Abstract
Polygonum cuspidatum Sieb. and Zucc. has been traditionally used as a member of many anti-inflammatory polyherbal formulations, but is now a widespread invasive neophyte in Europe and America. To discuss if the invasive variety is chemically identical to the native one in traditional medicine, the different constituents of the invasive variety compared to the native variety were isolated and their anti-inflammatory activity was tested. Resveratroloside and catechin-(4α→8)-catechin, the newly found constituents in the invasive variety, have similar nitric oxide (NO) inhibition potency as that of piceid (the major constituent of P. cuspidatum), but the newly found major constituent, i.e., piceatannol glucoside, showed no apparent effect. On the other hand, as a marker, the total content of resveratrol in the methanol root extract after glucosidase hydrolysis was measured and compared between the invasive and native varieties. The total content of resveratrol measured in the root extracts of the Swiss sample was about 2.5 times less than that of the Chinese one. This study brings attention to the point that when the invasive variety of P. cuspidatum is used in traditional medicine, the chemical difference should be kept in mind.
Keywords: Anti-inflammatory, Invasive neophyte, Polygonum cuspidatum, Piceatannol glucoside, Resveratrol
INTRODUCTION
Polygonum cuspidatum Sieb. and Zucc. (Polygonaceae) is a traditional Chinese medicine for the treatment of various inflammatory diseases, hepatitis, tumors, and diarrhea, and is officially listed in the Chinese Pharmacopoeia.[1] But it has gained much notoriety in Europe and North America (where it is known as Mexican bamboo, Japanese bamboo, or Japanese knotweed) as an invasive neophyte (introduced after 1500), due to its virtually indestructible growing characteristics: A fast-growing, robust perennial herb that emerges early in the spring and forms dense thickets up to 9 feet in height. The thickets are so dense that they can reduce the diversity of plant species and significantly alter natural habitats.[2] Thus, it makes the resource of this plant very abundant. Why the plant became invasive is an elusive ecological issue. The presence of different metabolites might confer ecological advantages to the introduced varieties.[3]
The comparison of chromatographic fingerprints of root samples P. cuspidatum from China and Switzerland using high performance liquid chromatography simultaneously coupled to ultraviolet detection and electrospray ionization mass spectrometry (HPLC/UV/ESI-MS) showed that P. cuspidatum collected in Switzerland (present as an invasive neophyte) was obviously different from the samples of P. cuspidatum from China with respect to piceatannol glucoside, resveratroloside, and some proanthcyanidins, although piceid was still the major constituent. No piceatannol was detected in the roots of P. cuspidatum from China, but could be found obviously in P. cuspidatum from Switzerland; meanwhile, the relative contents of resveratroloside and some proanthcyanidins increased.[4] It is generally accepted that multiple constituents are responsible for the therapeutic effect of plants.[5] Can the invasive variety present in Switzerland still be used like the native traditional medicine from China? With this question, we isolated the major different compounds from the invasive variety of P. cuspidatum and evaluated their anti-inflammatory activity using lipopolysaccharide (LPS)-stimulated bone marrow derived macrophage (BMDM) cell assay.
Nitric oxide (NO) is recognized as a mediator and regulator in pathological reactions, especially in acute inflammatory responses. NO is derived from the oxidation of L-arginine through three isoforms of nitric oxide synthase (nNOS, eNOS, and iNOS). iNOS mainly exists in macrophages, and is expressed by stimulation with interferon-gamma, tumor necrosis factors, or LPS. Pro-inflammatory agents, such as LPS, can significantly increase NO production in macrophages through activation of iNOS.[6]
Secondly, P. cuspidatum is one of the important natural sources of resveratrol. Resveratrol has numerous pharmacological properties and is responsible for many activities of P. cuspidatum, specially anti-inflammatory activity,[7] hepatoprotection,[8] antibacterial activity,[9,10] etc. Actually, piceid is present to a greater extent than its aglycone, resveratrol, in P. cuspidatum,[4] but hydrolysis of this glycosylated derivative can occur in small intestine and liver, which would enhance the amount of the biologically active resveratrol.[11] However, the invasive and native varieties of P. cuspidatum were different in the presence and relative content of resveratrol glucosides (piceatannol glucoside, resveratroloside, and piceid); so, it is difficult to say which variety of P. cuspidatum is better for medicinal use. To compare the two varieties with respect to resveratrol, the total content of resveratrol in the two varieties was measured and compared after enzymatic hydrolysis of their extracts (to convert the glucosides into resveratrol).
