Abstract
Excessive oxidative stress induced by reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive metabolites of carcinogens alters cellular homeostasis, leading to genetic/epigenetic changes, genomic instability, neoplastic transformation, and cancer initiation/progression. As a protective mechanism against oxidative stress, antioxidant/detoxifying enzymes reduce these reactive species and protect normal cells from endo-/exogenous oxidative damage. The transcription factor nuclear factor-erythroid 2 p45 (NF-E2)-related factor 2 (Nrf2), a master regulator of the antioxidative stress response, plays a critical role in the expression of many cytoprotective enzymes, including NAD(P)H:quinine oxidoreductase (NQO1), heme oxygenase-1 (HO-1), UDP-glucuronosyltransferase (UGT), and glutathione S-transferase (GST). Recent studies demonstrated that many dietary phytochemicals derived from various vegetables, fruits, spices, and herbal medicines induce Nrf2-mediated antioxidant/detoxifying enzymes, restore aberrant epigenetic alterations, and eliminate cancer stem cells (CSCs). The Nrf2-mediated antioxidant response prevents many age-related diseases, including cancer. Owing to their fundamental contribution to carcinogenesis, epigenetic modifications and CSCs are novel targets of dietary phytochemicals and traditional Chinese herbal medicine (TCHM). In this review, we summarize cancer chemoprevention by dietary phytochemicals, including TCHM, which have great potential as a safer and more effective strategy for preventing cancer.
Keywords: Cancer chemoprevention, Cancer stem cells, Epigenetics, Nrf2, Phytochemicals, Traditional Chinese herbal medicine
Oxidative stress and the antioxidant defense system
Hydroxyl peroxide, superoxide, and hydroxyl radicals, generally known as reactive oxygen species (ROS), are the metabolites of oxygen in normal cells, whereas nitrite, nitrate, and peroxynitrite, referred to as reactive nitrogen species (RNS), are the byproducts of nitric oxide (NO) metabolism.[1] Mitochondria-catalyzed electron transport reactions, UV light irradiation, X-rays, gamma rays, chronic inflammation, lipid peroxidation, and environmental pollutants are the common stimuli for ROS/RNS induction.[2,3] Maintaining a reasonable level of ROS/RNS in the body is essential for normal physiological processes, including cellular senescence and programmed cell death, which are beneficial anti-tumorigenic functions.[4,5] However, high levels of ROS/RNS generate oxidative stress, a critical trigger of genomic instability, defects in DNA damage repair, transformation of normal cells to premalignant cells, enhanced proliferation and survival of malignant cells, and subsequent cancer development.[6,7] Oxidative stress also has a significant association with many other chronic diseases such as neurodegenerative diseases [Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS)], cardiovascular disease, diabetes, and inflammatory diseases.[5,8,9]
Non-enzymatic and enzymatic antioxidant regulation
Oxidative stress is counteracted by enzymes that inhibit the generation of ROS or by direct scavenging of free radicals by organic molecules.[5] Vitamin C (l-ascorbate), vitamin E, carotenoids, selenium, flavonoids, and thiol antioxidants such as glutathione, thioredoxin, and lipoic acid are the non-enzymatic antioxidants.[4,10,11] However, at high concentrations, some of these molecules, such as vitamin C and vitamin E, induce oxidative stress, thereby increasing ROS levels.[12]
Enzymatic antioxidants include superoxide dismutases (SODs), catalase, and glutathione peroxidase. Three isoforms of SODs (SOD1–SOD3) are the major antioxidant defense against (O2−▪), and all three isoforms require catalytic metals (Cu or Mn) for their activation.[13,14] The enzyme catalase degrades and reduces hydrogen peroxide.[15] Glutathione peroxidases include glutathione S-transferases (GSTs) and glutathione peroxidases (GPx), which are important for protecting the living organisms from free radical-induced oxidative damage.[16,17]
Phase I and phase II enzymes are closely associated with xenobiotic metabolism and are also involved in antioxidant activity. The phase I drug metabolic enzymes, which belong to the larger cytochrome P450 enzyme family, catalyze reactions through oxidation, reduction, hydrolysis, cyclization, and decyclization,. By contrast, phase II conjugating enzymes play crucial cytoprotective roles against carcinogens and ROS by catalyzing conjugation reactions involving glucuronic acid, sulfation, and glutathione to inactivate or detoxify harmful substrates by increasing their solubility or facilitating their excretion.[18,19,20] Most polyphenolic antioxidants exert their activity through phase II enzymes.[21]
Nrf2-related antioxidant regulation
When the cellular redox status of cells is altered by ROS/RNS, some ROS/RNS-sensitive regulatory transcription factors, such as nuclear factor-erythroid 2 p45 (NF-E2)-related factor 2 (Nrf2), nuclear factor-kappaB (NF-κB), and hypoxia-inducible factor-1alpha (HIF-1alpha), are modified with subsequent activation. Many phase II enzymes as well as some detoxifying genes, such as glutathione S-transferase (GST), peroxiredoxin1 (Prxl), γ-glutamate cysteine ligase (γ-GCLC and γ-GCLM), heme oxygenase-1 (HO-1), and NAD(P)H:quinine oxidoreductase (NQO1), are inducible and activated by Nrf2, a key orchestrator of antioxidant signaling.[1,20,22,23]
Nrf2 is a basic leucine zipper-containing transcription factor that activates phase II/detoxifying and many other genes through the cis antioxidant response element (ARE), which contains a conserved sequence (5′-A/G TGA C/T NNNGC A/G-3′, where N can be any nucleic acid).[24,25,26,27,28,29,30] Keap1 (Kelch-like ECH-associating protein 1), an interacting protein of Nrf2, serves as an adaptor that bridges Nrf2 and Cul3 for protein ubiquitination.[31] The sulfhydryl residues in Keap1 are sensitive to electrophiles, and ROS cause cellular redox status changes, making Keap1 a primary redox sensor,[32,33] although Nrf2 itself may also be a redox sensor that regulates its subcellular localization through its MESTA motif.[34] [Figure 1] shows the schematic structure of Nrf2 and Keap1 and the mechanism of Nrf2 activation.
Figure 1.
