Abstract
Pracparatum mungo (Lu-Do Huang) is a traditional Chinese functional medicine made from the natural fermentation of mung bean (Lǜ Dòu) mixed with other Chinese medicines. It has been recognized as having liver protecting and detoxifying effects. As mung beans have been verified to possess anti-inflammatory, antioxidant, antipyretic, and whitening actions, the present research utilized the in vitro, ex vivo, and in vivo experimental models to investigate the antioxidant and melanin inhibiting effects of P. mungo on the skin. The in vitro experiment revealed that P. mungo methanol extract (PMME) and P. mungo ethanol extract (PMEE) possess the capacity to clear α,α-diphenyl-2-picrylhydrazyl (DPPH) radicals and inhibit tyrosinase activity. The ex vivo experiment indicated that PMEE can promote the growth of MDCK cells and increase the enzymatic activities of superoxide dismutase (SOD) and catalase in MDCK cells. On the other hand, PMME and PMEE can suppress the proliferation of A375 cells, and PMEE can reduce the enzymatic activities of SOD and catalase in A375 cells. The in vivo results showed that P. mungo can enhance the enzymatic performance of SOD, Catalase, and glutathione peroxidase (GPx) in the liver. The results also showed that P. mungo has antioxidant characteristics and can inhibit tyrosinase activity, thereby promoting the growth of skin tissues and suppressing the proliferation of A375 cells, and thus enhancing the effects that the antioxidant enzymatic performance has on the liver. These results can be applied in the development of tyrosinase inhibitors or antioxidants used for the inhibition of melanin biosynthesis or for auto-oxidation in further industrial applications, particularly those relating to functional food or cosmetic compositions.
Keywords: Anti-melanogenesis, Antioxidant, Pracparatum mungo, Tyrosinase inhibitor
INTRODUCTION
Melanin production is principally responsible for skin color and plays a significant role in protecting the skin from ultraviolet (UV) light; however, overproduction and accumulation of melanin can result in various dermatological disorders including melisma, freckles, age spots, and sites of actinic damage or other hyperpigmentations.[1,2] Thus, melanogenic inhibitors have become increasingly important ingredients in medication[3] and cosmetics[4] to prevent hyperpigmentation. Tyrosinase is a copper-containing enzyme catalyzing the oxidation of o-diquinones and o-diphenols in the first stages of melanin biosynthesis. This enzyme is also responsible for the undesired browning reactions in damaged fruits during post-harvest handling and processing.[5] Many tyrosinase inhibitors are topically used for treating localized hyperpigmentations such as lentigo, nevus, ephelides, melisma, and post-inflammatory states in humans.[6,7]
Previous studies of cultured murine and human skin cells[8,9,10,11,12] and human skin[13,14,15] have shown that photodamage involves the generation of reactive oxygen species (ROS) and the depletion of endogenous antioxidant networks. To minimize ROS-induced injuries, skin possesses antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD). Exposure to UV light and oxidative stress caused by excessive ROS are casually linked to skin disorders,[16] indicating that antioxidants may benefit skin health. It is also known that ROS play significant roles in the regulation of melanocyte proliferation and melanogenesis, while ROS scavengers and inhibitors may down-regulate hyperpigmentation and UV-induced melanogenesis.[17]
Since ancient times, mung beans (Lǜ Dòu) have been known for their diuretic and detoxifying effects and have been commonly used to relieve summer heat. Previous research findings have shown that mung beans possess anti-inflammatory[18,19,20] and antioxidant activities,[21,22,23] the ability to adapt to warm environments[24] and to reduce lipogenesis,[25] and whitening effects.