliferation and induced apoptosis in hepatoma cells, leading to possible anti-tumor application of LBP.[27,28] Another study exhibited the protective effects of LBP on high-fat diet induced liver oxidative stress injury through the restoration of antioxidant enzyme activities and the reduction of oxidative stress products [e.g. malondialdehyde (MDA)].[29] In an alcohol-induced liver injury rat model, LBP co-treatment with the administration of ethanol significantly ameliorated the liver injury. Underlying mechanism involved alleviation of oxidative stress and reduction of lipid accumulation in the liver.[30] We also found that in a carbon tetrachloride (CCl4)-induced acute mouse liver injury model, pre-treatment with 1 mg/kg and 10 mg/kg LBP before the intoxication of CCl4 obviously improved hepatic histology, reduced oxidative stress, alleviated hepatic inflammation/chemoattraction, and promoted liver regeneration partly through an NF-κB–dependent pathway.[31] Due to its beneficial properties in the amelioration of oxidative stress and inflammation, it is reasonable to speculate its role in NAFLD progression. To test this hypothesis, we applied our newly established voluntary NAFLD rat in which the energy percentage from the fat is only 30% to co-treat with LBP.[32] Eight-week induction of NAFLD in the rat introduced typical clinical symptoms of fatty liver disease, such as fat deposition, fibrosis, increased serum aminotransferase level, oxidative stress, inflammation, and apoptosis. Co-treatment with LBP (1 mg/kg, daily oral feeding) effectively improved hepatic histology, reduced fat accumulation, fibrosis, oxidative stress, inflammation, and apoptosis partially through modulating the transcriptional factors NF-κB and activator protein-1 (AP-1). In addition, long-term uptake of LBP did not show obvious adverse effect on healthy rat liver (Xiao et al., submitted manuscript). Therefore, we concluded that LBP holds great potential in the treatment of NAFLD with low side effects.
Garlic (大蒜 Dà Suàn)
Garlic, or Allium sativum, is a species in the onion genus Allium. It has a very long history (over 6,000 years) as both culinary and medicinal uses in Asia, Egypt, and the Mediterranean regions. In terms of liver diseases, a recent paper reported the protective effect of oral consumption of whole garlic attenuates high-fat diet caused abnormal lipid profile through AMP-activated protein kinase (AMPK) pathway.[33] In a fructose-fed rat model, administration of raw garlic homogenate along with the diet induction improves insulin resistance, oxidative stress, and lipid metabolism.[34] Aged black garlic, when given along with chronic ethanol administration in Sprague-Dawley rat, effectively reduced hepatic oxidative stress through the enhancement of antioxidant enzymes and the decrease of cytochrome P450 system activity.[35] Diallyl trisulfide from garlic has also been found to play protective roles in CCl4-induced acute liver damage.[36] This result is consistent with our findings that aged garlic derived S-allylmercaptocysteine (SAMC) alleviated CCl4-induced hepatic necro-inflammation and oxidative stress. SAMC also promoted liver regenerative ability after acute injury.[37] Thus, based on these observations, we evaluated the protective property of SAMC against NAFLD in our clinically relevant high-fat diet model. Similar to the results of LBP, our high-fat diet recipe induced evident NAFLD symptoms in rats after an 8-week feeding. SAMC co-treatment also potently attenuated NAFLD features including steatosis, fibrosis, oxidative stress, and inflammation. These beneficial effects were partially mediated by kinase- and transcriptional factor-dependent pathways.[38] In addition, we demonstrated that SAMC could also inhibit apoptosis while promoting hepatic macroautophagy, contributing to further protection against NAFLD-induced chronic liver injury (Xiao et al., article in press).
