Abstract
The caterpillar fungus Ophiocordyceps sinensis (syn.† Cordyceps sinensis), which was originally used in traditional Tibetan and Chinese medicine, is called either “yartsa gunbu” or “DongChongXiaCao (冬蟲夏草 Dōng Chóng Xià Cǎo)” (“winter worm-summer grass”), respectively. The extremely high price of DongChongXiaCao, approximately USD $20,000 to 40,000 per kg, has led to it being regarded as “soft gold” in China. The multi-fungi hypothesis has been proposed for DongChongXiaCao; however, Hirsutella sinensis is the anamorph of O. sinensis. In Chinese, the meaning of “DongChongXiaCao” is different for O. sinensis, Cordyceps spp.,‡ and Cordyceps spƒ. Over 30 bioactivities, such as immunomodulatory, antitumor, anti-inflammatory, and antioxidant activities, have been reported for wild DongChongXiaCao and for the mycelia and culture supernatants of O. sinensis. These bioactivities derive from over 20 bioactive ingredients, mainly extracellular polysaccharides, intracellular polysaccharides, cordycepin, adenosine, mannitol, and sterols. Other bioactive components have been found as well, including two peptides (cordymin and myriocin), melanin, lovastatin, γ-aminobutyric acid, and cordysinins. Recently, the bioactivities of O. sinensis were described, and they include antiarteriosclerosis, antidepression, and antiosteoporosis activities, photoprotection, prevention and treatment of bowel injury, promotion of endurance capacity, and learning-memory improvement. H. sinensis has the ability to accelerate leukocyte recovery, stimulate lymphocyte proliferation, antidiabetes, and improve kidney injury. Starting January 1st, 2013, regulation will dictate that one fungus can only have one name, which will end the system of using separate names for anamorphs. The anamorph name “H. sinensis” has changed by the International Code of Nomenclature for algae, fungi, and plants to O. sinensis.
Keywords: Bioactive Ingredients, Cordyceps sinensis, DongChongXiaCao, Hirsutella sinensis, Ophiocordyceps sinensis
INTRODUCTION
The caterpillar fungus Ophiocordyceps sinensis (syn. Cordyceps sinensis) is one of the entomogenous Ascomycetes and parasitizes the larvae of Lepidoptera to form the well-known traditional Tibetan medicine “yartsa gunbu” or, in traditional Chinese medicine, “DongChongXiaCao (冬蟲夏草 Dōng Chóng Xià Cǎo)”(“winter worm-summer grass,” [Figure 1]). DongChongXiaCao is a well-described remedy that has been used in traditional Chinese medicine for over 700 years.[1] The wild fungus, which possesses a plant-like fruiting body and originates from dead caterpillar that fill with mycelia [Figure 2], has generally been called C. sinensis or Cordyceps spp. (“ChongCao” in Chinese) due to its insect-shape appearance. O. sinensis (previously named C. sinensis) is a slow-growing fungus and needs to be grown at a comparatively low temperature, i.e., below 21°C. Both temperature and growth rate are crucial factors that identify O. sinensis from other similar fungi.[2] In recent decades, curative and health-care products derived from the so-called “Cordyceps” are extremely popular in China in various forms such as capsules, oral liquids, and drinks.[3] Most of these products, derived from submerged mycelial O. sinensis cultures [Figure 3], are the popular merchandise items on the market.
Figure 1.

Wild DongChongXiaCao (black part: the fruiting body; brown part: the caterpillar corpus)
Figure 2.
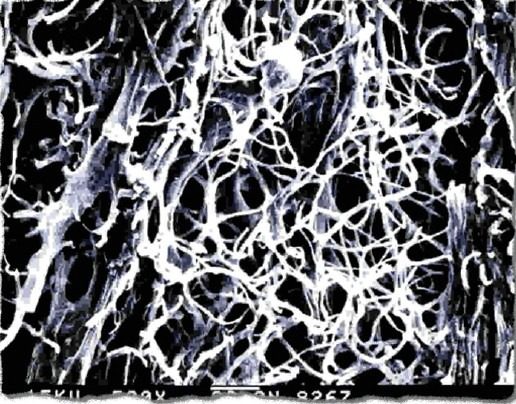
Scanning electron micrograph of mycelia filling the inside of a fruiting body of wild DongChongXiaCao
Figure 3.

Scanning electron micrograph of mycelia from the medium of a submerged culture of Ophiocordyceps sinensis
It has been shown that O. sinensis can be used to treat conditions such as hyposexuality, night sweats, hyperglycemia, hyperlipidemia, asthenia, arrhythmias, and other heart, respiratory, renal and liver diseases.[3] Although “natural O. sinensis specimens” have significant pharmaceutical effects, the commercial cultivation of this fungus on larvae of moth to produce fruiting body has not yet been successful.[4] Therefore, the biology of O. sinensis remains a secret, and its commercial cultivation is still a dream.[5]
In the past years, several new names have been proposed for O. sinensis-like species from alpine regions, such as O. gansuënsis, O. crassispora, O. kangdingensis, O. multiaxialis, O. nepalensis, and others, but there is not sufficient to distinguish these species from O. sinensis.[6] Fungi other than O. sinensis originating from natural O. sinensis specimens could be important resources for the development of alternative products.[7] For example, it is claimed that the Bailing Capsule, a cultured product isolated from DongChongXiaCao, has a similar chemical composition to natural O. sinensis; in addition, the Bailing Capsule possesses anti-inflammatory, antihypoxia, and antitumor properties and has the ability to regulate the endocrine system, enhance immune function, and protect the kidneys, lung, liver, and other organs.[8]
As a traditional oriental medicine, DongChongXiaCao is endemic to alpine habitats on the Tibetan Plateau, located predominantly in Tibet, the Tibetan autonomous prefectures of neighboring provinces, and the high Himalayas.[9] In recent years, DongChongXiaCao has been regarded as the Himalayan Viagra, which has caused the price to reach USD $6.77 per piece of wild medicine.[10] Over the past 10 years, its value has increased dramatically. For example, collectors must pay as much as USD $12,500 per kg for top-quality material.[11] In 2008-2009, the price of C. sinensis crude drug was around USD $13,000 per kg, which caused it to be regarded as “soft gold” in China.[12] Furthermore, it is believed that the price of this fungus reached USD $20,000 to 40,000 per kg in the international market.[13] As of August 2012, the price per gram of wild DongChongXiaCao in Beijing is up to CNY ͵698 [Figure 4], or USD $111,560 per kg. This price already surpasses that of real gold. According to the government statistics for 2004, 50,000 kg of this drug were collected, which contributed more than USD $225 million to the Tibet Autonomous Region's GDP,[14] these data suggested that about 40% of the rural cash income in the Tibet Autonomous Region comes from DongChongXiaCao collection.
Figure 4.

Label showing the precious price (CNY ¥698 per gram) of wild DongChongXiaCao sold in August, 2012 in Beijing, China
REVIEW ARTICLES AND SPECIAL REPORTS OF CORDYCEPS
Since 1998, there have been more than 25 reviews or special reports published discussing Cordyceps, and 14 of them have focused on C. sinensis. For example, these studies have emphasized: terminology, life strategy, and ecology;[5] traditional uses and medicinal potential in Sikkim;[10] the reliability of fungal materials;[2] ecology, trade, and development in Tibet;[15] production and sustainability on the Tibetan Plateau and in the Himalayas;[16] origin of scientific name, morphological characteristics, micromorphological characteristics of the teleomorph, identification, hosts, and synonymy;[6] ethnomycological use, collection, discovery, protection, and the range of diseases treated in Northern Yunnan Province in China;[9] host spectrum, distribution, artificial rearing host, infection technology, and substitute products;[17] clinical efficacy for chronic kidney diseases;[18] markers and analytical methods for quality control;[19] history, use, and implications;[20] pharmacological functions;[21] safety, effects on the nervous system, glucose metabolism, effects on the respiratory, hepatic, cardiovascular, immune systems, immunological disease, inflammatory conditions, cancer, and diseases of the kidney;[22] and in vitro and in vivo studies, open-label and double-blinded clinical trials on the respiratory, renal, hepatic, cardiovascular, immunological, and nervous systems, and in its effects on cancer, glucose metabolism, inflammatory conditions, and toxicological studies.[23] Two papers have focused on C. militaris, placing emphasis on: biological aspects including the host range, mating system, cytology and genetics, insect- and noninsect nutritional requirements, environmental influence on stroma development, and commercial development;[24] and active principles and culture techniques.[25] The other publications focus on Cordyceps, examining: pharmacological functions and development of products;[3] production, isolation, purification, structure elucidation, and pharmacological action of polysaccharide;[1] chemical constituents;[26] preparations and chemical structures of polysaccharide;[27] taxonomic concepts, preparations, apoptosis, chemical constituent profiling, hosts, and poisoning;[28] history, medicinal uses, chemical composition, and cultivation;[29] pharmacological actions;[30] and the pharmacological basis of “Yin-nourishing (養陰Yǎng Yīn)” and “Yang-invigorating (壯陽 Zhuàng Yáng)” actions.[31]
TERMINOLOGY OF “DONGCHONGXIACAO” WITH REGARD TO CORDYCEPS
“DongChongXiaCao” is commonly known as “yarsa gumba” in Tibetan, because “yarsa” means winter and “gumba” means summer. “Gunba” or “gonba” has also been used to replace “gumba,” and the fungus is named “keera jhar” (insect herb) in the Indian mountains.[13]
However, the term “DongChongXiaCao” in Chinese has been recognized as having different meanings, as follows:
The traditional Chinese medicine originating from O. sinensis (syn. C. sinensis).
The health food originating from O. sinensis.
The fungus O. sinensis.
The fungi Cordyceps spp.
The fungus Cordyceps sp.
The fungi Ophiocordyceps spp.
The fungus Ophiocordyceps sp.
The wild and crude medicine that has a caterpillar shape with fruiting body.
The mycelia of O. sinensis derived from submerged culture.
The mycelia of H. sinensis derived from submerged culture.
The traditional Chinese medicine originating from the larvae of Hepialidae (Lepidoptera) infected by O. sinensis.
The traditional Chinese medicine originating from the larvae of Thitarodes (syn. Hepialus) infected by O. sinensis.
The traditional Chinese medicine originating from the larvae of T. armoricanus (syn. H. armoricanus) infected by O. sinensis.
The traditional Chinese medicine originating from the larvae of Hepialidae (Lepidoptera) infected by Ophiocordyceps spp.
The traditional Chinese medicine originating from the larvae of Hepialidae (Lepidoptera) infected by Cordyceps spp.
The term “ChongCao” (meaning Insect Grass) in Chinese is the abbreviation of “DongChongXiaCao,” which related products is popular in the market in Taiwan. For consumers, it is confusing whether “ChongCao” is equivalent to “DongChongXiaCao,” the traditional Chinese medicine “DongChongXiaCao,” or the fungi Cordyceps spp. In addition, C. militaris, a type species of Cordyceps, has been regarded as a substitute for “DongChongXiaCao” and is named “North DongChongXiaCao,” “BeiChongCao,” “ChongCao mycelium,” or “ChongCao fruiting body.” There is no doubt that O. sinensis is not equivalent to the traditional Chinese medicine “DongChongXiaCao,” because the latter has a caterpillar shape with a fruiting body. In the market, natural products with caterpillar shapes and fruiting bodies that are called “DongChongXiaCao” are extensively distributed; however, the origins of most of these microorganisms and their hosts remain uncertain. In the scientific view, the traditional Chinese medicine wild “DongChongXiaCao” is not strictly equivalent to O. sinensis unless the species has been identified. In fact, for most literature, the term “C. sinensis” used in the materials section might refer to wild DongChongXiaCao or to its fruiting bodies or cultured mycelia. Dong and Yao[2] reviewed 152 papers from PubMed on O. sinensis since 1998 and found that at least 116 papers (over three-quarters) used unreliable, uncertain, or unspecified materials, including so-called cultivated fruit bodies that were apparently not O. sinensis strains, based on temperature and growth period.
RENAMING, ANAMORPH, AND GENETIC DIVERSIFICATION OF C. SINENSIS
Renaming of C. sinensis
The colony characteristics of O. sinensis cultures are significant different from Cordyceps spp. Mostly Cordyceps species possess brightly colored and fleshy stromata. The family of Cordycipitaceae has been validated based on the type species of Cordyceps, C. militaris, and a new family, Ophiocordycipitaceae, based on the Genus Ophiocordyceps Petch, the majority of whose species produce dark pigments and tough to pliant stromata, often possesses aperithecial apices including C. sinensis.[32] Based on the publication referenced above, C. sinensis has been transferred to Ophiocordyceps and has been renamed O. sinensis.