MATERIALS AND METHODS
General
The 1H and 13C spectra were recorded in CD3OD at 500 and 125 MHz, respectively, on a Varian Inova 500 MHz spectrometer (Palo Alto, CA, USA) with trimethylsilane as the internal standard. HR-MS spectra were recorded on a Waters Micromass (Manchester, UK) Liquid Chromatography Time of Flight (LCT) time of flight (TOF) mass spectrometer coupled to an AcquityTM Ultra Performance LC system. Medium pressure liquid chromatography (MPLC) was carried out on an RP-18 column (15-25 μm; 450 mm ×50 mm i.d., Merck (Darmstadt, Germany)) using a slow CH3CN-H2O gradient. HPLC-UV/DAD was carried out on an HP-1100 Agilent system (Hewlett-Packard, Palo Alto, CA, USA) with an Xterra®C18 column (5 μm, 3.5 × 150 mm; Waters, Milford, MA, USA) using 0.5% acetic acid in water (A) and acetonitrile (B) gradient in 60 min (10-40% B in 40 min, then 40-100% B in 20 min). The UV detector was set at 290 nm. The optical rotations were measured with a Perkin Elmer 241 MC Polarimeter.
Plant material
Three batches of roots of P. cuspidatum were collected in Lausanne (Vaud, Switzerland) and identified by Dr. Andrew Marston, University of Geneva. Three batches of Chinese samples of roots of P. cuspidatum were collected in Shandong, Shanxi, and Yunnan Province, respectively, and identified by Prof. Xuesen Wen, Department of Pharmacognosy, Shandong University. A voucher specimen (no. 2009012 and 2009013, respectively) was deposited in the Laboratory of Pharmacognosy and Phytochemistry, University of Geneva. The roots were washed, cut, dried at room temperature, away from direct sunlight, and ground into powder.
Extraction and isolation
The dried and powered roots of P. cuspidatum (65 g) extracted by successive macerations in solvents of increasing polarity (Dichloromethanol (DCM), MeOH) (3 × 24 h) on a shaker at room temperature yielded, after removing the solvent by rotary evaporator under vacuum and lyophilizing, 0.3 g of crude DCM extract (0.5%) and 11.0 g (16.9%) of crude MeOH extract. MeOH extract (6.0 g) was fractionated by MPLC with a slow CH3CN-H2O step gradient to obtain 320 fractions. Fractions were pooled according to the HPLC/UV trace at 254/290 nm to yield 44.0 mg of piceid (1), 38.0 mg of resveratroloside (2), 16.0 mg of piceatannol glucoside (3), 20.0 mg of (−)-catechin (4), 13.0 mg of (−)-epicatechin (5), 11.0 mg of procyanidin B3 [catechin-(4α→8)-catechin] (6), 34.0 mg of vanicoside B (7), as well as four crystals of anthraquinones, emodin-8-O-glucoside (4.0 mg), physcion-8-O-glucoside (5.0 mg), emodin (12.0 mg), and physicion (5.0 mg).
The structure of purified compounds [Figure 1] was elucidated by direct comparisons of their spectral data (1HNMR, 13CNMR, COSY, TOCSY, HSQC, HMBC, and HR-MS) with those found in literature.[12,13,14,15,16,17,18,19,20,21]
Figure 1.