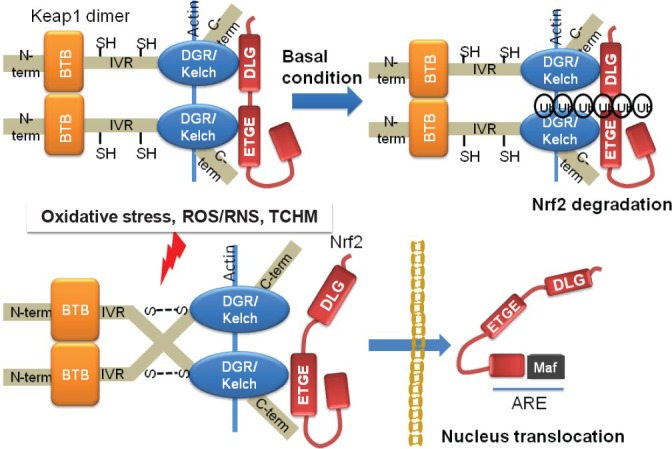
Hypothetical mechanism of Nrf2 activation.
Keap1 is dimerized through the BTB domain and is anchored to the actin cytoskeleton via the DGR/Kelch region. Nrf2 binds to the DGR/Kelch region of the Keap1 dimer via a high-affinity ETGE (hinge) motif and a low-affinity DLG (latch) bAU2 motif. The two-site binding exposes the Ub-acceptor site(s) in Nrf2. Under normal conditions, ubiquitinated Nrf2 is degraded by the proteasome, which maintains the equilibrium between synthesis and degradation of the Nrf2 protein in the cell. Once Keap1 is exposed to oxidants or electrophilic compounds, cysteine thiol groups in the IVR region of Keap1 interact with oxidative stress, inducing the formation of disulfide bonds. Disulfide bond formation results in a conformational change that renders Keap1 unable to bind to Nrf2, which then translocates to the nucleus. In this stage, the Ub-acceptor site is not easily accessible. The ubiquitination and proteasomal degradation of Nrf2 are impeded. The released Nrf2, in heterodimeric combination with other transcription factors such as small Maf, binds to the ARE regulatory region of phase II genes and enhances their transcription
Nrf2-related inflammatory pathway regulation
Up to 20% of human cancer is triggered by chronic inflammation, and NF-κB is a key orchestrator of innate immune/inflammatory regulation.[35] With exposure to various stimuli, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, H2O2, lipopolysaccharide (LPS), or microbial infection, IκB proteins are subject to proteasome-mediated degradation as a consequence of phosphorylation at serine and threonine by IκB kinases (IKKs) within the IKK complex.[36,37] The degradation of IκBs leads to nuclear translocation of NF-κB to activate downstream target genes including different inflammatory cytokines and chemokines, adhesion molecules, enzymes [such as cyclooxygenase 2 (COX-2) and NO synthase], and many other stress response genes.[37,38,39,40] NF-κB activation has been observed in many cancer types. For example, suppression of the NF-κB pathway by deletion of IKKb, an upstream regulator of NF-κB, leads to inhibition of cancer cell proliferation and a dramatic decrease in tumor incidence in a colitis-associated cancer model.[41,42,43,44]
Potential interfaces and significant crosstalk are associated with Nrf2 and NF-κB signaling. Compared with wild-type mice, we and others have observed that in Nrf2-KO mice, inflammatory-related signals such as TNF, IL-1, COX-2, and iNOS attenuate expression in primary peritoneal macrophages upon stimulation with LPS after pretreatment with sulforaphane (SFN).[45,46]
Chemopreventive effects of phytochemical compounds
Phytochemicals possess potential anti-cancer effects
Dissecting the chemopreventive effects of dietary compounds and phytochemicals extracted from herbal medicines, particularly the mechanisms of their antioxidant activities, is an important area of research. For example, isothiocyanates, such as phenethyl isothiocyanate (PEITC) and SFN, have been purified from cruciferous vegetables, and other dietary compounds, such as curcumin and dibenzoylmethane (DBM), exhibit potential anti-cancer effects in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice and the derivative tumor cell line TRAMP C1. Curcumin or PEITC, either alone or in combination, significantly decreases the incidence of prostate tumor formation.[47] Vitamin E is a generic name for structurally related tocopherols and tocotrienols. We and others have shown that gamma-tocopherol (gamma-T) enriched mixed tocopherol activates the expression of Nrf2 and suppresses prostate intraepithelial neoplasia (PIN) and tumor development in the TRAMP prostate cancer mouse model, corresponding to inhibition of the expression of proliferating cell nuclear antigen (PCNA), COX-2, and estrogen receptor α (ERα), and the induction of apoptosis.[48,49] Compounds identified in some herbal medicines also exhibit antioxidant activities. For example, the three common ginsenosides present in ginseng, Rb1 (Rb1), ginsenoside Rg1 (Rg1), and ginsenoside 20(S)-protopanaxatriol (20S), induce Nrf2 ARE in HepG2-C8 cells stably transfected with an ARE luciferase reporter gene.[50]
Potential antioxidant responses regulated by chemopreventive compound treatment
The potential mechanisms of these phytochemicals in chemoprevention may include 1) attenuating oxidative stress by serving as direct antioxidants or inducing Nrf2, as has been shown for curcumin, vitamin E, epigallocatechin-3-gallate (EGCG), and synthetic triterpenoid CDDO-Me;[51,52,53,54,55,56] 2) anti-inflammatory activities; and 3) cell cycle and apoptosis regulation. In HT-29 human colon cancers and RAW 264.7 murine macrophages, PEITC suppressed inflammation by inhibiting pro-inflammatory mediator mediators and cytokines (iNOS, COX-2, IL-1b, IL-6, and TNF-α). PEITC also suppressed LPS-induced phosphorylation and degradation of IkBa and decreased nuclear translocation of p65.[57] DBM, for example, blocks the growth and progression of prostate cancer in TRAMP mice and arrests TRAMP-C1 cells at the G2-M phase of the cell cycle. The expression of phosphorylated retinoblastoma, c-myc, cyclin D1, cyclin A, phosphorylated Akt, phosphorylated PDK-1, and phosphorylated S6 was also significantly reduced by DBM.[58]
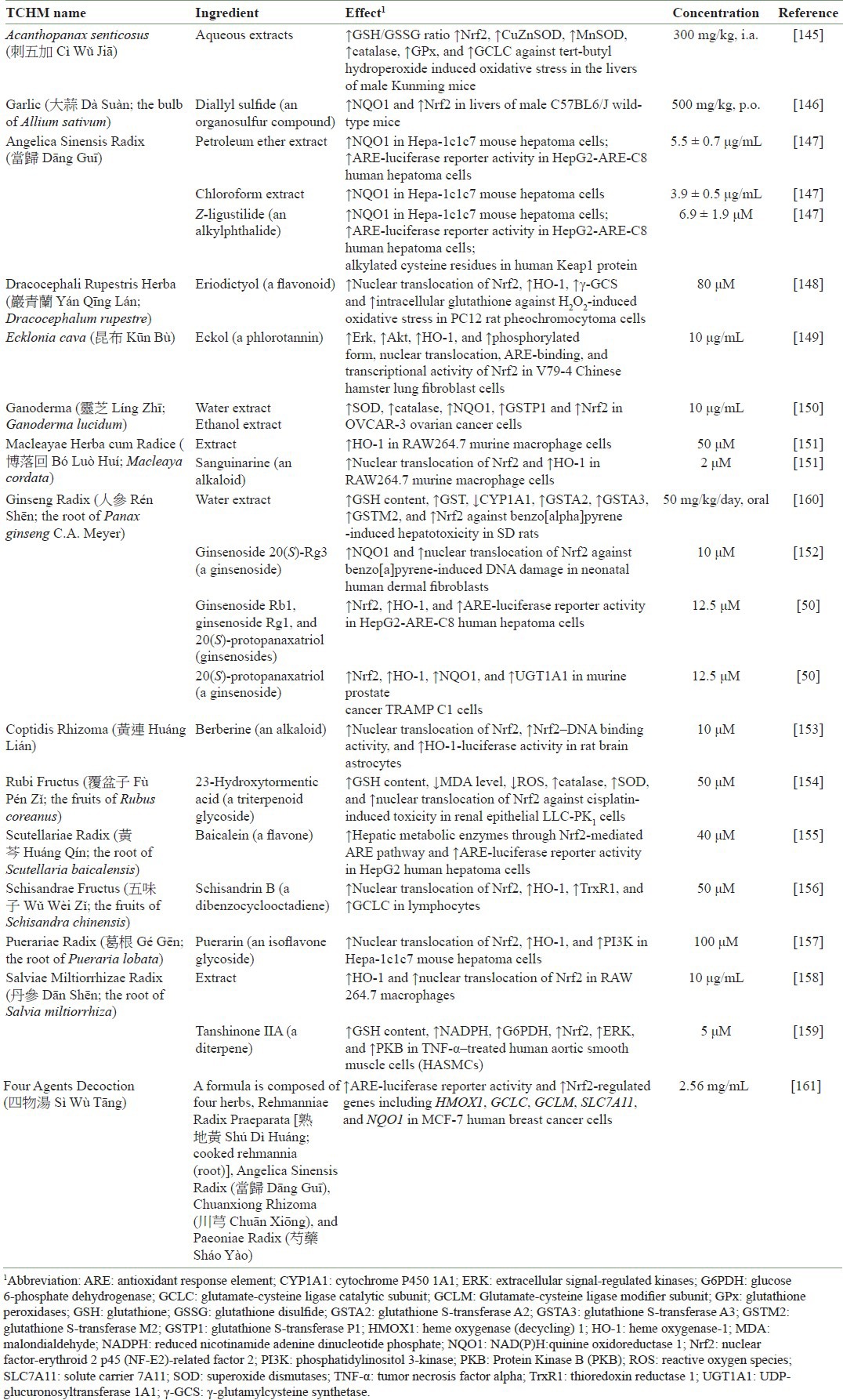
