Abstract
Mulberry (桑葚子 sāng shèn zǐ), a traditional Chinese medicine (TCM) in Taiwan, has many bioactive substances, including polyphenol and anthocyanins compounds. Over the past decade, many scientific and medical studies have examined mulberry fruit for its antioxidation and antiinflammation effects both in vitro and in vivo. This review thus focuses on the recent advances of mulberry extracts (MEs) and their applications in the prevention and treatment of human cancer, liver disease, obesity, diabetes, and cardiovascular disease. The ME modulates several apoptotic pathways and matrix metalloproteinases (MMPs) to block cancer progression. Mulberry can increase detoxicated and antioxidant enzyme activities and regulate the lipid metabolism to treat hepatic disease resulting from alcohol consumption, high fat diet, lipopolysaccharides (LPS) and CCl4 exposure. Of the various compounds in ME, cyanidin 3-glucoside (C3G) is the most abundant, and the active compound studied in mulberry research. Herein, the antioxidant and antiinflammatory actions of C3G to improve diabetes and cardiovascular disease are also discussed. These studies provide strong evidence ME may possess the bioactivity to affect the pathogenesis of several chronic diseases.
Keywords: Chemoprevention effects, Cyanidin-3-glucoside, Mulberry extracts (ME), Traditional Chinese medicine (TCM)
INTRODUCTION
Mulberry (桑葚子 Sang Shèn Zǐ) belongs to the genus Morus of the family Moraceae [Figure 1]. It is widely distributed in Asia, Europe, North America, South America, and Africa. For more than 5000 years, mulberry has been planted for sericulture and has been a valuable resource. The fruit is commonly eaten, often dried, or made into wine, fruit juice, jam, and canned food. Mulberry can grow in a wide range of climatic, topographical, and soil conditions, which can affect the chemical composition and nutritional status of plants. Studies have been reported on the chemical composition and nutritional potentials of some mulberry species worldwide.[1,2,3,4,5,6]
Figure 1.

The fruits of mulberry
The deep colored mulberry fruits are rich in phenolic compounds, including flavonoids, anthocyanins, and carotenoids.[7,8,9] They represent one of the most widely distributed classes of flavonoids in plants. Such natural substances extracted from plants have been shown to have greater antioxidant and antiinflammatory effects and have been used for health maintenance and disease management since the beginning of recorded history.[10] Mulberry is traditionally used in Chinese medicines as a pharmaceutical for antifever diuretics, liver protection, eyesight improvement, blood pressure reduction, and cardiovascular disease prevention. Dietary mulberry has been reported to have not only antioxidative, antiinflammatory, antitumor, and antidiabeticeffects, but also cardiovascular, hepato-, and neuro-protectiveproperties[11,12,13,14,15,16] [Figure 2]. This review will highlight the current understanding of the mulberry and discuss the mechanism of its chemopreventive effects.
Figure 2.
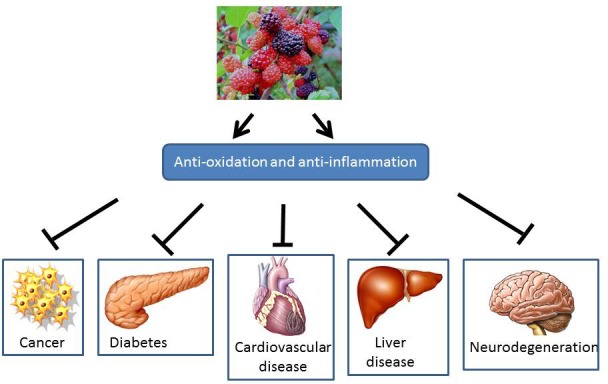
Summary of biological activity of ME on various diseases
Mulberry composition and effectiveness
The proximate composition of mulberry fruits was reported by Imran et al.[17] [Table 1]. The moisture contents were in the range of 78.03–82.4 g/100 g fresh weight (FW). The ash contents ranged between 0.46 and 0.87 g/100 g dry weight (DW). The total lipid contents were in the range of 0.48–0.71 g/100 g DW. The total protein contents of the fruit samples were small, varying between 0.96 and 1.73 g/100 g DW. The crude fiber contents of the fruit samples varied widely between 0.57 and 11.75 g/100 g DW. The total carbohydrate contents showed slight variations among the studied fruits samples. The carbohydrate concentration was found to be in the range of 13.83–17.96 g/100 g DW. Interestingly, the main sugars identified in the analyzed mulberry were glucose and fructose, with sucrose not being detected. The calorific value, calculated on a dry weight basis, ranged between 64.11 and 84.22 kcal/100 g. The overall results showed the fruit samples could be a potential source of lipids, proteins, fibers, carbohydrates, and hence energy.
Table 1.