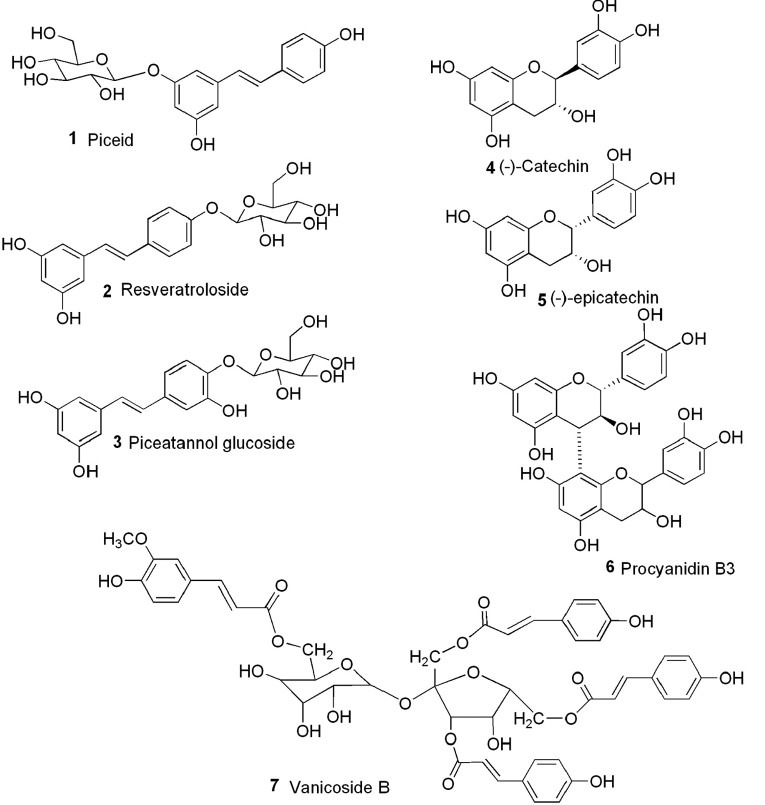
Structures of the compounds isolated from the methanol extract of the roots of P. cuspidatum(invasive variety from Switzerland)
Bone marrow derived macrophage cell cultures and treatment with compounds followed by LPS stimulation
Murine bone marrow cells were differentiated into macrophages after culturing at 6 × 105 cells per well in a 96-well plate in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 20% L929 cell-conditioned medium as a source of granulocyte-macrophage colony-stimulating factor. After 6 days of culture, the cell preparation contained 99% of adherent macrophages representing about 10% of the total cells initially put into culture.
BMDM cells were treated or not with various concentrations (25, 12.5, 6.25, 3.125, 1.5625, and 0.78 μg/ml) of pure compounds for 30 min. Cells were then stimulated with Escherichia coli (serotype 055:B5) LPS (1 μg/ml) for 24 h. The supernatant was harvested for nitrite determination.
NO determination
NO accumulation in cell culture medium was measured as an indicator of NO production by Griess reagent (1% sulfanilamide and 0.1% naphtylethylenediamide in 2.5% phosphoric acid). In brief, the cultured supernatant (50 μl) was mixed with 50 μl of Griess reagent for 5 min. The absorbance was measured at 570 nm and nitrite concentration was determined by comparison with reference to a sodium nitrite standard curve.
Statistical analysis
Assays were done in triplicate. Data are expressed as mean ± standard deviation for the number of experiments.
Hydrolysis of the plant extract and quantitative measurement of the content of resveratrol with HPLC
Acid hydrolysis and enzyme hydrolysis of resveratrol glucosides
To choose the method of hydrolysis fit for the extract, both acid hydrolysis and enzyme hydrolysis were performed on piceid firstly to convert it into resveratrol. Acid hydrolysis: Piceid (3.4 mg) in 0.02 M HCl (10 ml) was heated at 90°C for 4 h (300 r.p.m.), then the solution was cooled down and the pH was adjusted to 7.0 with NH4OH. The solution (750 ml) was mixed with the same volume of MeOH before injecting 10 μl for HPLC analysis. Enzyme hydrolysis: Piceid (1.0 mg) with β-glucosidase from almonds (1 mg, 8.9 U/mg, from Sigma (St. Louis, MO, USA)) in 1 ml NaOAc buffer (pH 5.5) was incubated at 37°C, 900 r.p.m. for 4 h. The reaction was stopped by directly adjusting the concentration to 10 ml with MeOH. After centrifugation, the supernatant (10 μl) was injected for HPLC analysis.
