Abstract
Hyperlipidemia and oxidation play major roles upon cardiovascular diseases (CVDs). C-phycocyanin (CPC), the major component in blue-green algae, possesses antiinflammatory and radical scavenging properties. Herein we aimed to investigate the effect of CPC upon lipid metabolism and its antioxidant effects. Golden Syrian hamsters were randomly assigned to five groups: (1) control; (2) 0.2% cholesterol; (3) 0.2% cholesterol+ 1% lopid; (4) 0.2% cholesterol+ 0.25% CPC; and (5) 0.2% cholesterol+ 1.25% CPC. All animals were sacrificed after 8-week feeding. Serum cholesterol, triglyceride (TG), low-density lipoprotein (LDL), glutamate-oxaloacetate transaminase (GOT), and glutamate-pyruvate transaminase (GPT) were examined. The diene conjugation in the Cu2+-mediated oxidation of LDL was measured. The protein levels of several antioxidative enzymes including catalase (CAT), superoxide dismutases (SOD), and glutathione peroxidase (GPx) of liver were assayed. HepG2 cells were cultured in medium containing various concentrations of CPC (0, 1, 15, and 30 μM). The mRNA concentrations of LDL receptor, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase, SOD-1 and GPx of HepG2 cells in each group were analyzed. CPC was effective in lowering serum cholesterol, total cholesterol (TC), TG, LDL, GOT, and GPT. CPC was found to decrease the malondialdehyde (MDA) equivalents and delay the diene conjugation in the Cu2+-mediated oxidation of LDL. CPC increase the enzyme expressions of CAT, SOD, and GPx. CPC concentrations were positively correlated with the mRNA level of LDL receptor while the mRNA levels of HMG CoA reductase, SOD-1, and GPx in HepG2 cells were not affected. The lipid-lowering and antioxidation effects of CPC suggest its roles in prevention of CVD and atherosclerotic formation.
Keywords: Atherosclerosis, C-phycocyanin, Cholesterol, Lipid, Reactive oxygen species
INTRODUCTION
The association of hyperlipidemia and oxidation with cardiovascular disease (CVD) has been well established. Increased lipid or oxidant level in blood might be an important risk factor for the development of CVD. Patients with hypercholesterolemia, hypertriglycemia, impaired antioxidation have a high incidence of CVD as well as increased morbidity and mortality.[1] Hyperlipidemia or compromised oxidation might interrupt the endothelial dysfunction.[2] Recent studies also suggested the immunity impairment as an early mechanism for ischemia-reperfusion affair for CVD.[3]
Reactive oxygen species (ROS) play important roles in the development of CVD.[4] Abnormal ROS production might interfere with vascular function. Free radicals have beneficial or detrimental effects upon vascular wall, which depend on their nature and concentration.[5] ROS such as superoxide anion, hydroxyl radical, and hydrogen peroxide are crucial in inflammatory responses, where they participate in physiological processes such as the arachidonic acid cascade and phagocytosis.[6] The equilibrium between the amounts of ROS produced and scavenged is related with the gamete cell stability and damage.[5] Numerous antioxidants are related with the ROS detoxification, including superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx). SOD and GPx could dispose hydrogen peroxide and other ROS.[7]
C-phycocyanin (CPC), a blue colored, fluorescent protein purified from blue-green algae, is often used as a dietary nutritional supplement. CPC has been associated with antiinflammatory and radical scavenging properties.[8] CPC has also been demonstrated to improve serum lipids and may reduce the risk of CVD.[9] Numerous clinical effects of CPC have been demonstrated, including antiinflammatory activity,[10] antiapoptosis,[11] antioxidant,[12] antiradiation,[13] etc. However, little is known about the molecular mechanisms of CPC in these aspects. Furthermore, its roles upon cardiovascular system, lipid metabolism, and ROS remain to be explored.
The clinical application of CPC and its mechanism through which CPC improves CVD have yet to be fully elucidated. In this survey, we designed to determine the effects of CPC upon the cholesterol, lipid, and ROS metabolism. We aimed to investigate the effects of CPC on the lipid and ROS metabolism of hamster fed with a high cholesterol diet. To the best of our knowledge, this survey is the first report in these related aspects.
MATERIALS AND METHODS
Animal preparation
A total of 56 male Golden Syrian hamsters (7 weeks old) were randomly assigned to five groups including (1) control; (2) 0.2% cholesterol; (3) 0.2% cholesterol+ 1% lopid; (4) 0.2% cholesterol+ 0.25% CPC; and (5) 0.2% cholesterol+ 1.25% CPC. The ambient temperature was maintained between 22 and 24°C and the animals were kept on an artificial 12-h light–dark cycle. All protocols were approved by the Institutional Animal Care and Use Committee of China Medical University, Taichung, Taiwan. After 8-week feeding, all hamsters were sacrificed.