The anamorph of C. sinensis
Twenty-two names spanning 13 genera associated with the anamorph of C. sinensis have been described.[33] However, H. sinensis and C. sinensis belong to different stages of the life cycle of the same organism: H. sinensis is the anamorph of C. sinensis, rather than Paecilomyces sinensis or other species.[34] The rDNA ITS sequences of C. sinensis collected from different geographical regions are almost identical and are significantly different from substitutes.[35] H. sinensis has been confirmed as the anamorph of C. sinensis by both DNA sequences and microcyclic conidiation, and additionally, two species, C. multiaxialis and C. nepalensis, were shown to share identical or almost identical ITS sequences with C. sinensis.[36]
The relationship between teleomorphs of Cordyceps spp. and their presumed anamorphs has been investigated by analyzing 5.8S and ITS rDNA sequences. Both sequence analyzes demonstrated that H. sinensis is the anamorph of C. sinensis.[37] When analyzing the sequences of ITS1, ITS2 and 5.8S rDNA regions, some species with different names had similar morphologies to C. sinensis, which suggested that these species might be synonymous with C. sinensis.[38] Based on the results of the 5.8S rDNA and ITS region analyses, it is clear that the ITS sequences within C. sinensis are highly homologous, regardless of the geographical origin. The evolutionary distance values between C. sinensis and H. sinensis were found to be the same as those for C. sinensis from different geographic regions, and C. sinensis should only have H. sinensis as its asexual stage, regardless of sample origin.[39] Even though many studies indicate that H. sinensis is the only anamorph of C. sinensis, molecular evidence demonstrates the existence of both H. sinensis and Paecilomyces hepiali DNA in the caterpillars and fruiting bodies of natural C. sinensis, strongly supports the multi-fungi hypothesis for natural C. sinensis.[40]
Genetic diversification of C. sinensis
A high diversity in the fungal community structure occurs in natural O. sinensis.[5] The significant genetic divergence in O. sinensis was found to be greater among southern isolates than among northern isolates in China.[41] The genetic similarity indices range from 0.282 to 0.782, which indicate that there is a high level of diversity among natural C. sinensis samples.[42] In addition, a total of 141 markers, 99.3% of which were polymorphic, were identified in 180 individual samples of natural C. sinensis from 18 populations, and these 18 populations can be divided into five groups based on genetic distance and by grouping pattern matches with geographic distributions along the latitudinal gradient.[43] It has been observed that differences in medicinal effects among C. sinensis populations may be attributed to the existence of genetically differentiated chemotypes in morphotaxon.[44]
CHEMICAL CONSTITUENTS, PROXIMATE COMPOSITION AND VOLATILE COMPOUNDS
Chemical constituents
The chemical constituents of natural Cordyceps include cordycepic acid, glutamic acid, amino acids, polyamines, cyclic dipeptides, saccharides and sugar derivatives, sterols, nucleotides and nucleosides, 28 saturated and unsaturated fatty acids, fatty acid derivatives and other organic acids, vitamins, and inorganic elements.[13] Palmitic acid, linoleic acid, oleic acid, stearic acid, and ergosterol are the main components of natural and cultured Cordyceps, these fatty acids, as well as 14 investigated compounds, can be used to discriminate the hierarchical cluster, as the palmitic acid and oleic acid contents in natural Cordyceps are significantly higher than those in the cultured Cordyceps.[45]
Proximate composition and others
Significant differences in proximate composition, such as for protein, fat, carbohydrate, and moisture content, were observed between the corpus and the fruiting body of wild DongChongXiaCao and the fermented mycelia of C. sinensis. There is a conspicuously high carbohydrate content of 39.4% for fermented mycelia, whereas carbohydrates comprise 24.20% of the corpus and 24.9% of the fruiting body in wild DongChongXiaCao.[46] Fermented mycelia had lower protein and fat contents (14.8 and 6.63%, respectively) than the corpus (29.1 and 8.62%, respectively) and the fruiting body (30.4 and 9.09%, respectively) of wild DongChongXiaCao.[46] However, Smironv et al,[47] showed that the mycelia of C. sinensis had high protein (29%) and low lipid contents (7%), and, similar to other studies. They also indicated that the mycelia were rich in endopolysaccharides (EPS, 15%), phospholipids (up to 28% of total lipids), and unsaturated fatty acids (C18:1 - up to 44%; C18:2 - 53% of total fatty acids).
The compositions of natural fruiting bodies of C. sinensis (NFCS) and mycelia from submerged cultures (MSMC) and shaking cultures (MSKC) of H. sinensis were compared by Li et al.[48] They indicated that the crude fat, crude protein, total, and essential amino acid contents could be ranked in the following descending order: MSKC > MSMC > NFCS, and additionally, unsaturated fatty acids in MSMC account for 65.9% of total fatty acids, which is noticeably lower than for NFCS (86.9%) and MSKC (76.5%). The total content of four nucleosides (adenosine, guanosine, uridnine, and inosine) in MSMC (6.20 mg/g) was significantly higher than NFCS (1.80 mg/g) and MSKC (1.60 mg/g).[48] Moreover, the sugar and protein contents of EPS from Hirsutella sp. were 92.7 and 5.2%, respectively, and the monosaccharide components of EPS are mannose, galactose, and glucose with a molar ratio of 4.0:8.2:1.0, and its molecular weight is 23 kDa.[49]
Volatile compounds
From the mycelia of H. sinensis cultured with solid-state media (SSM) and submerged fermentation (SF), 51 volatile compounds were identified, and phenols, acids, and alkanes were the major classes of compounds, while butylated hydroxytoluene was the most abundant volatile compound and accounted for 47.38% and 46.12% of the total volatile compounds in mycelia cultured by SSM and SF, respectively.[50]
BIOACTIVE INGREDIENTS AND BIOACTIVITIES
A review of the literature regarding the bioactive ingredients and bioactivities of O. sinensis reveals that eight types of materials have been used, including (1) the crude powder, (2) the crude powder of the fruiting body, (3) the extracts from the crude powder, (4) the extracts from the crude powder of the fruiting body, (5) the crude powder of mycelia, (6) mycelial extracts, (7) the supernatants of submerged fermented cultures and (8) the whole broth of submerged fermented cultures. The diversity of these materials brings up the major concern of whether the knowledge we have acquired regarding O. sinensis is applicable to wild “Dong Chong Xia Cao” or the fermented mycelia or mycelial fermentation products (including cultured broth) of O. sinensis, H. sinensis, or other species.
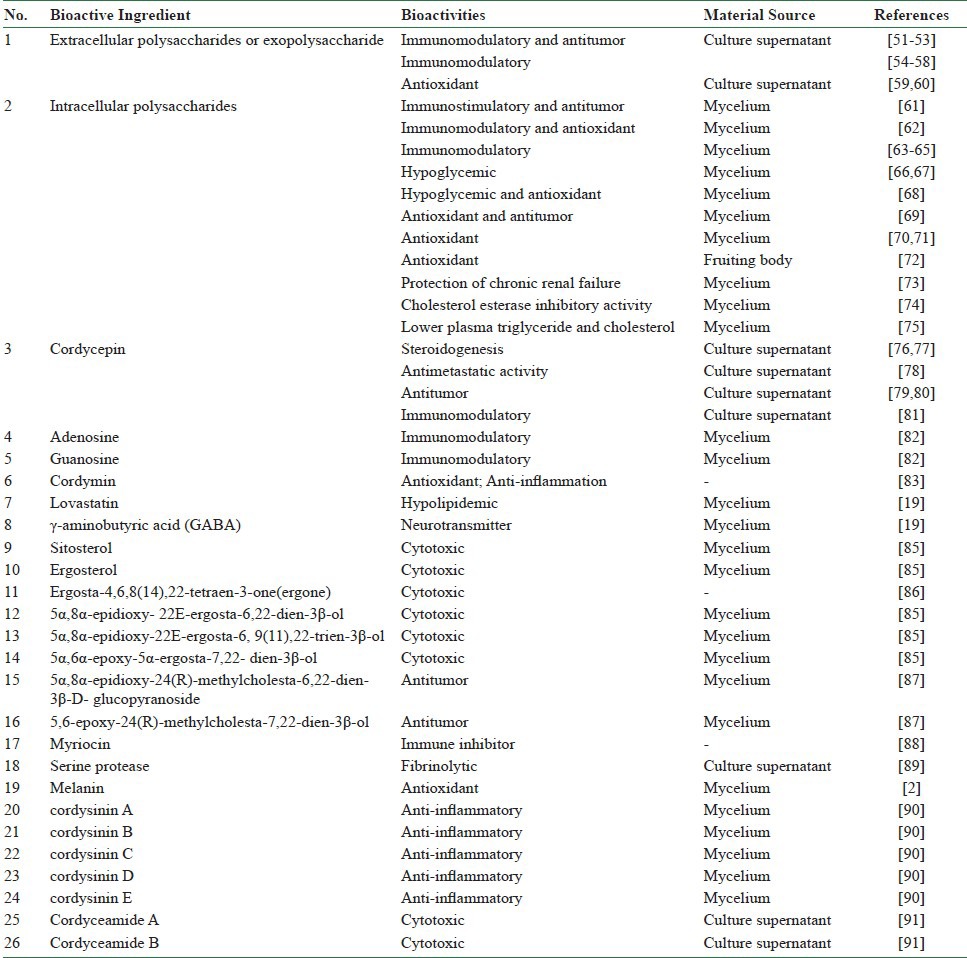
Over 20 bioactive ingredients from mycelia, culture supernatants, or fruiting bodies have been published, as shown in Table 1. In summary, these ingredients include (1) extracellular polysaccharides, (2) intracellular polysaccharides, (3) cordycepin, (4) adenosine, (5) guanosine, (6) cordymin, (7) lovastatin, (8) γ-aminobutyric acid (GABA), (9) sitosterol, (10) ergosterol, (11) ergosta-4,6,8(14),22-tetraen-3-one(ergone), (12) 5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol, (13) 5α, 8α- epidioxy-22E-ergosta-6,9(11),22-trien-3β-ol, (14) 5α,6α-epoxy-5α-ergosta-7,22-dien-3β-ol, (15) 5α,8α-epidioxy- 24(R)-methylcholesta-6,22-dien-3β-D-glucopyranoside, (16),6-epoxy-24(R)-methylcholesta-7,22-dien-3β-ol, (17) myriocin, (18) melanin, (19) cordysinin A, (20) cordysinin B, (21) cordysinin C, (22) cordysinin D, (23) cordysinin E, and (24) serine protease.
Table 1.
Bioactive ingredients, bioactivities and material sources of O. sinensis
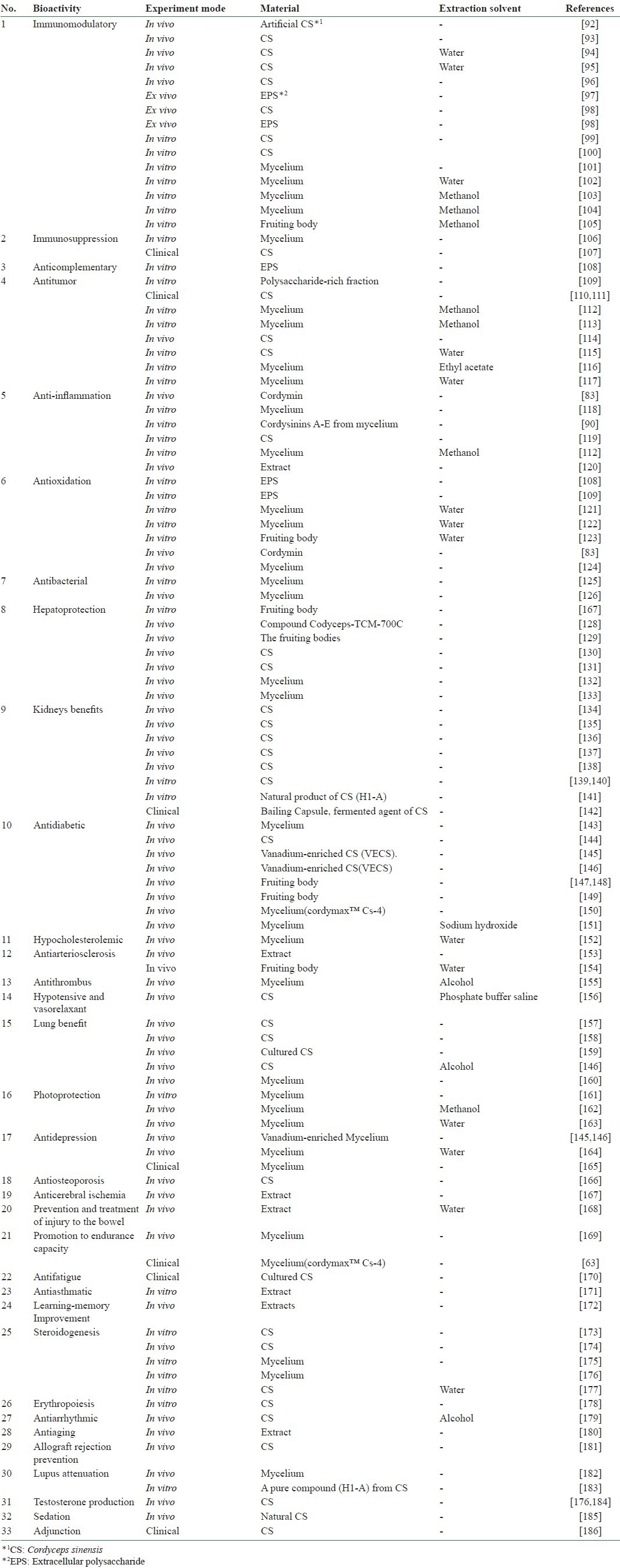
The bioactivities of O. sinensis, which have been identified from materials derived from different preparations or extracts that were tested in in vitro, in vivo, or ex vivo studies, are shown in Table 2. Over 30 different bioactivities have been reported for O. sinensis, including (1) immunomodulatory, (2) immunosuppressive, (3) anticomplementary, (4) antitumor, (5) anti-inflammatory, (6) antioxidant, (7) antibacterial, (8) hepatoprotection, (9) kidney benefitting, (10) antidiabetes, (11) hypocholesterolemia, (12) antiarteriosclerosis, (13) antithrombus, (14) hypotension and vasorelaxant, (15) lung benefitting, (16) photoprotection, (17) antidepression, (18) antiosteoporosis, (19) anticerebral ischemia, (20) antifatigue, (21) antiasthma, (22) steroidogenesis, (23) erythropoiesis, (24) antiarrhythmia, (25) antiaging, (26) testosterone production, (27) sedation, and (28) adjunction, as well as the ability to do the following: (29) prevent and treat injury to the bowel, (30) promote endurance capacity, (31) improve learning-memory, (32) prevent allograft rejection, and (33) attenuate lupus.
Table 2.
