Abstract
According to the prediction of the 2008 World Health Organization (WHO) report, depression will be the highest burden disease by the year 2030. Daylily flower (金針花 Jīn Zhēn Huā ; the flower of Hemerocallis fulva) is traditionally used for soothing in Chinese dietary therapy. The major flavonoid of daylily flowers, rutin, is also characterized to be an antidepressant. In this study, we investigated the antidepressant effects of ethanol extract of daylily flowers (DFEtoH) and rutin by forced swimming test (FST) and neurotransmitter metabolism of brain regions (frontal cortex, hippocampus, striatum, and amygdala). Results show that either short- or long-term tests, the extract and rutin significantly reduce the immobility time and increased swimming time of FST, which are compared with the vehicle (P < 0.05). The extract and rutin also increase the serotonin, norepinephrine, and dopamine concentration of these brain regions (P < 0.05). In long-term tests, the daylily flowers extract markedly increased serotonin concentration and reduced serotonin turnover rate in these brain regions but not frontal cortex. In conclusion, present data illustrated that DFEtoH does have antidepressant-like effects possibly via the regulation of serotonergic system. Moreover, rutin might be playing a very important role in the antidepressant-like effects of DFEtoH.
Keywords: Antidepressant, Daylily, Forced swimming test, Hemerocallis fulva, Serotonergic system
INTRODUCTION
According to the 2008 World Health Organization (WHO) report, major depressive disorder (MDD) had overtake acquired immune deficiency syndrome (AIDS), cardiovascular disease, and cancer and ranked in the top three of high burden and disability diseases in the world, first in middle-and-high-income countries, and predicted to be the world's top first by the year 2030.[1] However, this disability brings serious economic loss in the modern society. In the United States, MDD caused 77.4 billion USD in 1997 and elevated to 97.3 billion USD in 2008.[2,3] Suicidal behaviors are the most terrible result of MDD patients. Suicide had been one of the 10 leading death causes for 13 years since 1997 in Taiwan. Research showed that among these suicidal victims, as high as 97–100% are mentally disordered, and depression accounted for 87.1%.[4] Thus, in order to improve social encumbrances and reduce suicidal rate, antidepressant and pathological researches should be actively pushed forward.
Unfortunately, the exact pathology of this severe psychiatric disorder is not investigated clearly. So far, many pathological hypotheses were established upon physiopsychological researches, which includes neuroplastiticy reduction induced by hypothalamic–pituitary–adrenal (HPA) axis over activation and chronic inflammation.[5,6,7] Clinical investigations also showed that the cerebral or plasma neurotransmitters were reduced in patients with depression.[8,9,10] Many studies indicate that these pathological hypothesis will lead to neuroplasticity reduction, which may be the major cause of depression.[7,11]
Antidepressant is the major therapy for MDD patients. Clinical observation shows that the depressive symptoms were developed by these drugs. The pharmacology of these medicines is inhibition of neurotransmitter reuptake or blocking metabolic enzymes in order to increase neurotransmitter concentration in synapse. Moreover, in vitro studies showed that these antidepressants may also enhance neuroplasticity.[12] Although antidepressant may be the most efficient therapy, these drugs will lead to many troublesome side-effects such as nausea, tremor, vomiting, diarrhea, and psychomotor activation.[13,14,15] Alternatively, several in vitro studies show many natural products having neural protective effect. In vivo investigation also indicates that some functional foods and Chinese medicines can improve depression-like behaviors. Thus, these functional foods could be valuable for the development of antidepressants.
Daylily flower (金針花 Jīn Zhēn Huā ; the flower of Hemerocallis fulva) is traditionally used for soothing in Chinese dietary therapy. The flowers of daylily are rich in variety of antioxidants such as carotenoids, flavonoids, anthocyanin, and so on.[16,17] Recent studies indicated that daylily flowers greatly improved sleeping[18] and memory,[19] along with potent antioxidant ability. The alcohol extracts of daylily flowers exhibited better biological activities than the aqueous extract.[17,20,21] Research analysis of methanol extract of daylily flowers showed that rutin is a key component, as high as 19%, which exhibited the best antioxidant activity among those components isolated by this extraction method.[16] Using tail suspension test as a behavioral model of depression-like behavior, studies revealed that mice treated with rutin showed a significant reduction of immobility time, suggesting a potential antidepressant effect of rutin.[22] The above-mentioned studies suggest rutin as a candidate antidepressant chemical, and could be the major active component in extract of daylily flowers.
The most widely used behavioral model for evaluating antidepressant effect is forced swimming test (FST), which was established by Porsolt in 1979.[23] As a homologous model, the extent of depression is determined through observing and measuring rats’ behavior in an inescapable transparent water barrel in a defined period of time. In this procedure, the accumulated time during which the rats remained motionless and no struggling attempt to swim, it defined as the immobility time and it represented behavioral despair, which imitated in human depression. Longer immobility time indicated more serious depression or depression-like behavior. Recent neuronal studies indicate that FST increases corticosterone excitation and further lead to new born neuron cell (BrdU+ cell) reduction and dysregulation of neurotransmissions.[24,25,26] Previous studies showed that immobility behavior induced by the FST can be largely improved by various antidepressants and electro-convulsive therapy (ECT).[27,28,29,30,31] Therefore, FST is a suitable model to induce depression-like behavior for testing the effect or mechanism of antidepressants.