The proximate composition of mulberry fruits
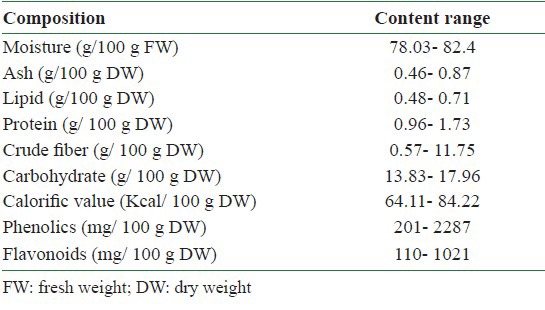
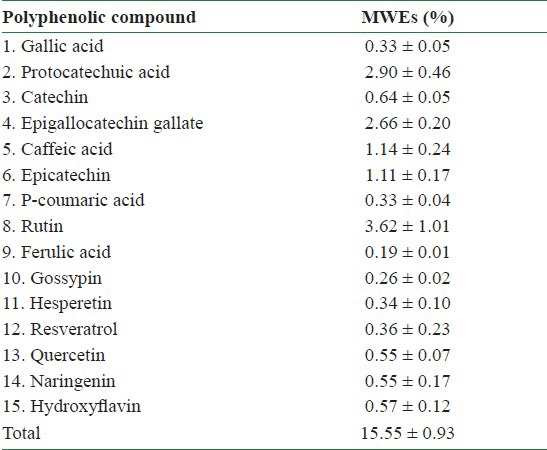
Phenolic acids constitute about one-third of the dietary phenols and are present in plants in free and bound forms.[18] For the different species of mulberry the yield of extract (%), total phenolics and total flavonoids of 6.9–54.0%, 201–2287 mg/100 g DW and 110–1021 mg/100 g DW, respectively, varied significantly as fruit maturity progressed[19] [Table 1]. Among the flavonols, the content of myricetin was found to be high in Morus alba (88 mg/100 g DW). The amount of quercetin was as high in Morus laevigata (145 mg/100 g DW) at the fully ripened stage. M. laevigata and M. nigra contained p-coumaric acid and vanillic acid while M. macroura and M. alba contained p-hydroxy-benzoic acid and chlorogenic acid as the major phenolic acids. Overall, a trend to an increase in the percentage of extraction yield, total phenolics and total flavonoids, flavonols and phenolic acids was observed as maturity progressed from unripened to fully ripened stages. The nutrient compositions of ME were analyzed in our laboratory as shown in [Table 2].[20] Analysis of ME revealed the presence of gallic acid (0.31%), protocatechuic acid (2.92%), catechin (0.54%), epigallocatechingallate (2.68%), caffeic acid (1.10%), epicatechin (1.21%), p-coumaric acid (0.35%), rutin (3.22%), ferulic acid (0.27%), gossypin (0.26%), hesperetin (0.34%), resveratrol (0.35%), quercetin (0.50%), naringenin (0.52%), and hydroxyflavin (0.58%) [Table 3]. Further, HPLC/ESI/MS/MS (High performace liquid chromatography/ electrospray ionization/ Tandem mass spectrometry) analysis of mulberry fruits revealed the presence of four anthocyanins recognized as cyanidin 3-O-glucoside (C3G), cyanidin 3-O-rutinoside (C3R), pelargonidin 3-O-glucoside (P3G), and pelargonidin 3-O-rutinoside (P3R).[21,22]
Table 2.
Composition of Mulberry water extracts
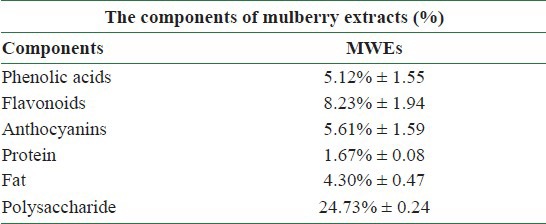
Table 3.
HPLC chromatograms assay
The bioactivity of the uptake of anthocyanins from mulberry in the intestine was investigated. In the animal model, anthocyanin-rich extract from wild mulberry was orally administered to Wistar rats, and their concentrations were determined in plasma, kidney, and the gastrointestinal (GI) tract.[23] The total cyanidin level reached a maximum concentration at 15 minutes after oral administration in the plasma and kidney. C3G and C3R were found in plasma as glucuronides, as sulfates of cyanidin, and as unchanged forms. The increase in the plasma anthocyanins level resulted in a significant increase in antioxidant capacity, whereas only 0.11% of cyanidin glycosides were absorbed after 8 hours of administration.
Antioxidation and antiinflammation
Among the phytochemicals in fruit, phenolic acids and flavonols are regarded as major functional food components and are thought to contribute to the health effects of fruit-derived products due to the prevention of various diseases associated with oxidative stress.[24] Many studies suggest flavonoids, a family of compounds with a C6-C3-C6 skeleton structure, display several biological activities, including antiallergic, antiviral, antitumor, and antiinflammatory action and antioxidant activity.[25] HPLC analysis showed that the main flavonols rich in mulberry fruit were rutin, morin, quercetin, and myricetin [Figure 3]. These flavonols are reported to be effective antioxidants.[26] For instance, morin significantly reduced the tissue level of cyclosporin, a potent immunosuppressive agent with narrow therapeutic range, and dramatically decreased the nitric oxide production by the activated macrophages.[27] In addition, C3G isolated from mulberry fruits inhibited the cerebral ischemic damage caused by oxygen glucose deprivation in PC12 cells.[28] Such anthocyanin-rich fruits have shown concentration-dependent antioxidant activity (inhibition of copper-induced peroxidation of liposome).[29] Black mulberry juice inhibited the human cytochrome CYP3A activity in the pooled human liver microsome system.[30] These reports support the possibility mulberry juice contains several components with antioxidant activity. Different species of Morus were analyzed for phenolic compounds and antioxidant activity. The total phenolic compounds of the sugar-free extracts (SFEs) were much higher than crude extracts containing sugars.[31] The SFEs from the Morusnigra displayed greater total antioxidant activities than those from other species of Morus.
Figure 3.