Enzymatic hydrolysis of extract solution and quantitative measurement of the content of resveratrol with HPLC
Powdered roots (2 g) of P. cuspidatum were macerated in 30 ml 50% MeOH, shaking at 120 r.p.m. for 24 h. The extract solution was filtered and adjusted to 50 ml with 50% MeOH. The extract solution (10 ml) was concentrated with rotovapor and suspended in 2 ml NaOAc buffer (pH 5.5). Then, 500 μl of solution in buffer was mixed with β-glucosidase (20 mg) in 500 μl NaOAc buffer (pH 5.5) and incubated at 37°C, 900 r.p.m for 4 h. The reaction was stopped by directly adjusting the concentration to 5.0 ml with MeOH. After centrifugation, the supernatant (10 μl) was injected for HPLC analysis, where the UV detector was set at 290 nm. A series of concentrations of resveratrol (from Sigma) were analyzed for constructing calibration curve.
The content of resveratrol in the plant material = the concentration measured (g/l) × dilute coefficient/mass of the plant material (g)
Here, the dilute coefficient of the three steps is 100.
RESULTS AND DISCUSSION
NO inhibitory assay
To discuss the anti-inflammatory activity of the invasive neophyte P. cuspidatum, the constituents distinguished from the native variety and of relative high content (referring specially to compounds 1, 2, 3, 6, and 7 in Figure 1) were isolated and tested using NO inhibitory assay at concentration without cytotoxicity. The inhibitory effects of the compounds on NO production in LPS-stimulated BMDM cells are shown in Figure 2. The NO levels in tested cells with (activated group) and without (untreated) LPS stimulation were 48.23 ± 5.95 μM and 9.35 ± 0.42 μM, respectively. After LPS-stimulated cells were co-incubated with 25 μg/ml compounds, among the three stilbene analogs, both resveratroloside (2) and pieatannol glucoside (3) showed significant inhibition at 25 μg/ml, while the activity of piceid (1) was not apparent. However, at a lower concentration (below 12.5 μg/ml), 1 and 2 showed similar but significant inhibition (about 50%). Further reducing the concentration weakly influenced the activity. However, 3 gave an obvious dose-dependent decrease in activity and showed no more activity at concentrations below 12.5 μg/ml. We further compared the NO inhibitory power of piceid with that of resveratrol. At the concentration of 12.5 μg/ml, resveratrol showed a little stronger power (60% inhibition) than piceid (data not shown on the graph).
Figure 2.
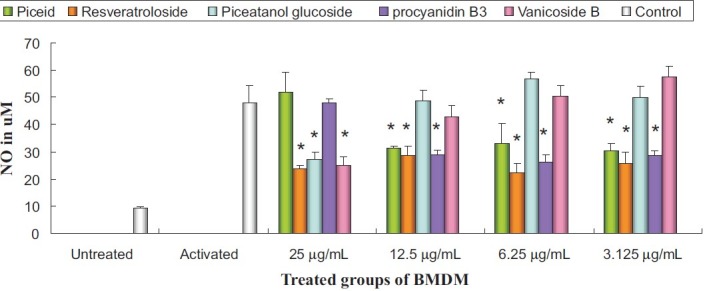
Inhibitory effects of isolated compounds on nitric oxide production in LPS-stimulated BMDM cells. The data are representative of the three experiments and expressed as mean ± SEM. Columns with asterisks (*) show statistically significant difference (P <</i>0.05)
The phenylpropanoid vanicoside B (7) showed statistically significant inhibition at 25 μg/ml and dose-dependent decrease in activity (no more activity at concentrations below 6.25 μg/ml). The procyanidin catechin-(4μ→8)-catechin (6) did not show significant inhibition at 25 μg/ml. But after dilution, a similar phenomenon was observed as piceid showed significant inhibition (about 50%) at concentration below 12.5 μg/ml and a lower concentration influenced the activity slightly. The abnormal dose-dependent relation of piceid and procyanidin may be due to the dual effect of polyphenols, which can be pro-oxidant in certain environments.[22]
Total content of resveratrol in the plants after hydrolysis
Acid hydrolysis or enzyme hydrolysis
To convert piceid to resveratrol, both acid hydrolysis (20% v/v HCl in the solution) and enzyme hydrolysis (7 U β-glucosidase/mg extract) were attempted. However, there are at least four adducts present in acid hydrolysis, which will influence the content of resveratrol. By contrast, enzyme hydrolysis can well convert piceid into resveratrol without visible adducts [Figure 3].