Green tea (綠茶 Lǜ Chá)
Green tea is one of the best documented plants that have been used in the prevention of liver diseases. It has also received much attention in the last 20 years. Green tea is a kind of tea made solely from the leaves of plant Camellia sinensis. It originated from China and then spread to other Asian countries, such as Japan, Korea, and Vietnam. Recently, it has also spread to Western countries where black tea is traditionally consumed. Studies using extracts from green tea found the beneficial or even therapeutic effects of green tea on liver diseases. Chen et al., used a pure form of epigallocatechin-3-gallate (EGCG), the major polyphenol of green tea, in mice treated with CCl4. They found that EGCG showed a dose-dependent ameliorative effect on CCl4-induced liver injury, oxidative stress, and inflammation at both histological and biochemical levels.[39] Another recent study demonstrated that EGCG potently inhibited the entry of hepatitis C virus (HCV) in hepatoma cell lines and primary human hepatocytes through blocking both extracellular virions and cell-to-cell spread.[40] In alcohol-induced rat liver injury model, co-treatment of whole green tea extracts with ethanol administration effectively attenuated hepatic oxidative stress through cytochrome P450 2E1 (CYP2E1) and reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase systems.[41] Moreover, in obese mice, typical NASH features, including lipid accumulation, oxidative stress, nitrative stress, and inflammatory response, were attenuated by green tea extract administration.[42] Our recent study suggested that 85% pure extract of (−) EGCG reduced the severity of liver injury in an experimental model of NAFLD associated with lower concentration of pro-fibrogenic, oxidative stress, and pro-inflammatory mediators, partly through modulating the activities of transforming growth factor/SMAD (TGF/SMAD), phosphoinositide 3-kinase/Akt/forkhead box protein O1 (PI3K/Akt/FoxO1) and NF-κB pathways. Therefore, green tea polyphenols and EGCG are a useful supplement in the prevention of NAFLD (Xiao et al., submitted manuscript).
Resveratrol
Resveratrol (RSV; 3, 5 4′-trihydroxystilbene) is a phytoalexin extracted from red grapes. It is one of the most well-documented and accepted herbal derivatives in the world due to its strong capacity against oxidation and inflammation.[43] In recent years, many groups have found the very promising phytochemical properties of RSV in the treatment of NAFLD. Co-treatment with RSV promoted the phosphorylation of AMPK, leading to the suppression of lipogenic genes (SREBP-1c and FAS), after NAFLD was induced with high-fat diet.[44] This effect was further supported by the studies that followed in which treatment of RSV attenuated oxidative stress and inflammation in vivo.[45] The effects of RSV on dysregulated lipid metabolism during NAFLD were found to be through sirtuin 1 (SIRT1) pathway[46,47] and the up-regulation of hepatic low-density lipoprotein receptor.[48] Regarding its potent effects on oxidative stress and inflammation, as well as its wide availability around the world, RSV is a significant candidate for the daily prevention of fatty liver diseases.
Milk thistles
Both silybin and silymarin are derivatives of plant milk thistle (Silybum marianum), a flowering plant of the daisy family. In the past 10 years, more than 10,000 papers have been published on the antioxidative, chemopreventive, and hepatoprotective effects of these substances. In the liver cells, the common beneficial effects of silybin and silymarin include antioxidative effects, direct/indirect effects on inflammation and fibrosis, as well as modulation of metabolic pathways. For example, in the presence of oxidative stress and nitrosative stress, silybin inhibits the formation of free radicals and nitric oxide, phosphorylates adenosine diphosphate to increase the content of adenosine triphosphate, decreases the content of MDA, and recovers the basal expression levels of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx).[49,50] The anti-inflammatory effects of silybin and silymarin are from their interference with NF-κB–controlled transduction cascade. Consistent with the antioxidant activity, silybin and silymarin inhibit NF-κB activation and translocation through suppression of upstream phosphorylation and degradation of IκBα.[51] A recent basic study exhibited that crude extract of Silybum marianum played anti-apoptotic and anti-inflammatory roles in the treatment of experimental steatohepatitis, when rats were fed with methionine and choline deficient (MCD) diet for 8 weeks. The beneficial effects of S. marianum were partly found to be through the mitogen-activated protein kinase (MAPK) pathway.[52] Another interesting clinical study found that when compared with the therapeutic effects on chronic hepatitis C patients, the effects of silymarin were better on NAFLD patients due to their higher flavonolignan plasma concentrations and more extensive enterohepatic cycling.[53]
Other derivatives and decoctions
Unlike the modern Western medicine, traditional Chinese medicine prefers herbology, meaning the combination of different medical herbs into one recipe or prescription.[54] With the help from advanced technologies in chemistry, pharmacology, and experimental biology, the effective monomers of many Chinese medicine formulae have been identified. One of the most attractive monomers is berberine, an alkaloid isolated from the Chinese herb Coptidis rhizoma (黃連 Huáng Lián, the root of Coptis chinensis). In laboratory studies, berberine is considered to possess an anti-steatotic effect due to its ability to re-activate AMPK[55] and to up-regulate low-density lipoprotein receptor expression through the extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) pathways.[56,57] In addition, berberine is shown to reduce hepatic inflammatory responses. In db/db mice, addition of berberine down-regulated the expression of pro-inflammatory cytokines [e.g. tumor necrosis factor (TNF)-α and interleukin (IL)-6] through the modulation of NF-κB signaling pathway.[55,58] However, to date, there is no direct study examining the effects of berberine treatment on NAFLD.
