Abstract
Hericium erinaceus a culinary and medicinal mushroom is a well established candidate for brain and nerve health. Ganoderma lucidum, Grifola frondosa and Sarcodon scabrosus have been reported to have neurite outgrowth and neuronal health benefits. The number of mushrooms, however, studied for neurohealth activity are few compared to the more than 2 000 species of edible and / or medicinal mushrooms identified. In the on-going search for other potent culinary and / or medicinal mushrooms, indigenous mushrooms used in traditional medicines such as Lignosus rhinocerotis and Ganoderma neo-japonicum are also being investigated. Further, the edible mushroom, Pleurotus giganteus can be a potential candidate, too. Can these edible and medicinal mushrooms be tapped to tackle the health concerns of the aging population which is projected to be more than 80-90 million of people age 65 and above in 2050 who may be affected by age-related neurodegenerative disorders. Scientific validation is needed if these mushrooms are to be considered and this can be achieved by understanding the molecular and biochemical mechanisms involved in the stimulation of neurite outgrowth. Though it is difficult to extrapolate the in vitro studies to what may happen in the human brain, studies have shown that there can be improvement in cognitive abilities of the aged if the mushroom is incorporated in their daily diets.
Keywords: Neuronal health, Mushrooms, Hericium erinaceus, Pleurotus giganteus, Lignosus rhinocerotis, Ganoderma neo-japonicum, Neurite outgrowth, Nerve regeneration
INTRODUCTION
The macrofungi - the mushrooms are eukaryotic, non-photosynthetic, and aerobic organisms that form characteristic fruiting bodies. Belonging to the Fifth Kingdom, all mushrooms are heterotrophic, and they assimilate nutritive substances by absorption of simple molecules as nutrients after complex organic polymers such as celluloses are degraded by extracellular enzymes secreted by them. The mushrooms are taxonomically classified in two different groups: Basidiomycetes, which comprise many of the known genera and Ascomycetes. Edible and medicinal mushrooms, which can be commercially produced, are cultivated on lignocellulosic agricultural residues such as straw, wood chips, and sawdust.[1]
Mushrooms have been used by humans since thousands of years as food and/or as medicine. More than 14,000 species of mushrooms are recognized, and among them, approximately 2000 are identified as edible.[2] Of the 2000 edible mushrooms in 30 genera, 270 species are now considered as potential therapeutic or preventative agents that may ensure wellness of humans.[3] Today, the increasing consumption of mushrooms can be attributed not only to the pleasant flavor and aroma of culinary mushrooms but also to the current search for natural products, which are free of pesticides; food as nutraceuticals or functional food with high vitamins and protein contents as well bioactive activities including antioxidant activities.[4] The culinary mushrooms can be excellent components in our daily diet, because they are rich in proteins, fibers, carbohydrates, vitamins, and minerals. While at the same time, mushrooms are low in calories and fats including cholesterol. In the recent years to unravel the mysteries of mushrooms, especially those used in traditional medicines, mushrooms are being investigated for their many ethnomycological claims of medicinal value. The mushrooms are being developed as nutraceuticals or nutriceuticals to garner the essence of mushrooms and to make consumption easy. Further, scientific validation of traditional knowledge bears evidence of the many positive effects of consuming mushrooms fresh or processed on human health.[2] In the words of Chang:[5]
‘Without leaves, without buds, without flowers;
Yet they form fruit.
As a Food, as a tonic, as a medicine;
The entire creation is precious.’