The aims of this study are (1) to perform content analysis of rutin in DFEtoH; (2) to analyze monoamine neurotransmitters and their metabolites in the brain; (3) to investigate the antidepressant effect of DFEtoH in short-term and long-term treatment using FST; and (4) to further understand whether rutin is the major active component in extract of daylily flowers.
MATERIALS AND METHODS
Preparation of ethanol extract from dried daylily flowers
The dried daylily flowers we used were purchased from Taimali, the main production site of daylily in Taiwan, which was processed by the low-sulfur manufacturing methods, and was genetically identified as Hemerocallis fulva. In small amount preparation method for short-term test, 1 kg powdered daylily flowers was added to 20 L 95% ethanol and stirred for 24 hours, filtered and then condensed at low pressure following by air dry at 37°C for fully removing ethanol, and stored at -20°C. The final extraction efficiency is 16.2%. For large-scale preparation used for long-term test, 10 kg dried daylily flowers powder was mixed with 100 L 95% ethanol and undergoing sonication for 3 hours to yield extract, filtered and then condensed at low pressure following by air dry at 37°C for fully removing ethanol, and stored at -20°C until use. The final extraction efficiency is 10%.
High performance liquid chromatography (HPLC) condition for analyzing rutin content of ethanol extract of daylily flowers extracted by large-scale preparation method
The DFEtoH was dissolved in methanol, filtered through a 0.45 μm membrane, then run through HPLC for analysis. Parameters: RP-18 column; water phase contained 5% methanol, 0.2% acetic acid and water; organic phase was 100% methanol. Flow rate at 20 μl/min; detection wavelength at 254 nm; Peak ABC as the data analysis software. Rutin standard was purchased from Sigma. After calculation, the result showed that each gram of daylily ethanol extract contained 7.5 mg of rutin.
Experimental animals and treatment
Animals were purchased from the Lestco Biotechnology company (Yilan, Taiwan) and maintained in animal room whose environment was controlled at12 hour/12 hour light/dark cycle and temperature at 23±2°C, unlimited water and food pellet (PMI Feed, St. Louis, MO, USA), weighed daily for calculating food intake. Short-term phase study used 6-week-old male SD rats, randomly divided into six groups: normal control (NC), negative control (C), fluoxetine (F), low dosage (D), medium dosage (MD), and high dosage (HD) of DFEtoH, after prefeeding for 1 week, at 7 weeks old, experiment was performed. All samples were dissolved in 0.5% carboxylmethyl cellulose (CMC) and fed to rats once daily for 7 successive days by oral gavage with 0.5% CMC, 18 mg/kg fluoxetine, DFEtoH for 3, 15, and 30 g/kg bw raw compound after appropriate calculation according to extraction efficiency. Besides the NC group, all the other five groups underwent FST after 7 days of oral gavage. For long-term experiment, 5-week-old male SD rats were randomly divided into six groups: negative control (C), fluoxetine (F), low dosage DFEtoH (D), medium dosage (MD), high dosage (HD) and rutin (R). After prefeeding for 2 weeks, experiment was performed when animals were 7 weeks old. The rats were treated with 0.5% CMC, 18 mg/kg fluoxetine, DFEtoH for 3, 15, and 30 g/kg bw raw compound after appropriate calculation according to extraction efficiency and rutin, equivalent to the high dosage group by oral gavage once daily for 28 days, and followed by FST. All animals were sacrificed immediately after FST and removed brain for analyzing the content of neurotransmitters. Fluoxetine (Prozac®) was purchased from Lily (Taiwan).
Forced swimming test
Our FST was modified from the method established by Porsolt et al. in 1977.[27] A transparent glass barrel with diameter of 21 cm was filled with water until 30 cm high, so that the rat cannot reach the bottom. After oral gavage at the last day, day 7 or day 28, all rats except the NC group were placed in the barrel for 15-min pretest, followed by towel drying and drying apparatus warmed, then placed back into the cage. After 24 hours, a similar 5-min test was performed, and tape recorded. Rat behaviors can be distinguished as struggling, swimming, and immobility. The struggling was defined as animal vigorously swam vertical. The swimming was defined as swimming slowly and circling around the barrel. Immobility time was the index of rat behavioral despair, and its duration will be used for determining the extent of depression.
Analysis of brain monoamine neurotransmitters by HPLC
Extraction of monoamines from brain tissues
After FST, all animals were anesthetized by CO2 and sacrificed by decapitation. The brain was removed immediately, divided into frontal cortex, striatum, hippocampus, and amygdala on ice according to Glowinsk's method,[32] and stored in -80°C. The method of monoamine extraction from brain tissue was modified from Cheng.[33] Extraction solvent was modified into 10-7M ascorbic acid, 1.5 mg/100 ml pargyline, and 50 pg/μl isoproterenol in 0.1N HCl. All brain tissues were homogenized with 5 ml extraction solvent, then centrifuged at 10000 × g for 20 min at 4°C. The supernatant was stored in -80°C for further analysis.
Analyzing monoamine by HPLC
Supernatant of brain tissues was filtered through a 0.45 μm membrane, analyzed for serotonin (5-HT), dopamine (DA), norepinephrine (NE), 5-hydroxyindoleacetic acid (5-HIAA), 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) contents using HPLC coupled by electrochemical detector (ECD). All standards were purchased from Sigma. Column: Alltech Apollo C18, 5 μ, 4.6 × 250 mm; guard column: Meta Guard C18-A, Polaris, 5 μ, 7.5 × 4.6 mm; data analysis software: Peak ABC; electroprobe: C-KCl reference electrode (BAS, West Lafayette, IN, USA); pump: Pump PM-92E (BAS, West Lafayette, IN, USA); electrochemical detector: Amperometric Detector LC-4C (BAS, West Lafayette, IN, USA).