[26] Sun Si-miao (Sūn Sī Miǎo), a famous doctor of the Tang dynasty (Táng), known as the “King of Medicine” (Yào áng), wrote in his Supplement to the Formulas of a Thousand Gold Worth (Bèi Jí Qiān Jīn Yào Fāng) that mung bean cures chills and fevers (Hán Rè), heat stroke (Rè Zhòng), ends diarrhea (Xiè Lì) and sudden afflux dysentery (Cù Pì), and is good for the fullness and distention of urine (Xiǎo Biàn Zhàng Mǎn). Mung beans can not only be used as a medicine but also as an edible vegetable (sprouts, leguminous crop, or pulse). Li Shi-zhen (Lǐ Shí Zhēn), a herbalist from the Ming Dynasty (Míng), praised the mung bean as “an object of real property which, when added to a dish as a vegetable, will bring tranquility throughout the valley.” As food, mung beans can be made into things such as bean porridge, bean rice, bean wine, and bean cakes which, when eaten regularly, will produce results that cannot even be stated. When thought of as a vegetable, people most often use the sprout form of the mung bean. Wang Shi-xiong (Wáng Shì Xióng), a renowned physician and dietitian of the Qing Dynasty (Qīng), once reportedly exclaimed, sprouts as vegetables what a refreshingly beautiful tast
For thousands of years, Pracparatum mungo, a derivative of mung beans, has been known as a “sacred detoxification medicine” that is widely used for its liver detoxifying properties. P. mungo is a natural food product refined from Phaseolus mungo beans of the plants in the Leguminosae family. The refinement process involves natural fermentation and soaking in a complex traditional Chinese medicine preparation. There are a variety of purposes for P. mungo as a traditional Chinese medicine, such as removing impurities, adjusting smells, reducing or eliminating toxicities or side effects, easing excessive medicinal properties, whitening skin, and inducting medicines into affected channels to enhance their efficacy. The traditional manufacturing process of P. mungo is quite unique because the concepts of yin and yang (Yīn Yáng) and the Five Elements (Wu Xing) (Wǔ Xíng) have been incorporated. The overall process can roughly be divided into four stages. In the first stage, Mountains, the process begins in the White Dew period in early autumn. Chinese herbal medicine is added to freshly selected mung beans and the mixture is placed within a living 3-5-year-old, high mountain green bamboo stem for approximately 120 days through various solar periods such as the White Dew, Autumnal Equinox, Cold Dew, Frost's Descent, Beginning of Winter, Slight Snow, Great Snow, and Lesser Cold periods. In the Rivers stage, during the Lesser Cold period, the entire section of living green bamboo containing the mung beans is excised, placed in boys’ urine (specifically urine from prepubescent boys because the mung bean possesses cold characteristics that require the yang energy of boys to neutralize it and moderate the medicinal property of the P. mungo) and covered. The beans are then allowed to ferment naturally for approximately 120 days. By the beginning of summer, during the Grain Full period, the beans are rinsed in a stream of water deep in the mountains for approximately 120 days. The third stage, or Day stage, occurs by the beginning of autumn during the White Dew and Autumnal Equinox periods. During this stage, the bamboo section is split open and the fully fermented mung beans that have a complete bean shape and beautiful appearance and color are removed. The beans are then soaked in Chinese herbal soup once every 5-7 days and dried in the morning, with the herb species being changed in accordance with the 24 solar terms. In the fourth stage, or Night stage, the mung beans are dried at dawn but exposed to dew water at night for several months until the Grain Rain period. Each day/night alternating cycle of the preparation lasts for approximately 120 days and for a total of approximately 480 days.
P. mungo (also known as Lu-Do Huang) is a traditional and functional food that is made from mung beans (Ph. mungo L.). Previous studies have shown that mung beans contain natural antioxidants and can offer protection against injuries due to heat stress.[24,27] While there is one study discussing the modern applications of P. mungo for its hepatoprotective effects associated with increase in SOD and glutathione (GSH) levels, there is no scientific evidence showing the ability of P. mungo to protect skin. In this study, we used in vitro, ex vivo, and in vivo examinations to investigate the inhibition of melanogenesis and antioxidant properties of P. mungo extracts. These results can be used to develop tyrosinase inhibitors or antioxidants to inhibit melanin biosynthesis and auto-oxidation, and could have further industrial applications, especially in functional food or cosmetic compositions.