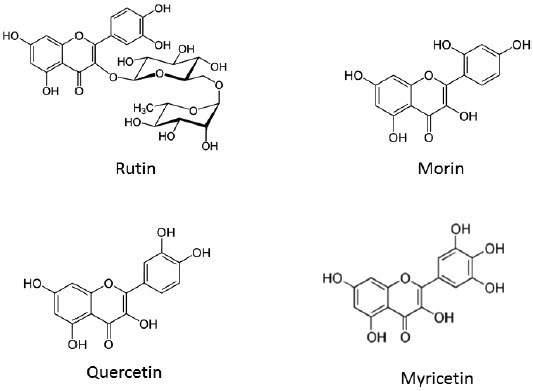
The structures of main flavonols rich in mulberry fruit
Supplementation of ME could protect the liver from age-associated antioxidant decline and protect the brain from cognitive deficits in SAMP8 mice.[32] Anthocyanins and water extracts from mulberry fruit can scavenge free radicals, inhibit low-density lipoprotein (LDL) oxidation, and have beneficial effects on blood lipid and atherosclerosis.[33,34] In addition, the serum and liver content of thiobarbituric acid-related substances, a lipid peroxidation product, significantly decreased, while the superoxide dismutase (SOD) of red blood cell and liver, as well blood glutathione peroxidase (GSH-Px) activities significantly increased.[35] Antistress activity is tightly coupled with antioxidant activity. Interestingly, after administration of mulberry juice one or two weeks before the stress loading, lipid peroxidation was completely blocked. Administration of mulberry juice after the stress loading, without preadministration, was also protective.[36]
According to a study published in the “Journal of Medicinal Food,” mulberry is an excellent source of C3G, traditionally used for treating inflammatory conditions such as rheumatoid arthritis. During this animal research, mulberry fruit extract provided antiinflammatory benefits for arthritic rats.[37] Yang et al. reported that mulberry fruit increased NO production and tumor necrosis factor-alpha (TNF-α) secretion in RAW 264.7 and peritoneal macrophages, co-stimulatory molecule expression in peritoneal macrophages, and interferon-γ (IFN-γ) expression in splenocytes. Further studies indicated that mulberry fruit could significantly induce the phosphorylation of the signal molecules of mitogen-activated protein kinases (MAPKs) and the degradation of IkBα, which finally led to the activation and nuclear translocation of nuclear factor-kB (NF-kB) for the target gene expression. All those notions disclosed that the aqueous extract of mulberry fruit is a TLR4 activator, which induces a Th1 immune response as a consequence of induction of cytokines secretion, especially TNF-α and IFN-γ.[38]
Anticancer effects
There is a growing realization that a lower incidence of cancer is probably due to the consumption of polyphenol rich diets. Consequently, a systematic dissection of the chemopreventive potential of polyphenolic compounds in recent years has clearly supported their health benefits, including anticancer properties. Many studies in cultured cells, animal models, and human clinical trials have illustrated the protective role of dietary polyphenols against different types of cancers.[39,40,41] Various mechanistic explanations for their chemopreventive efficacy include their ability to interrupt or reverse the carcinogenesis process by actingon intracellular signaling network molecules involved in the initiation and/or promotion of cancer, or their potential to arrest or reverse the progression stage of cancer.[42,43]
Six cancer cell lines; AGS, MCF7, SW742, SKLC6, A375, and PLC/PRF/5 exposed to nine novel candidate herbal extracts and cytotoxic drug were performed. The IC50 related to candidate herbal extracts were calculated in a range from 22.2 to 99.9 μg/ml, the minimum IC50 related to M. alba extract and maximum IC50 related to C. limon extract (bulk extract) on AGS, indicating the better chemopreventive efficacy of mulberry.[44] Mulberry anthocyanins, cyanidin 3-rutinoside, and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line by regulating the activation of c-Jun and NF-κB.[45] Mulberry fruit extracts result in human glioma cell death in vitro through the reactive oxygen species (ROS)-dependent mitochondrial pathway and glioma tumor growth in vivo via reduction of tumor cell proliferation and the induction of apoptosis.[46] By targeting p38/p53 and the c-jun pathways, mulberry anthocyanins (MACs) suppressed gastric cancer cell survival and tumorigenesis, and induced apoptotic death in vitro and in vivo.[47] Moreover, MACs could mediate B16-F1 cell metastasis by reduction of matrix metalloproteinase-2 and -9 (MMP-2, MMP-9) activities involving the suppression of the Ras/PI3K signaling pathway. Besides, B16-F1 melanoma cells were also injected into the right groin of C57BL/6 mice, and they were fed with MACs at the same time. The hematoxylin-eosin stain (H and E stain) and immunohistochemistry stain showed that the MACs inhibited the metastasis of B16-F1 cells in vivo.[12] Effects of ME on the acquired capabilities of cancer development through various mechanisms are summarized in [Figure 4]. Several cancer trials on antiangiogenesis, immune regulation, and tumor microenvironment modulation have been completed but not yet published.
Figure 4.
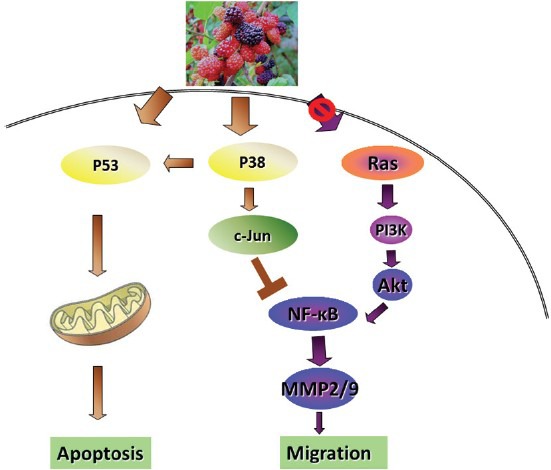
Effects of ME on inhibiting cancer development through various mechanisms
A major impediment in the anticancer chemotherapy process is the multidrug resistance (MDR) phenomenon. Combinational drug therapy has a long history and its root can be found in traditional Chinese medicines (TCMs). IC50 values generated by single anticancer drug treatments and several concentrations of ME in combination with doxorubicin on human fetal fibroblast HF2 cells, findings showed that the IC50 values induced by a single anticancer drug (doxorubicin) are much lower than that by combinational treatments.[48] According to the above studies, ME has potential as a cancer chemopreventive agent against apoptosis evasion, insensitivity to antigrowth signals, tissue metastasis, and angiogenesis. Moreover, it can be used in combinational drug therapy by regulating the immune status of the tumor microenvironment.
