Abstract
Stereospermum suaveolens is a folk remedy for the treatment of diabetes and liver disorders in southern parts of India. In the present study, the protective effect of the ethyl acetate fraction of ethanol extract from S. suaveolens against hepatic oxidative stress was evaluated in streptozotocin (STZ)-induced diabetic rats for 14 days. The ethyl acetate fraction was administered orally to the STZ diabetic rats at the doses of 200 and 400 mg/kg. Blood glucose level was measured according to glucose oxidase method. In order to determine hepatoprotective activity, changes in the levels of serum biomarker enzymes such as aspartate transaminase (AST), alanine transaminase (ALT), and serum alkaline phosphatase (SALP) were assessed in the ethyl acetate fraction treated diabetic rats and were compared with the levels in diabetic control rats. In addition, the antioxidant activity of ethyl acetate fraction was evaluated using various hepatic parameters such as thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT). It was found that administration of ethyl acetate fraction (200 and 400 mg/kg) produced a significant (P < 0.001) fall in fasting blood glucose level, TBARS, bilirubin, AST, ALT, and SALP, while elevating the GSH levels, and SOD and CAT activities in diabetic rats. Histopathologic studies also revealed the protective effect of ethyl acetate fraction on the liver tissues of diabetic rats. It was concluded from this study that the ethyl acetate fraction from ethanol extract of S. suaveolens modulates the activity of enzymatic and nonenzymatic antioxidants and enhances the defense against hepatic oxidative stress in STZ-induced diabetic rats.
Keywords: Antioxidant enzymes, Ethyl acetate fraction, Histopathology, Serum biomarker enzymes, Stereospermum suaveolens, STZ-induced diabetic rats
INTRODUCTION
Diabetes mellitus is a multifaceted serious illness involving endocrine pancreas with multiple complications and affecting more than 285 million people worldwide, and is considered as one of the three leading causes of death in the world.[1] In diabetes, oxidative stress has been found to be mainly due to an increased production of reactive oxygen species and a sharp reduction of antioxidant defenses.[2] A number of clinical studies suggest that the antioxidants in plants are key factors in reducing the incidence of diabetic complications.[3,4] Finding new natural sources of antioxidants with potential antidiabetic activity can be useful to future therapy against diabetic mellitus.
Stereospermum suaveolens Roxb. (Bignoniaceae), popularly known as padiri in Tamil, is a large deciduous tree found throughout the moist parts of India. Traditionally, a decoction of the root is used for the treatment of inflammation, pain, fever, and asthma.[5,6] The flowers mixed with honey are given orally for the control of hiccups. In southern India, the bark is used in folk medicine for the treatment of diabetes.[5] The root extract is known to possess anticancer activity due to the presence of lapachol.[5,7] Previous phytochemical studies showed the presence of lapachol, dehydro-α-lapachone,[8] sterekunthal B, sterequinone C, and stereochenols A and B[9,10] in the bark, and stereolensin,[11] scutellarein, 6-hydroxy luteolin,[12] dinatin (4,5,7-trihydroxyl-6-methoxyflavon), and dinatin-7-glucuroniside[13] in the leaves. Previous studies in our laboratory showed that crude ethanol extract and ethyl acetate fraction of S. suaveolens have antihyperglycemic effects in streptozotocin (STZ)-induced diabetic rats.[14,15] No reports are available on the antioxidant activity of ethyl acetate fraction in the liver. Thus, the objective of the present study was to further explore our previous data by focusing now on the protective effect of S. suaveolens ethyl acetate fraction against oxidative stress in the liver of STZ-induced diabetic rats.
MATERIALS AND METHODS
Chemicals
STZ, trichloroacetic acid (TCA), thiobarbituric acid (TBA), reduced glutathione (GSH), Tris-HCl, sodium dodecyl sulfate (SDS), nitro blue tetrazolium (NBT), reduced nicotinamide adenine dinucleotide (NADH), and phenazine methosulfate (PMS) were purchased from SISCO Research Laboratory, Mumbai, India. Glibenclamide was obtained from Prudence Pharma Chem, Ankeshwara, Gujarat, India. The solvents and chemicals used were of analytical grade.
Plant material
The plant was identified and authenticated by the Dr. Mohan, Scientist, Tropical Botanical Garden and Research Institute, Palode, Thiruvananthapuram district, Kerala, India, and a voucher specimen (TBS-1) has been deposited in our laboratory for further reference. The bark of S. suaveolens (Roxb.) DC was collected during October 2006 from Palode forest, Tiruvananthapuram district. The bark of the plant was dried under shade and powdered with a mechanical grinder. The powdered plant material was then passed though sieve No. 40 and stored in an air-tight container for future use.