Parameters for analysis of NE, DA, DPOAC: electrochemical detector: range 10.0 nA, filter 0.1 Hz, AppE cell 0.75 V; mobile phase: 20.5 g NaH2PO4, 185 mg EDTA, 130 mg SOS (1-octanesulfonic acid, sodium salt) and 107 ml methanol per liter; flow rate: 500 μl/min. Parameters for analysis of 5-HT, 5-HIAA, HVA: electrochemical detector: range 5.0 nA, filter 0.1 Hz, AppE cell 0.75 V; mobile phase: 20.5 g NaH2PO4, 185 mg EDTA, 130 mg SOS and 200 ml methanol per liter; flow rate: 500 μl/min.
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) and Duncan's new multiple range test for comparing differences between groups by using SAS statistical software, version 9.0.
RESULTS
Rutin content of daylily flowers ethanol extract
Rutin content is analyzed by HPLC and used standard to quantify. The peak of rutin in each sample will appear for about 12 min after injection [Figure 1]. Rutin concentration of small-scale and large-scale extraction are 7.85 ± 0.23 and 7.45 ± 0.17 (mg/g ethanol extract).
Figure 1.
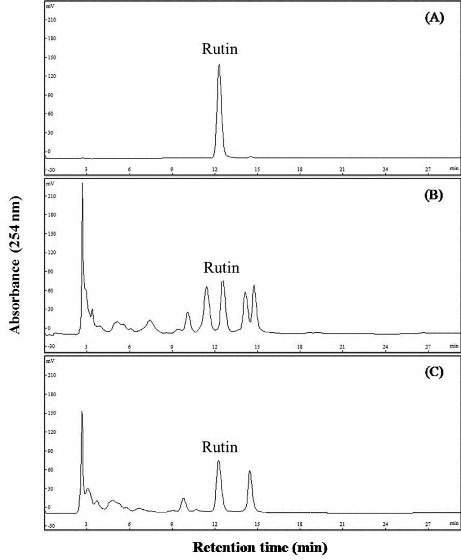
The chromatogram of rutin standard and DFEtoH. (A) Rutin (B) large-scale extraction (C) small-scale extraction
Antidepressant effect
In this study, FST is used to investigate the antidepressant effect of daylily flowers ethanol extract. Rat behaviors can be distinguished as struggling, swimming, and immobility. The duration of immobility behavior is related to the despair thinking and the depression-like level. The results revealed that all rats treated with the DFEtoH had a significant reduction of immobility time in both short-term and long-term experiments. Groups of medium dosage, high dosage, and rutin had similar effect as fluoxetine in long-term treatment. Further investigation of swimming behavior, all treatment groups, except for low dosage in long-term experiment, had increased swimming time without affecting struggling time [Figure 2].
Figure 2.
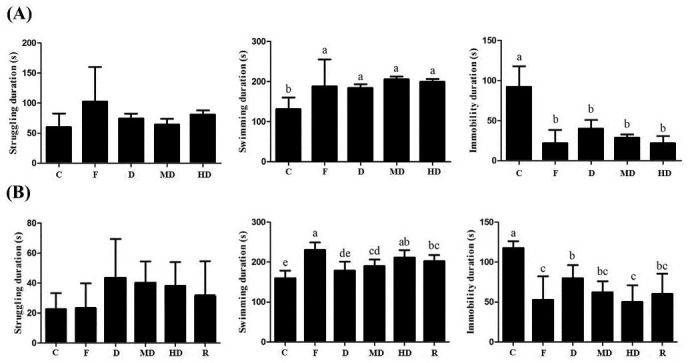
The rat behaviors under forced swimming test. (A) Short-term experiment (B) long-term experiment. C: negative control; F: fluoxetine; D: low dosage of ethanol extract of daylily flowers; MD: medium dosage of DFEtoH; HD: high dosage of DFEtoH; R: rutin. abcd data not sharing the same letter are significantly different from one another in each group (P < 0.05) by ANOVA and Duncan's multiple range test
Neurotransmitter determination and metabolic rate
Neurotransmitters reduction and metabolic rate increasing are discovered in MDD patients and depress-like induced animals. In this study, monoamines, the major mood-related neurotransmitters are detected by HPLC.
In the acute experiments, serotonin content of amygdala of each daylily flowers treatments (D, MD, and HD) were significantly increased, which were compared to FST-induced rats and had no difference with normal control. However, there was no difference in frontal cortex and hippocampus [Table 1A]. The metabolic rate of serotonin of depression-like rats was higher than normal control. After daylily flowers extract or antidepressant treating, the metabolic rate was significant reduced [Figure 3A]. Norepinephrine concentration of high dosage group in amygdala was significantly higher than the negative control. In this dosage, norepinephrine in amygdala was not different between normal rats [Table 1B]. Dopamine concentration of all dosage was significant difference in striatum. Furthermore, the calculation of the ratio of dopamine and its metabolite in the striatum, the metabolic rate of dopamine was reduced [Figure 3B]. But in other brain regions, there is no difference between daylily flowers treatments and normal control [Table 1C]. These results can indicate that the DFEtoH can influence some neurotransmitters in brain in short time.
Table 1.