Figure 3.
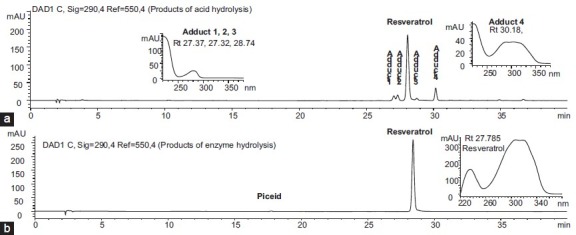
HPLC-DAD chromatographs of hydrolyzed products of piceid after (a) acid hydrolysis and (b) enzyme hydrolysis. HPLC-UV/DAD was carried out on an HP-1100 Agilent system with an Xterra® C18 column (5 μm, 3.5 × 150 mm) using 0.5% acetic acid in water and acetonitrile gradient in 60 min (10-40% acetonitrile in 40 min, then 40-100% acetonitrile in 20 min)
Figure 4 shows the HPLC profile of the extract solutions of roots of two P. cuspidatum samples before and after enzyme hydrolysis.
Figure 4.
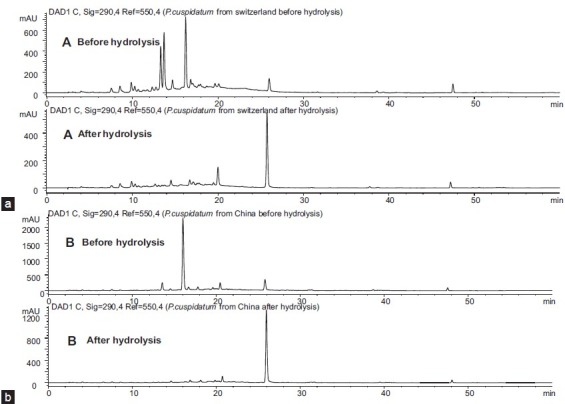
Chromatography of the extract solution of roots before and after enzyme hydrolysis: (a) P. cuspidatum (Swiss) and (b) P. cuspidatum (Chinese). HPLC-UV/DAD was carried out on an HP-1100 Agilent system with an Xterra® C18 column (5 μm, 3.5 × 150 mm) using 0.5% acetic acid in water and acetonitrile gradient in 60 min (10-40% acetonitrile in 40 min, then 40-100% acetonitrile in 20 min)
Measurement of the content of resveratrol after enzyme hydrolysis
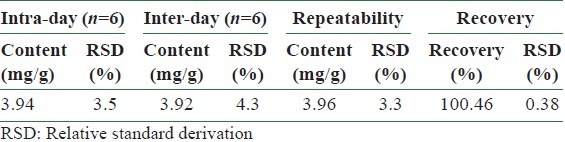
The method of quantitative analysis was validated in terms of linearity, limits of detection and quantification (LODs and LOQs) at 290 nm [Table 1], precision, repeatability, and recovery test [Table 2].
Table 1.