Bioactivities, experimental modes, materials and extraction solvents of O. sinensis
It has been found that most local folk/traditional healers use Cordyceps to treat 21 ailments, including erectile dysfunction, female aphrodisia, malignant tumors, bronchial asthma, bronchitis, diabetes, cough and cold, jaundice, alcoholic hepatitis, and others.[10] The traditional uses of caterpillar fungus among the Bai, Naxi, Lisu, and Tibetan people living in the mountainous Northern Yunnan Province are to improve eyesight, to treat calcium deficiency (specific to children), diabetes and associated nephropathy, and indigestion (specific to children), to speed up labor parturition, and to strengthen the immune system.[9] Moreover, C. sinensis exhibits broad biological and pharmacological actions in hepatic, renal, cardiovascular, and immunological systems, and possesses anticancer activity as well.[21]
Polysaccharides
Evidence indicates that Cordyceps polysaccharides may effectively improve immune system function and possess liver protection, antihyperglycemia, hyperlipidemia, antitumor and antioxidant activities.[1]
Extracellular polysaccharides
There are several different Extracellular polysaccharides (EPS) from O. sinensis that are based on their molecular weights (MW). EPS obtained from submerged O. sinensis culture supernatant with MW ranging from 5 to 200 kDa has antioxidant and immunomodulatory activities and may attenuate renal failure, described as follows. The EPS designated EPS-1 with an average MW of 38 kDa was hydrolyzed in diluted sulfuric acid solution at pH 1 and 90°C to yield two major MW fractions: 3.0 kDa and 30 kDa possessed high (30-80%) antioxidant and radical-scavenging activities.[60] An acidic polysaccharide AEPS-1, fractionated from the EPS produced by C. sinensis Cs-HK1 in mycelial culture, was composed of glucopyranose (Glcp) and pyrano-glucuronic acid (GlcUp) in an 8:1 M ratio plus a trace amount of mannose, which has an average MW of 36 kDa and significantly stimulated the release of several major cytokines that may have immunomodulatory properties.[57]
The polysaccharide CPS-2, mostly composed of α-(1→4)-d-glucose and α-(1→3)-d-mannose and branched with α-(1→4, 6)-d-glucose every 12 residues, has an average MW of 43.9 kDa and has been shown to significantly relieve renal failure.[73] A polysaccharide with a MW of 82 kDa that was isolated from the culture medium of Cordyceps, namely cordysinocan, and contained glucose, mannose, and galactose in a 2.4:2:1 ratio induced cell proliferation and the secretion of interleukin (IL)-2, IL-6, and IL-8.[54] In another study, EPS consisting of mannose, glucose, and galactose in a ratio of 23:1:2.6 with a MW of about 104 kDa had the ability to stimulate cytokine expression in immunocytes.[56] Moreover, EPS composed of polysaccharide-protein complexes with a β-D-glucan backbone and a wide range of MWs (5 kDa to more than 200 kDa) had moderate antioxidant activities.[59]
Intracellular polysaccharides
Intracellular polysaccharides (IPS), extracted from O. sinensis mycelia and containing 1,3-β-D-, 1,3-α-D-, or 1,4-α-D-glucan with 1,6-branched chains, had MWs ranging from 7.7 to 1180 kDa depending on the extract conditions. Structural analyses showed that insoluble polysaccharides with 1, 3-β-D-glucan contained some 1, 6-branched chains with an average particle diameter of 1.5 μm.[187] The D-glucan consisted of a backbone with (1→4)-D-glucosyl residues and carried a single (1→6)-linked D-glucosyl residue; and these α-D-glucosidic linkages were present in the polysaccharide with short exterior chains.[188] The neutral mannoglucan with a MW of 7.7 k Da consisted of Man and Glc units in the molar ratio of 1:9, a α-D-glucan backbone with (1→4)- and (1→3)-linkages, and side chains of α-D-(1→6)-Manp, which attached to the backbone via O-6 of α-(1→3)-Glcp residues.[189]
The polysaccharide CS-F10, purified from a hot water extract, was composed of galactose, glucose, and mannose in a molar ratio of 43:33:24, and its MW was estimated to be about 15 kDa, which has a comb-type structure with α-D-glucopyranosyl residues on the termini of the side-chains and characteristic sugar residues, such as 1, 5-linked β-D-galactofuranosyl residues.[190] Hot water extracts of mycelia (WIPS) and alkaline extracts of mycelia (AIPS) were characterized as α-d-glucans with a backbone of (1→4)-linked α-D-Glcp and similar MWs (WIPS 1180 kDa; AIPS 1150 kDa); and WIPS had a short branch of (1→6)-linked α-D-Glcp, and AIPS was a linear glucan, which is distinct from the branched structures of most glucans from medicinal fungi.[61]
IPS from C. sinensis mycelia with MWs ranging from 8.1 to 460 kDa have antioxidant, anti-inflammatory, immunomodulatory, hypoglycemic, and hypocholesterolemic activities, described as following. Antioxidant polysaccharide CPS1 was found to be a glucomannogalactan with a monosaccharide composed of glucose, mannose, and galactose in a ratio of 2.8:2.9:1, and its total carbohydrate content and average MW were 99.0% and 8.1 kDa, respectively.[72] The anti-inflammatory polysaccharide fraction CME-1, has a MW of 27.6 kDa and containing mannose and galactose in a ratio of 4:6.[191] The hypoglycemic polysaccharides obtained from a hot-water extract and alkaline extracts were found to be composed of galactose, glucose, and mannose in a ratio of 62:28:10 with a MW 45 kDa.[67] In addition, the isolated IPS composed of D-Glc, D-Man, L-Ara, and D-Gal in a molar ratio of 8:90:1:1 with an average MW of 83 kDa has been shown to have immunomodulatory potential.[65] A polysaccharide with MW 210 kDa may protect against free radical-induced neuronal cell toxicity.[192] Another polysaccharide with a MW of 210 kDa that was isolated and named CSP-1 is composed of glucose, mannose, and galactose in the ratio of 1:0.6:0.75 and was shown to have strong antioxidation activity and the abilities to decrease blood glucose and insulin secretion in diabetic animals.[68] The heteropolysaccharide PS-A, composed of D-glucose, D-galactose, and D-mannose in a molar ratio of 2:1:1 with a MW of 460 kDa, was shown to possess strong inhibitory activity against cholesterol esterase and may be a potential agent to control hypercholesterolemia.[74]
Nucleosides
It has been demonstrated that the nucleoside contents, including uracil, uridine, hypoxanthine, inosine, guanosine, adenosine, adenine, and cordycepin, of natural and cultured Cordyceps could be separated into two individual sub-groups, which suggested that the chemical characteristics of the cultured mycelia of different fungal strains isolated from natural C. sinensis were similar but were different from natural mycelia.[193] The perithecium of C. sinensis was found to have considerably higher amounts of total nucleosides and nucleobases compared to other parts of this fungus.[194] The content of the four active nucleosides, specifically adenosine, guanosine, cytidine, and thymidine, in C. sinensis was lower than that of cultured Cordyceps.[195] Moreover, there is a positive correlation between nucleoside content in C. sinensis and the growth altitude.[46]
Adenosine
Adenosine was abundant in the fruiting body and was considerably more abundant than in the corpus of natural DongChongXiaCao and the mycelia of C. sinensis.[196] It has been shown that the amount of adenosine in Cordyceps ranges from 0.28 to 14.15 mg/g.[197] The concentration of adenosine 2.45 ± 0.03 mg/g in C. militaris fruiting bodies was found to be higher than those of 1.643 ± 0.03 mg/g in natural C. sinensis, while the content of the fermented mycelia 1.592 ± 0.03 mg/g of C. militaris was similar to those of natural C. sinensis.[198]
Cordycepin
Studies have shown that cordycepin is abundant in cultured C. militaris (2.28 ± 0.84 mg/g) and sparse in natural C. sinensis; however, there is an undetectable amount in cultured C. sinensis.[199] The amount of cordycepin in Cordyceps was found to range from 0.006 mg/g to 6.36 mg/g.[196] In cultured mycelia and the fruiting body of Cordyceps, cordycepin contents were lower and varied from 0.006 to 1.64 mg/g.[196,198] The cordycepin content of the mycelia of C. sinensis cultured in potato dextrose agar (PDA) medium and Finger millet medium were 0.075 mg/g and 0.021 mg/g, respectively; however, the cordycepin content in natural C. sinensis was higher than in the mycelia cultured with PDA medium.[200] It has been indicated that the concentration of cordycepin in C. militaris fruiting bodies is higher than in natural C. sinensis, and the concentration of cordycepin in fermented C. militaris mycelia is similar to that in natural C. sinensis: the mean cordycepin contents in the fruiting bodies of C. militaris and C. sinensis were found to be 2.65 and 1.64 mg/g, respectively, and the content in C. militaris mycelia was 1.59 mg/g.[198] In addition, cultured mycelia and natural specimens of C. sinensis contained similar amounts of cordycepin.[42] When extracted with 50% methanol-chloroform, the cordycepin content of cultured mycelia varied from 0.002% to 0.029% (i.e., 0.02-0.29 mg/g) in twenty-one isolates, and the cordycepin content of natural Dong Chong Xia Cao varied from 0.004% to 0.006% (i.e., 0.04-0.06 mg/g).[201]
Mannitol
Mannitol is the so-called “ChongCao Acid” in Chinese and is also improperly named as “cordycepic acid.” When searching for the terms “mannitol” plus “Cordyceps” in the Scopus database, a limited number of articles can be found. Li et al,[202] indicated that the D-mannitol contents in natural fruiting bodies of C. sinensis, mycelia from shaking cultures, and fruiting bodies from artificial cultivation of H. beakdumountainsi were 8.9, 11.5, and 10.2%, respectively. For in vitro-cultured C. sinensis, the amount of D-mannitol yielded was almost the same as that in natural samples.[42] Moreover, when C. sinensis was cultured with millet, the D-mannitol content achieved levels as high as that in C. militaris fruiting bodies.[203] There was no obvious difference in the amount of nucleosides between cultured O. sinensis mycelia and natural products; however, natural products were shown to have a significantly higher D-mannitol content compared with submerged culture mycelia.[204]
Likewise, when searching for the terms “cordycepic acid” plus “Cordyceps” in the Scopus database, a limited articles can be found. The chemical constituents of natural Cordyceps, including cordycepic acid, have been described.[13] Dong et al.[205] showed that the superoxide dismutase activity and the content of cordycepic acid in the fruiting bodies of C. militaris were dependent on the sodium selenite concentration in the culture medium. It has been reported that C. sinensis has many bioactive components, such as 3′-deoxyadenosine, cordycepic acid, and Cordyceps polysaccharides.[3] In addition, yields of cordycepic acid from C. jiangxiensis, C. taii, and C. gunnii were found to be 11.81%, 8.72%, and 4.73%, respectively, of the dry weight of mycelia.[206]
Amino acids
The total amount of amino acids in fermented mycelia was determined to be 9.23%, which is lower than that in wild DongChongXiaCao (18.1%) but is similar to that in the fruiting body of wild DongChongXiaCao.[46] The three principal amino acids in the corpus-fruiting body are glutamic acid, aspartic acid, and arginine, and their contents are 2.64–2.66%, 1.70–1.84%, and 1.53–1.60%, respectively.[46] A mixture with 18 synthetic amino acids to mimic the amino acid composition in natural C. sinensis showed the same sedative action as natural C. sinensis.[185]
Sterols
The ergosterol content in the stroma of C. sinensis was found to be approximately 0.92 g/L and was about three times higher than that found in the sclerotium.[207] The fruiting body (CsA) and the host caterpillar (CsB) of C. sinensis had similar ergosterol compositions, but the level of ergosteryl esters in CsB was much higher than in CsA, these data indicated that CsA and CsB might exist in different growth phases and have different physiological functions for the growth and multiplication of C. sinensis.[208] It has been shown that ergosta-4,6,8(14),22-tetraen-3-1 may induce G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells.[86] 5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol, 5α,8α-epidioxy-22E-ergosta-6,9(11),22-trien-3β-ol, and 5α,6α-epoxy-5α-ergosta-7,22-dien-3β-ol isolated from the ethyl acetate fraction with a peroxide ring or an epoxide ring had substantial cytotoxic activity.[209] In addition, 5α,8α-epidioxy-24(R)-methylcholesta-6, 22-dien-3β-D-glucopyranoside, and 5,6-epoxy-24(R)-methylcholesta-7,22-dien-3β-ol from the methanol extract of C. sinensis had antitumor activity.[87]
Aurantiamides
Two aurantiamides, a new class of potent analgesic and anti-inflammatory agents, named cordyceamides A and B were isolated from the culture liquid of C. sinensis along with one known compound, aurantiamide acetate. The structures of these compounds were elucidated as N-benzoyl-l-tyrosinyl-l-phenylalaninol acetate and N-benzoyl-l-tyrosinyl-l-p- hydroxyphenylalaninol acetate by 1D and 2D-NMR techniques and by the comparison with the literature.[91]
Peptides
Cyclodipeptides
A cyclodipeptide named cordycedipeptide A, which is a natural compound that was isolated from the culture liquid of C. sinensis, had cytotoxic activity against L-929, A375, and Hela cells.[210]
Cordymin
The peptide cordymin from C. sinensis, which has a neuroprotective effect in the ischemic brain due to inhibited inflammation and increased antioxidant activity related to lesion pathogenesis, can be used as a potential preventive agent against cerebral ischemia-reperfusion injury.[83]
Myriocin
Myriocin (also known as the antibiotic ISP-1) is a new type of immune inhibitor extracted from C. sinensis, it was shown to significantly inhibit the upregulated expression of cyclin D1 induced by high concentrations of glucose, restoring the expression of cyclin D1.[88]
Melanin
The antioxidant activity of melanin, derived from a black pigment, was isolated from the fermentation broth of O. sinensis and showed much stronger scavenging abilities for 1,1-diphenyl-2-picrylhydrazyl (DPPH•) and ferrous ion chelation compared to the mycelial water extract.[211]
Lovastatin, γ-aminobutyric acid (GABA), and ergothioneine
Lovastatin, GABA, and ergothioneine are secondary metabolites of fungal growth. Chen et al.[84] indicated that mycelia of C. sinensis contained 1365 mg/kg of lovastatin, 220.5 mg/kg GABA, and detectable ergothioneine and had different hypolipidemia, hypotension, and antioxidant activities.