Many of those phytochemicals activate multiple signaling pathways. The detailed molecular events of Nrf2 activation by various phytochemicals remain unclear. Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERKs), c-Jun amino-terminal kinases (JNKs), and protein 38 (p38), and other kinases such as phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) may play a role in this activation.[59,60,61,62] Phytochemicals such as SFN and PEITC induce the phosphorylation of ERK1/2, JNK1/2, and c-Jun.[63] ERK and JNK have positive effects on ARE-mediated activities and Nrf2 transactivation,[5,62,64,65] while phosphorylation of Nrf2 by p38 increases Keap1/Nrf2 binding and therefore inhibits Nrf2 activity.[66] PKC directly phosphorylates Nrf2 at serine 40,[60,67,68,69] and PI3K increases Nrf2 nuclear translocation.[61,70,71,72] However, when dissecting the phosphorylation sites of Nrf2 in detail, MAPK had only a slight effect on Nrf2 translocation and activity.[73] Thus, Keap1–Nrf2 signaling regulation by MAPK may be cell-type dependent, and the indirect effect on Keap1 or cofactors such as CBP may be more important in the regulation of this antioxidant pathway. [Table 1] shows the effects of various traditional Chinese herbal medicine (TCHM) on Nrf2 induction.
Table 1.
Examples of the effect of TCHM on the activation of Nrf2
Future and novel targets for TCHM
Epigenetics
In recent years, evidence has shown that epigenetic alterations such as DNA methylation, histone modifications, and non-coding microRNAs (miRNAs) consistently contribute to carcinogenesis.[74,75] DNA methylation was the first epigenetic alteration observed in cancer cells and represents the most common molecular alteration in the origin of many cancers.[76,77] DNA methylation occurs at the 5′ position of the cytosine residue within CpG dinucleotides through the addition of a methyl group by DNA methyltransferases (DNMTs), including DNMT1, DNMT3A, and DNMT3B.[78,79] Although CpG dinucleotides are under-represented in the human genome, short regions rich in CpG content exist that are known as CpG islands, most of which are found in the proximal promoter regions of approximately half of human genes, where these CpG islands are generally unmethylated.[80] Thus, the hypermethylation of CpG islands leads to transcriptional silencing of tumor suppressors and other genes with important biological functions; global hypomethylation causes genomic instability and inappropriate activation of oncogenes and transposable elements.[81,82] In this context, many cancer-related genes, such as hMLH1, MGMT (DNA repair), p16INK4a, p15INK4b, p14ARF (cell cycle), death-associated protein kinase (DAPK) (apoptosis), CDH1, CDH13 (cell cadherin), Nrf2, and GSTP1 (detoxification) are inactivated by hypermethylation; genes such as HRAS, CAGE, cyclin D2, maspin, MN/CA9, S100/A4, HPV16, 14-3-3δ, and CT are activated by hypomethylation.[76,78,83]
Histone modification is also commonly recognized as a cause of tumor-suppressor gene inactivation via the post-translational modifications (i.e. acetylation, methylation, phosphorylation, ubiquitination, sumoylation, and ADP-ribosylation) of the amino-terminal tails of histones.[84,85] The most common histone modifications are acetylation/deacetylation and methylation/demethylation, which are mediated by histone acetyltransferase (HAT) and histone deacetylase (HDAC) enzymes, respectively, in combination with histone variants and ATP-dependent chromatin remodeling.[86] Thus, HATs transfer acetyl groups from acetyl-CoA to the ε-amino group of lysine (K) residues in histone tails (open chromatin and gene activation), whereas HDACs remove histone acetyl groups by catalyzing their transfer to coenzyme A (CoA) (condensed chromatin and gene inactivation).[87] For instance, the loss of acetylated H4-lysine 16 (H4K16ac) as well as the overexpression of HDACs such as HDAC1, HDAC2, and HDAC6 has been commonly reported during tumorigenesis in various types of cancer.[88,89] Histone methylation occurs at lysine and arginine residues.[86] This mechanism is regulated by histone methyltransferases (HMTs) and demethylases (HDMs), leading to either activation or repression depending on the residues modified and the type of modification present.[75,90] Methylation of histone H3–lysine 4 (H3–K4), H3–K36, or H3–K79 is associated with transcriptionally active chromatin, whereas methylation of H3–K9, H3–K27, or H4–K20 is associated with transcriptionally repressed chromatin, the two main silencing mechanisms in mammalian cells.[74,91] In this context, cancer cells display widespread changes in histone methylation patterns, and changes in H3–K9 and H3–K27 methylation patterns have been observed in various forms of cancer.[86]
The miRNAs are small, endogenous non-coding RNAs (20–22 nucleotides) that are now recognized as an important component of epigenetic gene regulation in mammals, which control an array of cellular processes such as differentiation, development, hematopoiesis, cell cycle regulation, and immunity.[92,93] Different cancer studies have shown that miRNAs interact with genes in diverse cellular pathways, resulting in differential gene expression profiles of normal and tumor tissues and among tumor types.[82,94] For instance, miRNAs such as miR-221 and miR-22 are highly expressed in different cancers (e.g. human thyroid, papillary carcinomas) targeting and down-regulating p27 (Kip1). Likewise, the miR-17-92 oncogenic cluster targets E2F1 (a cell cycle and apoptosis regulator), BIM (a pro-apoptotic gene that counteracts the anti-apoptotic activity of genes such as BCL2), and PTEN (a negative regulator of the oncogenic pro-survival PI3K/AKT signaling pathway)[[94]]. The down-regulation of let-7 and miR-15/miR-16 miRNAs, which target the RAS and BCL2 oncogenes, respectively, has also been described.[82] Another down-regulated miRNA is miR-126, which inhibits cancer cell growth, proliferation, adhesion, and invasion.[95] Other examples of miRNAs are miR-21, which is associated with tumor cell invasiveness and resistance to apoptosis, and miR-122, which is associated with tumor angiogenesis and cancer cell migration/invasion inhibition.[94,96]