MATERIALS AND METHODS
Preparation of P. mungo methanol (PMME) and ethanol (PMEE) extracts
P. mungo (Lu-Do Huang) was purchased from a local company (Eight Princes Biotechnology Co. Ltd., Cha-yi, Taiwan) and then powdered. The powder (100 g) was extracted with ethanol (90% ethanol, 1000 ml, two extractions) and methanol (100% methanol, 1000 ml, two extractions) for 24 h at 4°C and then centrifuged at 500 ×g for 20 min. The extract was filtered and then evaporated to dryness under reduced pressure in a rotary evaporator; the eventual yield was more than 10 g of methanol extract (PMME) and ethanol extract (PMEE). The PMME and PMEE were then lyophilized (EYMA Freeze Dryer, FDU-540). The final 0.93 g of PMME and 0.98 g of PMEE were dissolved in phosphate-buffered saline (PBS) for long-term storage at −20°C.
Tyrosinase inhibition assay
Aliquots of 40 μl of PMME and PMEE at different concentrations (2000, 4000, and 8000 μg/ml) were placed into a 96-well multiplate. Each well contained 40 μl of 15 mM l-3,4-dihydroxyphenylalanine (L-dopa) solution. The reactions were carried out by shaking at 37°C for 10 min. Subsequently, 40 μl of tyrosinase (30 U/ml) was added into each reaction mixture, and the mixtures were further incubated with shaking at 37°C for 20 min. A wavelength of 490 nm was used to determine each reaction, and the tyrosinase inhibition rates were calculated using the following formula. The inhibition rate for Vit C 250 μg/ml was designated as 100%, which was determined by dividing it by the tyrosinase inhibition rates determined for different concentrations of PMME and PMEE to yield the percentage tyrosinase inhibition rate values. The values were expressed as mean ± standard deviations (SD).
DPPH free radical scavenging assay
While auto-oxidation of lipids leads to a free radical chain reaction that accelerates the oxidation process, the lipid oxidation chain reaction is inhibited when the free radicals are scavenged by hydrogen donors of antioxidants. In antioxidant experiments, α,α-diphenyl-2-picrylhydrazyl (DPPH, C18H12N6O5) is the common free radical employed to assess the hydrogen donor provision by an antioxidant. DPPH is a stable free radical containing an odd electron that displays a strong absorbance at the visible light wavelength of 517 nm when dissolved in methanol. However, this absorbance is reduced when DPPH binds to another free radical or is cleared by an antioxidant. A low absorbance value indicates that the antioxidant being tested possesses strong activity against DPPH. Both PMME and PMEE were dissolved in ethanol in an appropriate manner to obtain a series of dilutions (0.030-1 mg/ml). The radical-scavenging activity against the stable DPPH was determined spectrophotometrically following the procedure of Espín.[28] The radical-scavenging rate for Vit C 25 μg/ml was designated as 100%, which was determined by dividing it by the radical-scavenging rates determined for different concentrations of PMME and PMEE to yield the percentage radical-scavenging rate values. The values were expressed as Mean ± SD.
Cell culture
Human malignant melanoma A375 cell line and Maddin-Darby canine kidney (MDCK) cell line were purchased from Bioresources Collection and Research Center (BRBC, Taiwan). The A375 cell line was cultured using 10% fetal bovine serum (FBS) supplemented with DMEM culture medium containing final concentrations of 4 mM l-glutamine, 4.5 g/l glucose, 1.5 g/l NaHCO3, and 1 mM sodium pyruvate. Both cell lines were cultured in an incubator under 37°C and 5% CO2. The MDCK cell line was maintained in Minimum Essential Medium (Gibco-BRL, USA) and supplemented with 10% FBS and antibiotics.