Measurement of plasma lipid level
The blood samples were collected in EDTA-coated glass tubes and centrifuged at 1000×g for 15 min at 4°C. The plasma was then separated and the total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) concentrations were assayed by commercial kits (Randox, San Diego, CA, USA). The protocols are described briefly as follows. TG detection: Serum was processed by enzymatic GPO-PAP method by using lipoproteinlipase, glycerol-kinase, glycerol-3-phosphate oxidase, and peroxidase to form a final product 4-(o-benzo-quinone-monoimido) phenazone with pink red color. The concentration of TG can then be determined at 500 nm. TC detection: Serum was processed by enzymatic CHOD-PAP method. Olyethylene-glycol-(p-(1,1,3,3,-tetramethy-butyl)-phenyl)-ether and polyethyleneglycol monoalkyl ether were used to release cholesterol from lipoprotein release. Cholesterol was then hydrolyzed by cholesterol esterase followed by reaction with cholesterol oxidase to produce H2O2, which was then catalyzed by peroxidase and reacted with 4-aminoantipyrin and phenol to yield pale red color Quinoneimine. The concentration of TC can then be determined at 500 nm. HDL-C detection: The VLDL and LDL in the plasma were precipitated by phosphotungstic acid and Mg2+ followed by 1000×g centrifugation for 15 min. The concentration of HDL in the supernatant was determined by the enzymatic CHOD-PAP method as described in the TC assay. LDL detection: The LDL in the plasma was precipitated by polyvinylsulfate followed by 1000×g centrifugation for 15 min. The cholesterol level other than LDL in the supernatant was determined by enzymatic DHOD-PAP method. The LDL concentration can then be determined by subtracting the supernatant cholesterol from TC level.
Copper-induced oxidation of LDL
LDL was then isolated by single-step nonequilibrium density gradient ultracentrifugation (Beckman TL-100, Beckman, Palo Alto, CA, USA) using a TLV-100 vertical rotor at 100,000 rpm for 30 min at 10°C. The isolated LDL fraction was desalted by passing it through a gel filtration column (Econo-Pac 10 DG, Bio Rad Laboratories, CA, USA). LDL was collected in 0.7 ml of phosphate buffered saline (PBS). The protein concentration of the eluate was measured using bovine serum albumin as standard. For the oxidation experiment, the LDL preparations were diluted with PBS to contain 0.05 g/l protein (≈0.1 μmol/l LDL). Oxidation was started by adding 10 μl of freshly prepared 0.167 mmol/l CuSO4 to 1.0 ml of LDL solution in a 1 cm quartz cuvette. Oxidation was determined as the production of hydroperoxides with conjugated double bonds (conjugated dienes) by continuously (at 1 min intervals for 8 h) monitoring the change in absorbance at 234 nm at 37°C. Several indices were obtained from the absorbance versus time curves: the initial absorbance of the sample was recorded to assess the baseline level of conjugated dienes (BDC, μmol/l) formed in the circulation prior to the isolation of LDL. The lag time (min) was determined from the intercept of lines drawn through the linear portions of the lag phase and the propagation phase.
TBARS levels
Malondialdehyde in plasma was determined by a modification of the method of Huang et al.[14] Briefly, the assay mixture consisted of 25 μl of the plasma, 2.5 μl of 60 mM CuSO4, and 22.5 μl of H2O and was incubated for 4 hours at 37°C. The mixture was then mixed with 0.35 ml of 20% trichloroacetic acid (TCA) and 0.35 ml of 0.67% thiobarbituric acid (TBA) and was heated for 30 min at 70°C. After centrifugation at 10,000 rpm for 2.5 min, the supernatant was assayed spectrophotometrically at 540 nm. Since other aldehydic compounds in the sample may be measured, the plasma malondialdehyde (MDA) content was expressed as thiobarbituric acid-reactive substances (TBARS, MDA equivalent) in nM.
Measurement of enzymatic activities of SOD, CAT, and GSH-Px in liver
Liver proteins for enzymatic activity assay were extracted from tissue treated with or without CPC according to the previously published protocols.[15] SOD activity was assayed by a commercial kit Ransod (Randox, San Diego, CA, USA). Enzyme activity of SOD was defined as one U to inhibit the reducing rate of 2-(-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride by 50%. The SOD activity is denoted as U/mg protein. GSH-Px activity was assayed by a commercial kit Ransel (Randox, San Diego, CA, USA). Enzyme activity of GSH-Px was defined as one U to oxidize 1 μmol nicotinamide adenine dinucleotide phosphate (NADPH) in 1 min. The GSH-Px activity is denoted as mU/mg protein. The assay of CAT was based on the protocol published by Aebi.[16] The CAT activity is denoted as U/mg protein.