Preparation of the crude plant extract and ethyl acetate fraction
The shade-dried coarse powder bark of S. suaveolens (500 g) was packed in the Soxhlet extraction apparatus and extracted with 1.5 l of 95% ethanol at a temperature of 40-50°C for 72 h. The extract was filtered and then concentrated to dryness in a rotary evaporator under reduced pressure at a temperature of 40°C. Then the crude ethanol extract of S. suavolens (EESS) (100 g) was dissolved in distilled water (500 ml) and fractionated with ethyl acetate.
The resultant black color residue was stored in a desiccator for use in subsequent experiments and considered as the ethyl acetate fraction. The yield of the ethyl acetate fraction was 16.52% w/w. Weighed amount of ethyl acetate fraction was suspended in 5% dimethyl sulfoxide (DMSO) in normal saline prior to oral administration.
Qualitative phytochemical analysis
Preliminary phytochemical screening was performed for the ethyl acetate fraction of EESS.[16,17,18]
Animals
Male Wistar albino rats (weighing 180-200 g) and male Swiss albino mice (20-25 g) were purchased from M/S Ghosh Enterprises, Kolkata, India. The animals were randomly grouped (n = 6), housed in polyacrylic cages (38 × 23 × 10 cm), and maintained under standard laboratory conditions (25 ± 2°C) with dark and light cycle (14/10 h). They were allowed free access to standard dry pellet diet (Hindustan Lever, Kolkata, India) and water ad libitum. The rats and mice were acclimatized to laboratory conditions for 1 week before commencement of the experiment. Ethical clearance was obtained from Jadavpur University Animals Ethical Committee for using animals in the present study.
Acute toxicity study
An acute oral toxicity study was performed as per Organisation for Econamic Co-operation and Development (OECD)-423 guidelines.[19] Male Swiss albino mice (20-25 g) were randomly distributed to six groups of three each. The animals were fasted overnight and the ethyl acetate fraction was administered orally at a dose of up to 2000 mg/kg body weight. Mortality and general behavior such as grooming, sedation, hyperactivity, loss of righting reex, respiratory rate, and convulsions of the animals were observed periodically for 72 h. The animals were observed continuously for the initial 2 h and intermittently for the next 6 h and then again at 24, 48, and 72 h, following drug administration.
Induction of diabetes
Rats were fasted for 16 h before the induction of diabetes with STZ. A freshly prepared solution of STZ (50 mg/kg) in 0.1 M cold citrate buffer (pH 4.5) was injected intraperitoneally in a volume of 1 ml/kg,[20,21] and the control rats were injected with citrate buffer alone. In order to control hypoglycemia during the first day after STZ administration, diabetic rats were given 5% glucose solution orally. Hyperglycemia was confirmed by the elevated fasting glucose levels in blood, determined at 48 h and then on day 6 after injection. Rats with moderate diabetes exhibiting fasting blood glucose levels in the range of 260-325 mg/100 ml were selected for the studies.
Treatment schedule
Rats were fasted for 16 h and divided into five groups of six each.[20]
The animals were treated orally, once daily, for 14 consecutive days, 7 days after STZ injection. The groupings are as follows:
Group I,nondiabetic control: 5% DMSO in normal saline (5 ml/kg)
Group II,STZ-diabetic control: 5% DMSO in normal saline (5 ml/kg)
Group III,STZ-diabetic rats: ethyl acetate fraction (200 mg/kg)
Group IV,STZ-diabetic rats: ethyl acetate fraction (400 mg/kg)
Group V,STZ-diabetic standard: glibenclamide (0.5 mg/kg).
Fasting blood glucose level of each animal was determined on days 1, 4, 7, 10, and 15 after the initiation of treatment. On the 15th day, blood was collected from the overnight-fasted rats by retro-orbital bleeding, using microcapillary technique. Serum was separated and used for the determination of biochemical parameters such as aspartate transaminase (AST), alanine transaminase (ALT), serum alkaline phosphatase (SALP), bilirubin, and total proteins (using Automated Span Diagnostic Reagents, Mumbai, India).
Assessment of antioxidant activity
The rats were sacrificed on the 15th day after collection of blood samples and their livers were excised immediately, washed in ice-cold phosphate-buffered saline (pH 7.4), blotted dry, and weighed. A 10% w/v of liver homogenate was prepared in 0.15 M Tris-HCl buffer (pH 7.4). The homogenate was centrifuged at 2000 × g for 20 min at 4°C to remove the cell debris and then the supernatant was centrifuged (REMI C-24) at 12,000 × g for 1 h at 4°C. The supernatant obtained was used for the determination of lipid peroxidation,[19] reduced GSH,[22] superoxide dismutase (SOD),[23] and catalase (CAT).[24]
Histopathologic study
The fragments from the liver tissues were preserved in 10% neutral formalin solution and embedded in paraffin wax. Histological sections were prepared, stained with Hematoxylin (H) and Eosin (E), mounted and observed under a light microscope.