In the therapy of Chinese medicine, herbal prescriptions of NAFLD are formulated from several kinds of herbs, based on the symptoms of patients. So, it is relatively difficult to figure out which component from the formula is the main effective monomer for the therapy of NAFLD. Actually, under most circumstances, the therapeutic properties of herbal formulae largely depend on the additive or synergistic effects of these monomers. In recent years, the functions of several Chinese medicine formulae in NAFLD treatment have been investigated in both pre-clinical and clinical studies. This aspect of information has been well reviewed by Dong et al.[59]
PERSPECTIVES AND FUTURE DIRECTIONS
Since the pathogenesis of NAFLD is attributed to multiple and parallel levels of pathological events, from upstream insulin resistance to downstream apoptosis/autophagy, effective therapies for NAFLD should target one or several of these events. To date, LBP, garlic derivatives, green tea, resveratrol, and milk thistle derivatives are the most promising reagents that can target most of the pathological changes during NAFLD development. Some of them even have clinical trial data to support their possible therapeutic function for NAFLD (e.g. LBP and milk thistle). The detailed experimental achievements of these derivatives are summarized in Table 1. The major problems in the widespread use of these derivatives are the shortage of detailed mechanistic pre-clinical studies and clinical trials evaluating their long-term side effects. Indeed, isolation of more potent anti-NAFLD monomers or Chinese medicine formulae is also necessary to effectively use these herbal agents in the daily prevention and therapy of chronic liver diseases and other related metabolic syndromes.
Table 1.
Summary of the beneficial properties of common herbal derivatives against NAFLD-induced hepatic injury from recent studies

REFERENCES
- 1.Alkhouri N, Lopez R, Berk M, Feldstein AE. Serum retinol-binding protein 4 levels in patients with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2009;43:985–9. doi: 10.1097/MCG.0b013e3181a0998d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–31. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol. 2002;97:1496–500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: An autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–10. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–7. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 7.Oshibuchi M, Nishi F, Sato M, Ohtake H, Okuda K. Frequency of abnormalities detected by abdominal ultrasound among Japanese adults. J Gastroenterol Hepatol. 1991;6:165–8. doi: 10.1111/j.1440-1746.1991.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Fan JG, Shao Y, Zeng MD, Wang JR, Luo GH, et al. Prevalence of nonalcoholic fatty liver among administrative officers in Shanghai: An epidemiological survey. World J Gastroenterol. 2003;9:1106–10. doi: 10.3748/wjg.v9.i5.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 11.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204–10. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–15. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 13.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–54. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 15.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–9. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 16.Kakizaki S, Takizawa D, Yamazaki Y, Nakajima Y, Ichikawa T, Sato K, et al. Nonalcoholic fatty liver disease in Japanese patients with severe obesity who received laparoscopic Roux-en-Y gastric bypass surgery (LRYGB) in comparison to non-Japanese patients. J Gastroenterol. 2008;43:86–92. doi: 10.1007/s00535-007-2130-0. [DOI] [PubMed] [Google Scholar]
- 17.Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: The pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299–314. doi: 10.2174/156652409787847191. [DOI] [PubMed] [Google Scholar]