Cell culture
Human hepatocellular carcinoma cell line (HepG2) were purchased from Food Industry Research and Development Institute (Hsinchu, Taiwan) and cultured in Dulbecco's modified eagle medium (DMEM) for further study. DMEM medium was composed with 10,000 units/ml penicillin/streptomycin, NaHCO3, and 10 % fetal bovine serum (FBS). The culture of HepG2 cells were performed as previously described.[17] The addition of different concentration of CPC (0.5% FBS, 1, 15, 30 μM) in each culture cells was made to observed the influences of CPC upon HepG2 cell growth. After 2 week culture, the HepG2 cells were harvested.
Real-Time PCR analysis
Total RNAs of HepG2 cells were extracted with Trizol reagent (Invitrogen). One microgram of each total RNA was reverse transcribed into cDNA using the RNA PCR kit (Invitrogen) using oligo (dT) primer. The reaction was carried out at 50°C for 60 min. The reverse transcriptase was inactivated with incubation at 98°C for 5 min and kept at 4°C.
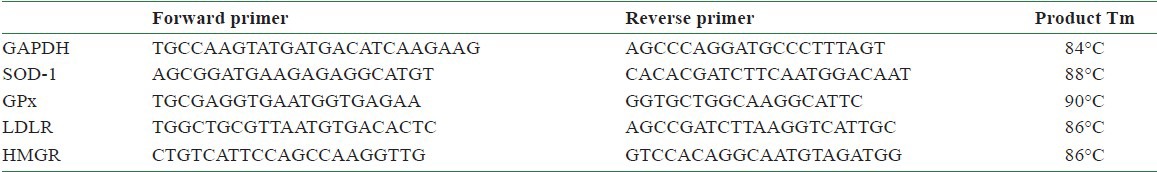
The following real-time polymerase chain reaction (PCR) was performed according to the manufacture protocol (Boehringer Mannheim). The reverse transcription reaction products were measured by PCR analysis, using mRNA encoding rat b-actin as an internal standard. All PCR reactions were performed as follows: 1 μl of cDNA was used for amplification with 1 U Taq DNA polymerase (Qiagen, Hilden, Germany), 5 μl PCR buffer, 5 μl 10 mM dNTPs (each), and 1 pmol of each specific upstream and downstream primer. The individual primer sequences and PCR conditions were designed as shown in [Table 3 (16)]. The real-time PCR for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), SOD1, GPx, low-density lipoprotein receptor (LDLR), and HMG-CoA reductase (or 3-hydroxy-3-methyl-glutaryl-CoA reductase or HMGCR) was performed as previously described.[17] The primer pairs (forward and reverse primers) used in real-time PCR to determine the pharmacological effects of CPC on 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase, SOD-1, and GPx were shown [Table 1].
Table 3.
Lag time of serum LDL oxidation in hamsters fed with various concentration of CPC diet
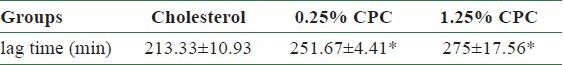
Table 1.
The primer pairs used in real-time PCR to determine the pharmacological effects of CPC
Statistical analysis
All data in each group are presented as mean ± standard deviation (SD). The lipid levels and mRNA levels for GAPDH, SOD-1, GPx, LDLR, and HMGR in each group were determined and compared. The SAS system (version 8.1, SAS Institute Inc., Cary, North Carolina, USA) with unpaired Student's t-test was utilized for statistical analyses. A P-value <0.05 was considered statistically significant.
RESULTS
CPC effectively inhibits lipid profiles
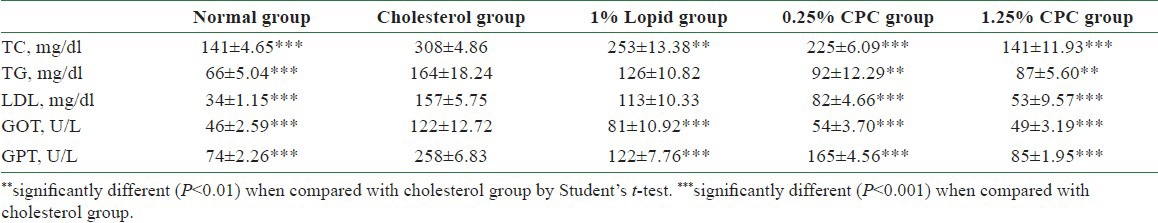
CPC was effective in lowering serum TC, TG, and LDL [Table 2] in hamsters. In comparing the cholesterol group, we observed the facts that CPC at both concentrations (0.25% and 1.25%) effectively inhibited TC, TG, and LDL serum levels [Table 2].