As the recognition of mushrooms as possible food or supplements that can improve health and enhance quality of life increases, numerous products are marketed with claims often based on ethnomycological knowledge. In an attempt to scientifically validate the numerous claims of the health benefits of mushrooms, related research are being actively undertaken world-wide. However, at this moment, many of the studies on the efficacy of medicinal mushrooms that are available to the public are based on animal studies (usually in mice) or cultured cells. There is a need to show efficacy in humans via clinical trials.[6] In China, complementary and alternative medicine is permitted as an alternative therapy for life-debilitating diseases, and mushroom nutriceuticals or mushroom functional foods are included in cancer and other life-threatening diseases management. One of the many diseases that threaten humans, neurodegenerative diseases can be very traumatic as one ages. Neurohealth is the concern for the predicted silver tsunami to hit humans – the aging tsunami is projected to be 80-90 million of 65 –plus population in 2050.[7]
Why the concern? A large percentage of this aging population (projected at about 80%) will have at least one chronic health-related diseases. Alzheimer and neurodegenerative diseases are high on the list of chronic diseases of the aged. Alzheimer's disease is primarily a disorder of aging with loss of cognitive function. This disease is characterized biologically by the death of neurons in the forebrain, hippocampus, and cerebral cortex accompanied by the presence of amyloid deposition. Amyloid β protein participates in the induction of neuronal cell death and neuritic changes. The symptoms caused by the death of cholinergic neurons are treated with drugs that enhance neurotransmission at cholinergic synapses. The drugs include Aricept[R] by Pfizer, Exelon[R] by Novartis, Reminyl[R] by Janssen, and Cognex[R] by First Horizon. These drugs only delay further deterioration and do not reverse the damage done to cognitive functions. Another drug memantine (Forest Laboratories), which blocks the receptor for the glutamate neurotransmitter which may be responsible for the neurotoxicity in Alzheimer's disease, had temporary beneficial effects, too. We have to find preventative agents. In neurodegenerative diseases, once the clinical symptoms are manifested, it will be only possible to arrest or delay further degeneration process. The strategy will be to look for agents that slow down and prevent further damage to the vital cells involved. The search is now for small molecules that can cross the brain-blood and induce the production of nerve growth factor (NGF), a family of proteins responsible for maintenance, survival, and regeneration of neurons during adult life.
It has been shown that NGF absence caused an Alzheimer's-like symptom in the adult brain of mice. Prevention will be definitely better than cure. The preventative agent/s have to be part of our diet. We may have to turn to traditional knowledge that taps nature for medicine to prevent or reduce the severity of nerve-related diseases as we age. Many plants and spices such as turmeric are said to help. For example, in India, it is noted that Alzheimer among the older generation is not in alarming numbers. The regular consumption of spices including turmeric and pepper may be the reason, and currently, this is actively been studied.
Mushrooms for nerve and brain health
Can mushrooms help here? Almost all culinary mushrooms are noted for their polysaccharide components, which play a role in stimulation of immune system and possible applications in cancer management. Many edible genera, however, are also noted for their specific activities. Among the special activities, a number of mushrooms including Sarcodon scabrosus, Ganoderma lucidum, Grifola frondosa, and Hericium erinaceus are reported to have activities related to nerve and brain health. In this paper, the potentials of mushrooms as nutraceutical or nutriceuticals to help in the reduction or even prevention of age-related neurodegenerative diseases are discussed. Sarcodon scabrosus, a bitter mushroom, contains cyathane diterpenoids termed scabronines.[8] When rat pheochromocytoma cells (PC12) were treated with scabronines A and G, there was neurite outgrowth.[8] Extracts of G. lucidium contained neuroactive compounds that induced neuronal differentiation and prevented NGF-dependent apoptosis of rat pheochromocytoma PC12 neuronal cells. These effects are probably mediated through the Ras/Erk and cAMP-response element-binding protein (CREB) signaling pathways, because G. lucidum extracts induced phosphorylation of Erk 1, Erk 2, and CREB.[9]