Because epigenetic modifications are reversible, developing drugs that control epigenetic regulation represents a very promising and attractive avenue for treating or preventing cancers, including the development of functional foods or supplements as nutrition-based epigenetic modulators for cancer.[74,97] While HDAC (e.g. vorinostat, belinostat, romidepsin, and panobinostat) and DNMT (e.g. 5-azacitidine and 5-aza-20-deoxycytidine) inhibitors have been utilized at different phases of clinical trials,[98,99] the development of HDAC or DNMT inhibitors as anticancer drugs has been hindered by their adverse side effects.[75,100] However, many plant secondary metabolites extracted as natural products from fruits, vegetables, teas, spices, and traditional medicinal herbs have regulatory effects on the epigenetic machinery, thereby regulating multiple cancer-related pathways [e.g. NFκB, activator protein 1 (AP-1), signal transducers and activators of transcription (STAT3), Nrf2, peroxisome proliferator-activated receptor-γ (PPARγ), estrogen receptor, liver X receptor (LXR), and hypoxia inducible factor-1 (HIF-1)] and epigenetic cofactors (miRNAs) both in vitro and in vivo.[83,97,101]
Curcumin, which is found in turmeric, functions as a strong anticancer agent in different cancer models through the modulation of DNMT, HAT/HDAC, and miRNAs that target the Nrf2, Neurog-1, RARβ2, PTEN, and P53 pathways.[102,103,104,105] Similarly, EGCG from green tea epigenetically controls several molecular cancer targets such as RARβ, hTERT, GSTP1, p16, MGMT, hMLH1, MAGE-A1, Alu, LINE, BCLl2, IL-6, IL-12, NF-κB, and NOS-2 by DNA methylation, chromatin modification, and miRNA regulation.[87,106,107] Genistein from soybeans is another natural compound that controls the epigenetic machinery in many cancers both in vitro and in vivo. Genistein has been reported to be a DNMT inhibitor at the targets p16, BCLl2, RARβ, MGMT, CDKN2A, GSTP1, HMGN5, BTG3 and hTERT, and BRCA1;[74,108,109] a chromatin modification inducer at p21, p16, PTEN, CCLD, p53, FOXA3, SIRT1, BTG3, hTERT, and RARb;[110,111] and an miRNA activator at ZEB1, ZBTB10, and EGFR.[112,113] Finally, isothiocyanates from broccoli, broccoli sprouts, and wasabi also exhibit broad effects on epigenetic mechanisms, such as DNMT inhibition activity at GSTP1 and HDAC inhibition on p21 and GSTP1.[114,115,116] Although these individual dietary phytochemicals have consistently shown great potential in the prevention and treatment of cancers, additional studies are still needed to elucidate the synergistic effects of the combined use of dietary components on the coordinated crosstalk between different molecular cellular pathways and epigenetic machinery as well as to analyze the safety profile of doses, route of administration, organ specificity, and bioavailability of these bioactive components in human clinical studies.[117]
Cancer stem cells
Tumors consist of phenotypically and functionally different subtypes of cancer cells that may have distinct origins at the time of tumor initiation.[118,119] Although the origins of cells that cause cancer are largely unknown, studies have speculated that a certain subset of cancer cells with the capability of self-renewal and continuous differentiation may be responsible for the growth and spread of tumors.[120,121] Many studies have demonstrated the existence of cancer stem cells (CSCs) in several human cancers. The CSC model asserts that a small distinct population of tumorigenic cells that is capable of self-renewal and perpetual proliferation initiates and develops cancer.[122,123] Tumors following the CSC model contain intrinsically different subpopulations of tumorigenic and nontumorigenic cells organized in a hierarchy.[124,125,129] Acute myeloid leukemia was the first cancer to support the CSC model. Only a small population of cells contributed to the formation of tumors when transplanted into immunocompromised mice. These leukemia-initiating cells were enriched for the specific surface marker profile CD34+CD38−.[124,125] Subsequent studies were performed to demonstrate the role of CSCs in solid tumor formation. In the same xenograft model, a very low density of cells presenting CD44+CD24−/low initiated breast tumors.[126] Since these initial findings of the existence of CSCs were published, subsequent research has revealed evidence of CSCs in other human cancers such as colon, pancreatic, and ovarian cancer.[127,130,131] In the CSC model, there are two different types of cancer cells: tumorigenic and nontumorigenic. Through continuous self-renewal and differentiation, the minor population of tumorigenic CSCs gives rise to phenotypically diverse nontumorigenic cancer cells that are thought to compose the bulk of tumors but have little capacity to contribute to the progression of cancer.[120,132,133] Based on the CSC model, even though drug/radiation treatments result in the shrinkage of tumors, failure to eliminate tumorigenic CSCs may cause the recurrence of tumors because the few CSCs that survived from treatment can initiate the tumor again.[134,135] Thus, increasing evidence emphasizes the importance of the ability of both drugs and bioactive food components to modify the self-renewal capabilities of CSCs.[83,136]
Natural dietary compounds and TCHM targeting CSCs