Calibration curves, detection limits, and quantification limits of resveratrol (n=3) by HPLC-DAD (290 nm)
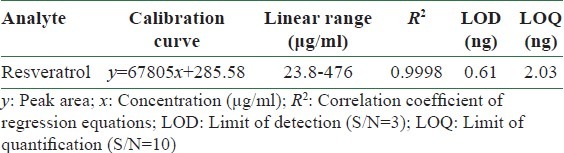
Table 2.
Intra- and inter-day variability and repeatability for resveratol in roots of P. cuspidatum from Switzerland
The quantitative analysis results showed that the total contents of resveratrol in the Swiss samples were 3.94 mg/g, 3.76 mg/g, and 4.11 mg/g, respectively, and the total contents of resveratrol in the Chinese samples were 10.74 mg/g (Shandong), 10.42 mg/g (Shanxi), and 10.01 mg/g (Yunnan) [Table 3].
Table 3.
Total contents of resveratrol in the Swiss samples and the Chinese samples (mg/g)

This is the first report about the anti-inflammatory activity of the invasive variety of P. cuspidatum. To summarize, the invasive variety of P. cuspidatum should have anti-inflammatory potency because the three compounds 1, 2, and 6 have activities at relatively lower concentrations (below 12.5 μg/ml). Concerning the newly found resveratrol glucosides in the invasive variety of P. cuspidatum, the activity of 2 is similar to that of piceid (1), but the other one, i.e., piceatannol glucoside (3), showed no apparent activity.
To find whether the invasive variety of P. cuspidatum can be used as the native one for anti-inflammatory medicine, the total content of effective constituents should be compared. Thus, in this study, enzymatic hydrolysis of the extracts was employed to convert piceid and resveratroloside into resveratrol, and the total content of resveratrol was compared between the two varieties. The result showed that resveratrol content in the Swiss samples is lower (about 2.5 times) than in the Chinese ones.
Considering the results of this work, we propose that the invasive variety should not be completely used as the native one, although both have anti-inflammatory potency. The alternative use of this variety in traditional medicine should keep the difference in mind.
ACKNOWLEDGMENT
The authors express gratitude to Dr. Olleros M. L. and Prof. Garcia I. in Faculty of Medicine, University of Geneva, for the test of NO inhibition. The first author expresses gratitude to Prof. A. Marston for his advice and manuscript revision. The authors thank the Swiss National Science Foundation (grant no. 200020-107775 to Prof. K. Hostettmann), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China (to Dr. Peihong Fan), National Science Foundation of China (grant no. 81001616), and Technology Development Project of Shandong Province (No. 2010GSF10223) for financial support.
REFERENCES
- 1.Pharmacopoeia of the People's Republic of China. Beijing, China: China Chemical Industry Press; 2000. China Pharmacopoeia Committee. [Google Scholar]
- 2.Nature Conservancy of Vermont. Invasive exotic fact sheet: Japanese knotweed. Montpelier VT. 1998 [Google Scholar]
- 3.Kourtev PS, Ehrenfeld JG, Huang WZ. Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol Biochem. 2002;34:1207–18. [Google Scholar]
- 4.Fan P, Hay AE, Marston A, Lou H, Hostettmann K. Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. and Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim Biochem Syst Ecol. 2009;37:24–34. [Google Scholar]
- 5.Yi T, Zhang H, Cai Z. Analysis of Rhizoma Polygoni Cuspidati by HPLC and HPLC-ESI/MS. Phytochem Anal. 2007;18:387–92. doi: 10.1002/pca.993. [DOI] [PubMed] [Google Scholar]