Cell viability assay (MTT assay)
Cells were seeded into a 96-well multiplate at a concentration of 2 × 104cells/well and incubated for a day. Different concentrations of PMME and PMEE were added to the cells, and the cells were incubated for another 24 h. Subsequently, a 4 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was added to each well and the reaction mixtures were incubated at 37°C and in 5% CO2 for 4 h. The MTT compounds were converted into purple formazan by succinate dehydrogenase in the mitochondria. Then, the MTT solutions were removed and DMSO was added. After incubating the mixtures for 10 min, absorbance readings at 540 nm were determined for each well using an enzyme-linked immunosorbent assay (ELISA) reader.
SOD activity
Cells were seeded into a six-well multiplate at a concentration of 2 × 104cell/well and allowed to grow for 1 day. Cells were subsequently cultured in the presence of different concentrations of PMME and PMEE for 48 h. Post incubation, the total proteins were collected and SOD activity was determined using a Superoxide Dismutase Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). Total proteins were quantified using a Biorad Protein Assay kit (Biorad, USA). Absorbance readings at 450 nm were determined for each sample using Genios Spectra FLUOR plus (TECAN, Austria). The SOD activity was determined as units/mg protein.
CAT activity
Cells were seeded into a six-well multiplate at a concentration of 2 × 104cell/well and allowed to grow for 1 day. Cells were subsequently cultured in the presence of different concentrations of PMME and PMEE for 48 h before the total proteins were collected. The CAT activity was determined using a Catalase Assay Kit (Cayman Chemical Company). Total proteins were quantified using a Biorad Protein Assay kit (Biorad, USA). Absorbance readings at 540 nm were determined for each sample using Genios Spectra FLUOR plus (TECAN, Austria). The CAT activity was expressed as units/mg protein.
Glutathione peroxidase (GPx) concentration
Cells were seeded into a six-well multiplate at a concentration of 2 × 104cell/well and allowed to grow for 1 day. Cells were subsequently cultured in the presence of different concentrations of PMME and PMEE for 48 h before the total proteins were collected. The GPx content in total protein count was determined using a Glutathione Peroxidase Assay Kit (Cayman Chemical Company). Total proteins were quantified using a Biorad Protein Assay kit (Biorad, USA). Absorbance readings at 340 nm were determined for each sample using Genios Spectra FLUOR plus (TECAN, Austria). The GPx concentration was expressed as μmoles of GPx/mg protein.
Animals
Four-week-old female BALB/c mice were purchased from the National Laboratory Animal Center (Taiwan) and kept at an ambient temperature of 22°C under a 12-h light cycle (6:00 a.m.-6:00 p.m.). All animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Test mice were orally administered different dosages of P. mungo powder every day and were divided into four groups (10 in each group), namely, control, low dose (25 mg P. mungo powder/day), medium dose (50 mg P. mungo powder/day), and high dose (100 mg P. mungo powder/day).[29] The duration of the experiment was 7 weeks. At the end of 6 weeks of treatment, the animals were sacrificed and their livers were dissected, washed with saline, weighed for analysis, and frozen in liquid nitrogen for storage at −80°C for future analysis.
Statistical analysis
All analyses were performed in duplicates, and the resulting mean with SD values were reported. Analysis was done using one-way variance (ANOVA) and Tukey's multiple comparison tests. P values < 0.05 were regarded as significant (*P < 0.05, **P < 0.01, *P < 0.0001).
RESULTS
In vitro tyrosinase inhibition and antioxidant activity
Tyrosinase inhibition activity
Tyrosinase is an important enzyme for melanin production. Hence, to evaluate the skin whitening effects of PMME and PMEE, their ability to suppress melanin production was investigated. Tyrosine inhibition rates of the different extracted materials at different concentrations were measured.