Cordysinins
Five cordysinins, A-E, from the mycelia of C. sinensis have been identified and have been shown to have anti-inflammatory activities, and 1-(5-Hydroxymethyl-2-furyl)-β-carboline was shown to most significantly inhibit superoxide anion generation and elastase release.[90]
SHAKING CULTURE AND SUBMERGED FERMENTATION OF O. SINENSIS
The maximal production of mycelia, EPS, and exo-biopolymer are important concerns for producers, especially for industry. Several factors that may affect the production of O. sinensis in shaking culture and submerged fermentation, including strain, medium, and culture conditions, have been discussed in different studies. The amounts of mycelial biomass, EPS, and exo-biopolymer range from 11.10 to 62.3 g/l, 0.43 to 3.21 g/l and 22.0 to 28.4 g/l, respectively, described as follows.
It has been observed that Tween 80 can exhibit a remarkable effect on EPS production by increasing EPS yield more than two-fold at 1.5% (w/v); the effects of Tween 80 can probably be attributed to the stimulation of EPS biosynthesis and release from fungal cells.[212] In addition, evidence has shown that palmitic acid may significantly increase the production of biomass and extracellular polysaccharides to 11.10 g/l and 0.43 g/l, respectively.[191] Adding ammonium to the mycelial culture of strain Cs-HK1 may enhance intracellular cordycepin accumulation, which may be attributed to the uptake of ammonia for nucleoside synthesis, and EPS production, which may be attributed to the increased uptake of glucose for EPS biosynthesis.[213]
The optimal medium for the production of mycelia and exo-biopolymers by strain CS 16 is 2% sucrose, 0.9% yeast extract, 0.3% K2HPO4, and 0.4% CaCl2, and this medium was shown to yield maximal mycelia and exo-biopolymer productions of 54.0 g/l and 28.4 g/l, respectively, in shaking-flask cultures.[214] In bioreactor cultures, rapid differentiation and cell lysis occurred when agitation speed was increased. When the agitation speed was maintained at 350 rpm, 62.3 g/l of mycelia and 22.0 g/l of exo-biopolymers were obtained from strain CS 16 in submerged fermentation.[215] In shaking cultures, the maximum polysaccharide production was 3.05 and 3.21 g/l in a flask and a 5-L jar fermentor, respectively, when fungi were supplied with optimal medium.[216] It is believed that the optimal medium for mycelial growth is 50 g/l sucrose, 10 g/l peptone, and 3 g/l yeast extract, which would produce over 22 g/l of mycelial biomass after 40 days of submerged culture at a temperature below 20°C.[217]
CULTURE AND BIOACTIVITY OF HIRSUTELLA SINENSIS OR HIRSUTELLA SP.
There is little literature published on H. sinensis, the anamorph of O. sinensis. This might be due to geographic limitations, difficulty in isolation and cultivation, and slow growth under low temperatures.
Culture
It was shown that most strains of H. sinensis prefer to grow on Sabouraun's dextrose agar, and some of the strains prefer potato-dextrose agar as the medium for optimal development.[218] The optimized conditions and medium for submerged fermentation of Hirsutella sp. have been described as follows: initial pH 5.5, potato extract 20% (w/v), sucrose 2.5%, peptone 0.5%, K2HPO4 0.2%, MgSO4 0.05%, and fermentation for 4 days, the highest production of EPS and mycelial biomass may reach 2.17 and 10.06 g/l, respectively.[49]
Bioactivity
H. sinensis extract (HSW) was shown to have a remarkable action in vitro and in vivo in a model of kidney injury;[219] the extract downregulated transforming growth factor-β1 in renal tissue, antagonized tubular epithelial-myofibroblast transdifferentiation and renal interstitial fibrosis, and improved renal function in chronic aristolochic acid nephropathy rats.[220] Furthermore, the fermented mycelia of H. sinensis significantly prevented spontaneous type 1 diabetes in non-obese diabetic mice.[221] Additionally, H. sinensis was shown to significantly increase animal survival after a lethal dose of radiation, accelerate leukocyte recovery, and stimulate immune lymphocyte proliferation.[222]
ECOLOGY AND HOSTS
O. sinensis is confined to the Tibetan Plateau and its surrounding regions, including Tibet, Gansu, Qinghai, Sichuan, and Yunnan provinces in China, in certain areas of the southern flank of the Himalayas, and in the countries of Bhutan, India, and Nepal. These regions are at altitudes of over 3,000 m. The fungus is distributed from the southernmost site in Yulong Naxi Autonomous County in northwestern Yunnan Province to the northernmost site in the Qilian Mountains in Qilian County, Qinghai Province and from the east edge of the Tibetan Plateau in Wudu County, Gansu Province to the westernmost site in Uttarakhand, India.[202] The yearly yield of C. sinensis in the Naqu district, which is the principal growth zone in Tibet, is 7000 kg, and the elevation of the distribution area is from 5000 m to 4100 m, and the ecological geographical distribution is mainly affected by vegetation, soil, temperature, and humidity.[223]
As hosts, the larvae of Thitarodes spp. play a vital role in supplying nutritional materials for the growth of O. sinensis. In the Tibetan Plateau, more than 40 species of the genus Thitarodes have been recorded since 1958.[224] According to another publication, 57 taxa are recognized as potential hosts of O. sinensis, including 1 Bipectilus, 1 Endoclita, 1 Gazoryctra, 12 Hepialus, 2 Magnificus, 3 Pharmacis, and 37 Thitarodes.[225] The recorded altitude ranges of the recognized potential host insects were found to vary from 2800 to 5100 m, and the distribution areas of these species covered 26 provinces in China and more than 12 other countries.[225] Due to long-term adaptive evolution, O. sinensis and its host insects have developed a living requirement for special high altitude-associated climates and soil conditions, such as sub-ambient atmosphere and soil temperatures and specific solar radiation, barometric pressure, and hypoxic conditions.[224] Studies show that there is genetic differentiation of C. sinensis among different latitudes.[226] The interspecific genetic differentiations are obvious in Hepialu, and the genus Hepialus might be considered the polyphyletic origin for Cytb sequences, which have abundant variations among the host insects of C. sinensis at both the specific and generic levels.[227]
CONCLUSIONS
O. sinensis is a complex fungus with multiple biological functions. Over 20 bioactive ingredients have been found in O. sinensis, such as extracellular polysaccharides, intracellular polysaccharides, cordycepin, adenosine, and sterols, derived from its mycelia, culture supernatant, or fruiting body. In addition, over 30 bioactivities have been indicated, including immunomodulatory, antitumor, anti-inflammation, and antioxidant activities, in preparations or solvent extracts in in vitro, in vivo, or ex vivo studies. However, few publications have given regard to the bioactive ingredients, bioactivities, and the medium and culture conditions of O. sinensis and H. sinensis, which must be incubated for more than 10 days at low temperatures (below 21°C). The slow growth rate at low temperature is a critical characteristic of O. sinensis and H. sinensis. It has been demonstrated that these fungi grow poorly at temperatures above 21°C and stop growing at 25°C or above.
The fungus O. sinensis is not equivalent to the traditional Chinese medicine DongChongXiaCao; the latter develops a fruiting body following the occupation of a dead caterpillar and grows wild in nature. The host caterpillar provides nutrients for O. sinensis, resulting in the formation of a fruiting body in the appropriate climate and environment. The morphology of O. sinensis in submerged culture can be microscopically visualized as mycelia, without the fruiting body. The relationship between O. sinensis and its host in the traditional Chinese medicine DongChongXiaCao, as well as its pathobiology and ecology, still remains unclear.
The mysterious caterpillar fungus O. sinensis has been renamed since 2007; however, many publications still use the name C. sinensis. When the names “Ophiocordyceps sinensis” and ”Cordyceps sinensis” are searched for in article titles, abstracts, and keywords in the Scopus database from the year 2008 to 2012, 84 and 255 articles can be found, respectively. Even though it has been confirmed that H. sinensis is the anamorph of C. sinensis, most articles still use the terms “Cordyceps sinensis,” “Cordyceps,” and “Cordyceps” to refer to the traditional Chinese medicine DongChongXiaCao. Among all of these publications, not one successfully obtained fruiting bodies from caterpillars artificially infected with O. sinensis or H. sinensis. The key identification characteristics for O. sinensis are the existence of a perithecium, ascus, and ascospore in the fruiting body. If the fruiting body cannot be formed from caterpillars infected by O. sinensis or H. sinensis, these key characteristics cannot be identified by microscope. Beginning January 1st, 2013, regulations will allow one fungus to have only one name, and the system of permitting separate names for anamorphs will end.[228] The anamorph name H. sinensis will be changed by the International Code of Nomenclature for algae, fungi, and plants (formerly called the International Code of Botanical Nomenclature)[229] to O. sinensis.
Notification
The term “Cordyceps sinensis” has been renamed to its synonym “Ophiocordyceps sinensis” by Sung et al. in 2007. In the discussion, “Cordyceps sinensis” is still used to represent “Ophiocordyceps sinensis” out of respect to the original authors of the articles that we cited.
Cordyceps spp. indicates any species that belongs to the genus Cordyceps.
Cordyceps sp. indicates the unidentified species that belong to the genus Cordyceps
REFERENCES
- 1.Zhong S, Pan H, Fan L, Lv G, Wu Y, Parmeswaran B, et al. Advances in research of polysaccharides in Cordyceps species. Food Technol Biotechnol. 2009;47:304–12. [Google Scholar]
- 2.Dong CH, Yao YJ. On the reliability of fungal materials used in studies on Ophiocordyceps sinensis. J Ind Microbiol Biotechnol. 2011;38:1027–35. doi: 10.1007/s10295-010-0877-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Gong Z, Su Y, Lin J, Tang K. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61:279–91. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang S, Wang M, Bai F, Liu X. High diversity of the fungal community structure in naturally-occurring Ophiocordyceps sinensis. PLoS ONE. 2010;5:1–8. doi: 10.1371/journal.pone.0015570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Li E, Wang C, Li Y, Liu X. Ophiocordyceps sinensis, the flagship fungus of China: Terminology, life strategy and ecology. Mycology. 2012;3:2–10. [Google Scholar]
- 6.Shrestha B, Zhang W, Zhang Y, Liu X. What is the Chinese caterpillar fungus Ophiocordyceps sinensis (Ophiocordycipitaceae)? Mycology. 2010;1:228–36. [Google Scholar]
- 7.Jin C, Wu X, Chen G. Clinical application of Jinshuibao capsule. Cap Med. 2005;12:42–3. [Google Scholar]
- 8.Xu H, Li S. Pharmacological effects of Bailing capsule and its application in lung disease research. Zhongguo Zhong Yao Za Zhi. 2010;35:2777–81. [PubMed] [Google Scholar]
- 9.Chen J, Lee S, Cao Y, Peng Y, Winkler D, Yang D. Ethnomycological use of medicinal chinese caterpillar fungus, Ophiocordyceps sinensis (Berk.) G. H. Sung et al. (ascomycetes) in Northern Yunnan Province, SW China. Int J Med Mushrooms. 2010;12:427–34. [Google Scholar]
- 10.Panda AK, Swain KC. Traditional uses and medicinal potential of Cordyceps sinensis of Sikkim. J Ayurveda Integr Med. 2011;2:9–13. doi: 10.4103/0975-9476.78183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon PF, Hywel-Jones NL, Maczey N, Norbu L, Tshitila, Samdup T, et al. Steps towards sustainable harvest of Ophiocordyceps sinensis in Bhutan. Biodivers Conserv. 2009;18:2263–81. [Google Scholar]
- 12.Au D, Wang L, Yang D, Mok DK, Chan AS, Xu H. Application of microscopy in authentication of valuable Chinese medicine I-Cordyceps sinensis, its counterfeits, and related products. Microsc Res Tech. 2012;75:54–64. doi: 10.1002/jemt.21024. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S. Trade of Cordyceps sinensis from high altitudes of the Indian Himalaya: Conservation and biotechnological priorities. Curr Sci. 2004;86:1614–9. [Google Scholar]