- 18.Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539–50. doi: 10.3748/wjg.v13.i34.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polyzos SA, Kountouras J, Zavos C, Deretzi G. Nonalcoholic fatty liver disease: Multimodal treatment options for a pathogenetically multiple-hit disease. J Clin Gastroenterol. 2012;46:272–84. doi: 10.1097/MCG.0b013e31824587e0. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz Y. Review article: Is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815–23. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]
- 21.Gossard AA, Lindor KD. Current therapies for nonalcoholic fatty liver disease. Drugs Today (Barc) 2011;47:915–22. doi: 10.1358/dot.2011.47.12.1688530. [DOI] [PubMed] [Google Scholar]
- 22.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 2012;57:157–66. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Firenzuoli F, Gori L. Herbal medicine today: Clinical and research issues. Evid Based Complement Alternat Med. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang RC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–52. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Amagase H, Sun B, Nance DM. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food. 2009;12:1159–65. doi: 10.1089/jmf.2008.0300. [DOI] [PubMed] [Google Scholar]
- 27.Chao JC, Chiang SW, Wang CC, Tsai YH, Wu MS. Hot water-extracted Lycium barbarum and Rehmannia glutinosa inhibit proliferation and induce apoptosis of hepatocellular carcinoma cells. World J Gastroenterol. 2006;12:4478–84. doi: 10.3748/wjg.v12.i28.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Chen H, Huang J, Li Z, Zhu C, Zhang S. Effect of Lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005;76:2115–24. doi: 10.1016/j.lfs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu HT, He XJ, Hong YK, Ma T, Xu YP, Li HH. Chemical characterization of Lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int J Biol Macromol. 2010;46:540–3. doi: 10.1016/j.ijbiomac.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Cheng D, Kong H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011;16:2542–50. doi: 10.3390/molecules16032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao J, Liong EC, Ching YP, Chang RC, So KF, Fung ML, et al. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139:462–70. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 32.Tipoe GL, Ho CT, Liong EC, Leung TM, Lau TY, Fung ML, et al. Voluntary oral feeding of rats not requiring a very high fat diet is a clinically relevant animal model of non-alcoholic fatty liver disease (NAFLD) Histol Histopathol. 2009;24:1161–9. doi: 10.14670/HH-24.1161. [DOI] [PubMed] [Google Scholar]
- 33.Lee MS, Kim IH, Kim CT, Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141:1947–53. doi: 10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- 34.Padiya R, Khatua TN, Bagul PK, Kuncha M, Banerjee SK. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr Metab (Lond) 2011;8:53. doi: 10.1186/1743-7075-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MH, Kim MJ, Lee JH, Han JI, Kim JH, Sok DE, et al. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J Med Food. 2011;14:732–8. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 36.Hosono-Fukao T, Hosono T, Seki T, Ariga T. Diallyl trisulfide protects rats from carbon tetrachloride-induced liver injury. J Nutr. 2009;139:2252–6. doi: 10.3945/jn.109.109611. [DOI] [PubMed] [Google Scholar]
- 37.Xiao J, Liong EC, Ling MT, Ching YP, Fung ML, Tipoe GL. S-allylmercaptocysteine reduces carbon tetrachloride-induced hepatic oxidative stress and necroinflammation via nuclear factor kappa B-dependent pathways in mice. Eur J Nutr. 2012;51:323–33. doi: 10.1007/s00394-011-0217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao J, Ching YP, Liong EC, Nanji AA, Fung ML, Tipoe GL. Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur J Nutr. 2013;52:179–91. doi: 10.1007/s00394-012-0301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JH, Tipoe GL, Liong EC, So HS, Leung KM, Tom WM, et al. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am J Clin Nutr. 2004;80:742–51. doi: 10.1093/ajcn/80.3.742. [DOI] [PubMed] [Google Scholar]