Hericium erinaceus triggers neurite outgrowth and regeneration of damaged nerves
The culinary mushroom that has been extensively studied for its neurohealth properties is H. erinaceus (Lion's Mane mushroom). The polysaccharides in an aqueous extract of the Lion's mane mushroom could induce neuronal differentiation and promote neuronal survival.[10] The chemical profile of extracts of this mushroom has been actively studied and reported by Kawagishi and co-researchers. Extracts of H. erinaceus induced the expression of neurotrophic factors such as NGF in astrocytes.[11,12] In their studies, hericenones were isolated from the fruiting bodies of Lion's mane while erinacines were isolated from the mycelium of the mushroom. Endoplasmic reticulum stress - attenuating compounds and dilinoleoyl phosphatidylethanolamine, a phospholipid with linoleic acid, an unsaturated fatty acid, purified from extracts of dried fruiting bodies of H. erinaceus, reduce endoplasmic reticulum stress-induced cell death. This may reduce the risk of neurodegenerative disease-induced cell death.[13] Fujiwara et al.[14] investigated the neuroprotective effect of H. erinaceus on the ischemic brain damage in a middle cerebral artery occlusion model in mice. Hericium erinaceus (300 mg/kg, continuously fed for 14 days after surgery) significantly decreased the size of the cerebral infarcts one day after the occlusion. In another study, a double-blind trial was conducted with 50- to 80-year-old Japanese men and women diagnosed with mild cognitive impairment in order to examine the efficacy of oral administration of H. erinaceus, for improving cognitive impairment, using a cognitive function scale based on the revised Hasegawa Dementia Scale (HDS-R).[15] The subjects in the H. erinaceus group took four 250 mg tablets containing 96% of Yamabushitake dry powder three times a day for 16 weeks. Cognitive function scale scores increased with the duration of intake. Laboratory tests showed no adverse effect of H. erinaceus. The study suggested that H. erinaceus is effective in improving mild cognitive impairment.[15] Extracts of H. erinaceus induced phosphorylation of c-Jun N-terminal kinase (JNK) and its downstream substrate c-Jun, and increased c-Fos expression, suggesting that H. erinaceus promotes nerve growth factor gene expression via JNK signaling.[16] Furthermore, the efficacy of H. erinaceus in vivo has been examined. Mice given feed regime containing 5% (w/w) H. erinaceus dry powder for seven days showed an increase in the level of NGF mRNA expression in the hippocampus.[16]
These studies are very encouraging, and the consumption of H. erinaceus-fresh or processed is acceptable. There are many preparations available off the counter. However, there should be guidelines and monitoring to ensure safety, efficacy, and other parameters to ensure that these preparations are functional as claimed. The best option will be consumption of fresh mushrooms. The processing has been reported to affect the neurite stimulatory activity of H. erinaceus.[17,18] Cultivation techniques and conditions may affect the medicinal properties of mushrooms too. Though Hericium erinaceus is a temperate mushroom, it can be cultivated in Malaysia, a tropical country.
Hericium erinaceus (Plate 1a) cultivation in Malaysia is still in its infancy. The consumers at large do not know the potential of this rather strange-looking mushroom. Realizing the potential of this mushroom, research on the locally grown mushroom will help spearhead the growing as well as the consumption of this mushroom in Malaysia.
Plate 1.

Selected culinary and medicinal medicinal mushrooms with neurite outgrowth stimulatory activity. a: H. erinaceus; b: P. giganteus; c: G. lucidium; d: L. rhinocerotis; d: G.neo-japonicum
The stimulation of neurite extension using in vitro model using NG 108-15 cells by H. erinaceus grown in hot climate was investigated. Though the mushroom is now grown at higher temperatures, the unique property of this mushroom is retained. In fact, there was no difference in the neurite outgrowth stimulatory activity between the low- and high temperature-grown mushrooms.[19] However, the post-harvest processing technique does affect the activity.[17,18,19] This is very important to note, as the commercially available H. erinaceus is oven- dried to increase its shelf life. The extracts of the fruiting bodies dried in an oven was demonstrated to have lost their ability to stimulate neurite outgrowth [Figure 1].[19]
Figure 1.