- 14.Winkler D. Yartsa Gunbu (Cordyceps sinensis) and the fungal commodification of Tibet's rural economy. Econ Bot. 2008;62:291–305. [Google Scholar]
- 15.Winkler D. Cordyceps sinensis: A precious parasitic fungus infecting Tibet. Field Mycol. 2010;11:60–7. [Google Scholar]
- 16.Winkler D. Caterpillar fungus (Ophiocordyceps sinensis) production and sustainability on the Tibetan Plateau and in the Himalayas. Asian Med. 2009;5:291–316. [Google Scholar]
- 17.Liu F, Wu XL, Chen SJ, Yin DH, Zeng W, Zhong GY. Advances in studies on artificial culture of Cordyceps sinensis. Chin Tradit Herb Drugs. 2007;38:302–5. [Google Scholar]
- 18.Xu FL, Huan WY, Wu TX, Qiu X, Zhang H, Liu XH. Clinical efficacy of Cordyceps sinensis for chronic kidney diseases: A systematic review. Chin J Evid Based Med. 2006;6:804–8. [Google Scholar]
- 19.Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–84. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Canney S. Cordyceps sinensis animal, vegetable or both? J Chin Med. 2006;80:43–9. [Google Scholar]
- 21.Wang SY, Shiao MS. Pharmacological functions of Chinese medicinal fungus Cordyceps sinensis and related species. J Food Drug Anal. 2000;8:248–57. [Google Scholar]
- 22.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis Part II. J Altern Complement Med. 1998;4:429–57. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 23.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha B, Zhang W, Zhang Y, Liu X. The medicinal fungus Cordyceps militaris: Research and development. Mycol Prog. 2012;11:599–614. [Google Scholar]
- 25.Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia. 2010;81:961–8. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Liu JL. Advances in studies on chemical constituents of Cordyceps. Chin Tradit Herb Drugs. 2009;40:1157–60. [Google Scholar]
- 27.Xiao JH. Current status and ponderation on preparations and chemical structures of polysaccharide in fungi of Cordyceps (Fr.) Link. Chin Tradit Herb Drugs. 2008;39:454–60. [Google Scholar]
- 28.Paterson RRM. Cordyceps - A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69:1469–95. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holliday JC, Cleaver M. Medicinal value of the caterpillar fungi species of the genus Cordyceps (Fr.) link (Ascomycetes). A review. Int J Med Mushrooms. 2008;10:219–34. [Google Scholar]
- 30.Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509–19. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 31.Siu KM, Mak DH, Chiu PY, Poon MK, Du Y, Ko KM. Pharmacological basis of ‘Yin-nourishing’ and ‘Yang-invigorating’ actions of Cordyceps, a Chinese tonifying herb. Life Sci. 2004;76:385–95. doi: 10.1016/j.lfs.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Sung GH, Hywel-Jones NL, Sung JM, Luangsa-Ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y, Yao YJ. Names related to Cordyceps sinensis anamorph. Mycotaxon. 2002;84:245–54. [Google Scholar]
- 34.Chen YQ, Wang N, Qu LH, Li TH, Zhang WM. Determination of the anamorph of Cordyceps sinensis inferred from the analysis of the ribosomal DNA internal transcribed spacers and 5.8S rDNA. Biochem Syst Ecol. 2001;29:597–607. doi: 10.1016/s0305-1978(00)00100-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen YQ, Wang N, Zhou H, Qu LH. Differentiation of medicinal Cordyceps species by rDNA ITS sequence analysis. Planta Med. 2002;68:635–9. doi: 10.1055/s-2002-32892. [DOI] [PubMed] [Google Scholar]
- 36.Liu ZY, Yao YJ, Liang ZQ, Liu AY, Pegler DN, Chase MW. Molecular evidence for the anamorph-teleomorph connection in Cordyceps sinensis. Mycol Res. 2001;105:827–32. [Google Scholar]
- 37.Liu ZY, Liang ZQ, Liu AY, Yao YJ, Hyde KD, Yu ZN. Molecular evidence for teleomorph-anamorph connections in Cordyceps based on ITS-5.8S rDNA sequences. Mycol Res. 2002;106:1100–8. [Google Scholar]
- 38.Kinjo N, Zang M. Morphological and phylogenetic studies on Cordyceps sinensis distributed in southwestern China. Mycoscience. 2001;42:567–74. [Google Scholar]
- 39.Chen YQ, Hu B, Xu F, Zhang W, Zhou H, Qu LH. Genetic variation of Cordyceps sinensis, a fruit-body-producing entomopathogenic species from different geographical regions in China. FEMS Microbiol Lett. 2004;230:153–8. doi: 10.1016/S0378-1097(03)00889-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhu JS, Yao YS, Chen W, Zheng TY, Lu JH, Guo YL. Molecular co-existence of Paecitomyces hepiali and Hirsutella sinensis in caterpillar and fruiting bodies of Cordyceps sinensis. FASEB J. 2007;21:A1079. [Google Scholar]
- 41.Zhang Y, Xu L, Zhang S, Liu X, An Z, Wang M, et al. Genetic diversity of Ophiocordyceps sinensis, a medicinal fungus endemic to the Tibetan Plateau: Implications for its evolution and conservation. BMC Evol Biol. 2009;9:290. doi: 10.1186/1471-2148-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh R, Negi PS, Ahmed Z. Genetic variability assessment in medicinal caterpillar fungi Cordyceps spp. (Ascomycetes) in central himalayas, India. Int J Med Mushrooms. 2009;11:185–9. [Google Scholar]
- 43.Liang HH, Cheng Z, Yang XL, Li S, Ding ZQ, Zhou TS, et al. Genetic diversity and structure of Cordyceps sinensis populations from extensive geographical regions in China as revealed by inter-simple sequence repeat markers. J Microbiol. 2008;46:549–56. doi: 10.1007/s12275-008-0107-1. [DOI] [PubMed] [Google Scholar]
- 44.Stensrud Ø, Schumacher T, Shalchian-Tabrizi K, Svegården IB, Kauserud H. Accelerated nrDNA evolution and profound AT bias in the medicinal fungus Cordyceps sinensis. Mycol Res. 2007;111:409–15. doi: 10.1016/j.mycres.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Yang FQ, Feng K, Zhao J, Li SP. Analysis of sterols and fatty acids in natural and cultured Cordyceps by one-step derivatization followed with gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2009;49:1172–8. doi: 10.1016/j.jpba.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Hsu TH, Shiao LH, Hsieh C, Chang DM. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom DongChongXiaCao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chem. 2002;78:463–9. [Google Scholar]
- 47.Smirnov DA, Shcherba VV, Bisko NA, Poyedinok NL. Some Biologically Active Substances from a Mycelial Biomass of Medicinal Caterpillar Fungus Cordyceps sinensis (Berk.) Sacc. (Ascomycetes) Int J Med Mushrooms. 2009;11:69–76. [Google Scholar]
- 48.Li C, Li Z, Fan M, Cheng W, Long Y, Ding T, et al. The composition of Hirsutella sinensis, anamorph of Cordyceps sinensis. J Food Compost Anal. 2006;19:800–5. [Google Scholar]
- 49.Li R, Jiang XL, Guan HS. Optimization of mycelium biomass and exopolysaccharides production by Hirsutella sp in submerged fermentation and evaluation of exopolysaccharides antibacterial activity. Afr J Biotechnol. 2010;9:196–203. [Google Scholar]
- 50.Yu S, Zhang Y, Fan M. Analysis of volatile compounds of mycelia of Hirsutella sinensis, the anamorph of Ophiocordyceps sinensis. Appl Mech Mater. 2012;140:253–7. [Google Scholar]
- 51.Song D, He Z, Wang C, Yuan F, Dong P, Zhang W. Regulation of the exopolysaccharide from an anamorph of Cordyceps sinensis on dendritic cell sarcoma (DCS) cell line. Eur J Nutr. 2012 doi: 10.1007/s00394-012-0373-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Yoon TJ, Yu KW, Shin KS, Suh HJ. Innate immune stimulation of exo-polymers prepared from Cordyceps sinensis by submerged culture. Appl Microbiol Biotechnol. 2008;80:1087–93. doi: 10.1007/s00253-008-1607-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Yang J, Chen J, Hou Y, Han X. Immunomodulatory and antitumour effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumour-bearing mice. Biotechnol Appl Biochem. 2005;42:9–15. doi: 10.1042/BA20040183. [DOI] [PubMed] [Google Scholar]
- 54.Cheung JK, Li J, Cheung AW, Zhu Y, Zheng KY, Bi CW, et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J Ethnopharmacol. 2009;124:61–8. doi: 10.1016/j.jep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Kuo MC, Chang CY, Cheng TL, Wu MJ. Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: Enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl Microbiol Biotechnol. 2007;75:769–75. doi: 10.1007/s00253-007-0880-5. [DOI] [PubMed] [Google Scholar]
- 56.Sheng L, Chen J, Li J, Zhang W. An exopolysaccharide from cultivated Cordyceps sinensis and its effects on cytokine expressions of immunocytes. Appl Microbiol Biotechnol. 2011;163:669–78. doi: 10.1007/s12010-010-9072-3. [DOI] [PubMed] [Google Scholar]
- 57.Wang ZM, Peng X, Lee KL, Tang JC, Cheung PC, Wu JY. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011;125:637–43. [Google Scholar]
- 58.Zhang W, Li J, Qiu S, Chen J, Zheng Y. Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia. 2008;79:168–73. doi: 10.1016/j.fitote.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Leung PH, Zhao S, Ho KP, Wu JY. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2009;114:1251–6. [Google Scholar]
- 60.Yan JK, Li L, Wang ZM, Leung PH, Wang WQ, Wu JY. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chem. 2009;117:641–6. [Google Scholar]
- 61.Yan JK, Wang WQ, Li L, Wu JY. Physiochemical properties and antitumor activities of two α-glucans isolated from hot water and alkaline extracts of Cordyceps (Cs-HK1) fungal mycelia. Carbohydr Polym. 2011;85:753–8. [Google Scholar]
- 62.Chen SD, Lin SY, Lai YS, Cheng YH. Effect of Cordyceps sinensis adlay fermentative products on antioxidant activities and macrophage functions. Taiwan J Agric Chem Food Sci. 2008;46:223–33. [Google Scholar]
- 63.Chen S, Li Z, Krochmal R, Abrazado M, Kim W, Cooper CB. Effect of Cs-4® (Cordyceps sinensis) on exercise performance in healthy older subjects: A double-blind, placebo-controlled trial. J Altern Complement Med. 2010;16:585–90. doi: 10.1089/acm.2009.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W, Zhang W, Shen W, Wang K. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell Immunol. 2010;262:69–74. doi: 10.1016/j.cellimm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, Sun H, Qin F, Pan Y, Sun C. Effect of various extracts and a polysaccharide from the edible mycelia of Cordyceps sinensis on cellular and humoral immune response against ovalbumin in mice. Phytother Res. 2006;20:646–52. doi: 10.1002/ptr.1921. [DOI] [PubMed] [Google Scholar]
- 66.Huang ZJ, Ji H, Li P, Xie L, Zhao XC. Hypoglycemic effect and mechanism of polysaccharides from cultured mycelium of Cordyceps sinensis. J China Pharm Univ. 2002;33:51–4. [Google Scholar]
- 67.Kiho T, Hui J, Yamane A, Ukai S. Polysaccharides in Fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol Pharm Bull. 1993;16:1291–3. doi: 10.1248/bpb.16.1291. [DOI] [PubMed] [Google Scholar]
- 68.Li SP, Zhang GH, Zeng Q, Huang ZG, Wang YT, Dong TT, et al. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine. 2006;13:428–33. doi: 10.1016/j.phymed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Chen J, Zhang W, Lu T, Li J, Zheng Y, Kong L. Morphological and genetic characterization of a cultivated Cordyceps sinensis fungus and its polysaccharide component possessing antioxidant property in H22 tumor-bearing mice. Life Sci. 2006;78:2742–8. doi: 10.1016/j.lfs.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Wang G, Zhang J, Zhang G, Jia L, Liu X, et al. Extraction optimization and antioxidant activity of intracellular selenium polysaccharide by Cordyceps sinensis SU-02. Carbohydr Polym. 2011;86:1745–50. [Google Scholar]
- 71.Wang SH, Yang WB, Liu YC, Chiu YH, Chen CT, Kao PF, et al. A potent sphingomyelinase inhibitor from Cordyceps mycelia contributes its cytoprotective effect against oxidative stress in macrophages. J Lipid Res. 2011;52:471–9. doi: 10.1194/jlr.M011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Wang M, Ling Y, Fan W, Yin H. Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am J Chin Med. 2009;37:977–89. doi: 10.1142/S0192415X09007387. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Yin H, Lv X, Gao H, Wang M. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia. 2010;81:397–402. doi: 10.1016/j.fitote.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Kim SD. Isolation, structure and cholesterol esterase inhibitory activity of a polysaccharide, PS-A, from Cordyceps sinensis. J Appl Biol Chem. 2010;53:784–9. [Google Scholar]