- 40.Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54:1947–55. doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- 41.Chen KH, Li PC, Lin WH, Chien CT, Low BH. Depression by a green tea extract of alcohol-induced oxidative stress and lipogenesis in rat liver. Biosci Biotechnol Biochem. 2011;75:1668–76. doi: 10.1271/bbb.110163. [DOI] [PubMed] [Google Scholar]
- 42.Chung MY, Park HJ, Manautou JE, Koo SI, Bruno RS. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J Nutr Biochem. 2012;23:361–7. doi: 10.1016/j.jnutbio.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 44.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 45.Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. doi: 10.1186/1471-230X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colak Y, Ozturk O, Senates E, Tuncer I, Yorulmaz E, Adali G, et al. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17:HY5–9. doi: 10.12659/MSM.881749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang GL, Fu YC, Xu WC, Feng YQ, Fang SR, Zhou XH. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun. 2009;380:644–9. doi: 10.1016/j.bbrc.2009.01.163. [DOI] [PubMed] [Google Scholar]
- 48.Xin P, Han H, Gao D, Cui W, Yang X, Ying C, et al. Alleviative effects of resveratrol on nonalcoholic fatty liver disease are associated with up regulation of hepatic low density lipoprotein receptor and scavenger receptor class B type I gene expressions in rats. Food Chem Toxicol. 2013;52:12–8. doi: 10.1016/j.fct.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Täger M, Dietzmann J, Thiel U, Hinrich Neumann K, Ansorge S. Restoration of the cellular thiol status of peritoneal macrophages from CAPD patients by the flavonoids silibinin and silymarin. Free Radic Res. 2001;34:137–51. doi: 10.1080/10715760100300131. [DOI] [PubMed] [Google Scholar]
- 50.van Pelt JF, Verslype C, Crabbé T, Zaman Z, Fevery J. Primary human hepatocytes are protected against prolonged and repeated exposure to ethanol by silibinin-dihemisuccinate. Alcohol Alcohol. 2003;38:411–4. doi: 10.1093/alcalc/agg099. [DOI] [PubMed] [Google Scholar]
- 51.Gharagozloo M, Velardi E, Bruscoli S, Agostini M, Di Sante M, Donato V, et al. Silymarin suppress CD4+ T cell activation and proliferation: Effects on NF-kappaB activity and IL-2 production. Pharmacol Res. 2010;61:405–9. doi: 10.1016/j.phrs.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Aghazadeh S, Amini R, Yazdanparast R, Ghaffari SH. Anti-apoptotic and anti-inflammatory effects of Silybum marianum in treatment of experimental steatohepatitis. Exp Toxicol Pathol. 2011;63:569–74. doi: 10.1016/j.etp.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Schrieber SJ, Hawke RL, Wen Z, Smith PC, Reddy KR, Wahed AS, et al. Differences in the disposition of silymarin between patients with nonalcoholic fatty liver disease and chronic hepatitis C. Drug Metab Dispos. 2011;39:2182–90. doi: 10.1124/dmd.111.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White KP. A crash course in Chinese herbology for the psychopharmocological prescriber. Exp Clin Psychopharmacol. 2009;17:384–95. doi: 10.1037/a0016881. [DOI] [PubMed] [Google Scholar]
- 55.Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–9. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- 56.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Lim HJ, Park JH, Lee KS, Jang Y, Park HY. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem Biophys Res Commun. 2007;362:853–7. doi: 10.1016/j.bbrc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 58.Hsiang CY, Wu SL, Cheng SE, Ho TY. Acetaldehyde-induced interleukin-1beta and tumor necrosis factor-alpha production is inhibited by berberine through nuclear factor-kappaB signaling pathway in HepG2 cells. J Biomed Sci. 2005;12:791–801. doi: 10.1007/s11373-005-9003-4. [DOI] [PubMed] [Google Scholar]
- 59.Dong H, Lu FE, Zhao L. Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin J Integr Med. 2012;18:152–60. doi: 10.1007/s11655-012-0993-2. [DOI] [PubMed] [Google Scholar]