Treatment of nasopharyngeal sphere-derived cells with (−)EGCG, a major bioactive compound in green tea, failed to inhibit growth and apoptosis but induced the formation of a sphere, suggesting that EGCG potently eliminates the stem cell character of nasopharyngeal cancer cells.[137] EGCG inhibits the self-renewing capacity of human prostate cancer cell lines (PC-3 and LNCaP) containing a small population of CSCs presenting CD44+ CD133+. Furthermore, EGCG also inhibits the self-renewing capacity of CD44+α2β1+CD133+ CSCs isolated from human primary prostate tumors, as measured by spheroid formation in suspension. The inhibitory mechanism of EGCG on human prostate CSCs involves apoptosis, as was induced by activating caspase-3/7 and inhibiting the expression of Bcl-2, survivin, and XIAP.[138] Many studies have demonstrated the beneficial effect of cruciferous vegetables, such as broccoli and watercress, on cancer chemoprevention. SFN is a major active compound in cruciferous plants. It significantly decreases the growth of human pancreatic CSC-derived spheres by inhibiting the components of the sonic hedgehog (Shh) pathway and Gli transcription activity in vitro, suggesting the clonogenic depletion of the CSCs. SFN also inhibits downstream targets of Gli transcription by suppressing the expression of pluripotency-maintaining factors (Nanog and Oct-4) as well as PDGFRα and cyclin D1.[139] Treatment of a nonobese diabetic/severe-combined immunodeficient xenograft model with SFN inhibited the growth of breast CSCs and down-regulated the Wnt/β-catenin–related self-renewal pathway.[140] Curcumin is a well-known dietary phytochemical that is found in an Indian spice, turmeric, and has a large spectrum of chemoprevention activities. It suppresses mammosphere formation, reduces the proportion of aldehyde dehydrogenase-presenting cells, and inhibits Wnt signaling in breast stem/progenitor cells. However, curcumin is not toxic in differentiated cells, indicating that it could be a potential cancer prevention reagent for eliminating CSCs.[141]
Ginseng (人參 Rén Shēn; the root of Panax ginseng) is one of the best known Eastern traditional herbs, and research has demonstrated healthy beneficial properties of ginseng in humans. Ginsenoside F2, an active compound in ginseng that has been used in eastern Asia including Korea and China, induces apoptosis in breast CSCs via mitochondrial dysfunction. In addition, ginsenoside F2 induces the formation of acidic vesicular organelles, the recruitment of green fluorescent protein-light chain 3 (GFP-LC3)-II to autophagosomes, and elevation of Atg-7, suggesting that ginsenoside F2 initiates an autophagic progression in breast CSCs.[142] Celastrol, a triterpenoid from the plant Tripterygii Wilfordii Radix (雷公藤 Léi Gōng Téng; the root of Tripterygium wilfordii), effectively eradicated acute myeloid leukemia (AML) at the bulk, progenitor, and stem cell level, as demonstrated via chemical genomics methods such as gene expression-based high-throughput screening (GE-HTS) and the Connectivity Map.[143] Parthenolide (PTL) is a sesquiterpene lactone derived from the leaves of Tanacetum parthenium and is considered a main bioactive component in that herb. PTL induces the death of human leukemia stem cells in vitro without affecting normal hematopoietic cells.[144]
Many studies have been performed and are still ongoing to develop safer and more effective chemopreventive reagents. Owing to the critical role of CSCs in tumorigenesis, preventing the formation of or eliminating CSCs with dietary phytochemicals may be a safer and more efficient approach for combating strong malignancies. Thus, further studies to elucidate the physiological role of these dietary components in preventing the growth of CSCs are required.
CONCLUSIONS
In modern urbanized life, human beings are exposed to increased levels of various toxins, including environmental pollutants, dietary mutagens, carcinogens, microorganisms, and solar radiation. Accumulating evidence supports the effects of dietary phytochemicals, including TCHM, on ROS in health and diseases. Dietary phytochemicals have great potential not only for disease prevention, but also for improving the recovery from certain diseases and cancers by regulating various types of cellular damage caused by ROS. Dietary phytochemicals contribute to cellular protection by inducing phase II detoxifying/antioxidant enzymes such as GST, NAD(P)H quinine oxidoreductase 1 (NQO1), UDP-glucuronosyltransferase (UGT), and HO-1. Nrf2 plays an essential role in the transcriptional induction of phase II enzymes. Many studies have confirmed that various phytochemicals, including TCHM, contribute to cellular defensive mechanisms through the up-regulation of Nrf2. The restoration of various tumor-suppressor genes that are repressed by aberrant epigenetic alterations can be achieved by dietary phytochemical-induced epigenetic modifications. Studies of these natural compounds that modify the self-renewing capability and perpetual proliferation of CSCs have recently increased. Although the beneficial effects of dietary phytochemicals on human carcinogenesis are promising, effective, and safe, further studies of these natural dietary compounds are required. The elucidation of their biological functions, as well as their mechanisms of action, including which molecular targets in the signaling pathways are affected by phytochemicals, is needed to identify more effective and efficient chemopreventive solutions.
Keap1 is dimerized through the BTB domain and is anchored to the actin cytoskeleton via the DGR/Kelch region. Nrf2 binds to the DGR/Kelch region of the Keap1 dimer via a high-affinity ETGE (hinge) motif and a low-affinity DLG (latch) bAU2 motif. The two-site binding exposes the Ub-acceptor site(s) in Nrf2. Under normal conditions, ubiquitinated Nrf2 is degraded by the proteasome, which maintains the equilibrium between synthesis and degradation of the Nrf2 protein in the cell. Once Keap1 is exposed to oxidants or electrophilic compounds, cysteine thiol groups in the IVR region of Keap1 interact with oxidative stress, inducing the formation of disulfide bonds. Disulfide bond formation results in a conformational change that renders Keap1 unable to bind to Nrf2, which then translocates to the nucleus. In this stage, the Ub-acceptor site is not easily accessible. The ubiquitination and proteasomal degradation of Nrf2 are impeded. The released Nrf2, in heterodimeric combination with other transcription factors such as small Maf, binds to the ARE regulatory region of phase II genes and enhances their transcription.