- 75.Kiho T, Yamane A, Hui J, Usui S, Ukai S. Polysaccharides in fungi.XXXVI. 1 Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol Pharm Bull. 1996;19:294–6. doi: 10.1248/bpb.19.294. [DOI] [PubMed] [Google Scholar]
- 76.Leu SF, Poon SL, Pao HY, Huang BM. The in vivo and in vitro stimulatory effects of cordycepin on mouse Leydig cell steroidogenesis. Biosci Biotechnol Biochem. 2011;75:723–31. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 77.Pao HY, Pan BS, Leu SF, Huang BM. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C pathway. J Agric Food Chem. 2012;60:4905–13. doi: 10.1021/jf205091b. [DOI] [PubMed] [Google Scholar]
- 78.Kubo E, Sato A, Yoshikawa N, Kagota S, Shinozuka K, Nakamura K. Effect of Cordyceps sinensis on TIMP-1 secretion from mouse melanoma cell. Cent Eur J Biol. 2012;7:167–71. [Google Scholar]
- 79.Chen Y, Chen YC, Lin YT, Huang SH, Wang SM. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J Agric Food Chem. 2010;58:11645–52. doi: 10.1021/jf1028976. [DOI] [PubMed] [Google Scholar]
- 80.Yoshikawa N, Nakamura K, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Cordycepin and Cordyceps sinensis reduce the growth of human promyelocytic leukaemia cells through the Wnt signalling pathway. Clin Exp Pharmacol Physiol. 2007;34:S61–3. [Google Scholar]
- 81.Zhou X, Luo L, Dressel W, Shadier G, Krumbiegel D, Schmidtke P, et al. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am J Chin Med. 2008;3:967–80. doi: 10.1142/S0192415X08006387. [DOI] [PubMed] [Google Scholar]
- 82.Yu L, Zhao J, Zhu Q, Li SP. Macrophage biospecific extraction and high performance liquid chromatography for hypothesis of immunological active components in Cordyceps sinensis. J Pharm Biomed Anal. 2007;44:439–43. doi: 10.1016/j.jpba.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Liu YM, Cao W, Yao KW, Liu ZQ, Guo JY. Anti-inflammation and antioxidant effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab Brain Dis. 2012;27:159–65. doi: 10.1007/s11011-012-9282-1. [DOI] [PubMed] [Google Scholar]
- 84.Chen SY, Ho KJ, Hsieh YJ, Wang LT, Mau JL. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT - Food Sci Technol. 2012;47:274–8. [Google Scholar]
- 85.Matsuda H, Akaki J, Nakamura S, Okazaki Y, Kojima H, Tamesada M, et al. Apoptosis-inducing effects of sterols from the dried powder of cultured mycelium of Cordyceps sinensis. Chem Pharm Bull. 2009;57:411–4. doi: 10.1248/cpb.57.411. [DOI] [PubMed] [Google Scholar]
- 86.Zhao YY, Shen X, Chao X, Ho CC, Cheng XL, Zhang Y, et al. Ergosta-4,6,8(14),22-tetraen-3-one induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Biochim Biophys Acta. 2011;1810:384–90. doi: 10.1016/j.bbagen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Bok JW, Lermer L, Chilton J, Klingeman HG, Towers GH. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–8. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 88.Xiao ZH, Zhou JH, Wu HS. Effect of myriocin on the expression of cyclinD1 in high glucose-induced hypertrophy mesangial cells. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:677–9. [PubMed] [Google Scholar]
- 89.Li HP, Hu Z, Yuan JL, Fan HD, Chen W, Wang SJ, et al. A novel extracellular protease with fibrinolytic activity from the culture supernatant of Cordyceps sinensis: Purification and characterization. Phytother Res. 2007;21:1234–41. doi: 10.1002/ptr.2246. [DOI] [PubMed] [Google Scholar]
- 90.Yang ML, Kuo PC, Hwang TL, Wu TS. Anti-inflammatory principles from Cordyceps sinensis. J Nat Prod. 2011;74:1996–2000. doi: 10.1021/np100902f. [DOI] [PubMed] [Google Scholar]
- 91.Jia JM, Tao HH, Feng BM. Cordyceamides A and B from the culture liquid of Cordyceps sinensis (Berk.) Sacc. Chem Pharm Bull (Tokyo) 2009;57:99–101. doi: 10.1248/cpb.57.99. [DOI] [PubMed] [Google Scholar]
- 92.Zhong J, Zhang Y, Ding Z, Ye K. Effect of polysaccharide extract from artificial Cordyceps sinenisis on immune function of mouse. Zhongshan Da Xue Xue Bao Zi Ran Ke Xue Ban. 2011;50:99–102. [Google Scholar]
- 93.Shi B, Wang Z, Jin H, Chen YW, Wang Q, Qian Y. Immunoregulatory Cordyceps sinensis increases regulatory T cells to Th17 cell ratio and delays diabetes in NOD mice. Int Immunopharmacol. 2009;9:582–6. doi: 10.1016/j.intimp.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 94.Park DK, Choi WS, Park PJ, Kim EK, Jeong YJ, Choi SY, et al. Immunoglobulin and cytokine production from mesenteric lymph node lymphocytes is regulated by extracts of Cordyceps sinensis in C57Bl/6N mice. J Med Food. 2008;11:784–8. doi: 10.1089/jmf.2007.0550. [DOI] [PubMed] [Google Scholar]
- 95.Liu WC, Chuang WL, Tsai ML, Hong JH, Mcbride WH, Chiang CS. Cordyceps sinensis health supplement enhances recovery from taxol-induced leukopenia. Exp Biol Med. 2008;233:447–55. doi: 10.3181/0708-RM-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng Q. Effect of Cordyceps sinensis on cellular immunity in rats with chronic renal insufficiency. Zhonghua Yi Xue Za Zhi. 1992;72:27–9. 63. [PubMed] [Google Scholar]
- 97.Song D, Lin J, Yuan F, Zhang W. Ex vivo stimulation of murine dendritic cells by an exopolysaccharide from one of the anamorph of Cordyceps sinensis. Cell Biochem Funct. 2011;29:555–61. doi: 10.1002/cbf.1787. [DOI] [PubMed] [Google Scholar]
- 98.Huang Z, Jin J, Tong X, Yang Q, Gu B, Wang J, et al. The immunomodulatory effects of Cordyceps sinensis on dendritic cells derived from chronic myelogenous leukemia (CML) J Med Plant Res. 2011;5:5925–32. [Google Scholar]
- 99.Kawanishi T, Ikeda-Dantsuji Y, Nagayama A. Effects of two basidiomycete species on interleukin 1 and interleukin 2 production by macrophage and T cell lines. Immunobiology. 2010 doi: 10.1016/j.imbio.2009.10.005. 215516-20. [DOI] [PubMed] [Google Scholar]
- 100.Jordan JL, Sullivan AM, Lee TD. Immune activation by a sterile aqueous extract of Cordyceps sinensis: Mechanism of action. Immunopharmacol Immunotoxicol. 2008;30:53–70. doi: 10.1080/08923970701812332. [DOI] [PubMed] [Google Scholar]
- 101.Kuo CF, Chen CC, Lin CF, Jan MS, Huang RY, Luo YH, et al. Abrogation of streptococcal pyrogenic exotoxin B-mediated suppression of phagocytosis in U937 cells by Cordyceps sinensis mycelium via production of cytokines. Food Chem Toxicol. 2007;45:278–85. doi: 10.1016/j.fct.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Koh JH, Yu KW, Suh HJ, Choi YM, Ahn TS. Activation of macrophages and the intestinal immune system by an orally administered decoction from cultured mycelia of Cordyceps sinensis. Biosci Biotechnol Biochem. 2002;66:407–11. doi: 10.1271/bbb.66.407. [DOI] [PubMed] [Google Scholar]
- 103.Kuo YC, Tsai WJ, Wang JY, Chang SC, Lin CY, Shiao MS. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001;68:1067–82. doi: 10.1016/s0024-3205(00)01011-0. [DOI] [PubMed] [Google Scholar]
- 104.Chiu JH. Cordyceps sinensis increases the expression of major histocompatibility complex class ii antigens on human hepatoma cell line HA22T/VGH Cells. Am J Chin Med. 1998;26:159–70. doi: 10.1142/S0192415X9800021X. [DOI] [PubMed] [Google Scholar]
- 105.Kuo YC, Tsai WJ, Shiao MS, Chen CF, Lin CY. Cordyceps sinensis as an immunomodulatory agent. Am J Chin Med. 1996;24:111–25. doi: 10.1142/S0192415X96000165. [DOI] [PubMed] [Google Scholar]
- 106.Tang J, Tian D, Liu G. Immunosuppressive effect of Cordyceps CS-4 on human monocyte-derived dendritic cells in vitro. Am J Chin Med. 2010;38:961–72. doi: 10.1142/S0192415X1000838X. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Xue WJ, Tian PX, Ding XM, Yan H, Pan XM, et al. Clinical Application of Cordyceps sinensis on Immunosuppressive Therapy in Renal Transplantation. Transplant Proc. 2009;41:1565–9. doi: 10.1016/j.transproceed.2009.02.085. [DOI] [PubMed] [Google Scholar]
- 108.Choi JW, Ra KS, Kim SY, Yoon TJ, Yu KW, Shin KS, et al. Enhancement of anti-complementary and radical scavenging activities in the submerged culture of Cordyceps sinensis by addition of citrus peel. Bioresour Technol. 2010;101:6028–34. doi: 10.1016/j.biortech.2010.02.083. [DOI] [PubMed] [Google Scholar]
- 109.Ji NF, Yao LS, Li Y, He W, Yi KS, Huang M. Polysaccharide of Cordyceps sinensis enhances cisplatin cytotoxicity in non-small cell lung cancer H157 cell line. Integr Cancer Ther. 2011;10:359–67. doi: 10.1177/1534735410392573. [DOI] [PubMed] [Google Scholar]
- 110.Hao L, Wang Q, Kobayashi M, Tamesada M, Wang HJ. Effectiveness of Cordyceps sinensis alone or in combination with chemotherapy in patients with non-small cell lung cancer. Biotherapy. 2008;22:345–9. [Google Scholar]
- 111.Hao L, Wang Q, Wang B, Wang HJ. Clinical observation of Cordyceps combined with NP regimen in treatment of advanced non-small cell lung cancer. J Dalian Med Univ. 2007;29:563–5. [Google Scholar]
- 112.Rao YK, Fang SH, Tzeng YM. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J Ethnopharmacol. 2007;114:78–85. doi: 10.1016/j.jep.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 113.Kuo YC, Lin CY, Tsai WJ, Wu CL, Chen CF, Shiao MS. Growth inhibitors against tumor cells in Cordyceps sinensis other than cordycepin and polysaccharides. Cancer Invest. 1994;12:611–5. doi: 10.3109/07357909409023046. [DOI] [PubMed] [Google Scholar]
- 114.Jordan JL, Nowak A, Lee TD. Activation of innate immunity to reduce lung metastases in breast cancer. Cancer Immunol Immunother. 2010;59:789–97. doi: 10.1007/s00262-009-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kubo E, Yoshikawa N, Kunitomo M, Kagota S, Shinozuka K, Nakamura K. Inhibitory effect of Cordyceps sinensis on experimental hepatic metastasis of melanoma by suppressing tumor cell invasion. Anticancer Res. 2010;30:3429–33. [PubMed] [Google Scholar]
- 116.Wu JY, Zhang QX, Leung PH. Inhibitory effects of ethyl acetate extract of Cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine. 2007;14:43–9. doi: 10.1016/j.phymed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Nakamura K, Yamaguchi Y, Kagota S, Kwon YM, Shinozuka K, Kunitomo M. Inhibitory effect of Cordyceps sinensis on spontaneous liver metastasis of Lewis lung carcinoma and B16 melanoma cells in syngeneic mice. Jpn J Pharmacol. 1999;79:335–41. doi: 10.1254/jjp.79.335. [DOI] [PubMed] [Google Scholar]
- 118.Liu Z, Li P, Zhao D, Tang H, Guo J. Anti-inflammation effects of Cordyceps sinensis mycelium in focal cerebral ischemic injury rats. Inflammation. 2011;34:639–44. doi: 10.1007/s10753-010-9273-5. [DOI] [PubMed] [Google Scholar]
- 119.Li CY, Chiang CS, Tsai ML, Hseu RS, Shu WY, Chuang CY, et al. Two-sided effect of Cordyceps sinensis on dendritic cells in different physiological stages. J Leukoc Biol. 2009;85:987–95. doi: 10.1189/jlb.0908573. [DOI] [PubMed] [Google Scholar]
- 120.Chiou YL, Lin CY. The extract of Cordyceps sinensis inhibited airway inflammation by blocking NF-κB activity. Inflammation. 2012;35:985–93. doi: 10.1007/s10753-011-9402-9. [DOI] [PubMed] [Google Scholar]
- 121.Dong CH, Yao YJ. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis. Lebenson Wiss Technol. 2008;41:669–77. doi: 10.1016/j.lwt.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li SP, Li P, Dong TTX, Tsim KW. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine. 2001;8:207–12. doi: 10.1078/0944-7113-00030. [DOI] [PubMed] [Google Scholar]
- 123.Li SP, Su ZR, Dong TTX, Tsim KW. The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine. 2002;9:319–24. doi: 10.1078/0944-7113-00134. [DOI] [PubMed] [Google Scholar]
- 124.Zhang L, Chen SZ, Liu SS. Prosecutable function of Cordyceps sinensis extracts for hepatic mitochondrial oxidative injuries in diabetic mice. Chin J Clin Rehabil. 2006;10:132–4. [Google Scholar]
- 125.Hu Z, Ye M, Xia L, Tu W, Li L, Zou G. Purification and characterization of an antibacterial protein from the cultured mycelia of Cordyceps sinensis. Wuhan Da Xue Xue Bao Zi Ran Ke Xue Ban. 2006;11:709–14. [Google Scholar]
- 126.Kuo CF, Chen CC, Luo YH, Huang RY, Chuang WJ, Sheu CC, et al. Cordyceps sinensis mycelium protects mice from group A Streptococcal infection. J Med Microbiol. 2005;54:795–802. doi: 10.1099/jmm.0.45704-0. [DOI] [PubMed] [Google Scholar]
- 127.Wang BS, Lee CP, Chen ZT, Yu HM, Duh PD. Comparison of the hepatoprotective activity between cultured Cordyceps militaris and natural Cordyceps sinensis. J Funct Foods. 2012;4:489–95. [Google Scholar]