Hepatoprotection
Hepatocellular carcinoma (HCC) is the most common liver cancer, accounting for 90% of primary liver cancers. By far the most frequent risk factor for HCC is liver cirrhosis (LC), resulting from virus infection, alcohol consumption, nonalcoholic fatty liver disease (NAFLD), and some hereditary diseases.[49] Although the clinical diagnosis and management of early stage HCC has improved significantly, advanced HCC is a highly aggressive tumor with a poor or no response to common therapies. Epidemiologic evidence suggests that regular consumption of fruits and vegetables may reduce risk of some diseases, including cancer. Therefore, new effective and well-tolerated therapy strategies are urgently needed.
The ethanol-containing diet (ED) group developed fatty liver according to the lipid profile and liver histological findings. Compared with the control group, liver/body weight, serum triglyceride (TG) and total cholesterol (TC), liver TG and TC, serum alanine aminotransferase (ALT), and aspartic aminotransferase (AST) significantly increased in the ED group. In contrast, in the rats administered with TCM consisting of Astragalus membranaceus, M. alba, Crataegus pinnatifida, Alisma orientale, Salvia miltiorrhiza, and Pueraria lobata (2:2:2:2:1:1), liver/body weight, serum TG and TC, liver TG and TC, serum ALT and AST were significantly decreased, and the degree of hepatic lipid droplets was markedly improved compared with those in the ED group. TCM treatment causes significant reduction in alcohol-induced lipid hepatic accumulation, reversing fatty liver and liver damage, and can be used as a remedy for alcoholic fatty liver.[50]
Administration of a freeze-dried powder of mulberry fruit (MFP) to rats on a high-fat diet resulted in a significant decline in the levels of serum and liver TG, TC, serum LDL cholesterol, and a decrease in the atherogenicindex, while the serum high-density lipoprotein (HDL) cholesterol was significantly increased. In addition, the serum and liver content of thiobarbituric acid-related substances, a lipid peroxidation product, significantly decreased, while the SOD of red blood cell and liver, as well blood GSH-Px activities, significantly increased. No significant changes in the lipid profile in the serum and liver were observed in rats on a normal diet supplemented with MFP, but the blood and liver antioxidant status improved, as measured by SOD and GSH-Px activity, and lipid peroxidation was reduced. These beneficial effects of MFP on hyperlipidaemia rats might be attributed to its dietary fiber, fatty acids, phenolics, flavonoids, anthocyanins, vitamins, and trace elements content.[35] Our previous studies showed ME significantly reduced lipid accumulation, suppressed fatty acid synthesis, and stimulated fatty acid oxidation by stimulating adenosine monophosphate-activated protein kinase (AMPK).[20]
Oral administration of ME significantly reduced the lipid peroxidation triggered by CCl4, as shown by the reduced production of thiobarituric acid reactive substance (TBARS). The levels of serum AST, ALT, and alkaline phosphatase (ALP) were also reduced via cotreatment with ME compared with CCl4 treatment alone. Cotreatment with ME evidently reduced CCl4-induced liver weight and inhibited lipid deposition and fibrogenesis. ME attenuated the proinflammatory genes such as cyclooxygenase 2 (COX2), NF-κB, and inducible nitric oxide synthase (iNOS) expression. The current findings suggest that ME such as silymarin exhibit protective and curative effects against CCl4-induced liver damage and fibrosis via decreased lipid peroxidation and inhibited proinflammatory gene expression.[51] The similar hepatoprotective effects and molecular mechanisms of ME on acute liver failure induced by lipopolysaccharides (LPS) were proved in vivo.[52] Beside, ME suppressed oxidative stress to prevent the formation of hepatic malondialdehyde (MDA) in this study. The Morusnigra fruit juice also produced low MDA formation and showed a significant inhibitory effect on hepatocyte intoxication of 80%.[53]
Cytochrome P450 is a representative enzyme involved in hepatic drug metabolism, which is crucial for the elimination of many therapeutic drugs. Among the members of the P450 family, CYP3A is the most important enzyme and is involved in the majority of the P450-catalyzed metabolism.[54] It has been shown black mulberry is able to inhibit human CYP3A-catalyzed midazolam1-hydroxylation activity in liver microsomes, and the inhibitory effects are somewhat greater than those of pomegranate.[30,55] Satoh et al. also reported that black mulberry extract (ME) potently inhibits organic anion transporting polypeptide B(OATP-B) function at concentrations that seem to be physiologically relevant in vitro.[56] These results suggest that black mulberry may decrease the plasma concentrations of concomitantly ingested OATP-B substrate drugs or increase the plasma concentration levels of concomitantly ingested CYP3A substrate drugs.
In summary and as shown in [Figure 5], mulberry can be used to treat hepatic disease resulting from alcohol consumption, a high fat diet, LPS and CCl4 exposure through improving detoxicated and antioxidant enzymes and regulating the lipid metabolism.
Figure 5.