Statistical analysis
The experimental data were expressed as mean ± SEM. The data were analyzed using analysis of variance (ANOVA) and Dunnett's test. The results were considered statistically significant if P < 0.05.
RESULTS
Phytochemical screening
The qualitative phytochemical analysis of the ethyl acetate fraction revealed the presence of flavonoids, tannins, alkaloids, saponins, and glycosides.
Acute toxicity study
No mortality and toxic manifestations were observed up to the dose of 2000 mg/kg. Further dosing was not performed to estimate the LD50 (lethal dose) value. According to the OECD guidelines for the acute toxicity, an LD50 dose of 2000 mg/kg and above is categorized as unclassified, and hence, the drug is found to be safe. Based on the acute toxicity studies, the doses 200 and 400 mg/kg of the ethyl acetate fraction have been selected as the therapeutic dose.
Effect on blood glucose levels
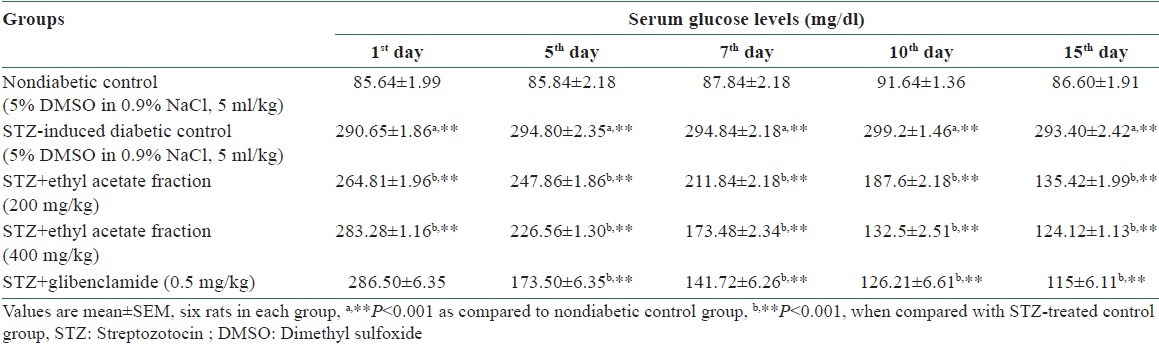
Oral administration of a dose of 200 and 400 mg/kg of the ethyl acetate fraction of EESS to STZ-induced diabetic rats significantly (P < 0.001) reduced the elevated fasting blood glucose levels, when compared to diabetic control rats. The effect of the ethyl acetate fraction of EESS on the fasting blood glucose levels in STZ-induced diabetic rats is shown in Table 1.
Table 1.
Effect of ethyl acetate fraction of Stereospermum suaveolens on the glucose level in STZ-induced diabetic rats
Effect on serum biomarkers
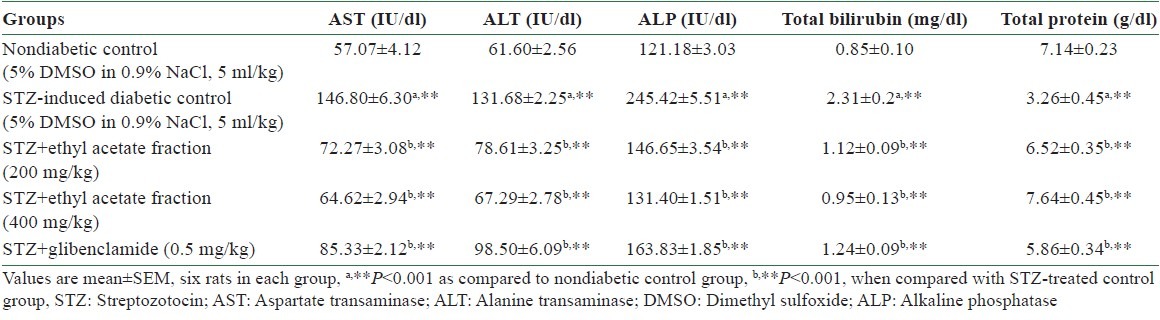
In the present study, AST, ALT, SALP, and bilirubin levels were elevated and total protein level was decreased in diabetic control rats when compared with nondiabetic control rats, and the results are depicted in Table 2. Oral administration of ethyl acetate fraction of EESS for 14 days significantly (P < 0.001) decreased serum biomarker enzymes and bilirubin levels and increased total protein level.