REFERENCES
- 1.Darley-Usmar V, Halliwell B. Blood radicals: Reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm Res. 1996;13:649–62. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- 2.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Tan AC, Konczak I, Sze DM, Ramzan I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer. 2011;63:495–505. doi: 10.1080/01635581.2011.538953. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–73. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 7.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 8.Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Cataldi A. Cell responses to oxidative stressors. Curr Pharm Des. 2010;16:1387–95. doi: 10.2174/138161210791033969. [DOI] [PubMed] [Google Scholar]
- 10.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 11.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 12.Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The role of vitamins in cancer: A review. Nutr Cancer. 2011;63:479–94. doi: 10.1080/01635581.2011.539315. [DOI] [PubMed] [Google Scholar]
- 13.Kojo S. Vitamin C: Basic metabolism and its function as an index of oxidative stress. Curr Med Chem. 2004;11:1041–64. doi: 10.2174/0929867043455567. [DOI] [PubMed] [Google Scholar]
- 14.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa M, Hashida M, Takakura Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv Drug Deliv Rev. 2009;61:319–26. doi: 10.1016/j.addr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Limon-Pacheco JH, Gonsebatt ME. The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10:287–97. doi: 10.2174/187152410793429683. [DOI] [PubMed] [Google Scholar]
- 17.Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–91. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansuy D. Brief historical overview and recent progress on cytochromes P450: Adaptation of aerobic organisms to their chemical environment and new mechanisms of prodrug bioactivation. Ann Pharm Fr. 2011;69:62–9. doi: 10.1016/j.pharma.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Boddupalli S, Mein JR, Lakkanna S, James DR. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: Perspectives in maintaining the antioxidant activity of vitamins a, C, and e. Front Genet. 2012;3:7. doi: 10.3389/fgene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: A promising strategy in cancer prevention. Bioessays. 2006;28:169–81. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 21.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–70. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res. 2007;67:546–54. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 23.Park YH, Kim SU, Lee BK, Kim HS, Song IS, Shin HJ, et al. Prx I suppresses K-ras-driven lung tumorigenesis by opposing redox-sensitive ERK/cyclin D1 pathway. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4421. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rushmore TH, Pickett CB. Glutathione S-transferases, structure, regulation, and therapeutic implications. J Biol Chem. 1993;268:11475–8. [PubMed] [Google Scholar]
- 25.Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280:20340–8. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 26.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–307. [PubMed] [Google Scholar]
- 27.Hu R, Saw CL, Yu R, Kong AN. Regulation of NF-E2-related factor 2 signaling for cancer chemoprevention: Antioxidant coupled with antiinflammatory. Antioxid Redox Signal. 2010;13:1679–98. doi: 10.1089/ars.2010.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 29.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 30.Shen G, Kong AN. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos. 2009;30:345–55. doi: 10.1002/bdd.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–86. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova AT, Holtzclaw WD, Wakabayashi N. Keap1, the sensor for electrophiles and oxidants that regulates the phase 2 response, is a zinc metalloprotein. Biochemistry. 2005;44:6889–99. doi: 10.1021/bi047434h. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–8. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 36.Inoue J, Kerr LD, Kakizuka A, Verma IM. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992;68:1109–20. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 37.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 38.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 39.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–83. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–32. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–68. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 44.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: From bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 45.Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76:967–73. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–15. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 47.Barve A, Khor TO, Hao X, Keum YS, Yang CS, Reddy B, et al. Murine prostate cancer inhibition by dietary phytochemicals--curcumin and phenyethylisothiocyanate. Pharm Res. 2008;25:2181–9. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smolarek AK, So JY, Thomas PE, Lee HJ, Paul S, Dombrowski A, et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERalpha, PPARgamma, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol Carcinog. 2012 doi: 10.1002/mc.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–9. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saw CL, Yang AY, Cheng DC, Boyanapalli SS, Su ZY, Khor TO, et al. Pharmacodynamics of ginsenosides: Antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25:1574–80. doi: 10.1021/tx2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- 52.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665–76. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 53.Lambert JD, Sang S, Hong J, Yang CS. Anticancer and anti-inflammatory effects of cysteine metabolites of the green tea polyphenol, (-)-epigallocatechin-3-gallate. J Agric Food Chem. 2010;58:10016–9. doi: 10.1021/jf102311t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sriram N, Kalayarasan S, Sudhandiran G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm Pharmacol Ther. 2009;22:221–36. doi: 10.1016/j.pupt.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–9. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 56.Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, et al. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009;620:138–44. doi: 10.1016/j.ejphar.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prawan A, Saw CL, Khor TO, Keum YS, Yu S, Hu L, et al. Anti-NF-kappaB and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem Biol Interact. 2009;179:202–11. doi: 10.1016/j.cbi.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khor TO, Yu S, Barve A, Hao X, Hong JL, Lin W, et al. Dietary feeding of dibenzoylmethane inhibits prostate cancer in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69:7096–102. doi: 10.1158/0008-5472.CAN-09-0597. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–41. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 60.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–74. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 61.Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–4. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 62.Zipper LM, Mulcahy RT. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci. 2003;73:124–34. doi: 10.1093/toxsci/kfg083. [DOI] [PubMed] [Google Scholar]
- 63.Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, Keum YS, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–45. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 64.Hu R, Shen G, Yerramilli UR, Lin W, Xu C, Nair S, et al. In vivo pharmacokinetics, activation of MAPK signaling and induction of phase II/III drug metabolizing enzymes/transporters by cancer chemopreventive compound BHA in the mice. Arch Pharm Res. 2006;29:911–20. doi: 10.1007/BF02973914. [DOI] [PubMed] [Google Scholar]