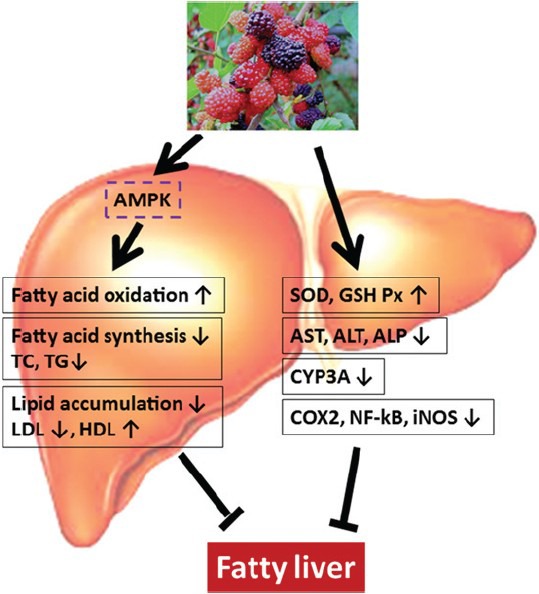
An overview of various targets of fatty liver shown to be regulated by ME
Antiobesity and cardiovascular functions
Obesity is associated with a great diversity of diseases, including metabolic syndrome and cardiovascular disease. There is a metabolic rationale linking the expanded abdominal or visceral fat depot to high TG, low HDL, high LDL dyslipidemia, and insulin resistance, which are often accompanied with impaired metabolic regulation in adipose tissue, leading to an overproduction of free fatty acid (FFA).[57] Using 6-week-old male hamsters, we investigated the antiobese effect of ME. After 12 weeks of treatment, ME lowered high-fat diet (HFD)-induced body weight and visceral fat, accompanied with hypolipidemic effects by reducing serum triacylglycerol, cholesterol, free fatty acid, and the LDL/HDL ratio. ME decreased hepatic lipids, thus protected livers from impairment. The hepatic peroxisome proliferator-activated receptor R and carnitine palmitoyltransferase-1 were elevated, while fatty acid synthase and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase were reduced by ME, indicating that ME regulated lipogenesis and lipolysis, which exerted the antiobese and hypolipidemic effects. Noticeably, ME showed both efficacy and safety in vivo. In conclusion, ME can be used to reduce body weight, serum, and lipids.[58]
Abundant lines of evidence suggest anthocyanins can reduce cardiovascular risk, improve inflammation, and protect against chemical toxicity and cerebral ischemic damage.[28,59,60,61] Two extracts, ME and MACs, exhibit antioxidative and antiatherosclerogensis abilities in vitro. Liu et al. showed that ME and MACs were able to inhibit the relative electrophoretic mobility (REM), ApoB fragmentation, and TBARS formation in Cu2+-mediated oxidation LDL. MWEs and MACs also had the ability of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging for reducing the formation of free radicals mediated by copper ions. Further, we observed that ME and MACs could decrease macrophage death induced by oxLDL. The ME and MACs could also inhibit the formation of foam cells. Both ME and MACs showed great ability to scavenge radicals, inhibit LDL oxidation, and decrease atherogenic stimuli in macrophages, while the efficacy of MACs is 10-fold greater than that of ME. It demonstrated that anthocyanins components in MEs acted to prevent atherosclerosis.[22]
Other research also reported that anthocyanins and water extracts from mulberry fruit can scavenge free radicals, inhibit LDL oxidation, and have beneficial effects on blood lipid and atherosclerosis.[62,63] Treatment of the freeze-dried powder of mulberry to rats on a high-fat diet resulted in a significant decline in the levels of serum and liver TG, TC, serum LDL, while the serum HDL significantly increased.[35] Morusnigra fruit has a protective action against lipid peroxidation of LDL induced by copper ions.[53] However, further investigations into the mechanisms of mulberry, which reduce cardiovascular risk, are needed.
Antidiabetes
Research on the capacities of mulberry for improving diabetes mostly focuses on the leaves and less on the fruits. However, the cyanidin-3-glucopyranoside (C3G) from mulberry fruit in regard to the improvement and protection of erectile function was examined.[64] Sprague-Dawley rats were divided into normal control, diabetes mellitus (DM), and DM with C3G concentrated material treatment (DM + C3G) groups. The DM group rats showed markedly lower erectile parameters than those in the control group, whereas rats in the DM + C3G group showed improved erectile function by minimizing corporal apoptosis and increasing the expression of endothelial nitric oxide synthase (eNOS) and neuronal NOS protein. A significant increase in 8-hydroxy-2-deoxyguanosine (8-OHdG) was shown in the DM group compared with the normal group. However, in the DM + C3G group, 8-OHdG was, statistically speaking, significantly reduced compared with the DM group. This study is the first to suggest that mulberry may be able to improve and protect erectile function under conditions of diabetes-induced oxidative stress.
Neuroprotective effects
Cerebral ischemia results in low oxygen and glucose supply and is accompanied by a decrease in the level of adenosine triphosphate (ATP) formation. Recent studies support the role of oxidative mechanisms in cerebral ischemia injury. C3G, which is an aglycon of anthocyanin, has free radical scavenging and inflammation suppressing activity, and protects against endothelial dysfunction.[65,66,67] Several studies in neuroprotective efficacy of ME containing C3G are summarized in [Table 4] and described below.
Table 4.
Studies showing neuroprotective of mulberry extracts
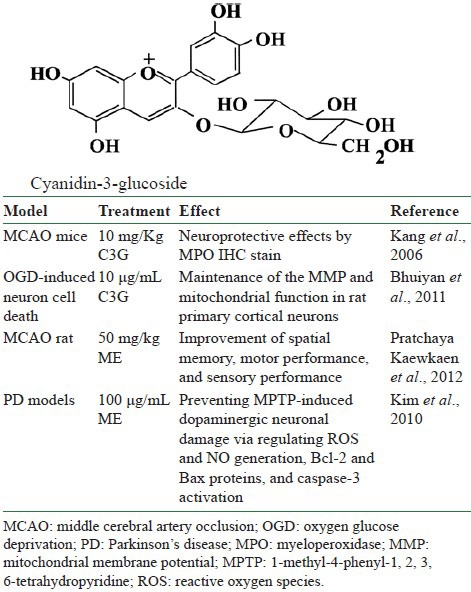
A 1% HCl–methanol mulberry fruit extract containing C3G was shown to have a cytoprotective effect on rat PC12 pheochromocytoma cells that had been exposed to hydrogen peroxide. The extract inhibited the cerebral ischemic damage caused by oxygen glucose deprivation (OGD) in PC12 cells. The neuroprotective effect of the mulberry fruit extract was further demonstrated in vivo using a mouse-brain-injury model with transient middle cerebral artery occlusion (MCAO). Compared with the control group, C3G had neuroprotective effects on the PC12 cells exposed to hydrogen peroxide in vitro and on cerebral ischemic damage in vivo.[28]
In another study, C3G fraction extracted from the M. alba was investigated for its neuroprotective effects against OGD and glutamate-induced cell death in rat primary cortical neurons.[68] A time-course study of OGD-induced cell death of primary cortical neurons at 7 days in vitro (DIV) indicated that neuronal death was OGD duration-dependent. It was also demonstrated that OGD for 3.5 h resulted in approximately 50% cell death, as measured by a LDH release assay. Treatments with mulberry C3G fraction maintained the mitochondrial membrane potential (MMP) in primary cortical neurons exposed to OGD as assessed by the intensity of rhodamine-123 fluorescence. These results therefore suggest that the neuroprotective effects of mulberry C3G fraction are mediated by the maintenance of the MMP and mitochondrial function in rat primary cortical neurons.