Table 2.
Effect of ethyl acetate fraction of Stereospermum suaveolens on serum biomarkers in STZ-induced diabetic rats
Effects on hepatic in vivo antioxidant activities
Lipid peroxidation
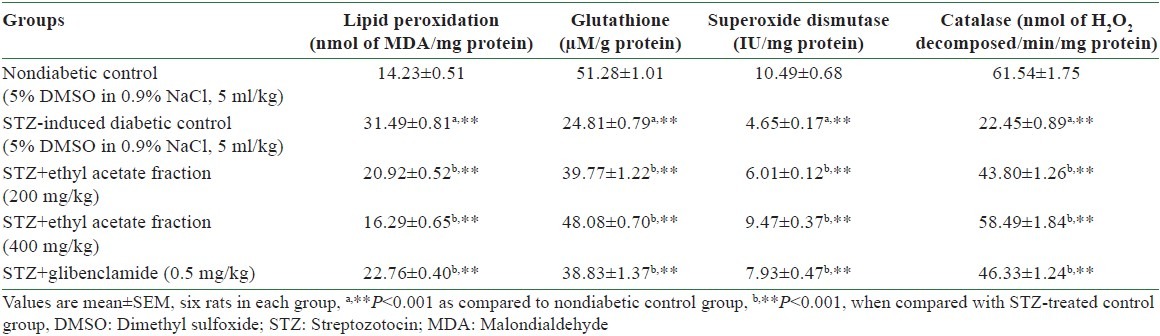
STZ-induced diabetic rats showed an increased level of thiobarbituric acid reactive substances (TBARS) in the liver [Table 3], as compared to nondiabetic rats. However, treatment of diabetic rats with ethyl acetate fraction significantly (P < 0.001) decreased the concentration of TBARS in the liver tissue as compared to the respective diabetic control rats.
Table 3.
Effect of ethyl acetate fraction of Stereospermum suaveolens on liver lipid peroxidation, glutathione, and antioxidants in STZ-induced diabetic rats
Reduced GSH content
The total GSH content decreased in STZ-induced diabetic rats as compared to non-diabetic rats. Administration of ethyl acetate fraction significantly (P < 0.001) increased the total GSH content in the liver when compared to the level in STZ-induced diabetic control rats, and the results are shown in Table 3.
SOD and CAT
In diabetic control rats, the activities of SOD and CAT were significantly (P < 0.001) decreased in the liver. The diabetic rats treated with ethyl acetate fraction exhibited a significant (P < 0.001) increase in the activities of SOD and CAT in the liver, and the results are shown in Table 3.
Histopathologic studies of liver
Figure 1 presents the liver section of nondiabetic control rat, which shows normal cellular architecture with distinct hepatic cells, sinusoidal spaces, and well brought-out central vein. The liver section of diabetic control rat shows several large areas of necrosis, severe inflammatory cell infiltration, fatty change, and sinusoidal dilation and congestion [Figure 2]. Histopathologic examination of the livers of rats treated with ethyl acetate fraction and glibenclamide maintained normal lobular architecture and had mild fatty change, feathery degeneration, and only slight neutrophil infiltration almost comparable to the normal [Figures 3–5].
Figure 1.
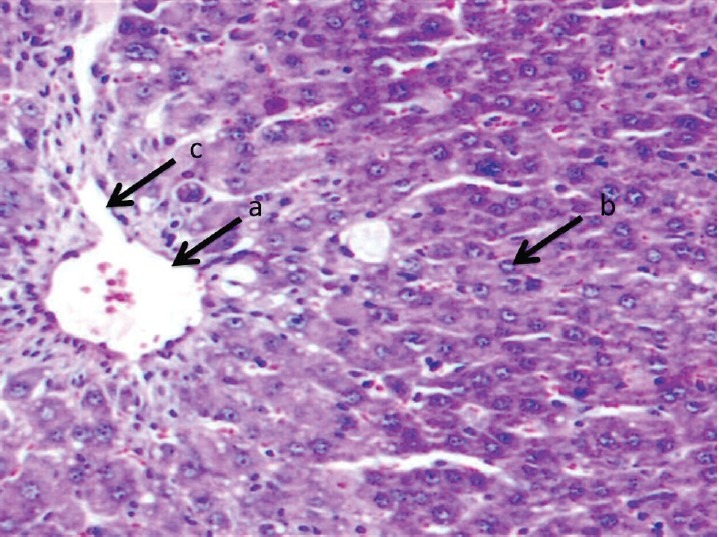
Liver section of nondiabetic control rat stained with Hematoxylin and Eosin shows normal hepatocytes with normal lobular architecture: (a) central vein; (b) normal hepatocytes; and (c) normal sinusoids
Figure 2.