- 65.Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, et al. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–60. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 66.Keum YS, Yu S, Chang PP, Yuan X, Kim JH, Xu C, et al. Mechanism of Action of Sulforaphane: Inhibition of p38 Mitogen-Activated Protein Kinase Isoforms Contributing to the Induction of Antioxidant Response Element-Mediated Heme Oxygenase-1 in Human Hepatoma HepG2 Cells. Cancer Res. 2006;66:8804–13. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 67.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A. 2000;97:12475–80. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–42. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 69.Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–82. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 70.Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–10. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 71.Lee JM, Hanson JM, Chu WA, Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001;276:20011–6. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- 72.Kang KW, Choi SH, Kim SG. Peroxynitrite activates NF-E2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: The role of nitric oxide synthase in rat glutathione S-transferase A2 induction. Nitric Oxide. 2002;7:244–53. doi: 10.1016/s1089-8603(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 73.Sun Z, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem Pharmacol. 2010;80:1771–92. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su ZY, Shu L, Khor TO, Lee JH, Fuentes F, Kong AN. A Perspective on Dietary Phytochemicals and Cancer Chemoprevention: Oxidative Stress, Nrf2, and Epigenomics. Top Curr Chem. 2013;329:133–62. doi: 10.1007/128_2012_340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 77.Arasaradnam RP, Commane DM, Bradburn D, Mathers JC. A review of dietary factors and its influence on DNA methylation in colorectal carcinogenesis. Epigenetics. 2008;3:193–8. doi: 10.4161/epi.3.4.6508. [DOI] [PubMed] [Google Scholar]
- 78.Esteller M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene. 2002;21:5427–40. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 79.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15:3938–46. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klose RJ, Bird AP. Genomic DNA methylation: The mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 82.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 83.Lee JH, Khor TO, Shu L, Su ZY, Fuentes F, Kong AN. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol Ther. 2013;137:153–71. doi: 10.1016/j.pharmthera.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–12. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 85.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Han ZG. Epigenetic analysis in the search for tumor suppressor genes. Epigenomics. 2010;2:489–93. doi: 10.2217/epi.10.29. [DOI] [PubMed] [Google Scholar]
- 87.Gerhauser C. Cancer Chemoprevention and Nutri-Epigenetics: State of the Art and Future Challenges. Top Curr Chem. 2013;329:73–132. doi: 10.1007/128_2012_360. [DOI] [PubMed] [Google Scholar]
- 88.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–84. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 89.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 90.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 92.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neelakandan K, Babu P, Nair S. Emerging roles for modulation of microRNA signatures in cancer chemoprevention. Curr Cancer Drug Targets. 2012;12:716–40. doi: 10.2174/156800912801784875. [DOI] [PubMed] [Google Scholar]
- 94.Brait M, Sidransky D. Cancer epigenetics: Above and beyond. Toxicol Mech Methods. 2011;21:275–88. doi: 10.3109/15376516.2011.562671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le XF, Merchant O, Bast RC, Calin GA. The Roles of MicroRNAs in the Cancer Invasion-Metastasis Cascade. Cancer Microenviron. 2010;3:137–47. doi: 10.1007/s12307-010-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–82. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 97.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res. 2012;65:565–76. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 98.Issa JP. DNA methylation as a therapeutic target in cancer. Clin Cancer Res. 2007;13:1634–7. doi: 10.1158/1078-0432.CCR-06-2076. [DOI] [PubMed] [Google Scholar]
- 99.Fenaux P, Ades L. Review of azacitidine trials in Intermediate-2-and High-risk myelodysplastic syndromes. Leuk Res. 2009;33(Suppl 2):S7–11. doi: 10.1016/S0145-2126(09)70227-9. [DOI] [PubMed] [Google Scholar]
- 100.Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112:981–9. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Plants against Cancer: A review on natural phytochemicals in preventing and treating cancers and their drug ability. Anticancer Agents Med Chem. 2012;12:1281–305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jha AK, Nikbakht M, Parashar G, Shrivastava A, Capalash N, Kaur J. Reversal of hypermethylation and reactivation of the RARbeta2 gene by natural compounds in cervical cancer cell lines. Folia Biol (Praha) 2010;56:195–200. doi: 10.14712/fb2010056050195. [DOI] [PubMed] [Google Scholar]
- 103.Shu L, Khor TO, Lee JH, Boyanapalli SS, Huang Y, Wu TY, et al. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011;13:606–14. doi: 10.1208/s12248-011-9300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khor TO, Huang Y, Wu TY, Shu L, Lee J, Kong AN. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol. 2011;82:1073–8. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 105.Vilas-Zornoza A, Agirre X, Martín-Palanco V, Martín-Subero JI, San José-Eneriz E, Garate L, et al. Frequent and simultaneous epigenetic inactivation of TP53 pathway genes in acute lymphoblastic leukemia. PLoS One. 2011;6:e17012. doi: 10.1371/journal.pone.0017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69:583–92. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 107.Lee YH, Kwak J, Choi HK, Choi KC, Kim S, Lee J, et al. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int J Mol Med. 30:69–74. doi: 10.3892/ijmm.2012.966. [DOI] [PubMed] [Google Scholar]
- 108.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–41. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 109.Volate SR, Muga SJ, Issa AY, Nitcheva D, Smith T, Wargovich MJ. Epigenetic modulation of the retinoid X receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse model of intestinal cancer. Mol Carcinog. 2009;48:920–33. doi: 10.1002/mc.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–60. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 111.Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, Kawamoto K, et al. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer Res. 2008;68:2736–44. doi: 10.1158/0008-5472.CAN-07-2290. [DOI] [PubMed] [Google Scholar]
- 112.Parker LP, Taylor DD, Kesterson J, Metzinger DS, Gercel-Taylor C. Modulation of microRNA associated with ovarian cancer cells by genistein. Eur J Gynaecol Oncol. 2009;30:616–21. [PubMed] [Google Scholar]
- 113.Sun Q, Cong R, Yan H, Gu H, Zeng Y, Liu N, et al. Genistein inhibits growth of human uveal melanoma cells and affects microRNA-27a and target gene expression. Oncol Rep. 2009;22:563–7. doi: 10.3892/or_00000472. [DOI] [PubMed] [Google Scholar]
- 114.Ma X, Fang Y, Beklemisheva A, Dai W, Feng J, Ahmed T, et al. Phenylhexyl isothiocyanate inhibits histone deacetylases and remodels chromatins to induce growth arrest in human leukemia cells. Int J Oncol. 2006;28:1287–93. [PubMed] [Google Scholar]