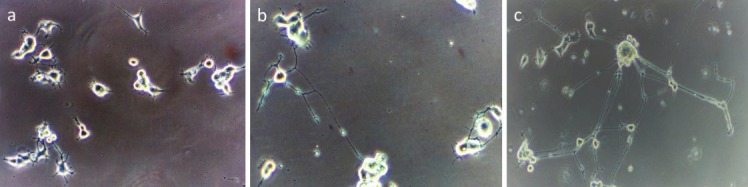
The effects of an aqueous extract of fruiting bodies of H. erinaceus grown in a tropical climate. a: Negative control (without treatment); b: NGF (20 ng/ml); c: Aqueous extract of H. erinaceus (extensive neurite outgrowth)
In another study, it was shown that extracts of H. erinaceus can enhance the activity of NGF in NG108-15 cells. A combination of 10 ng/ml NGF with 10 μg/ml H. erinaceus aqueous extract induced the highest percentage increase of 59.5 ± 5.0% neurite outgrowth compared to untreated neural cells (Lai et al., 2012, unpublished). The effect of neurite outgrowth stimulation was enhanced in this combined mixture when compared to the concentration of extract or NGF applied individually. The neurite outgrowth stimulation activity increased approximately 1.2-fold when compared to treatment with only 10 ng/ml NGF (Lai et al., 2012, unpublished). At higher concentrations, however, the enhancement of neurite stimulation activity of the aqueous mushroom extract and NGF was not observed. Further, early observations of an on-going study showed that the mushroom extract triggered NGF secretion in the presence of small amounts of NGF. Can this mushroom be then the secret to brain health? The direct extrapolation of in vitro results is not possible. Further studies, especially on mechanism of action at biochemical and molecular levels, are important. This far, there are no reports on toxicity due to long-term consumption of the mushroom. Further, a number of reported studies showed that this mushroom has potential to be developed into a functional food. The processing techniques, however, have to be optimized so that maximum activity will be retained to enhance nerve health and possibly nerve regeneration.
Peripheral nerve injury leads to changes at the axonal site of injury and remotely in the dorsal root ganglia (DRG) containing cell bodies of sensory afferent neurons. The regenerative ability of injured axons depends on their capacity to locally synthesize new proteins and to degrade others at the injury site autonomously from the cell body. Nerve crush injury is a well-established axonotmetic model in experimental regeneration studies to investigate the impact of various pharmacological treatments.[20,21] Further, axonal protein synthesis in the normal and injured peroneal nerves was examined. The results indicated that the intensity of nuclear ribonucleoprotein at the injured segments of crushed nerves in rats of treated groups was significantly higher than in negative control group. This suggests that local axonal protein synthesis machinery underlies growth cone initiation after injury. In addition, the principal signaling pathways that have been demonstrated to be involved in axon regeneration are protein kinase B (Akt) and mitogen-activated protein kinase (MAPK). Akt cascade plays a major role in mediating neurotrophin-promoted cell survival while MAPK cascade is involved in mediating neurite outgrowth. A subfamily of MAPK, extracellular signal-regulated kinases 1 and 2 (ERK1/2), controls cellular differentiation and proliferation. Double-labeling immunofluorescence studies showed that small neurons in L5 DRG ipsilateral to the crush injury in rats of treated groups expressed higher immunoreactivities for Akt and ERK1/2 compared to negative control group.[22] These data suggest that daily oral administration of aqueous extract of H. erinaceus fresh fruiting bodies could promote the regeneration of injured rat peripheral nerve in the early stages of recovery.[23] Hericium erinaceus, the mushroom associated with brain and nerve health, has been well-studied,[23] and preparations are available. However, there is a paucity of clinical trials to endorse its potential in mitigating / delaying neurodegenerative disorders. Further, this mushroom may not be the only one that has beneficial effects on nerve and brain health.