Table 2.
Serum concentrations of total cholesterol (TC), triacylglycerol (TG), low density lipoprotein (LDL), GOT, GPT levels affected by 8 weeks of CPC treatment
Assessment of liver functions in GOT and GPT serum activities
CPC significantly reduces glutamate-oxaloacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) [Table 2] in hamsters. In comparing the cholesterol group, we observed the facts that CPC at both concentrations (0.25% and 1.25%) effectively reduced the serum concentrations of GOT and GPT [Table 2].
CPC delays the copper-induced oxidation of LDL
Our results demonstrated that CPC delayed the diene conjugation in the Cu2+-mediated oxidation as lag time was found to be increased [Table 3]. The lag time for cholesterol, 0.25%, and 1.25% CPC groups were 213.3, 251.7, and 275 min, respectively.
CPC reduces TBARS levels in hamster plasma
The MDA content, a measure of lipid peroxidation, was assayed in the form of TBARS as previously described.[14] Cholesterol ingestion was associated with increase of MDA equivalent formation. CPC at 0.25% and 1.25% both significantly reduced the formation of TBARS (MDA equivalents) when compared with the cholesterol group [Figure 1].
Figure 1.
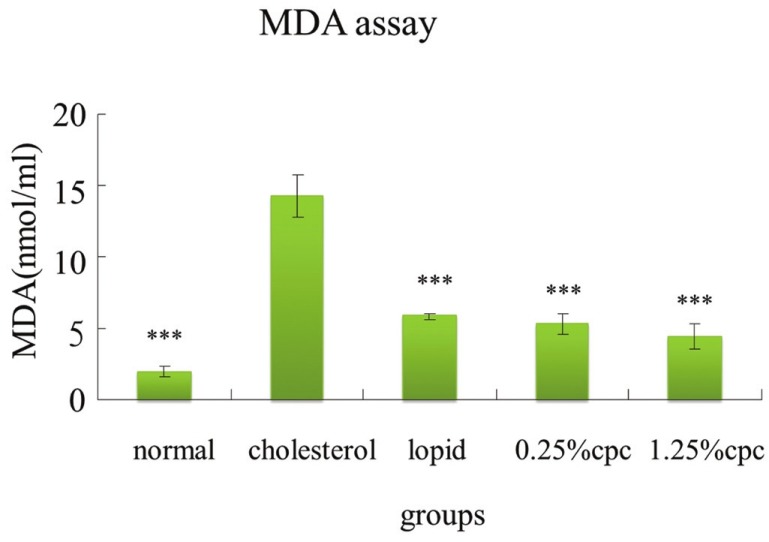
The inhibitory effects of CPC on MDA equivalent formation in hamsters fed with various diets. ***significantly different (P<0.001) when compared with cholesterol group
Measurement of antioxidant enzymes activities of SOD, GPx, and CAT in liver
The activities of SOD, GPx, and CAT of animals in hypercholesterolemia status decreased [Figures 2–4]. Our result demonstrated that CPC at 1.25% effectively increased the activities of SOD, GPx, and catalase, respectively [Figures 2–4].
Figure 2.
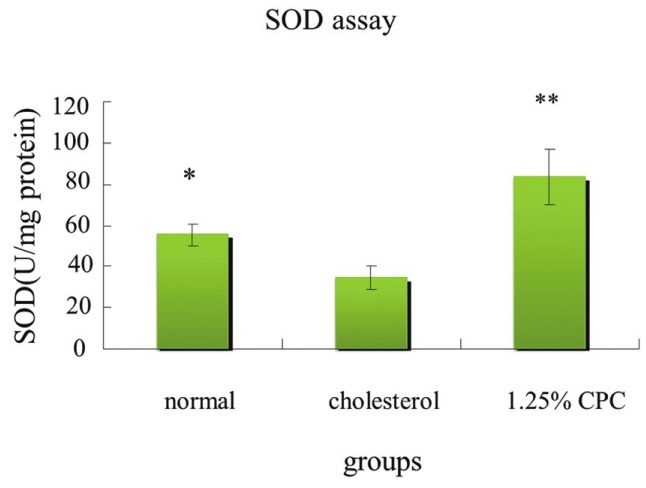
The effects of CPC on SOD enzyme activity of hamsters fed with various diets. *significantly different (P<0.05) when compared with cholesterol group by Student's t-test. **significantly different (P<0.01) when compared with cholesterol group
Figure 4.