- 128.Ko WS, Hsu SL, Chyau CC, Chen KC, Peng RY. Compound Cordyceps TCM-700C exhibits potent hepatoprotective capability in animal model. Fitoterapia. 2010;81:1–7. doi: 10.1016/j.fitote.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 129.Jung SH, Lee YS, Lim SS, Kim YS, Lee S, Shin KH. Hepatoprotective and antioxidant capacities of Paecilomyces japonica and Cordyceps sinensis in rats with CCl 4 -induced hepatic injury. Korean J Hortic Sci. 2009;27:668–72. [Google Scholar]
- 130.Li FH, Liu P, Xiong WG, Xu GF. Effects of Cordyceps sinensis on dimethylnitrosamine-induced liver fibrosis in rats. Zhong Xi Yi Jie He Xue Bao. 2006;4:514–7. doi: 10.3736/jcim20060515. [DOI] [PubMed] [Google Scholar]
- 131.Liu YK, Shen W. Inhibitive effect of Cordyceps sinensis on experimental hepatic fibrosis and its possible mechanism. World J Gastroenterol. 2003;9:529–33. doi: 10.3748/wjg.v9.i3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Manabe N, Azuma Y, Sugimoto M, Uchio K, Miyamoto M, Taketomo N, et al. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism and blood flow in dietary hypoferric anaemic mice. Br J Nutr. 2000;83:197–204. doi: 10.1017/s0007114500000258. [DOI] [PubMed] [Google Scholar]
- 133.Manabe N, Sugimoto M, Azuma Y, Taketomo N, Yamashita A, Tsuboi H, et al. Effects of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism in the mouse. Jpn J Pharmacol. 1996;70:85–8. doi: 10.1254/jjp.70.85. [DOI] [PubMed] [Google Scholar]
- 134.Zhong F, Liu X, Zhou Q, Hao X, Lu Y, Guo S, et al. 1H NMR spectroscopy analysis of metabolites in the kidneys provides new insight into pathophysiological mechanisms: Applications for treatment with Cordyceps sinensis. Nephrol Dial Transplant. 2012;27:556–65. doi: 10.1093/ndt/gfr368. [DOI] [PubMed] [Google Scholar]
- 135.Yu H, Zhou Q, Huang R, Yuan M, Ao X, Yang J. Effect of Cordyceps sinensis on the expression of HIF-1 a αand NGAL in rats with renal ischemia-reperfusion injury. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37:57–68. doi: 10.3969/j.issn.1672-7347.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 136.Wu R, Zhou Q, Lin S, Ao X, Chen X, Yang J. Effect of Cordceps sinensis on the expression of ICAM-1 and VCAM-1 in the kidney of spontaneously hypertensive rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:152–8. doi: 10.3969/j.issn.1672-7347.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 137.Song LQ, Si-Ming Y, Xiao-Peng M, Li-Xia J. The protective effects of Cordyceps sinensis extract on extracellular matrix accumulation of glomerular sclerosis in rats. Afr J Pharm Pharmaco. 2010;4:471–8. [Google Scholar]
- 138.Zhao X, Li L. Cordyceps sinensis in protection of the kidney from cyclosporine A nephrotoxicity. Zhonghua Yi Xue Za Zhi. 1993;73:410–2. 447. [PubMed] [Google Scholar]
- 139.Wang Y, Yin HP, Chen T, Wang M. Preliminary structural identification and protection on renal cell injury of acidic polysaccharide from Cordyceps sinensis. J China Pharm Univ. 2009;40:559–64. [Google Scholar]
- 140.Zhang XL, Bi-Cheng L, Al-Assaf S, Phillips GO, Phillips AO. Cordyceps sinensis decreases TGF-β1 dependent epithelial to mesenchymal transdifferentiation and attenuates renal fibrosis. Food Hydrocoll. 2012;28:200–12. [Google Scholar]
- 141.Lin CY, Ku FM, Kuo YC, Chen CF, Chen WP, Chen A, et al. Inhibition of activated human mesangial cell proliferation by the natural product of Cordyceps sinensis (H1-A): An implication for treatment of iga mesangial nephropathy. Transl Res. 1999;133:55–63. doi: 10.1053/lc.1999.v133.a94239. [DOI] [PubMed] [Google Scholar]
- 142.Guo SM, Zhong F, Zhou Q, Lu Y, Hao X, Wang WM, et al. Renal protective effect of Cordyceps sinensis on 5/6 nephrectomy-induced renal fibrosis in rats. J Shanghai Jiaotong Univ. 2012;32:1–8. [Google Scholar]
- 143.Kan WC, Wang HY, Chien CC, Li SL, Chen YC, Chang LH, et al. Effects of extract from solid-state fermented Cordyceps sinensis on type 2 diabetes mellitus. Evid Based Complement Alternat Med. 2012:1–10. doi: 10.1155/2012/743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.El Ashry FE, Mahmoud MF, El Maraghy NN, Ahmed AF. Effect of Cordyceps sinensis and taurine either alone or in combination on streptozotocin induced diabetes. Food Chem Toxicol. 2012;50:1159–65. doi: 10.1016/j.fct.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 145.Guo J, Li C, Wang J, Liu Y, Zhang J. Vanadium-enriched Cordyceps sinensis, a contemporary treatment approach to both diabetes and depression in rats. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/neq058. 450316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo JY, Han CC, Liu YM. A contemporary treatment approach to both diabetes and depression by Cordyceps sinensis, rich in vanadium. Evid Based Complement Alternat Med. 2010;7:387–9. doi: 10.1093/ecam/nep201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lo HC, Hsu TH, Tu ST, Lin KC. Anti-hyperglycemic activity of natural and fermented Cordyceps sinensis in rats with diabetes induced by nicotinamide and streptozotocin. Am J Chin Med. 2006;34:819–32. doi: 10.1142/S0192415X06004314. [DOI] [PubMed] [Google Scholar]
- 148.Lo HC, Tu ST, Lin KC, Lin SC. The anti-hyperglycemic activity of the fruiting body of Cordyceps in diabetic rats induced by nicotinamide and streptozotocin. Life Sci. 2004;74:2897–908. doi: 10.1016/j.lfs.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 149.Wang NN, Wang YJ, Zhang X. Effects of Cordyceps sinensis on blood glucose, plasma insulin, histology of liver and kidney in rats. Chin Pharm J. 2003;38:924–6. [Google Scholar]
- 150.Balon TW, Jasman AP, Zhu JS. A fermentation product of Cordyceps sinensis increases whole-body insulin sensitivity in rats. J Altern Complement Med. 2002;8:315–23. doi: 10.1089/10755530260128005. [DOI] [PubMed] [Google Scholar]
- 151.Ji H, Tu HH, Li NS, Liu GQ. Study on hypoglycemic activity of alkaline extract from cultural mycelium of Cordyceps sinensis. Chin Pharmacol Bull. 1993;9:386–9. [Google Scholar]
- 152.Koh JH, Kim JM, Chang UJ, Suh HJ. Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis. Biol Pharm Bull. 2003;26:84–7. doi: 10.1248/bpb.26.84. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y, Yang M, Gong S, Yang Y, Chen B, Cai Y, et al. Cordyceps sinensis extracts attenuate aortic transplant arteriosclerosis in rats. J Surg Res. 2012;175:123–30. doi: 10.1016/j.jss.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 154.Yamaguchi Y, Kagota S, Nakamura K, Shinozuka K, Kunitomo M. Inhibitory effects of water extracts from fruiting bodies of cultured Cordyceps sinensis on raised serum lipid peroxide levels and aortic cholesterol deposition in atherosclerotic mice. Phytother Res. 2000;14:650–2. doi: 10.1002/1099-1573(200012)14:8<650::aid-ptr675>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 155.Zhao Y. Inhibitory effects of alcoholic extract of Cordyceps sinensis on abdominal aortic thrombus formation in rabbits. Zhonghua Yi Xue Za Zhi. 1991;71:612–5. 642. [PubMed] [Google Scholar]
- 156.Chiou WF, Chang PC, Chou CJ, Chen CF. Protein constituent contributes to the hypotensive and vasorelaxant activities of Cordyceps sinensis. Life Sci. 2000;66:1369–76. doi: 10.1016/s0024-3205(00)00445-8. [DOI] [PubMed] [Google Scholar]
- 157.Chen M, Cheung FW, Chan MH, Hui PK, Ip SP, Ling YH, et al. Protective roles of Cordyceps on lung fibrosis in cellular and rat models. J Ethnopharmacol. 2012;143:448–54. doi: 10.1016/j.jep.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang YS, Feng YZ, Cao ZF, Gu ZL, Yang QY, Yang XT, et al. Effect of Cordyceps sinensis and Panax notoginseng compound extracts on Bleomycin-induced pulmonary fibrosis in rats and its mechanisms. Chin Tradit Herb Drugs. 2011;42:1766–72. [Google Scholar]
- 159.Xu H, Li S, Lin Y, Liu R, Gu Y, Liao D. Effectiveness of cultured Cordyceps sinensis combined with glucocorticosteroid on pulmonary fibrosis induced by bleomycin in rats. Zhongguo Zhong Yao Za Zhi. 2011;36:2265–70. [PubMed] [Google Scholar]
- 160.Lin XX, Xie QM, Shen WH, Chen Y. Effects of fermented Cordyceps powder on pulmonary function in sensitized guinea pigs and airway inflammation in sensitized rats. Zhongguo Zhong Yao Za Zhi. 2001;26:624–5. [PubMed] [Google Scholar]
- 161.Wong WC, Wu JY, Benzie IF. Photoprotective potential of Cordyceps polysaccharides against ultraviolet B radiation-induced DNA damage to human skin cells. Br J Dermatol. 2011;164:980–6. doi: 10.1111/j.1365-2133.2010.10201.x. [DOI] [PubMed] [Google Scholar]
- 162.Lin CC, Pumsanguan W, Koo MM, Huang HB, Lee MS. Radiation protective effects of Cordyceps sinensis in blood cells. Tzu Chi Med J. 2007;19:226–32. [Google Scholar]
- 163.Liu WC, Wang SC, Tsai ML, Chen MC, Wang YC, Hong JH, et al. Protection against radiation-induced bone marrow and intestinal injuries by Cordyceps sinensis, a Chinese herbal medicine. Radiat Res. 2006;166:900–7. doi: 10.1667/RR0670.1. [DOI] [PubMed] [Google Scholar]
- 164.Nishizawa K, Torii K, Kawasaki A, Katada M, Ito M, Terashita K, et al. Antidepressant-like effect of Cordyceps sinensis in the mouse tail suspension test. Biol Pharm Bull. 2007;30:1758–62. doi: 10.1248/bpb.30.1758. [DOI] [PubMed] [Google Scholar]
- 165.Morikubo K, Uchida M, Kume A, Tsunoo H, Tajima T, Nagata A. Effect of a mixture of mycelial extract of cultured Cordyceps sinensis and Ayamurasaki anthocyanin on the mental condition of middle-aged people. Jpn Pharmacol Ther. 2005;33:729–34. [Google Scholar]
- 166.Qi W, Wang PJ, Guo WJ, Yan YB, Zhang Y, Lei W. The mechanism of Cordyceps sinensis and strontium in prevention of osteoporosis in rats. Biol Trace Elem Res. 2011;143:302–9. doi: 10.1007/s12011-010-8829-4. [DOI] [PubMed] [Google Scholar]
- 167.Liu Z, Li P, Zhao D, Tang H, Guo J. Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Behav Brain Funct. 2010;6:61. doi: 10.1186/1744-9081-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marchbank T, Ojobo E, Playford CJ, Playford RJ. Reparative properties of the traditional Chinese medicine Cordyceps sinensis (Chinese caterpillar mushroom) using HT29 cell culture and rat gastric damage models of injury. Br J Nutr. 2011;105:1303–10. doi: 10.1017/S0007114510005118. [DOI] [PubMed] [Google Scholar]
- 169.Kumar R, Negi PS, Singh B, Ilavazhagan G, Bhargava K, Sethy NK. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J Ethnopharmacol. 2011;136:260–6. doi: 10.1016/j.jep.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 170.Nagata A, Tajima T, Uchida M. Supplemental anti-fatigue effects of Cordyceps sinensis (Tochu-Kaso) extract powder during three stepwise exercise of human. Jpn J Phys Fitness Sports Med. 2006;55:145–51. [Google Scholar]
- 171.Sun W, Yu J, Shi YM, Zhang H, Wang Y, Wu BB. Effects of Cordyceps extract on cytokines and transcription factors in peripheral blood mononuclear cells of asthmatic children during remission stage. Zhong Xi Yi Jie He Xue Bao. 2010;8:341–6. doi: 10.3736/jcim20100407. [DOI] [PubMed] [Google Scholar]
- 172.Gong MF, Xu JP, Chu ZY, Luan J. Effect of Cordyceps sinensis sporocarp on learning-memory in mice. Zhong Yao Cai. 2011;34:1403–5. [PubMed] [Google Scholar]
- 173.Chen YC, Huang BM. Regulatory mechanisms of Cordyceps sinensis on steroidogenesis in MA-10 mouse ley dig tumor cells. Biosci Biotechnol Biochem. 2010;74:1855–9. doi: 10.1271/bbb.100262. [DOI] [PubMed] [Google Scholar]
- 174.Leu SF, Chien CH, Tseng CY, Kuo YM, Huang BM. The in vivo effect of Cordyceps sinensis mycelium on plasma corticosterone level in male mouse. Biol Pharm Bull. 2005;28:1722–5. doi: 10.1248/bpb.28.1722. [DOI] [PubMed] [Google Scholar]
- 175.Chen YC, Huang YL, Huang BM. Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. Int J Biochem Cell Biol. 2005;37:214–23. doi: 10.1016/j.biocel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 176.Huang YL, Leu SF, Liu BC, Sheu CC, Huang BM. In vivo stimulatory effect of Cordyceps sinensis mycelium and its fractions on reproductive functions in male mouse. Life Sci. 2004;75:1051–62. doi: 10.1016/j.lfs.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 177.Wang SM, Lee LJ, Lin WW, Chang CM. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J Cell Biochem. 1998;69:483–9. doi: 10.1002/(sici)1097-4644(19980615)69:4<483::aid-jcb9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 178.Yu L, Guozhen C, Dezhao J, Ziqiang Z, Guolin C. Effect of Cordyceps sinensis on erythropoiesis in mouse bone marrow. Hunan Yi Ke Da Xue Xue Bao. 1990;15:43–7. [Google Scholar]