In an animal model, male Wistar rats were orally given MFP at doses of 2, 10, and 50 mg/kg BW at periods of 7 days before and 21 days after induced MCAO. The animals’ spatial memory, motor performance, and sensory performance were measured every 7 days before and after the operation until the end of the experiment. In addition, the density of survival neurons was determined using cresyl violet stain. The results showed that significant improvement in health conditions when using mulberry fruits after a single dose and 7 days of MCAO at doses of 2 and 10 mg/kg BW. For cognitive deficit condition, mulberry fruits can exert a neuroprotective effect at doses of 10 and 50 mg/kg BW after 21 days MCAO. A significant change in sensory recovery was observed at 50 mg/kg BW after MCAO 7 days. The mulberry fruits also improved the density of survival neurons in CA2 and CA3 of the hippocampus after 21 days of MCAO. All results in this study suggest that mulberry fruit has high potential against stroke conditions. However, the precise underlying mechanism still requires further investigation.[69]
Worldwide, as the population ages, the probability of people developing neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (PD) is increasing. Oxidative stress is thought to be one of the factors that reduce cognitive and motor performance in neurodegenerative disease. Kim et al. examined the protective effects of ME against neurotoxicity in in vitro and in vivo PD models.[70] In SH-SY5Y cells stressed with 6-hydroxydopamine (6-OHDA), ME significantly protected the cells from neurotoxicity. Other assays demonstrated that the protective effect of ME was mediated by its antioxidant and antiapoptotic effects, regulating reactive oxygen species and NO generation, Bcl-2 and Bax proteins, mitochondrial membrane depolarisation and caspase-3 activation. In mesencephalic primary cells stressed with 6-OHDA or 1-methyl-4-phenylpyridinium (MPPt), pretreatment with ME also protected dopamine neurons, showing a wide range of effective concentrations in MPPt-induced toxicity. In the sub-acute mouse PD model induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), ME showed a preventative effect against PD-like symptoms (bradykinesia) in the behavioral test and prevented MPTP-induced dopaminergic neuronal damage. These results indicate that ME have neuroprotective effects in vitro and in vivo, and may be useful in preventing or treating PD.
CONCLUSION
Recent researches have indicated that mulberry fruit has beneficial effects in reducing the risk of chronic disease. These results strongly reflect the chemoprevention potential of ME. Although several studies have established the potential of ME and were carried out in animals to prove the preventive potential of mulberry, a proper understanding of mechanisms that reduce the risk of disease remains elusive. Further testing of ME in humans is required to confirm these observations. Based on our understanding of the mechanisms for ME, we conclude that ME is unlikely to have a single target or receptor that is responsible for all the observed activities. Since information on the bioavailability of mulberry after consumption is limited in humans, studies on absorption, distribution, and the metabolism of mulberry polyphenols in animals and humans are needed. Future studies should not only focus on understanding the mechanisms, but also design strategies for the development of ME as better chemopreventive agents. Therefore, the advanced study will be needed to demonstrate that the single, pure component of ME has chemopreventive activity and to reveal the possible mechanism of action of these compounds, respectively. Over a dozen different cellular proteins and enzymes have been identified that are regulated by ME. In the future, microarray gene chip technology should be determined to show which genes are regulated by ME. How ME produces its chemopreventive effects is not fully understood, but they are probably mediated in part through the antioxidant and antiinflammatory action of the extracts. Although further studies are needed, mulberry appears to be a low-risk, inexpensive complementary therapy for a number of conditions. We suggest that naturally occurring agents such as ME could be developed as potent chemopreventive agents and as natural healthy foods for managing chronic disease, metabolic syndromes, or cancer.
ACKNOWLEDGEMENTS
The authors thank the National Science Council (NSC 99-2632-B-040-001-MY3, NSC 99-2320-B040-013-MY3, NSC-99-2321-B040-001 and NSC101-2622-B040-001-CC2) for supporting in this review.