The concentration of monoamines in four brain regions in acute test. (A) Serotonin (B) Norepinephrine (C) Dopamine
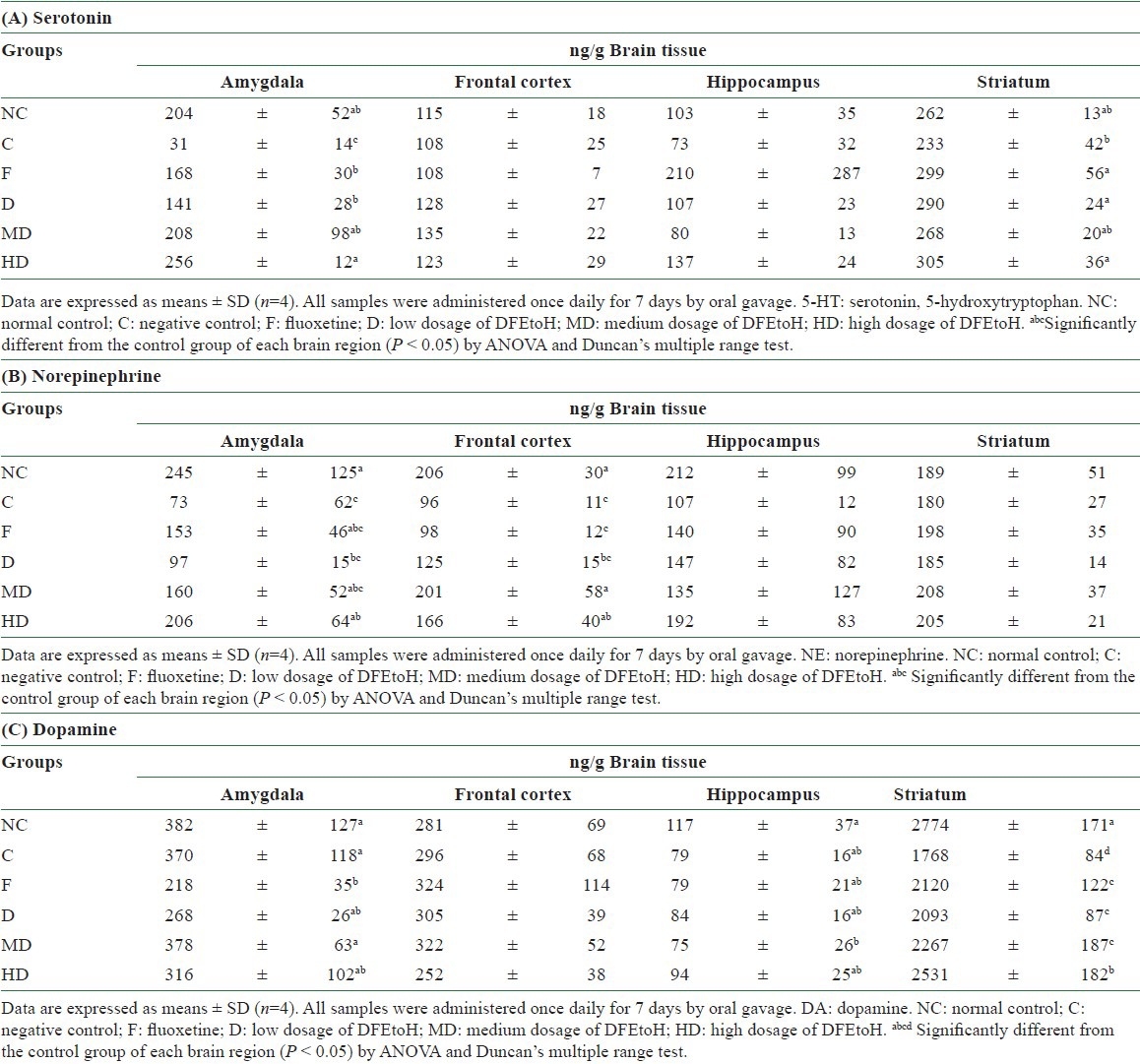
Figure 3.
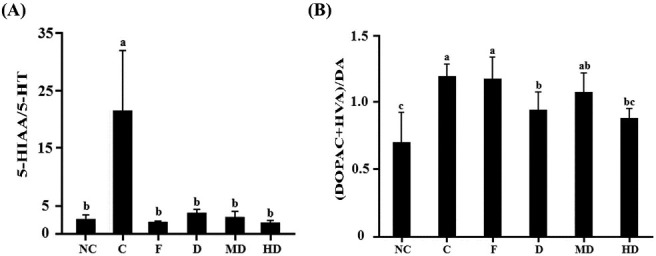
The metabolic rate of monoamines in acute experiment. (A) Serotonin in amygdala (B) dopamine in striatum. NC: normal control, C: negative control; F: fluoxetine; D: low dosage of DFEtoH; MD: medium dosage of DFEtoH; HD: high dosage of DFEtoH. abc data not sharing the same letter are significantly different from one another in each group (P < 0.05) by ANOVA and Duncan's multiple range test
There was difference between acute and chronic experiments for serotonin status. Unlike acute test, not only in amygdala but also in frontal cortex and hippocampus, MD and HD can significantly elevate serotonin level. Only high dosage treatment can affect serotonin concentration in striatum [Table 2A]. The results of metabolic rate are corresponding with neurotransmitter content. In amygdala and hippocampus, all dosage of extract can significantly reduce the metabolic rate of serotonin [Figure 4A and B]. In striatum, only high dosage can reduce metabolic rate [Figure 4C]. However, there is no effect in frontal cortex [Figure 4D]. All treatments can influence norepinephrine content in amygdala, but only HD group had significant difference in hippocampus. However, it was not similar to acute study, the ethonal extract cannot influence norepinephrine in frontal cortex and striatum [Table 2B]. For dopamine, there was no change in amygdala, hippocampus and frontal cortex but not in striatum [Table 2C]. Dopamine metabolic rate was also found to be reduced in each dosage groups [Figure 5].