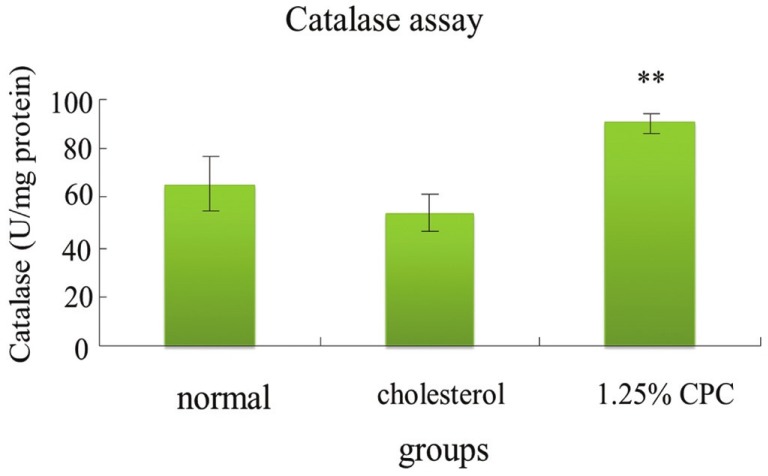
The activities of catalase in hamsters were increased by CPC treatment. **significantly different (P<0.01) when compared with cholesterol group
Figure 3.
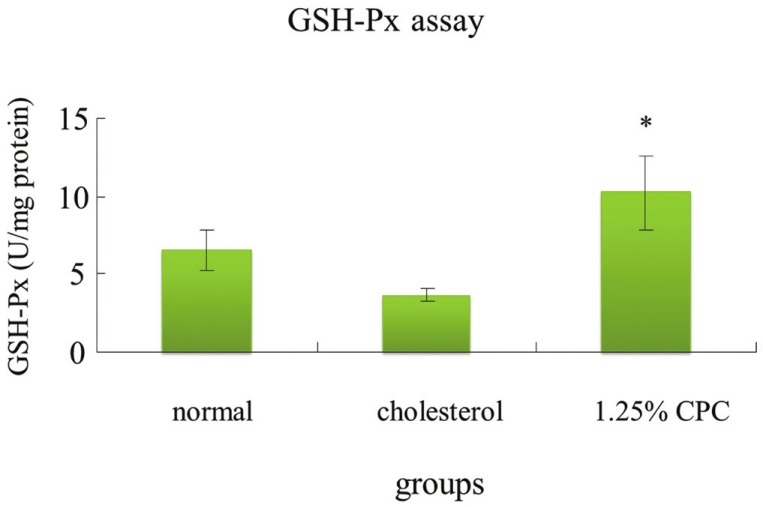
The activities of GSH-Px in hamsters were increased by CPC treatment. *significantly different (P<0.05) when compared with cholesterol group
CPC effects on LDL receptor, HMG CoA reductase, SOD-1, and GPx mRNA levels
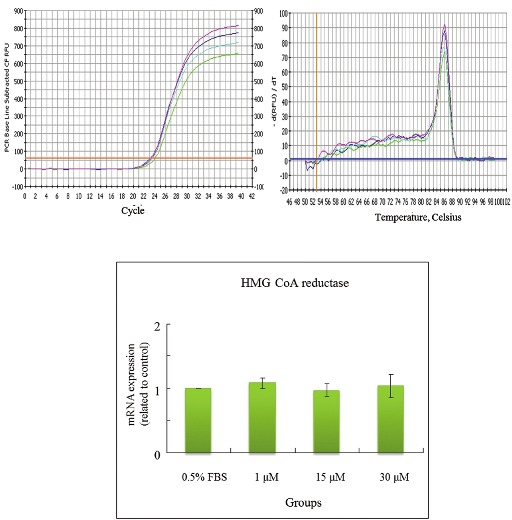
The concentrations of CPC addition were positively correlated with the mRNA levels of LDL receptor in HepG2cells [Figure 5]. CPC at 30 μM appeared to increase levels of mRNA of LDL receptor. The lower concentrations of CPC (1 and 15 μM) were nonsignificantly influencing upon LDL receptor. In contrast, the concentrations of CPC addition were not associated with the mRNA levels of HMG CoA reductase, SOD-1, and GPx in Hep G2 cells [Figures 6–8]. The concentrations of HMG CoA reductase, SOD-1, and GPx in each group were nonsignificantly different [Figures 6–8].
Figure 5.