REFERENCES
- 1.Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (M. nigra) mulberry fruits. Food Chem. 2007;103:1380–4. [Google Scholar]
- 2.Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007;102:1233–40. [Google Scholar]
- 3.Darias-Martin J, Lobo-Rodrigo G, Hernandez-Cordero J, Diaz-Diaz E, Diaz-Romero C. Alcoholic beverages obtained from black mulberry. Food Technol Biotechnol. 2003;41:173–6. [Google Scholar]
- 4.Elmacı Y, Altuğ T. Flavour evaluation of three black mulberry (Morus nigra) cultivars using GC/MS, chemical and sensory data. J Sci Food Agric. 2002;82:632–5. [Google Scholar]
- 5.Gao X, Ohlander M, Jeppsson N, Björk L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 2000;48:1485–90. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 6.Gerasopoulos D, Stavroulakis G. Quality characteristics of four mulberry (Morus sp.) cultivars in the area of Chania, Greece. J Sci Food Agric. 1997;73:261–4. [Google Scholar]
- 7.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–7. [Google Scholar]
- 8.Sass-Kiss A. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res Int. 2005;38:1023–9. [Google Scholar]
- 9.Zadernowski R, Naczk M, Nesterowicz J. Phenolic acid profiles in some small berries. J Agric Food Chem. 2005;53:2118–24. doi: 10.1021/jf040411p. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaswamy K, Raghuramulu N. Bioactive phytochemicals with emphasis on dietary practices. Indian J Med Res. 1998;108:167–81. [PubMed] [Google Scholar]
- 11.Andallu B, Suryakantham V, Lakshmi Srikanthi B, Reddy GK. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin Chim Acta. 2001;314:47–53. doi: 10.1016/s0009-8981(01)00632-5. [DOI] [PubMed] [Google Scholar]
- 12.Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J Agric Food Chem. 2008;56:9286–93. doi: 10.1021/jf8013102. [DOI] [PubMed] [Google Scholar]
- 13.El-Beshbishy HA, Singab AN, Sinkkonen J, Pihlaja K. Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci. 2006;78:2724–33. doi: 10.1016/j.lfs.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, et al. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J Agric Food Chem. 2007;55:5869–74. doi: 10.1021/jf062680g. [DOI] [PubMed] [Google Scholar]
- 15.Isabelle M, Lee BL, Ong CN, Liu X, Huang D. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J Agric Food Chem. 2008;56:9410–6. doi: 10.1021/jf801527a. [DOI] [PubMed] [Google Scholar]
- 16.Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–6. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 17.Imran M, Khan H, Shah M, Khan R, Khan F. Chemical composition and antioxidant activity of certain Morus species. J Zhejiang Univ Sci B. 2010;11:973–80. doi: 10.1631/jzus.B1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem. 2003;51:2866–87. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- 19.Mahmood T, Anwar F, Abbas M, Saari N. Effect of maturity on phenolics (phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from pakistan. Int J Mol Sci. 2012;13:4591–607. doi: 10.3390/ijms13044591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou TT, Hsu MJ, Chan KC, Huang CN, Ho HH, Wang CJ. Mulberry extract inhibits oleic acid-induced lipid accumulation via reduction of lipogenesis and promotion of hepatic lipid clearance. J Sci Food Agric. 2011;91:2740–8. doi: 10.1002/jsfa.4489. [DOI] [PubMed] [Google Scholar]
- 21.Pawlowska AM, Oleszek W, Braca A. Quali-quantitative analyses of Flavonoids of Morus nigra L. and Morus alba L. (Moraceae) fruits. J Agric Food Chem. 2008;56:3377–80. doi: 10.1021/jf703709r. [DOI] [PubMed] [Google Scholar]
- 22.Liu LK, Lee HJ, Shih YW, Chyau CC, Wang CJ. Mulberry anthocyanin extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J Food Sci. 2008;73:H113–21. doi: 10.1111/j.1750-3841.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 23.Hassimotto NM, Genovese MI, Lajolo FM. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr Res. 2008;28:198–207. doi: 10.1016/j.nutres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Lodovici M, Guglielmi F, Meoni M, Dolara P. Effect of natural phenolic acids on DNA oxidation in vitro. Food Chem Toxicol. 2001;39:1205–10. doi: 10.1016/s0278-6915(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 25.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 26.Lu Xi WL, Wei H, Yang Z, Wei W. Structure-activity relationship of flavonoids in antioxidant activity. Food Sci. 2006;27:233–7. [Google Scholar]
- 27.Fang SH, Hou YC, Chao PD. Pharmacokinetic and pharmacodynamic interactions of morin and cyclosporin. Toxicol Appl Pharmacol. 2005;205:65–70. doi: 10.1016/j.taap.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett. 2006;391:122–6. doi: 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 29.Hassimotto NM, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem. 2005;53:2928–35. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Yoon YJ, Shon JH, Cha IJ, Shin JG, Liu KH. Inhibitory effects of fruit juices on CYP3A activity. Drug Metab Dispos. 2006;34:521–3. doi: 10.1124/dmd.105.007930. [DOI] [PubMed] [Google Scholar]
- 31.Arfan M, Khan R, Rybarczyk A, Amarowicz R. Antioxidant activity of mulberry fruit extracts. Int J Mol Sci. 2012;13:2472–80. doi: 10.3390/ijms13022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih PH, Chan YC, Liao JW, Wang MF, Yen GC. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer's disease. J Nutr Biochem. 2010;21:598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Du Q, Zheng J, Xu Y. Composition of anthocyanins in mulberry and their antioxidant activity. J Food Compos Anal. 2008;21:390–5. [Google Scholar]
- 34.Chen CC, Hsu JD, Huang HP, Yang MY, Wang CJ. Mulberry extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. Food Chem. 2005;91:601–7. doi: 10.1021/jf030065w. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Yang L, Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol. 2010;48:2374–9. doi: 10.1016/j.fct.2010.05.074. [DOI] [PubMed] [Google Scholar]
- 36.Sakagami H, Asano K, Satoh K, Takahashi K, Terakubo S, Shoji Y, et al. Anti-stress activity of mulberry juice in mice. In Vivo. 2006;20:499–504. [PubMed] [Google Scholar]
- 37.Kim AJ, Park S. Mulberry extract supplements ameliorate the inflammation-related hematological parameters in carrageenan-induced arthritic rats. J Med Food. 2006;9:431–5. doi: 10.1089/jmf.2006.9.431. [DOI] [PubMed] [Google Scholar]