- 115.Wang LG, Beklemisheva A, Liu XM, Ferrari AC, Feng J, Chiao JW. Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer. Mol Carcinog. 2007;46:24–31. doi: 10.1002/mc.20258. [DOI] [PubMed] [Google Scholar]
- 116.Wang LG, Liu XM, Fang Y, Dai W, Chiao FB, Puccio GM, et al. De-repression of the p21 promoter in prostate cancer cells by an isothiocyanate via inhibition of HDACs and c-Myc. Int J Oncol. 2008;33:375–80. [PubMed] [Google Scholar]
- 117.Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010;1:101–16. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: Origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 119.Nowell PC. Mechanisms of tumor progression. Cancer Res. 1986;46:2203–7. [PubMed] [Google Scholar]
- 120.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 121.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–3. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 122.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000121. e1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 125.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 126.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 128.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 130.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–66. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 133.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: Cancer stem cells versus clonal evolution. Cell. 2009;138:822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 134.McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH, et al. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science. 2010;327:879–83. doi: 10.1126/science.1182378. [DOI] [PubMed] [Google Scholar]
- 135.Ginestier C, Wicinski J, Cervera N, Monville F, Finetti P, Bertucci F, et al. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle. 2009;8:3297–302. doi: 10.4161/cc.8.20.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim YS, Farrar W, Colburn NH, Milner JA. Cancer stem cells: Potential target for bioactive food components. J Nutr Biochem. 2012;23:691–8. doi: 10.1016/j.jnutbio.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin CH, Shen YA, Hung PH, Yu YB, Chen YJ. Epigallocathechin gallate, polyphenol present in green tea, inhibits stem-like characteristics and epithelial-mesenchymal transition in nasopharyngeal cancer cell lines. BMC Complement Alternat Med. 2012;12:201. doi: 10.1186/1472-6882-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava R. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Sonic Hedgehog Signaling Inhibition Provides Opportunities for Targeted Therapy by Sulforaphane in Regulating Pancreatic Cancer Stem Cell Self-Renewal. PLoS ONE. 2012;7:e46083. doi: 10.1371/journal.pone.0046083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–85. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mai TT, Moon J, Song Y, Viet PQ, Phuc PV, Lee JM, et al. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012;321:144–53. doi: 10.1016/j.canlet.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 143.Hassane DC, Guzman ML, Corbett C, Li X, Abboud R, Young F, et al. Discovery of agents that eradicate leukemia stem cells using an in silico screen of public gene expression data. Blood. 2008;111:5654–62. doi: 10.1182/blood-2007-11-126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–35. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang X, Hai CX, Liang X, Yu SX, Zhang W, Li YL. The protective effects of Acanthopanax senticosus Harms aqueous extracts against oxidative stress: Role of Nrf2 and antioxidant enzymes. J Ethnopharmacol. 2010;127:424–32. doi: 10.1016/j.jep.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 146.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, et al. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 147.Dietz BM, Liu D, Hagos GK, Yao P, Schinkovitz A, Pro SM, et al. Angelica sinensis and its alkylphthalides induce the detoxification enzyme NAD(P)H: Quinone oxidoreductase 1 by alkylating Keap1. Chem Res Toxicol. 2008;21:1939–48. doi: 10.1021/tx8001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lou H, Jing X, Ren D, Wei X, Zhang X. Eriodictyol protects against H(2)O(2)-induced neuron-like PC12 cell death through activation of Nrf2/ARE signaling pathway. Neurochem Int. 2012;61:251–7. doi: 10.1016/j.neuint.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 149.Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY, Kang MY, et al. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42:297–305. doi: 10.1016/j.biocel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 150.Hsieh TC, Wu JM. Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. Int J Mol Med. 2011;28:1065–9. doi: 10.3892/ijmm.2011.788. [DOI] [PubMed] [Google Scholar]
- 151.Vrba J, Orolinova E, Ulrichova J. Induction of heme oxygenase-1 by Macleaya cordata extract and its constituent sanguinarine in RAW264.7 cells. Fitoterapia. 2012;83:329–35. doi: 10.1016/j.fitote.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 152.Poon PY, Kwok HH, Yue PY, Yang MS, Mak NK, Wong CK, et al. Cytoprotective effect of 20S-Rg3 on benzo[a]pyrene-induced DNA damage. Drug Metab Dispos. 2012;40:120–9. doi: 10.1124/dmd.111.039503. [DOI] [PubMed] [Google Scholar]
- 153.Chen JH, Huang SM, Tan TW, Lin HY, Chen PY, Yeh WL, et al. Berberine induces heme oxygenase-1 up-regulation through phosphatidylinositol 3-kinase/AKT and NF-E2-related factor-2 signaling pathway in astrocytes. Int Immunopharmacol. 2012;12:94–100. doi: 10.1016/j.intimp.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 154.Kim YH, Choi JH, Rim HK, Kang HJ, Chang SG, Park JH, et al. 23-Hydroxytormentic acid and niga-ichgoside f(1) isolated from Rubus coreanus attenuate cisplatin-induced cytotoxicity by reducing oxidative stress in renal epithelial LLC-PK(1) cells. Biol Pharm Bull. 2011;34:906–11. doi: 10.1248/bpb.34.906. [DOI] [PubMed] [Google Scholar]
- 155.Qin S, Chen J, Tanigawa S, Hou DX. Gene expression profiling and pathway network analysis of hepatic metabolic enzymes targeted by baicalein. J Ethnopharmacol. 2012;140:131–40. doi: 10.1016/j.jep.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 156.Checker R, Patwardhan RS, Sharma D, Menon J, Thoh M, Bhilwade HN, et al. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-kappaB. Free Radic Biol Med. 2012;53:1421–30. doi: 10.1016/j.freeradbiomed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 157.Hwang YP, Jeong HG. Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol Appl Pharmacol. 2008;233:371–81. doi: 10.1016/j.taap.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 158.Lee SE, Jeong SI, Yang H, Jeong SH, Jang YP, Park CS, et al. Extract of Salvia miltiorrhiza (Danshen) induces Nrf2-mediated heme oxygenase-1 expression as a cytoprotective action in RAW 264.7 macrophages. J Ethnopharmacol. 2012;139:541–8. doi: 10.1016/j.jep.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 159.Zhang HS, Wang SQ. Nrf2 is involved in the effect of tanshinone IIA on intracellular redox status in human aortic smooth muscle cells. Biochem Pharmacol. 2007;73:1358–66. doi: 10.1016/j.bcp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 160.Gum SI, Jo SJ, Ahn SH, Kim SG, Kim JT, Shin HM, et al. The potent protective effect of wild ginseng (Panax ginseng C.A. Meyer) against benzo[alpha]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs. J Ethnopharmacol. 2007;112:568–76. doi: 10.1016/j.jep.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 161.Wen Z, Wang Z, Wang S, Ravula R, Yang L, Xu J, et al. Discovery of molecular mechanisms of traditional Chinese medicinal formula Si-Wu-Tang using gene expression microarray and connectivity map. PLoS One. 2011;6:e18278. doi: 10.1371/journal.pone.0018278. [DOI] [PMC free article] [PubMed] [Google Scholar]