- 6.Wang SY, Li XY, Xiao JH, Yang JC, Kao YT, Chang ST. Anti-inflammatory activity of Lindera erythrocarpa fruits. Phytother Res. 2008;22:213–6. doi: 10.1002/ptr.2289. [DOI] [PubMed] [Google Scholar]
- 7.Bralley EE, Greenspan P, Hargrove JL, Wicker L, Hartle DK. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J Inflamm (Lond) 2008;5:1. doi: 10.1186/1476-9255-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura Y, Ohminami H, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stilbene components of roots of Polygonum ssp.on liver injury in peroxidized oil-fed rats. Planta Med. 1983;49:51–4. doi: 10.1055/s-2007-969810. [DOI] [PubMed] [Google Scholar]
- 9.Hegde VR, Pu H, Patel M, Black T, Soriano A, Zhao W, et al. Two new bacterial DNA primase inhibitors from the plant Polygonum cuspidatum. Bioorg Med Chem Lett. 2004;14:2275–7. doi: 10.1016/j.bmcl.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Wu L, Song G, Liu K, Zhang X, Zhang Y. Study on chemical constituents of Polygonum cuspidatum Sieb.J. Shenyang Pharm Uni. 1999;16:17–20. [Google Scholar]
- 11.Henry-Vitrac C, Desmoulière A, Girard D, Mérillon JM, Krisa S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur J Nutr. 2006;45:376–82. doi: 10.1007/s00394-006-0609-8. [DOI] [PubMed] [Google Scholar]
- 12.Coskun M, Satake T, Hori K, Saiki Y, Tanker M. Studies on Turkish Rhamnus species. Part 5. Anthraquinone glycosides from Rhamnus libanoticus. Phytochemistry. 1990;29:2018–20. [Google Scholar]
- 13.Demirezer LO, Kuruuzum-Uz A, Bergere I, Schiewe HJ, Zeeck A. The structures of antioxidant and cytotoxic agents from natural source: Anthraquinones and tannins from roots of Rumex patientia. Phytochemistry. 2001;58:1213–7. doi: 10.1016/s0031-9422(01)00337-5. [DOI] [PubMed] [Google Scholar]
- 14.Foo LY, Lu Y, McNabb WC, Waghorn G, Ulyatt MJ. Proanthocyanidins from Lotus pedunculatus. Phytochemistry. 1997;45:1689–96. [Google Scholar]
- 15.Francis GW, Aksnes DW, Holt Ø. Assignment of the [1]H and [13]C NMR spectra of anthraquinone glycosides from Rhamnus frangula. Magn Reson Chem. 1998;36:769–72. [Google Scholar]
- 16.Mohri Y, Sagehashi M, Yamada T, Hattori Y, Morimura K, Kamo T, et al. An efficient synthesis of procyanidins.Rare earth metal Lewis acid catalyzed equimolar condensation of catechin and epicatechin. Tetrahedron Lett. 2007;48:5891–4. [Google Scholar]
- 17.Svedström U, Vuorela H, Kostiainen R, Tuominen J, Kokkonen J, Rauha JP, et al. Isolation and identification of oligomeric procyanidins from Crataegus leaves and flowers. Phytochemistry. 2002;60:821–5. doi: 10.1016/s0031-9422(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 18.Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–6. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann ML, Sneden AT. Vanicosides A and B, protein kinase C inhibitors from Polygonum pensylvanicum. J Nat Prod. 1994;57:236–42. doi: 10.1021/np50104a007. [DOI] [PubMed] [Google Scholar]
- 20.Zhong F, Li Y, Chen Y, Yang G. Isolation and antibacterial activities of emodin from Polygonum cuspidatum. J S-Cent Univ Natl. 2006;25:40–2. [Google Scholar]
- 21.Yeh SF, Chou TC, Liu TS. Effects of anthraquinones of Polygonum cuspidatum on HL-60 cells. Planta Med. 1988;54:413–4. doi: 10.1055/s-2006-962484. [DOI] [PubMed] [Google Scholar]
- 22.Lu NH, Chen PQ, Yang Q, Peng YY. Anti- and pro-oxidant effects of (+)-catechin on hemoglobin-induced protein oxidative damage. Toxico In vitro. 2011;25:833–8. doi: 10.1016/j.tiv.2011.02.003. [DOI] [PubMed] [Google Scholar]