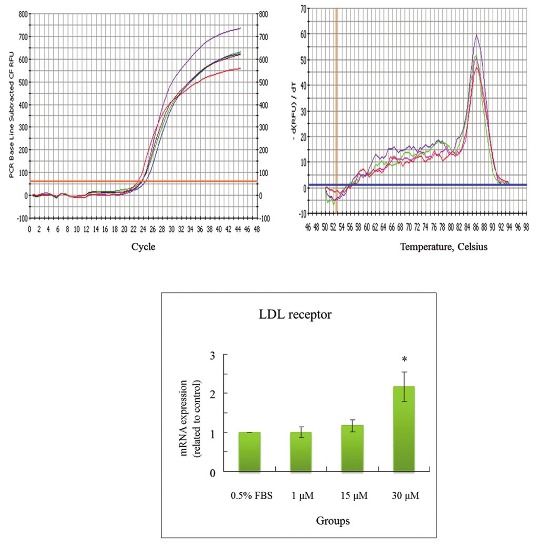
Effects of CPC on mRNA levels of LDL receptor in HepG2 cell. *significantly different (P<0.05) when compared with 0.5% FBS control group
Figure 6.
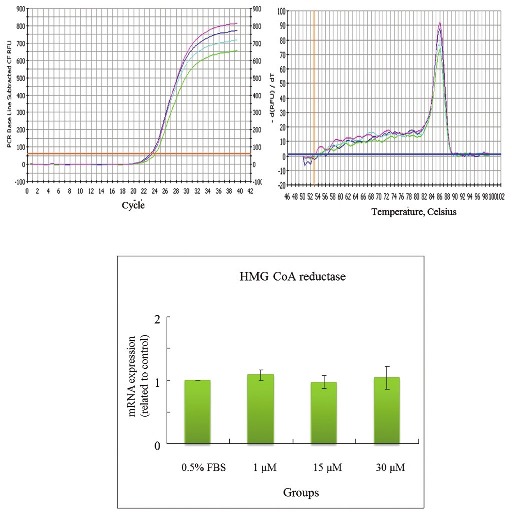
Effects of CPC on mRNA levels of HMG CoA reductase in HepG2 cell
Figure 8.
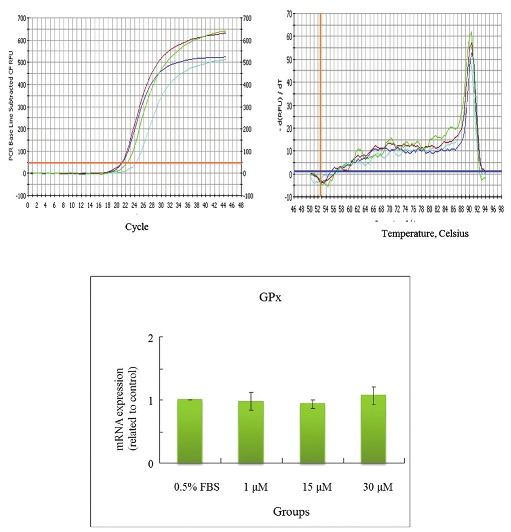
Effects of CPC on mRNA levels of GPx in HepG2 cell
DISCUSSION
CPC is one of the major biliproteins isolated from microalgae Spirulina. CPC has been found to have an antiinflammatory activity and exert beneficial effect in various diseases.[18] The inhibitory activity of CPC on nitric oxide (NO), inducible nitric oxide synthase (iNOS), or other lipopolysaccharide (LPS) is probably associated with suppressing the antiinflammatory activity.[10] CPC also play an antioxidant protector against cell lysis by peroxyl radicals.[19] The antiinflammatory effects of CPC might be associated with its inhibition of PGE2 and phospholipase A2 (PLA2) activities.[19] CPC also play a major role upon the protection against oxidative damage to DNA.[20]
Atherosclerosis is the main cause of arterial lesions and leads to arterial restenosis.[21] The regulation of vascular disorder involved may be complex and multifactor. Hyperlipidemia is associated with the mechanisms contributing to atherosclerosis atherosclerotic disease as well as the development of CVD. High blood lipid and oxidation of LDL have been known to play important roles in atherosclerosis.[22] Therefore, lowering blood lipid or prolonging the lag phase of LDL oxidation has potential therapeutic applications to prevent atherogenesis. In this study, Lopid has been chosen as our positive control group due to the fact that Lopid can lower TG levels.[23] Lopid has also been described to decrease the risk of coronary heart disease by increasing HDL-C and decreasing LDL-C.[24] Our data clearly presents that CPC delays the diene conjugation in the Cu2+-mediated oxidation as lag time was increased [Table 3].