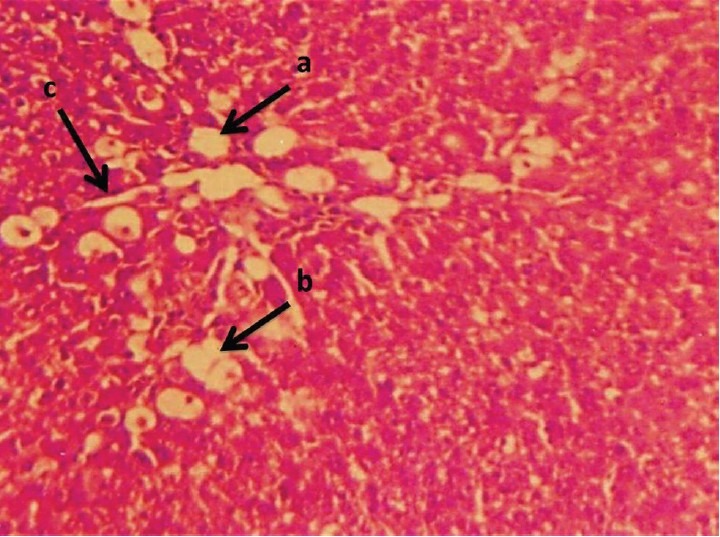
Liver section of STZ-induced diabetic control rat stained with Hematoxylin and Eosin is shown: (a) severe necrosis; (b) infiltrated inflammatory cell with fatty change; and (c) sinusoidal dilation
Figure 3.
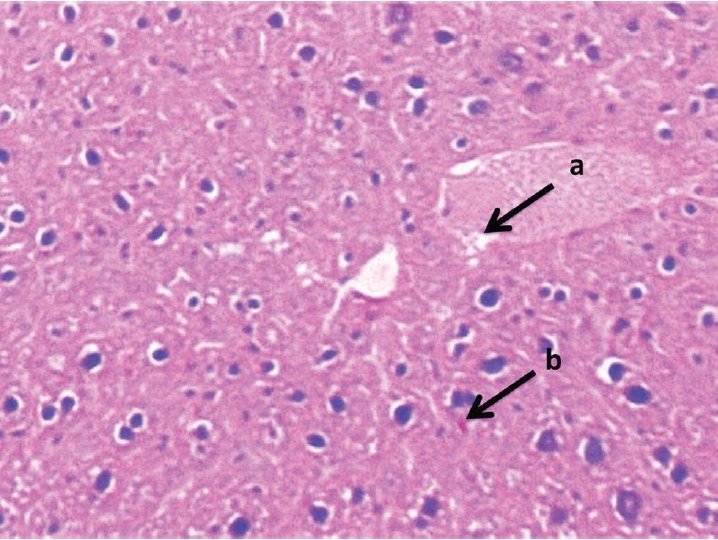
Liver section of ethyl acetate fraction (200 mg/kg) + STZ treated rat stained with Hematoxylin and Eosin is shown: (a) feathery degeneration; (b) slight neutrophil infiltration, almost comparable to the normal part
Figure 5.
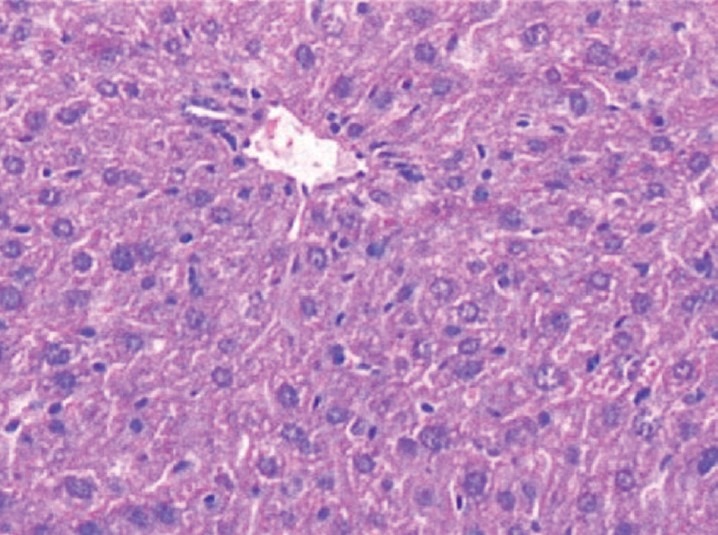
Liver section of glibenclamide + STZ treated rat stained with Hematoxylin and Eosin, showing mild degeneration with normal architecture
Figure 4.