Exploring neurotrophic mushrooms in traditional knowledge
To tap the indigenous knowledge in the utilization of the macrofungal diversity of Malaysia as food and medicine, the diversity of potential mushrooms used by the indigenous communities are being explored for neurohealth. Three mushrooms were selected – Lignosus rhinocerotis (Cooke) Ryvarden (Plate1d); Ganoderma neo-japonicum Imazeki (Plate1e) and Pleurotus giganteus (Berk.) Karunarathna and K.D. Hyde (Plate 1b)
Based on indigenous knowledge, selected mushrooms have been used to prepare infusions that are believed to improve vitality, increase alertness and overall wellness of the individual.[24] One such mushroom is Lignosus rhinocerotis.[25] The sclerotium of L. rhinocerotis (known as tiger milk mushroom) is taken daily or when needed in small amounts to ‘increase energy’ by the indigenous people of Malaysia. A national treasure mushroom, L. rhinocerotis, has been used to treat variety ailments by local and indigenous communities in Malaysia.[26] The mushroom is harvested in the wild and is believed in folklore that it grows where the tiger has spilled its milk. The mushroom, which is rarely encountered in the wild, has now been successfully domesticated.[25] In on-going in vitro investigations, an aqueous extract of the sclerotium of L. rhinocerotis induced neurite outgrowths of 24.4% and 42.1% at 20 μg/ml (w/v) of aqueous extract alone and a combination of 20 μg/ml (w/v) aqueous extract and 30 ng/ml (w/v) of NGF, respectively, in rat pheochromocytoma cells (PC-12Adh). Further, a combination of NGF and sclerotium extract had additive effects and enhanced neurite outgrowth.[27] Neuronal differentiation was confirmed by indirect immunofluorescence of neurofilament protein. To our knowledge, this is the first report on neurite-stimulating activities of L. rhinocerotis, and it may be a potential mushroom to develop nerve and brain health nutraceuticals or nutriceuticals. However, studies on the chemical components will shed light on this possibility. There are on-going studies to determine the chemical components involved and the mechanisms of activity that trigger neurite outgrowth.
Another interesting mushroom frequently used by indigenous communities is Ganoderma neo-japonicum. The mushroom was boiled, and the decoction was consumed to treat fever, epilepsy and to improve body strength (Tan et al., 2012, unpublished). The neurite outgrowth stimulation activity of aqueous extracts of the fruiting bodies of G. lucidum (Plate 1c) and G. neo-japonicum were investigated. Varying concentrations of the mushrooms and 50 ng/mL (w/v) of NGF as positive control was added to PC-12Adh cells. Aqueous extract of G. neo-japonicum showed the highest percentage [Figure 2] of neurite-bearing cells of 14.05 ± 0.66% at 50 μg/mL (w/v), followed by G. lucidum (12.08 ± 0.85%) at a higher concentration of 75 μg/mL (w/v). The percentage of neurite-bearing cells of G. neo-japonicum obtained with 50 μg/mL (w/v) was 73% higher compared to the positive control. It was proposed that G. neo-japonicum contained NGF-like bioactive compound/s for maintaining and rebuilding the neural communications network that may help to prevent neurodegenerative diseases (Syntyche Ling et al., 2012, unpublished) [Figure 3 and 4].
Figure 2.
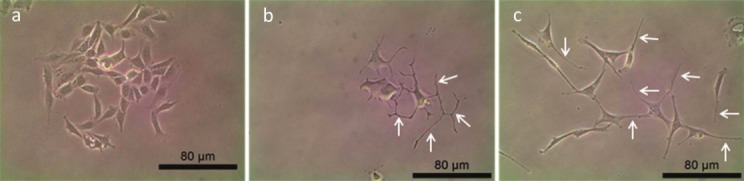
The morphology of PC-12Adh cells. Arrows indicate neurite extensions. a: Negative control - F-12K medium only; b: Positive control - 50 ng/mL (w/v) of NGF; c: 50 μg/mL of G. neo-japonicum
Figure 3.
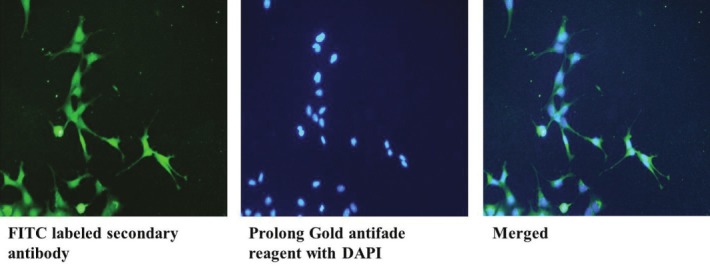
Neurofilament staining to confirm neurite outgrowth
Figure 4.