Table 2.
The concentration of monoamines in four brain regions in chronic test. (A) Serotonin (B) Norepinephrine (C) Dopamin
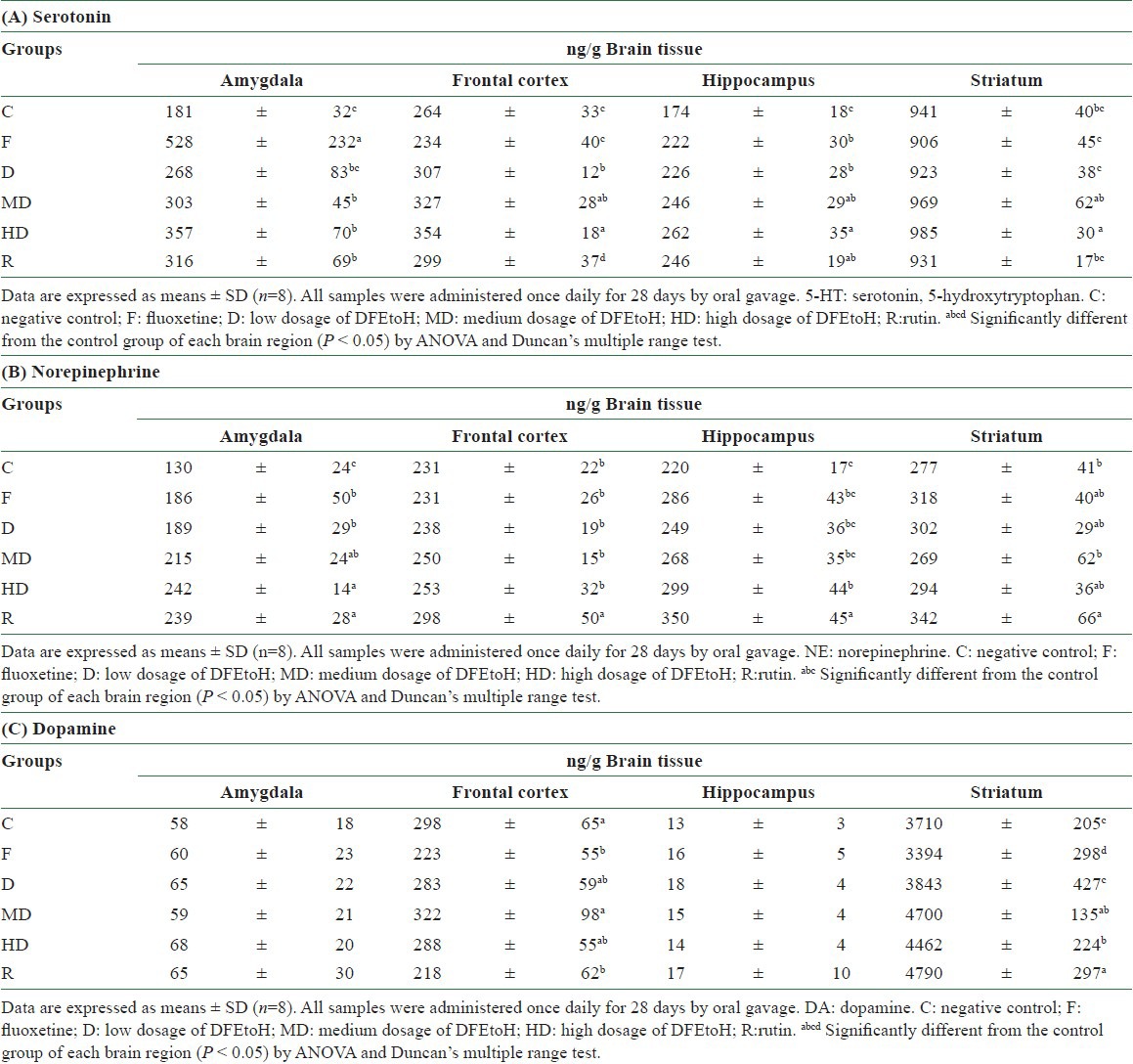
Figure 4.
The metabolic rate of serotonin in chronic experiment in each brain regions. (A) Amygdala (B) hippocampus (C) striatum (D) frontal cortex. C: negative control; F: fluoxetine; D: low dosage of DFEtoH; MD: medium dosage of DFEtoH; HD: high dosage of DFEtoH; R: rutin. abcd data not sharing the same letter are significantly different from one another in each group (P < 0.05) by ANOVA and Duncan's multiple range test
Figure 5.