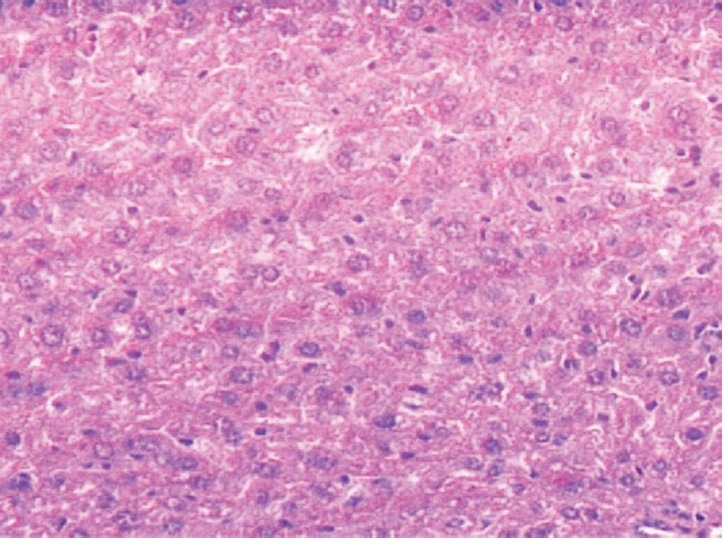
Liver section of ethyl acetate fraction (400 mg/kg) + STZ treated rat stained with Hematoxylin and Eosin, showing normal lobular architecture
DISCUSSION
Reactive oxygen species play an important role in the pathogenesis and development of complications of diabetes. In diabetes, hyperglycemia increases the generation of free radicals and the liver is overburdened due to oxidative stress, which may lead to anatomical changes with the impairment of antioxidant enzymes functions, thus affecting carbohydrate metabolism and the activities of serum biomarker enzymes.[23,24] Antioxidants originating from plants play an important role in inhibiting and scavenging the free radicals, thus providing protection to human beings against oxidative stress in diabetes. As a part of our ongoing research on the medicinal plant S. suaveolens, the present study establishes the protective effect of the ethyl acetate fraction from EESS on hepatic damage in STZ diabetic rats.
The STZ diabetic rats exhibited persistent hyperglycemia which is the main diabetogenic factor and contributes to the oxidative stress in the liver.[25]
The present data indicate that daily administration of ethyl acetate fraction (200 and 400 mg/kg) of EESS for 14 days significantly reduced hyperglycemia in STZ-induced diabetic rats when compared to diabetic control rats in a dose-dependent manner. This finding suggests that the ethyl acetate fraction of EESS had a potent antihyperglycemic activity in diabetic rats, which corresponded to the previous finding that the crude ethanol extract reduced blood glucose level in STZ diabetic rats.[14]
Assessment of liver function can be made by estimating the activities of serum biomarker enzymes such as AST, ALT, and SALP, which are originally present in higher concentration in the cytoplasm of hepatocytes. Serum bilirubin and the total protein levels, on the other hand, are related to the functions of hepatic cells.[26,27] The increased levels of AST, ALT, SALP, and serum bilirubin are conventional indicators of hepatocellular necrosis caused by oxidative stress in diabetes.[28] In the present study, there was a significant rise in serum biomarker levels such as AST, ALT, and SALP in diabetic rats, which could be related to excessive accumulation of amino acids (glutamate and alanine) in the serum of diabetic animals as a result of amino acid mobilization from protein stores.[29,30] Administration of fraction at the doses of 200 and 400 mg/kg significantly decreased the AST, ALT, and SALP toward normal level, indicating the ethyl acetate fraction of EESS preserved the structural integrity of the hepatocellular membrane and liver cell damage caused by oxidative stress associated with diabetes, which is confirmed by histopathologic studies.
Abnormalities in serum total proteins and bilirubin are very common in hepatic toxicity. The rise in the levels of serum bilirubin is the most sensitive and confirms the intensity of jaundice.[26] It was found that the ethyl acetate fraction treatment reduced elevated serum bilirubin and increased the level of total protein in STZ-induced diabetes, indicating that the ethyl acetate fraction may prevent hepatic injury.