Free radicals have beneficial or detrimental effects upon vascular functions, which depend on their nature and concentration. The generations of ROS, such as superoxide anion, hydrogen peroxide, and the hydroxyl radical can result in the damage to cell membranes. Lipid peroxidation triggers the loss of membrane integrity, causing increased cell permeability, enzyme inactivation, structural damage to DNA, and cell death.[25] Oxidative stress has been implicated in the development of vascular disorder.
Higher SOD might scavenge the generation of ROS as well as lower the risk of CVD.[4] A balance is maintained between the amount of ROS and that scavenged by antioxidant. Cellular damage arises when this equilibrium is disturbed, especially when the cellular scavenging systems cannot eliminate the increased ROS. Lipid peroxidation could damage the cell plasma membrane, which leads to loss of cytosolic components and cell death.
SOD is related with the scavenging of serum ROS. MDA, an end product of lipid peroxidation, represented the level of lipid peroxidation. GPx, a ROS scavenger, plays a role in protecting sperm membranes from the deleterious effects of lipid peroxidation.[26] GPx is related with the balance between the GSSG (reduced form of glutathione) and GSH (oxidized form of glutathione).[27] Glutathione acts as a free radical scavenger and improves the semen quality. Glutathione could inhibit the lipid peroxidation of the cell membrane.[27] Reduced glutathione could protect spermatozoa from oxidative stress.[28] Through the interaction with GPx, reduced glutathione can neutralize hydroxyl radicals and detoxify the peroxides.[27]
In this study, we observed that the CPC could effectively lower the serum levels of TC, TG, LDL, GOT, and GPT [Table 2]. We also observed its dose-dependent effect upon the elimination of TC, LDL, and GPT [Table 2]. Cholesterol/lipid might increase the level of ROS, whereas the CPC intake might compensate the cholesterol/lipid compromise, which might increase the expressions of SOD, GPx, and catalase and decrease the expression of MDA. Also, CPC delayed the diene conjugation in the Cu2+-mediated oxidation as lag time was increased [Table 3]. On the basis of the abovementioned data, we did not find differences between 0.25% CPC and 1.25% CPC groups [Tables 2 and 3]. Therefore, in the following studies we only chose 1.25% CPC for our studies. These findings suggest the clinical values of CPC upon the CVD individuals. We also observed that the concentrations of CPC addition were positively correlated with the mRNA concentration of LDL receptor in HepG2 cells [Figure 5]. In contrast, the CPC concentrations seem not to be associated with the mRNA concentration of other markers, such as HMG CoA reductase [Figure 6], SOD-1 [Figure 7], and GPx [Figure 8]. Although their correlation was nonstatistically different, their correlation might exist after larger series included.
Figure 7.
Effects of CPC on mRNA levels of SOD-1 reductase in HepG2 cell
In conclusion, CPC are useful in the lipid-lowering and antioxidation, which suggest their roles in prevention of atherosclerotic formation and CVD susceptibilities. This could provide the database about the clinical roles of CPC upon lipid, ROS metabolism, as well as atherosclerosis or CVD pathogeneses. Our finding suggested that CPC might improve lipid profiles associated with CVD and the improving the compromised antioxidation condition. However, the real roles of its metabolic products and other herb medicines upon lipid and oxidant metabolism as well as the development of atherosclerosis or CVD merit further surveys. Furthermore, the dysfunctions of lipid or ROS are not the single determiner for the CVD or atherosclerosis. Other clinical values or roles of CPC upon CVD need further surveys. Larger scale or multiple factor surveys about CPC is required to clarify its fundamental roles upon CVD. Though, its molecular mechanisms underlying the cholesterol-lowering effect of CPC were not known, however, it is likely that CPC might regulate the turnover rate of LDL as shown by upregulation of LDLR. Also, antioxidation effects of CPC might contribute to less development of lipid profiles.
ACKNOWLEDGEMENT
This work has been supported by grant NSC97-2320-B-039-013-MY3 from National Science Council in Taiwan.