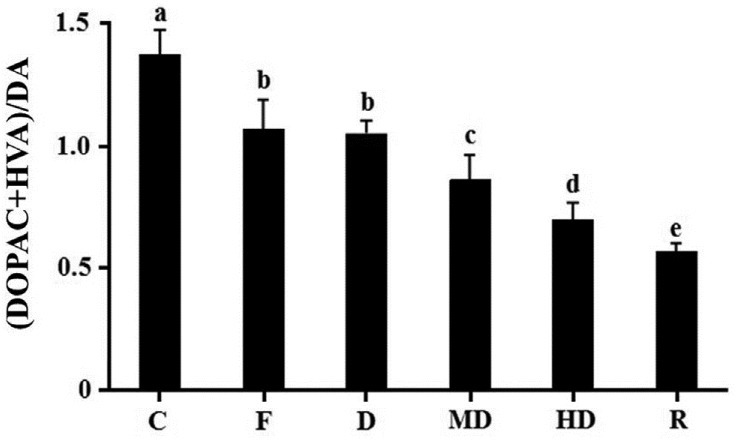
The metabolic rate of dopamine in chronic experiment in striatum. C: Negative control; F: fluoxetine; D: low dosage of DFEtoH; MD: medium dosage of DFEtoH; HD: high dosage of DFEtoH; R: rutin. abcde data not sharing the same letter are significantly different from one another in each group (P < 0.05) by ANOVA and Duncan's multiple range test.
DISCUSSION
For the antioxidant capacity, previous study shows that the ethanol extract is better than the water extract, which is according to the high phenolic compound containing rutin, catechin, and gallic acid.[17] Our previous experiment also indicates that these phenols are highly contained in ethanol extract but not in water extract (data not shown). Rutin is a well known phenolic compound, which exist in many herbals. The famous antidepressive functional food, St. John's wort claims that the rutin is abundant inside. The ethanol extract of Schinus molle L., a rutin-rich herbal, can reduce the immobility time of mice in the tail suspension test.[22] In this study, the extraction rate of small and large scale is 16.8% and 10%, rutin is 7.85% and 7.45% content in these extracts, respectively. Thus, we aim at investigating whether the DFEtoH, a rutin-rich plant, has antidepressive effect or not. To the best of our knowledge, this study is the first to investigate the antidepressive effect of DFEtoH. Thus, this study is a pioneer research of daylily flowers and its antidepressant function.
FST is a common model for antidepressant screening. In this model, rats are forced to swim for 15 min at first day and for 5 min 24 hours later. It mimics the high stress level in order to induce the depression-like behavior in rats, which is regarded as hopeless thinking of human being. Physiological studies show FST can cause over secretion of corticosterone.[25,34] This situation is similar to humans who are under stress and HPA axis dysregulation occurs. Normal concentration of corticosterone stimulates neuronal development.[35] However, in vitro studies show that over secretion of corticorsterone will lead calcium influx deucedly and finally cause neuronal cell death.[36,37,38] The reduction of neuroplasticity may be the convergent point of each depression hypothesis. The plasticity of neuron includes the increase of neuron numbers, structure remodeling, and neurogenesis.[39] However, studies show stress-induced neuroplastiticy in both human and animal models.[7,40,41] Accordingly, FST is a feasible stress-induced model for mimicking human-like depressive behavior and neuronal alteration. In this study, either in acute or in chronic experiments, the immobility time is significantly reduced by DFEtoH treating. In acute test, three dosages of extract have the same consequent as fluoxetine [Figure 2A]. Moreover, medium and high dosages have similar results as fluoxetine [Figure 2B]. This discovery represents that the DFEtoH have potential antidepressant activity. In other behaviors, the extract of daylily flowers only influences swimming duration but not struggling. Either in acute or in chronic tests, the swimming duration of treatment groups is significantly enhanced compared with negative control, and it also has similar effect as the positive control, the selective serotonin reuptake inhibitor (SSRI), fluoxetine [Figure 2]. Swimming is the one of three major behaviors of rats during FST. This behavior is controlled by serotonergic system. Many studies show that swimming behavior duration will be elevated by SSRI treated.[30,31,42] The last major behavior is struggling, which is controlled by noradrenergic system.[29,30] However, struggling behavior did not change by any treatment in this study [Figure 2]. This behavioral investigation indicates that the antidepressant effect of daylily flowers extract may result in affecting serotonergic neuron or serotonin concentration.
Clinical studies show that depression patients have lower monoamines content in cerebrum.[8,43,44] It may be caused due to overactivation of monoamine receptors or hypermetabolic rate.[45,46,47] Therefore, a common strategy for the treatment of MDD patients is to use antidepressants that alter monoamine oxidase (MAO) activity and monoamine reuptake. In this study, serotonin has got the most evidence, especially in amygdala. In this brain region, all dosages of daylily extract can increase serotonin content and have no difference between normal rats. In the chronic test, it is more effective on increasing serotonin concentration in these brain regions. The relationship between neurotransmitters and FST behaviors are mentioned as above. Thus, such alteration may reflect the observed behavioral change in rats in the FST. The extract of daylily flowers appears to have the least or no effect on the dopaminergic system, except the dopamine level in the striatum. Further, we detect the metabolites of these neurotransmitters in order to know the metabolic rate. Although the contributions of neurotransmitters and its metabolites in patients with depression are unclear, we can calculate the ration of monoamines and its metabolite as an index to evaluate the effect of antidepressants. For example, research indicates that the serotonin metabolic rate in rat hippocampus was blocked by 10 days treatment of fluoxetine (20 mg/kg/day) or other serotonin-type antidepressant treating.[48,49] Our study shows that serotogenic system is the most effective target of daylily flowers. In acute test, 82.5–86.6% serotonin metabolic rate in amygdala is blocked, which is comparable between extract and negative control [Figure 3A]. However, in chronic test, the serotonin metabolic rate is also reduced in amygdala, hippocampus, and striatum for 40–60%, 30–47.9%, and 18.3–40.7%, respectively. Based on these results, we suggest the antidepressant mechanism of daylily ethnol extract is through reducing monoamine oxidase activity or metabolism. Monoamine oxidase is the metabolic enzymes of monoamines in synapse and cleft. These enzymes could be classified into MAO-A and MAO-B. In vivo study shows that MAO-A mutant mice could represent similar behavior as MAO-A deficient human and the monoamines concentration are significantly higher than wide type. However, this deamination reaction results produce hydrogen peroxide in brain.[50] Oxidative stress can lead to neuron cell apoptosis and neuroplasticity reduction.[6,51] In vivo and clinical studies also indicate that monoamine oxidase hyperactivity was observed in some MDD patients or depression-like animals.[52,53] This situation may not only cause neurotransmitters reduction but also raise oxidative stress and lead to neurotoxicity. Some researches show that some phytochemicals have inhibition of MAO activity and neuroprotective function. Some of these phytochemicals are rich in ethanol of daylily flowers extract such as rutin, isoquercitrin, and quercetin.[54] The MAO-A inhibition function of quercetin has been discovered.[55] In this study, we investigated that the ethanol extract can highly affect the serotonergic system. Therefore, we surmise that the antidepressant effect may be contributed by MAO inhibitor function. However, the antidepressant effect of rutin is lower than the high dosage extract group in chronic test [Figure 2B]. It indicates that daylily flower extract may have other nutrients, which can affect the depressive-like behavior.