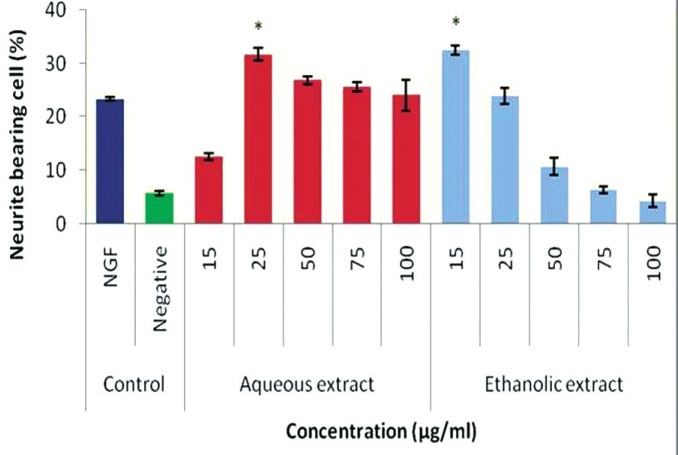
Effects of NGF and P. giganteus extracts on neurite outgrowth using PC12 as an in vitro model. * P < 0.05 compared to NGF (the positive control)[29]
Pleurotus giganteus (Berk.) Karunarathna and K.D. Hyde formerly known as Panus/Lentinus giganteus is a popular mushroom consumed by indigenous communities in Malaysia.[28] A variety domesticated in China is being grown in Malaysia, and it is a very popular culinary mushroom and has a nutritional profile that warrants its inclusion in human diets.[29] The fruiting bodies of P. giganteus contained per gm per 100 gm: 67.2 carbohydrate, 15.4 protein, and 33.3 dietary fiber. It was rich in minerals like magnesium and potassium. To our knowledge, the medicinal benefits, if any, are yet to be explored. Recently, it was reported to have hepato-protective effects.[30] An aqueous extract at 25 μg/ml had a significant (P < 0.05) effect (31.7 ± 1.1%) in stimulating neuronal differentiation compared to 50 ng/ml of NGF (28.3 ± 0.4%).[29] The high potassium level in the fruiting bodies and the presence of bioactive compounds (mainly triterpenoids) could be responsible for the neuronal stimulatory activity. The chemical identity responsible for the activity has to be identified. This may lead to treatment regimes in partially impaired cognitive functions in patients. Further, in the on-going study, it was observed that neurite outgrowth stimulated by P. giganteus was mediated via the “cross-talk” between MEK/ERKs and PI3K/Akt pathways. The mushroom may be a candidate for the development of functional food to reduce or prevent severity of age-related neurodegenerative diseases. Prevention is preferred for this traumatic disorder; once the symptoms are clinically apparent, the reversal is almost impossible.
CONCLUSION
The studies done by many researchers as well as on-going studies show that selected mushrooms do have neurotrophic properties that can be beneficial to humans. Regular consumption may promote nerve and brain health. This is particularly useful during injury (as in an accidents) or as we age. Further, there is a need to study the potential mushrooms to elucidate their mechanisms of activities as well as clinical trials. This far, only H. erinaceus has been extensively studied. The identification of more varieties of mushroom with these unique properties may contribute to identifying novel chemical agents that can have preventative or therapeutic functions.
ACKNOWLEDGEMENT
The authors thank the Ministry of Higher Education for High Impact Research (HIR) grant, F000002-21001; Ministry of Science, Technology and Innovation, Malaysia for SciFund grant 12-02-03-2050 and University of Malaya for supporting funds RG158/11AFR, RG136/10AFR, 66-02-03-0074.