Numerous studies have demonstrated that oxidative stress is a key pathogenic factor in the development of diabetic complications.[31,32] Oxidative stress induces the production of highly reactive oxygen species that are toxic to the cell, particularly the cell membrane in which these radicals interact with the lipid bilayer and produce lipid peroxides.[32]
Lipid peroxidation appears to be a key element in the production of secondary complications in diabetes. In the present study, the oxidative stress, measured as levels of malondialdehyde (MDA), was significantly lower (P < 0.001) in the liver of ethyl acetate fraction treated rats compared to that in diabetic control rats. This suggests that ethyl acetate fraction of EESS might protect the liver tissue from lipid peroxidation. Furthermore, enhanced oxidative stress due to diabetes may also result from a dysfunction in the defense system against free radicals, such as reduction in glutathione or inactivation of SOD and CAT.[33]
Glutathione, a tripeptide present in micromolar concentrations in all the cells, acts as an antioxidant and its decrease has been reported in diabetes mellitus.[33] GSH has an important role in the generation of cellular redox state and, consequently, the imbalance in reduced GSH to oxidized glutathione ratio is a putative indicator of cellular oxidative stress. The GSH levels were significantly (P < 0.001) decreased in the liver of diabetic control rats. Decreased glutathione levels in diabetes have been considered to be an indicator of increased oxidative stress.[34,35,36] Treatment with ethyl acetate fraction improved the reduced GSH level as compared to that in STZ diabetic control rats, suggesting strengthening of antioxidant defenses in liver.
The cellular radical scavenging systems include the enzymes such as SOD, which scavenges the superoxide ion by catalyzing its dismutation, and CAT, a heme protein enzyme that removes hydrogen peroxide.[37] Therefore, reduction in the activity of these enzymes (SOD, CAT) results in a number of deleterious effects due to the accumulation of superoxide anion radicals and hydrogen peroxide. These antioxidant enzyme levels significantly increased after the treatment of STZ-induced diabetic rats with the ethyl acetate fraction of EESS, indicating the free radical scavenging activity and its protective effect against hepatic tissue damage. The above in vivo antioxidant status reveals support to the protective action of ethyl acetate fraction against oxidative stress-induced alteration in the liver of diabetic rats.
Moreover, the phytochemical examination of ethyl acetate fraction of EESS indicates the presence of flavonoids, saponins, tannins, glycosides, and alkaloids. These phytochemicals have been proved to possess powerful antioxidant activities.[37,38,39] The observed antioxidant effect of this plant may be attributed to the presence of these bioactive principles.
CONCLUSION
The present research clearly indicates that the ethyl acetate fraction of S. suaveolens enhances the activity of hepatic antioxidant enzymes in STZ-induced diabetic rats, which play a key role in the defense mechanism against hepatic damage caused by free radicals during hyperglycemia. Further chemical and pharmacological investigations are in progress to isolate, identify, and characterize the active principle (s) as well as to pinpoint the exact molecular mechanism of ethyl acetate fraction of EESS involved in liver protection against oxidative stress in diabetes.
REFERENCES
- 1.Diabetes Atlas. 4th ed. Brussels: International Diabetes Federation; 2009. International Diabetes Federation (IDF) [Google Scholar]
- 2.Orhan N, Aslan M, Orhan DD, Ergun F, Yeşilada E. In-vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats. J. Ethnopharmacol. 2006;108:280–6. doi: 10.1016/j.jep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Gerber M, Boutron-Ruault MC, Hercberg S, Riboli E, Scalbert A, Siess MH. Food and cancer: State of the art about the protective effect of fruits and vegetables. Bull Cancer. 2002;89:293–312. [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: Their role in the prevention of cardiovascular diseases and cancer. Am J Med. 2002;113:71–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 5.Raw Materials. New Delhi: CSIR; 1976. The Wealth of India; pp. 1848–9. Anonymous. [Google Scholar]
- 6.Kirtikar KR, Basu BD. Indian Medicinal Plants. Dehradun, India: International Book Distributors; 1988. pp. 1848–9. [Google Scholar]
- 7.Ramachandran AG, Mohandoss S. 6-O-β-D-Glucosylscutellarein- A rare flavone glycoside from Stereospermum suaveolens. J Indian Chem. 1988;65:150–89. [Google Scholar]
- 8.Joshi KC, Bansal RK, Patni R. Chemical examination of the roots of Stereospermum suaveolens DC. J Indian Chem Soc. 1977;54:648–9. [Google Scholar]
- 9.Haque MR, Rahman KM, Begum B, Hasan CM, Rashid MA. Secondary metabolites from Stereospermum chelonoides. Univ Dhaka J Pharm Sci. 2005;4:61–4. [Google Scholar]