- 179.Mei QB, Tao JY, Gao SB, Xu GC, Chen LM, Su JK. Antiarrhythmic effects of Cordyceps sinensis (Berk.) Sacc. Zhongguo Zhong Yao Za Zhi. 1989;14:616–8. 640. [PubMed] [Google Scholar]
- 180.Ji DB, Ye J, Li CL, Wang YH, Zhao J, Cai SQ. Antiaging effect of Cordyceps sinensis extract. Phytother Res. 2009;23:116–22. doi: 10.1002/ptr.2576. [DOI] [PubMed] [Google Scholar]
- 181.Ding C, Tian P, Jia L, Li Y, Ding X, Xiang H, et al. The synergistic effects of C. sinensis with CsA in preventing allograft rejection. Front Biosci. 2009;14:3864–71. doi: 10.2741/3494. [DOI] [PubMed] [Google Scholar]
- 182.Chen JL, Chen YC, Yang SH, Ko YF, Chen SY. Immunological alterations in lupus-prone autoimmune (NZB/NZW) F1 mice by mycelia Chinese medicinal fungus Cordyceps sinensis-induced redistributions of peripheral mononuclear T lymphocytes. Clin Exp Med. 2009;9:277–84. doi: 10.1007/s10238-009-0043-3. [DOI] [PubMed] [Google Scholar]
- 183.Yang LY, Chen A, Kuo YC, Lin CY. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin Med. 1999;134:492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- 184.Huang BM, Hsiao KY, Chuang PC, Wu MH, Pan HA, Tsai SJ. Upregulation of steroidogenic enzymes and ovarian 17β-estradiol in human granulosa-lutein cells by Cordyceps sinensis Mycelium. Biol Reprod. 2004;70:1358–64. doi: 10.1095/biolreprod.103.022855. [DOI] [PubMed] [Google Scholar]
- 185.Zhang SS, Zhang DS, Zhu TJ, Chen XY. A pharmacological analysis of the amino acid components of Cordyceps sinensis Sacc. Yao Xue Xue Bao. 1991;26:326–30. [PubMed] [Google Scholar]
- 186.Ding C, Tian PX, Xue W, Ding X, Yan H, Pan X, et al. Efficacy of Cordyceps sinensis in long term treatment of renal transplant patients. Front Biosci (Elite Ed) 2011;3:301–7. doi: 10.2741/e245. [DOI] [PubMed] [Google Scholar]
- 187.Akaki J, Matsui Y, Kojima H, Nakajima S, Kamei K, Tamesada M. Structural analysis of monocyte activation constituents in cultured mycelia of Cordyceps sinensis. Fitoterapia. 2009;80:182–7. doi: 10.1016/j.fitote.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 188.Wu Y, Sun C, Pan Y. Studies on isolation and structural features of a polysaccharide from the mycelium of an Chinese edible fungus (Cordyceps sinensis) Carbohydr Polym. 2006;63:251–6. [Google Scholar]
- 189.Wu Y, Hu N, Pan Y, Zhou L, Zhou X. Isolation and characterization of a mannoglucan from edible Cordyceps sinensis mycelium. Carbohydr Res. 2007;342:870–5. doi: 10.1016/j.carres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 190.Kiho T, Ookubo K, Usui S, Ukai S, Hirano K. Structural features and hypoglycemic activity of a polysaccharide (CS- F10) from the cultured mycelium of Cordyceps sinensis. Biol Pharm Bull. 1999;22:966–70. doi: 10.1248/bpb.22.966. [DOI] [PubMed] [Google Scholar]
- 191.Wang XL, Liu GQ, Zhu CY, Zhou GY, Kuang SM. Enhanced production of mycelial biomass and extracellular polysaccharides in caterpillar-shaped medicinal mushroom Cordyceps sinensis CS001 by the addition of palmitic acid. J Med Plant Res. 2011;5:2873–8. [Google Scholar]
- 192.Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT, Lo CK, et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–13. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 193.Feng K, Wang S, Hu DJ, Yang FQ, Wang HX, Li SP. Random amplified polymorphic DNA (RAPD) analysis and the nucleosides assessment of fungal strains isolated from natural Cordyceps sinensis. J Pharm Biomed Anal. 2009;50:522–6. doi: 10.1016/j.jpba.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 194.Yuan JP, Zhao SY, Wang JH, Kuang HC, Liu X. Distribution of nucleosides and nucleobases in edible fungi. J Agric Food Chem. 2008;56:809–15. doi: 10.1021/jf0719205. [DOI] [PubMed] [Google Scholar]
- 195.Lei N, Du SS, Ni XM, Zhang WS, Guo ER, Li Q. Determination of nucleosides in natural Cordyceps sinensis and cultured Cordyceps by RP-HPLC. Chin Pharm J. 2006;41:948–51. [Google Scholar]
- 196.Ikeda R, Nishimura M, Sun Y, Wada M, Nakashima K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomed Chromatogr. 2008;22:630–6. doi: 10.1002/bmc.980. [DOI] [PubMed] [Google Scholar]
- 197.Li J, Feng CQ, Ni XM, Zhang WS. Determination of nucleosides of natural Cordyceps sinensis in Qinghai Province by capillary electrophoresis. Chin Pharm J. 2008;43:1105–7. [Google Scholar]
- 198.Huang L, Li Q, Chen Y, Wang X, Zhou X. Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr J Microbiol Res. 2009;3:957–61. [Google Scholar]
- 199.Yang FQ, Ge L, Yong JW, Tan SN, Li SP. Determination of nucleosides and nucleobases in different species of Cordyceps by capillary electrophoresis-mass spectrometry. J Pharm Biomed Anal. 2009;50:307–14. doi: 10.1016/j.jpba.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 200.Meena H, Mohsin M, Pandey HK, Negi PS, Ahmed Z. Estimation of cordycepin by improved HPLC method in the natural and cultured mycelia of high medicinal value Himalayan entomogenous fungus Cordyceps sinensis. Electron Environ Agric Food Chem. 2010;9:1598–603. [Google Scholar]
- 201.Varshney VK, Pandey A, Kumar A, Rathod D, Kannojia P. Chemical screening and identification of high cordycepin containing cultured isolate(s) of medicinal chinese caterpillar mushroom, Ophiocordyceps sinensis (Berk.) G.H. Sung et al. Int J Med Mushrooms. 2011;13:327–33. doi: 10.1615/intjmedmushr.v13.i4.20. [DOI] [PubMed] [Google Scholar]
- 202.Li Y, Wang XL, Jiao L, Jiang Y, Li H, Jiang SP, et al. A survey of the geographic distribution of Ophiocordyceps sinensis. J Microbiol. 2011;49:913–9. doi: 10.1007/s12275-011-1193-z. [DOI] [PubMed] [Google Scholar]
- 203.Lim L, Lee C, Chang E. Optimization of solid state culture conditions for the production of adenosine, cordycepin, and D-mannitol in fruiting bodies of medicinal caterpillar fungus Cordyceps militaris (L.:Fr.) Link (Ascomycetes) Int J Med Mushrooms. 2012;14:181–7. doi: 10.1615/intjmedmushr.v14.i2.60. [DOI] [PubMed] [Google Scholar]
- 204.Dong CH, Yao YJ. Comparison of some metabolites among cultured mycelia of medicinal fungus, Ophiocordyceps sinensis (Ascomycetes) from different geographical regions. Int J Med Mushrooms. 2010;12:287–97. [Google Scholar]
- 205.Dong JZ, Lei C, Ai XR, Wang Y. Selenium enrichment on Cordyceps militaris link and analysis on its main active components. Appl Biochem Biotechnol. 2012;166:1215–24. doi: 10.1007/s12010-011-9506-6. [DOI] [PubMed] [Google Scholar]
- 206.Xiao JH, Xiao DM, Xiong Q, Liang ZQ, Zhong JJ. Optimum extraction and high-throughput detection of cordycepic acid from medicinal macrofungi Cordyceps jiangxiensis, Cordyceps taii and Cordyceps gunnii. J Food Agric Environ. 2009;7:328–33. [Google Scholar]
- 207.Chen LC, Hseu RS, Guo JH. Determination of ergosterol and its derivatives in Cordyceps sinensis-associated products by high performance liquid chromatography and liquid chromatography-mass spectrometry. Chin J Anal Chem. 2011;39:1380–6. [Google Scholar]
- 208.Yuan JP, Wang JH, Liu X, Kuang HC, Zhao SY. Simultaneous determination of free ergosterol and ergosteryl esters in Cordyceps sinensis by HPLC. Food Chem. 2007;105:1755–9. [Google Scholar]
- 209.Matsuda H, Akaki J, Nakamura S, Okazaki Y, Kojima H, Tamesada M, et al. Apoptosis-inducing effects of sterols from the dried powder of cultured mycelium of Cordyceps sinensis. Chem Pharm Bull. 2009;57:411–4. doi: 10.1248/cpb.57.411. [DOI] [PubMed] [Google Scholar]
- 210.Jia JM, Ma XC, Wu CF, Wu LJ, Hu GS. Cordycedipeptide A, a new cyclodipeptide from the culture liquid of Cordyceps sinensis (Berk.) Sacc. Chem Pharm Bull. 2005;53:582–3. doi: 10.1248/cpb.53.582. [DOI] [PubMed] [Google Scholar]
- 211.Dong C, Yao Y. Isolation, characterization of melanin derived from Ophiocordyceps sinensis, an entomogenous fungus endemic to the Tibetan Plateau. J Biosci Bioeng. 2012;113:474–9. doi: 10.1016/j.jbiosc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 212.Liu YS, Wu JY. EVects of Tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus. J Ind Microbiol Biotechnol. 2012;39:623–8. doi: 10.1007/s10295-011-1066-9. [DOI] [PubMed] [Google Scholar]
- 213.Leung PH, Wu JY. Effects of ammonium feeding on the production of bioactive metabolites (cordycepin and exopolysaccharides) in mycelial culture of a Cordyceps sinensis fungus. J Appl Microbiol. 2007;103:1942–9. doi: 10.1111/j.1365-2672.2007.03451.x. [DOI] [PubMed] [Google Scholar]
- 214.Cha SH, Lim JS, Yoon CS, Koh JH, Chang HI, Kim SW. Production of mycelia and exo-biopolymer from molasses by Cordyceps sinensis 16 in submerged culture. Bioresour Technol. 2007;98:165–8. doi: 10.1016/j.biortech.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 215.Cha SH, Kim JC, Lim JS, Yoon CS, Koh JH, Chang HI, et al. Morphological characteristics of Cordyceps sinensis 16 and production of mycelia and exo-biopolymer from molasses in submerged culture. J Ind Engg Chem. 2006;12:115–20. [Google Scholar]
- 216.Hsieh C, Tsai MJ, Hsu TH, Chang DM, Lo CT. Medium optimization for polysaccharide production of Cordyceps sinensis. Appl Biochem Biotechnol. 2005;120:145–57. doi: 10.1385/abab:120:2:145. [DOI] [PubMed] [Google Scholar]
- 217.Dong CH, Yao YJ. Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. J Appl Microbiol. 2005;99:483–92. doi: 10.1111/j.1365-2672.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- 218.Barseghyan GS, Holliday JC, Price TC, Madison LM, Wassert SP. Growth and cultural-morphological characteristics of vegetative mycelia of medicinal caterpillar fungus Ophiocordyceps sinensis GH Sung et al. (Ascomycetes) Isolates from Tibetan Plateau (PR China) Int J Med Mushrooms. 2011;13:565–81. doi: 10.1615/intjmedmushr.v13.i6.90. [DOI] [PubMed] [Google Scholar]
- 219.Shen LH, An YT, Yang QY, Yang XT, Shen X, Ding H. In vitro and in vivo study of Hirsutella sinensis extract on kidney injury. Chin Pharmacol Bull. 2011;27:1537–40. [Google Scholar]
- 220.Chai JJ, Chen YP, Rui HL. Effects of Hirsutella sinensis on TGF-beta1 and Snail expressions and transdifferentiation of tubular epithelial-myofibroblast in renal tissue of rats with chronic aristolochic acid nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:325–9. [PubMed] [Google Scholar]
- 221.Shou QY, Fu HY, Chen FM, Zhou WM, Zhang CQ, Chen ML. Prevention of strain fermentation in Hirsutella sinensis on type 1 diabetes of non-obese diabetic mice. Chin Tradit Herb Drugs. 2010;41:1311–5. [Google Scholar]
- 222.Xun C, Shen N, Li B, Zhang Y, Wang F, Yang Y, et al. Radiation mitigation effect of cultured mushroom fungus Hirsutella sinensis (CorImmune) isolated from a Chinese/Tibetan herbal preparation - Cordyceps sinensis. Int J Radiat Biol. 2008;84:139–49. doi: 10.1080/09553000701797070. [DOI] [PubMed] [Google Scholar]
- 223.Chen S, Yin D, Li L, Zha X, Shuen J, Zhama C. Resources and distribution of Cordyceps sinensis in Naqu Tibet. Zhong Yao Cai. 2000;23:673–5. [PubMed] [Google Scholar]
- 224.Zhang G, Yu J, Wu G, Liu X. Factors influencing the occurrence of Ophiocordyceps sinensis. Shengtai Xuebao/ Acta Ecologica Sinica. 2011;31:4117–25. [Google Scholar]
- 225.Wang XL, Yao YJ. Host insect species of Ophiocordyceps sinensis: A review. ZooKeys. 2011;127:43–59. doi: 10.3897/zookeys.127.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hao JJ, Cheng Z, Liang HH, Yang XL, Li S, Zhou TS, et al. Genetic differentiation and distributing pattern of Cordyceps sinensis in China revealed by rDNA ITS sequences. Chin Tradit Herb Drugs. 2009;40:112–6. [Google Scholar]
- 227.Cheng Z, Geng Y, Liang H, Yang X, Li S, Zhu Y, et al. Phylogenetic relationships of host insects of Cordyceps sinensis inferred from mitochondrial cytochrome b sequences. Prog Nat Sci. 2007;17:789–97. [Google Scholar]
- 228.Hawksworth DL. A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. MycoKeys. 2011;1:7–20. doi: 10.5598/imafungus.2011.02.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McNeill1 J, Turland NJ. Major changes to the Code of Nomenclature-Melbourne, July 2011. Taxon. 2011;60:1495–7. [Google Scholar]