REFERENCES
- 1.Poppe J. Use of agricultural waste material in the cultivation of mushrooms. In: van Griensven LJ, editor. Science and Cultivation of Edible Fungi. Rotterdam / Brookfield: A.A. Balkema; 2000. pp. 3–24. [Google Scholar]
- 2.Chang ST, Buswell JA. Development of the world mushroom industry: Applied mushroom biology and international mushroom organizations. Int J Med Mushrooms. 2008;10:195–208. [Google Scholar]
- 3.Wasser SP. Medicinal mushrooms as a source of antitumour and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–74. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 4.Royse DJ. Foreword to the Fifth international conference on mushroom biology and mushroom products. Acta Edulis Fungi. 2005;12:1–2. [Google Scholar]
- 5.Chang ST. Bejing, China: 2010. A Collection of Publications on Mushroom Biology; p. 367. [Google Scholar]
- 6.Vikineswary S. Brain and nerve health: Can culinary and medicinal mushrooms help? Proceedings ‘The 15th Shizuoka Forum on Health and longevity Mushrooms - Achieving Healthy Longevity by Overcoming Dementia: drug Discovery and Medical Care’ Shizuoka, Japan. 2010 15-16 October 2010. [Google Scholar]
- 7.Acosta D, Wortmann M. World Alzheimer Report 2009. In: Jackson Prince M, Jackson J., editors. In Alzheimer's Disease International. London: 2009. pp. 1–92. [Google Scholar]
- 8.Obara Y, Nakahata N, Kita T, Takaya Y, Kobayashi H, Hosoi S, et al. Stimulation of neurotrophic factor secretion from 1321N1 human astrocytoma cells by novel diterpenoids, scabronines A and G. Eur J Pharmacol. 1999;370:79–84. doi: 10.1016/s0014-2999(99)00077-1. [DOI] [PubMed] [Google Scholar]
- 9.Cheung WM, Hui WS, Chu PW, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291–6. doi: 10.1016/s0014-5793(00)02317-6. [DOI] [PubMed] [Google Scholar]
- 10.Park YS, Lee HS, Won MH, Lee JH, Lee SY, Lee HY. Effect of an exo-polysaccharide from the culture broth of Hericium erinaceus on enhancement of growth and differentiation of rat adrenal nerve cells. Cytotechnology. 2002;39:155–62. doi: 10.1023/A:1023963509393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawagishi H, Shimada A, Shirai R, Okamoto K, Ojima F, Sakamoto H, et al. Erinacines A, B, and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994;35:1569–72. [Google Scholar]
- 12.Kawagishi H, Shimada A, Shizuki K, Mori H, Okamoto K, Sakamoto H, et al. Erinacine D, a stimulator of NGF-synthesis, from the mycelia of Hericium erinaceum. Heterocycl Comm. 1996;2:51–4. [Google Scholar]
- 13.Nagai K, Chiba A, Nishino T, Kubota T, Kawagishi H. Dilinoleoyl-phosphatidylethanolamine from Hericium erinaceum protects against ER stress-dependent Neuro2a cell death via protein kinase C pathway. J Nutr Biochem. 2006;17:525–30. doi: 10.1016/j.jnutbio.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara M, Egashira N, Mishima K. Neuroprotective effect of Hericium erinaceum. Foods and Food Ingredients. J Jpn. 2006:211. [Google Scholar]
- 15.Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother Res. 2009;23:367–72. doi: 10.1002/ptr.2634. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Obara Y, Hirota M, Azumi Y, Kinugasa S, Inatomi S, et al. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol Pharm Bull. 2008;31:1727–32. doi: 10.1248/bpb.31.1727. [DOI] [PubMed] [Google Scholar]
- 17.Vikineswary S, Wong KH, Kho YS, Abdullah N, Murali M, Kuppusamy UR. Mushrooms in healthy diet: Fresh or processed. In: Koutinas AA, Pandey A, Larroche C, editors. Current Topics on Bioprocesses in Food Industries. Chapter 32. New Delhi: Asiatech Publishers, Inc; 2008. pp. 494–501. [Google Scholar]