REFERENCES
- 1.Gotto AM, Jr, Moon JE. Management of cardiovascular risk: The importance of meeting lipid targets. Am J Cardiol. 2012;110(1 Suppl):3A–14A. doi: 10.1016/j.amjcard.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Fotis L, Agrogiannis G, Vlachos IS, Pantopoulou A, Margoni A, Kostaki M, et al. Intercellular adhesion melecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early stages of atherosclerosis in a rat model. In Vivo. 2012;26:243–50. [PubMed] [Google Scholar]
- 3.Zhang M, Carroll MC. Natural IgM-mediated innate autoimmunity: a new target for early intervention of ischemia-reperfusion injury. Expert Opin Biol Ther. 2007;7:1575–82. doi: 10.1517/14712598.7.10.1575. [DOI] [PubMed] [Google Scholar]
- 4.Jeremy JY, Shukla N, Muzaffar S, Handley A, Angelini GD. Reactive oxygen species, vascular disease and cardiovascular surgery. Curr Vasc Pharmacol. 2004;2:229–36. doi: 10.2174/1570161043385691. [DOI] [PubMed] [Google Scholar]
- 5.Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr Med Chem. 2012;19:2504–20. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacic P, Somanathan R. Unifying mechanism for eye toxicity: electron transfer, reactive oxygen species, antioxidant benefits, cell signaling and cell membranes. Cell Membr Free Radic Res. 2008;2:56–69. [Google Scholar]
- 7.Lu CY, Lee HC, Fahn HJ, Wei YH. Oxidative damage elicited by imbalance of free radical scavenging enzymes is associated with large-scale mtDNA deletions in aging human skin. Mutat Res. 1999;423:11–21. doi: 10.1016/s0027-5107(98)00220-6. [DOI] [PubMed] [Google Scholar]
- 8.Romay C, Armesto J, Remirez D, González R, Ledon N, García I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 9.Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, et al. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr. 2005;135:2425–30. doi: 10.1093/jn/135.10.2425. [DOI] [PubMed] [Google Scholar]
- 10.Cherng SC, Cheng SN, Tarn A, Chou TC. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007;81:1431–5. doi: 10.1016/j.lfs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Rimbau V, Camins A, Pubill D, Sureda FX, Romay C, González R, et al. C-phycocyanin protects cerebellar granule cells from low potassium/serum deprivation-induced apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:96–104. doi: 10.1007/s002100100437. [DOI] [PubMed] [Google Scholar]
- 12.Piñero Estrada JE, Bermejo Bescós P, Villar del Fresno AM. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco. 2001;56:497–500. doi: 10.1016/s0014-827x(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 13.Karpov LM, Brown II, Poltavtseva NV, Ershova ON, Karakis SG, Vasil’eva TV, et al. The postradiation use of vitamin-containing complexes and a phycocyanin extract in a radiation lesion in rats. Radiats Biol Radioecol. 2000;40:310–4. [PubMed] [Google Scholar]
- 14.Huang GJ, Deng JS, Chiu CS, Liao JC, Hsieh WT, Sheu MJ, et al. Hispolon Protects against Acute Liver Damage in the Rat by Inhibiting Lipid Peroxidation, Proinflammatory Cytokine, and Oxidative Stress and Downregulating the Expressions of iNOS, COX-2, and MMP-9. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/480714. 480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia E, Rao G, Van Remmen H, Heydari AR, Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J Nutr. 1995;125:195–201. doi: 10.1093/jn/125.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Jackson KG, Maitin V, Leake DS, Yaqoob P, Williams CM. Saturated fat-induced changes in Sf 60-400 particle composition reduces uptake of LDL by HepG2 cells. J Lipid Res. 2006;47:393–403. doi: 10.1194/jlr.M500382-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Romay Ch, González R, Ledón N, Remirez D, Rimbau V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4:207–16. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 19.Romay C, González R. Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J Pharm Pharmacol. 2000;52:367–8. doi: 10.1211/0022357001774093. [DOI] [PubMed] [Google Scholar]
- 20.Bhat VB, Madyastha KM. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem Biophys Res Commun. 2001;285:262–6. doi: 10.1006/bbrc.2001.5195. [DOI] [PubMed] [Google Scholar]
- 21.Smith TP. Atherosclerosis and restenosis: An inflammatory issue. Radiology. 2002;225:10–2. doi: 10.1148/radiol.2251020750. [DOI] [PubMed] [Google Scholar]
- 22.Victor VM, Apostolova N, Herance R, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: Mitochondria-targeted antioxidants as potential therapy. Curr Med Chem. 2009;16:4654–67. doi: 10.2174/092986709789878265. [DOI] [PubMed] [Google Scholar]
- 23.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 24.Roy A, Pahan K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol Immunotoxicol. 2009;31:339–51. doi: 10.1080/08923970902785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez JG, Storey BT. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989;23:77–90. doi: 10.1002/mrd.1120230108. [DOI] [PubMed] [Google Scholar]
- 27.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 28.Baker HW, Brindle J, Irvine DS, Aitken RJ. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65:411–9. doi: 10.1016/s0015-0282(16)58109-6. [DOI] [PubMed] [Google Scholar]