- 10.Haque MR, Rahman KM, Iskander MN, Hasan CM, Rashid MA. Stereochenols A and B, two quinones from Stereospermum chelonoides. Phytochemitry. 2006;67:2663–5. doi: 10.1016/j.phytochem.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran AG, Kotiyal JP. Stereolensin- A new flavone glucoside from Stereospermum suaveolens. Indian J Chem. 1979;18B:188–9. [Google Scholar]
- 12.Sankara Subramanian S, Nagarajan S, Sulochana N. Flavonoids of the leaves of Stereospermum suaveolens. Curr Sci. 1972;41:102–3. [Google Scholar]
- 13.Ghani A. Medicinal plants of Bangladesh.chemical constituents and uses. 1st ed. Dhaka: Asiatic Society of Bangladesh; 1998. p. 390. [Google Scholar]
- 14.Balasubramanian T, Lal MS, Sarkar M, Chatterjee TK. Antihyperglycemic and antioxidant activities of medicinal plant Stereospermum suaveolens in streptozotocin-induced diabetic rats. J Diet Suppl. 2009;6:227–51. doi: 10.1080/19390210903070780. [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanian T, Chatterjee TK, Senthilkumar GP, Mani T. Effect of ethyl acetate fraction of Stereospermum suaveolens extract in streptozotocin induced diabetic rats. ScientificWorld Journal. 2012;2012:413196. doi: 10.1100/2012/413196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horbone JB. Phytochemical methods. London: Chapman and Hall; 1988. pp. 60–6. [Google Scholar]
- 17.Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. Pune: Nirali Prakashan; 1998. pp. 122–8. [Google Scholar]
- 18.Trease GE, Evans WC. Pharmacognosy. East Bourne: ELBS Publication; 2002. pp. 93–194. 336-7. [Google Scholar]
- 19.Ecobichon DJ. The basis of toxicology testing. New York: CRC Press; 1977. p. 43. [Google Scholar]
- 20.Siddique O, Sun Y, Lin JC, Chien YW. Facilitated transdermal transport of insulin. J Pharm Sci. 1989;76:341–5. doi: 10.1002/jps.2600760416. [DOI] [PubMed] [Google Scholar]
- 21.Nagappa AN, Thakurdesai PA, Venkat Rao N, Singh J. Antidiabetic activity of Terminalia catappa Linn fruits.J. Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Ellman G. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:131–2. [PubMed] [Google Scholar]
- 25.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. Vol. 2. New York: Academic Press; 1974. pp. 674–84. [Google Scholar]
- 26.Bolkent S, Yanardag R, Karabulut-Bulan O, Ozsoy-Sacan O. The morphological and biochemical effects of glibornuride on rat liver in experimental diabetes. Hum Exp Toxicol. 2004;23:257–64. doi: 10.1191/0960327104ht444oa. [DOI] [PubMed] [Google Scholar]
- 27.Koyuturk M, Tunali S, Bolkent S, Yanardag R. Effects of vanadyl sulfate on liver of streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2005;104:233–47. doi: 10.1385/BTER:104:3:233. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006;79:1578–84. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Drotman RB, Lawhorn GT. Serum enzymes are indicators of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 30.Achilya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi ghrita against carbon tetrachloride induced hepatic damage in rats. J Ethnopharmacol. 2004;90:229–32. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 31.Colev V, Badescu M, Paduraru I, Mândreci I, Bohotin C. The zinc-metabolic disorder relation in experimental diabetes mellitus. Rom J Intern Med. 1994;32:71–5. [PubMed] [Google Scholar]
- 32.Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Isumer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: Effects of glycemic control. Clin Biochem. 2001;34:65–70. doi: 10.1016/s0009-9120(00)00199-5. [DOI] [PubMed] [Google Scholar]
- 33.Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. The effect of verapamil on the antioxidant defence system in diabetic kidney. Clin Chim Acta. 2002;322:105–12. doi: 10.1016/s0009-8981(02)00167-5. [DOI] [PubMed] [Google Scholar]
- 34.Kesavulu MM, Rao BK, Giri R, Vijaya J, Subramanyam G, Apparao C. Lipid peroxidation and antioxidant enzyme status in type 2 diabetics with coronary heart disease. Diabetes Res Clin Pract. 2001;53:33–9. doi: 10.1016/s0168-8227(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 35.Arai K, Maguchi S, Fujii S, Ishibashi H, Oikawa K, Taniguchi N. Glycation and inactivation of human Cu-Zn-superoxide diamutase. Identification of the in vitro glycated sites. J Biol Chem. 1997;262:16969–72. [PubMed] [Google Scholar]
- 36.Rotruck JT, Pope AL, Ganther HE, Swanson AB. Selenium: Biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 37.Anuradha CV, Selvam R. Effect of oral methionine on tissue lipid peroxidation and antioxidants in alloxan-induced diabetic rats. J Nutr Biochem. 1993;4:212–7. doi: 10.1016/0955-2863(90)90027-i. [DOI] [PubMed] [Google Scholar]
- 38.Bolzan AD, Bianchi MS. Genotoxicity of STZ. Mutation Res. 2002;512:121–34. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 39.Patel DK, Kumar R, Prasad S, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:316–22. doi: 10.1016/S2221-1691(11)60051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