In conclusion, the DFEtoH can provide rutin and other phenolic compounds, which can reduce the immobility behavior. This effect may through blocking monoamine oxdase and elevate the synaptic neurotransmitter concentration. However, the clearly mechanism should be investigated in the future.
ACKNOWLEDGEMENT
The authors thank the Agriculture and Food Agency, Council of Agriculture Yuan, Taiwan (100AS-3.1.3-FD-Z1) for financially supporting this study.
REFERENCES
- 1.WHO. Global Burden of Disease 2004 update. 2008 [Google Scholar]
- 2.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: How did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–75. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 3.Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev. 2008;30:1–14. doi: 10.1093/epirev/mxn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng AT. Mental illness and suicide. A case-control study in east Taiwan. Arch Gen Psychiatry. 1995;52:594–603. doi: 10.1001/archpsyc.1995.03950190076011. [DOI] [PubMed] [Google Scholar]
- 5.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 6.Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–6. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 8.Gao HQ, Zhu HY, Zhang YQ, Wang LX. Reduction of cerebrospinal fluid and plasma serotonin in patients with post-stroke depression: A preliminary report. Clin Invest Med. 2008;31:E351–6. doi: 10.25011/cim.v31i6.4921. [DOI] [PubMed] [Google Scholar]
- 9.Aberg-Wistedt A. A double-blind study of zimelidine, a serotonin uptake inhibitor, and desipramine, a noradrenaline uptake inhibitor, in endogenous depression. I. Clinical findings. Acta Psychiatr Scand. 1982;66:50–65. doi: 10.1111/j.1600-0447.1982.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 10.Quintana J. Platelet serotonin uptake dynamic changes in depression: Effects of long-term imipramine treatment and clinical recovery. J Affect Disord. 1989;16:233–42. doi: 10.1016/0165-0327(89)90078-5. [DOI] [PubMed] [Google Scholar]
- 11.Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–9. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 12.Piubelli C, Vighini M, Mathe AA, Domenici E, Carboni L. Escitalopram affects cytoskeleton and synaptic plasticity pathways in a rat gene-environment interaction model of depression as revealed by proteomics. Part II: Environmental challenge. Int J Neuropsychopharmacol. 2011;14:834–55. doi: 10.1017/S1461145710001306. [DOI] [PubMed] [Google Scholar]
- 13.Khawam EA, Laurencic G, Malone DA., Jr Side effects of antidepressants: An overview. (6-61).Cleve Clin J Med. 2006;73:351–3. doi: 10.3949/ccjm.73.4.351. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla P, Cipriani A, Hotopf M, Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: A meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38:69–77. doi: 10.1055/s-2005-837806. [DOI] [PubMed] [Google Scholar]
- 15.Campillos M, Kuhn M, Gavin AC, Jensen LJ, Bork P. Drug target identification using side-effect similarity. Science. 2008;321:263–6. doi: 10.1126/science.1158140. [DOI] [PubMed] [Google Scholar]
- 16.Cichewicz RH, Nair MG. Isolation and characterization of stelladerol, a new antioxidant naphthalene glycoside, and other antioxidant glycosides from edible daylily (hemerocallis) flowers. J Agric Food Chem. 2002;50:87–91. doi: 10.1021/jf010914k. [DOI] [PubMed] [Google Scholar]
- 17.Que F, Mao L, Zheng X. In vitro and in vivo antioxidant activities of daylily flowers and the involvement of phenolic compounds. Asia Pac J Clin Nutr. 2007;16(Suppl 1):196–203. [PubMed] [Google Scholar]
- 18.Uezu E. Effects of Hemerocallis on sleep in mice. Psychiatry Clin Neurosci. 1998;52:136–7. doi: 10.1111/j.1440-1819.1998.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 19.Uezu E. A philological and cxperirnental investigation of the effects of Hemerocallis as food in man and ddY mice. Bull Coll Educ Unit Ryukyrrs. 1997;51:231–38. [Google Scholar]
- 20.Bor JY, Chen HY, Yen GC. Evaluation of antioxidant activity and inhibitory effect on nitric oxide production of some common vegetables. J Agric Food Chem. 2006;54:1680–6. doi: 10.1021/jf0527448. [DOI] [PubMed] [Google Scholar]
- 21.Fu M, Mao L. In vitro antioxidant activities of five cultivars of daylily flowers from China. Nat Prod Res. 2008;22:584–91. doi: 10.1080/14786410701592828. [DOI] [PubMed] [Google Scholar]