- 38.Yang XY, Park GS, Lee MH, Chang IA, Kim YC, Kim SY, et al. Toll-like receptor 4-mediated immunoregulation by the aqueous extract of Mori Fructus. Phytother Res. 2009;23:1713–20. doi: 10.1002/ptr.2818. [DOI] [PubMed] [Google Scholar]
- 39.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 40.Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila) 2010;3:549–59. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, et al. Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–39. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manson MM. Cancer prevention - the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9:11–8. doi: 10.1016/s1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 43.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 44.Sardari S, Shokrgozar MA, Ghavami G. Cheminformatics based selection and cytotoxic effects of herbal extracts. Toxicol In Vitro. 2009;23:1412–21. doi: 10.1016/j.tiv.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–59. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 46.Jeong JC, Jang SW, Kim TH, Kwon CH, Kim YK. Mulberry fruit (Moris fructus) extracts induce human glioma cell death in vitro through ROS-dependent mitochondrial pathway and inhibits glioma tumor growth in vivo. Nutr Cancer. 2010;62:402–12. doi: 10.1080/01635580903441287. [DOI] [PubMed] [Google Scholar]
- 47.Huang HP, Chang YC, Wu CH, Hung CN, Wang CJ. Anthocyanin-rich Mulberry extract inhibit the gastric cancer cell growth in vitro Food Chemistry and xenograft mice by inducing signals of p38/p53 and c-jun. Food Chem. 2011;129:1703–9. [Google Scholar]
- 48.Ghavami G, Sardari S, Ali Shokrgozar M. Cheminformatics-based selection and synergism of herbal extracts with anticancer agents on drug resistance tumor cells-ACHN and A2780/cp cell lines. Comput Biol Med. 2011;41:665–74. doi: 10.1016/j.compbiomed.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 49.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 50.Kwon HJ, Kim YY, Choung SY. Amelioration effects of traditional Chinese medicine on alcohol-induced fatty liver. World J Gastroenterol. 2005;11:5512–6. doi: 10.3748/wjg.v11.i35.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu LS, Ho HH, Lin MC, Chyau CC, Peng JS, Wang CJ. Mulberry water extracts (MWEs) ameliorated carbon tetrachloride-induced liver damages in rat. Food Chem Toxicol. 2012;50:3086–93. doi: 10.1016/j.fct.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 52.Ou TT, Kuo CY, Chyau CC, Lee HJ, Peng JS, Wang CJ. Improvement of lipopolysaccharides-induced hepatic injuries and inflammation with mulberry extracts. J Sci Food Agric. 2012 doi: 10.1002/jsfa.5984. doi: 10.1002/jsfa.5984. [DOI] [PubMed] [Google Scholar]
- 53.Naderi GA, Asgary S, Sarraf-Zadegan N, Oroojy H, Afshin-Nia F. Antioxidant activity of three extracts of Morus nigra. Phytother Res. 2004;18:365–9. doi: 10.1002/ptr.1400. [DOI] [PubMed] [Google Scholar]
- 54.Guengerich FP. In vitro techniques for studying drug metabolism. J Pharmacokinet Biopharm. 1996;24:521–33. doi: 10.1007/BF02353478. [DOI] [PubMed] [Google Scholar]
- 55.Hidaka M, Okumura M, Fujita K, Ogikubo T, Yamasaki K, Iwakiri T, et al. Effects of pomegranate juice on human cytochrome p450 3A (CYP3A) and carbamazepine pharmacokinetics in rats. Drug Metab Dispos. 2005;33:644–8. doi: 10.1124/dmd.104.002824. [DOI] [PubMed] [Google Scholar]
- 56.Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–23. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 57.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 58.Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, Wang CJ. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59:2663–71. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- 59.Lazzè MC, Savio M, Pizzala R, Cazzalini O, Perucca P, Scovassi AI, et al. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis. 2004;25:1427–33. doi: 10.1093/carcin/bgh138. [DOI] [PubMed] [Google Scholar]
- 60.Meyer C, Blissett J, Oldfield C. Sexual orientation and eating psychopathology: the role of masculinity and femininity. Int J Eat Disord. 2001;29:314–8. doi: 10.1002/eat.1024. [DOI] [PubMed] [Google Scholar]
- 61.Tsuda T, Majumder K, Linask KK. Differential expression of flectin in the extracellular matrix and left-right asymmetry in mouse embryonic heart during looping stages. Dev Genet. 1998;23:203–14. doi: 10.1002/(SICI)1520-6408(1998)23:3<203::AID-DVG6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 62.Du Q, Zheng J, Xu Y. Composition of anthocyanins in mulberry and their antioxidant activity. J Food Compos Anal. 2008;21:390–5. [Google Scholar]
- 63.Chen CC, Liu LK, Hsu JD, Huang HP, Yang MY, Wang CJ. Mulberry extract inhibits the development of atherosclerosis in holesterol-fed rabbits. Food Chem. 2005;91:601–7. [Google Scholar]
- 64.Ha US, Koh JS, Kim HS, Woo JC, Kim SJ, Jang H, et al. Cyanidin-3-O-beta-D-glucopyranoside concentrated materials from mulberry fruit have a potency to protect erectile function by minimizing oxidative stress in a rat model of diabetic erectile dysfunction. Urol Int. 2012;88:470–6. doi: 10.1159/000336136. [DOI] [PubMed] [Google Scholar]
- 65.Kahkonen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem. 2003;51:628–33. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- 66.Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, et al. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73:1097–114. doi: 10.1016/s0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- 67.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–9. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 68.Bhuiyan MI, Kim HB, Kim SY, Cho KO. The neuroprotective potential of cyanidin-3-glucoside fraction extracted from mulberry following oxygen-glucose deprivation. Korean J Physiol Pharmacol. 2011;15:353–61. doi: 10.4196/kjpp.2011.15.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaewkaen P, Tong-Un T, Wattanathorn J, Muchimapura S, Kaewrueng W, Wongcharoenwanakit S. Effects of mulberry fruit powder in animal model of stroke. Am J Agric Biol Sci. 2012;7:322–9. doi: 10.1155/2012/263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim HG, Ju MS, Shim JS, Kim MC, Lee SH, Huh Y, et al. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson's disease models. Br J Nutr. 2010;104:8–16. doi: 10.1017/S0007114510000218. [DOI] [PubMed] [Google Scholar]