- 18.Wong KH, Vikineswary S, Abdullah N, Kuppusamy UR, Naidu M. Effects of cultivation techniques and processing on antimicrobial and antioxidant activities of Hericium erinaceus (Bull.: Fr.) Pers. extracts. Food Technol Biotechnol. 2009;47:47–55. [Google Scholar]
- 19.Wong KH, Vikineswary S, Naidu M, Keynes R. Activity of aqueous extracts of Lion's Mane Mushroom Hericium erinaceus (Bull.: Fr.) Pers (Aphyllophoromycetideae) on the Neural Cell Line NG108-15. Int J Med Mushrooms. 2007;9:57–65. [Google Scholar]
- 20.Wong KH, Naidu M, David RP, Abdulla MA, Kuppusamy UR. Functional recovery enhancement following injury to rodent peroneal nerve by lion's mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers Aphyllophoromycetideae) Int J Med Mushrooms. 2009;11:225–36. [Google Scholar]
- 21.Wong KH, Naidu M, David P, Abdulla MA, Abdullah N, Kuppusamy UR, et al. Peripheral nerve regeneration following crush injury to rat peroneal nerve by aqueous extract of medicinal mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae) Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/neq062. 580752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong KH, Naidu M, David P, Vikineswary S. Hericium erinaceus (Bull.:Fr.) Pers., a medicinal mushroom, activates peripheral nerve regeneration mechanisms. Chin J Integr Med. doi: 10.1007/s11655-014-1624-2. [In Press] [DOI] [PubMed] [Google Scholar]
- 23.Wong KH, Naidu M, David P, Bakar R, Vikineswary S. Neuroregenerative potential of Lion s Mane mushroom, Hericium erinaceus (Bull.:Fr.) Pers., (Higher Basidiomycetes), in the treatment of peripheral nerve injury (Review) Int J Med Mushrooms. 2012;14:427–46. doi: 10.1615/intjmedmushr.v14.i5.10. [DOI] [PubMed] [Google Scholar]
- 24.Vikineswary S, Noorlidah A, Normah I, Tan YH, Fauzi D, Jones EB. Edible and medicinal mushrooms. In Malaysian Fungal Diversity. In: Jones EB, Hyde KD, Vikineswary S, editors. Mushroom Research Centre, University of Malaysia and Ministry of National Resources and Environment. Malaysia: Kuala Lumpur; 2007. pp. 287–305. [Google Scholar]
- 25.Tan CS, Ng ST, Vikineswary S, Lo FP, Tee CS. Genetic markers for identification of a Malaysian medicinal mushroom, Lignosus rhinocerus (Cendawan Susu Rimau) Acta Hortic. 2010;859:161–8. [Google Scholar]
- 26.Tan CS. Lignosus rhinocerus (Cooke) Ryvarden. Encyclopedia of Life. [Last accessed in 2009]. Available from: http://www.eol.org/pages/192772 .
- 27.Eik LF, Naidu M, David P, Wong KH, Shin YS, Vikineswary S. Lignosus rhinocerus (Cooke) Ryvarden: a medicinal mushroom.that stimulates neurite outgrowth in PC-12 cells. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/320308. 320308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SS, Chang YS, Noraswati MN. Utilization of macrofungi by some indigenous communities for food and medicine in Peninsular Malaysia. For Ecol Manage. 2009;257:2062–5. [Google Scholar]
- 29.Phan CW, Wong WL, David P, Naidu M, Vikineswary S. In: Pleurotus giganteus (Berk) Karunarathna, Hyde KD: Nutritional value and in vitro neurite outgrowth activity rat pheochromocytoma cells. BMC Complement Altern Med. 2012;12:102. doi: 10.1186/1472-6882-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong WL, Abdulla MA, Chua KH, Kuppusamy UR, Tan YS, Vikineswary S. Hepatoprotective effects of Panus giganteus (Berk.) Corner against thioacetamide (TAA)-induced liver injury in rats. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/170303. 170303. [DOI] [PMC free article] [PubMed] [Google Scholar]