- 22.Machado DG, Bettio LE, Cunha MP, Santos AR, Pizzolatti MG, Brighente IM, et al. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur J Pharmacol. 2008;587:163–8. doi: 10.1016/j.ejphar.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: Effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57:201–10. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- 24.Llorens-Martin MV, Rueda N, Martinez-Cue C, Torres-Aleman I, Florez J, Trejo JL. Both increases in immature dentate neuron number and decreases of immobility time in the forced swim test occurred in parallel after environmental enrichment of mice. Neuroscience. 2007;147:631–8. doi: 10.1016/j.neuroscience.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 25.Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–18. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 26.Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotox Res. 2004;6:73–8. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 28.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 29.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–44. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 30.Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–6. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 31.Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–7. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- 32.Glowinsk J, Iversen LL. Regional studies of catecholamines in rat brain. 1. Disposition of [3H]norepinephrine [3H]dopamine and [3H]dopa in various regions of brain. J Neurochem. 1966;13:655–69. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheng FC, Kuo JS, Shih Y, Lai JS, Ni DR, Chia LG. Simultaneous measurement of serotonin, catecholamines and their metabolites in mouse-brain homogenates by high-performance liquid-chromatography with a microbore column and dual electrochemical detection. J Chromatogr B Biomed. 1993;615:225–36. doi: 10.1016/0378-4347(93)80336-3. [DOI] [PubMed] [Google Scholar]
- 34.Pinter O, Domokos A, Mergl Z, Mikics E, Zelena D. Do stress hormones connect environmental effects with behavior in the forced swim test? Endocr J. 2011;58:395–407. doi: 10.1507/endocrj.k10e-375. [DOI] [PubMed] [Google Scholar]
- 35.Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57:561–70. doi: 10.1016/j.brainresrev.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Elliott EM, Sapolsky RM. Corticosterone impairs hippocampal neuronal calcium regulation-possible mediating mechanisms. Brain Res. 1993;602:84–90. doi: 10.1016/0006-8993(93)90245-i. [DOI] [PubMed] [Google Scholar]
- 37.Talmi M, Carlier E, Rey M, Soumireu-Mourat B. Modulation of the in vitro electrophysiological effect of corticosterone by extracellular calcium in the hippocampus. Neuroendocrinology. 1992;55:257–63. doi: 10.1159/000126123. [DOI] [PubMed] [Google Scholar]
- 38.Mao QQ, Ip SP, Ko KM, Tsai SH, Zhao M, Che CT. Peony glycosides protect against corticosterone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2009;29:643–7. doi: 10.1007/s10571-009-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 41.Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- 42.Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Inter-individual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology (Berl) 2008;198:333–40. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 44.Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: Implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog Brain Res. 2008;172:233–64. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- 45.Kahn RS, Wetzler S, Asnis GM, Papolos D, van Praag HM. Serotonin receptor sensitivity in major depression. Biol Psychiatry. 1990;28:358–62. doi: 10.1016/0006-3223(90)90663-m. [DOI] [PubMed] [Google Scholar]
- 46.Coper H, Fähndrich E, Gebert A, Helmchen H, Honecker H, Müller-Oerlinghausen B, et al. Depression and monoamine oxidase. Prog Neuropsychopharmacol. 1979;3:441–63. doi: 10.1016/0364-7722(79)90000-6. [DOI] [PubMed] [Google Scholar]
- 47.Brandon S. Monoamine oxidase inhibitors in depression. Br Med J. 1982;285:1594–5. doi: 10.1136/bmj.285.6355.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuller RW, Wong DT. Inhibition of serotonin reuptake. Fed Proc. 1977;36:2154–8. [PubMed] [Google Scholar]
- 49.Mazarati A, Siddarth P, Baldwin RA, Shin D, Caplan R, Sankar R. Depression after status epilepticus: Behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131:2071–83. doi: 10.1093/brain/awn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Sanchez-Sellero I, Cruz-Landeira A, Lamas ML. Inhibition of brain monoamine oxidase activity by the generation of hydroxyl radicals: potential implications in relation to oxidative stress. Life Sci. 2001;69:879–89. doi: 10.1016/s0024-3205(01)01178-x. [DOI] [PubMed] [Google Scholar]
- 51.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 52.Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–16. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 53.Kato M, Iwata H, Okamoto M, Ishii T, Narita H. Focal cerebral ischemia-induced escape deficit in rats is ameliorated by a reversible inhibitor of monoamine oxidase-a: Implications for a novel animal model of post-stroke depression. Biol Pharm Bull. 2000;23:406–10. doi: 10.1248/bpb.23.406. [DOI] [PubMed] [Google Scholar]
- 54.Mazzio EA, Harris N, Soliman KF. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Med. 1998;64:603–6. doi: 10.1055/s-2006-957530. [DOI] [PubMed] [Google Scholar]
- 55.Chimenti F, Cottiglia F, Bonsignore L, Casu L, Casu M, Floris C, et al. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: Extraction, biological analysis, and computational study. J Nat Prod. 2006;69:945–9. doi: 10.1021/np060015w. [DOI] [PubMed] [Google Scholar]