In the current study, 250 μg/ml Vit C was used as the control group. Tyrosinase inhibition rates displayed by 2000, 4000, and 8000 μg/ml of PMME were 76.74 ± 1.29%, 107.61 ± 4.1 8%, and 174.84 ± 3.29%, respectively, of that displayed by the equivalence of 250 μg/ml Vit C [Figure 1]. Concentrations of 2000, 4000, and 8000 μg/ml PPME displayed tyrosinase inhibition rates that were 76.60 ± 3.06%, 135.91 ± 7.69%, and 176.61 ± 4.27%, respectively, of that the equivalence of 250 μg/ml Vit C. This finding shows that tyrosinase inhibition rates are elevated by increases in PMME and PMEE concentrations. The data also reveal that while PMME at a concentration of 8000 μg/ml displays the highest tyrosinase inhibition rate than the other groups, both the concentrations of 4000 and 8000 μg/ml of PMEE showed higher tyrosinase inhibition rates than that shown by 250 μg/ml Vit C. Therefore, the results indicate that PMME and PMEE showed better suppression rate of melanin production than 250 μg/ml Vit C.
Figure 1.
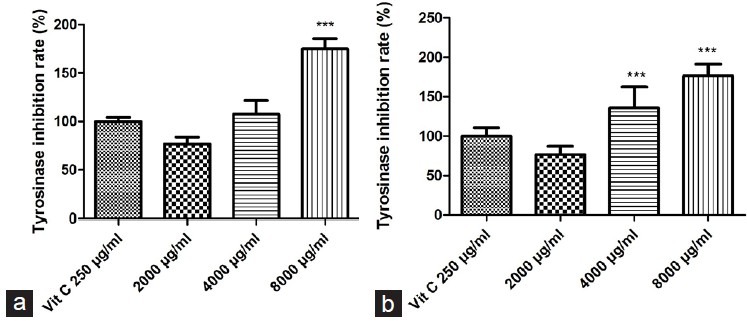
. Effects of PMME and PMEE on tyrosinase inhibition rates, in comparison with 250 μg/ml Vit C: (a) PMME; (b) PMEE (***P < 0.0001, compared with control)
Measurement of DPPH radical-scavenging capacity
Figure 2 shows that increase in concentrations of PMME and PMEE enhances the scavenging activity against DPPH free radicals in a dose–response manner. In the PMEE and PMME groups, there was significantly increased free radical scavenging activity, respectively, as compared to the normal control group. The free radical scavenging activity of both types of extract was 174.84 ± 3.23% and that of 25 μg/ml Vit C was 176.61 ± 4.29%. This finding also indicates that both PMME and PMEE have better DPPH free radical scavenging activity than 25 μg/ml Vit C.
Figure 2.
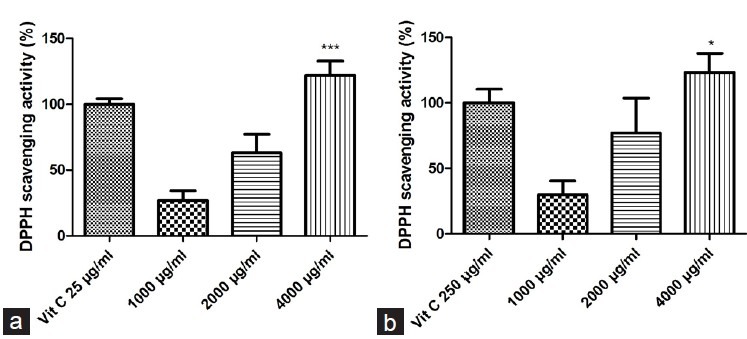
. Effects of PMME and PMEE on DPPH radical scavenging activity, in comparison with 25 μg/ml Vit C: (a) PMME; (b) PMEE (*P < 0.05, ***P < 0.0001, compared with control)
Ex vivo antioxidant activities evaluation
Determination of effect of PMME and PMEE on cell viability
To determine the effect of PMME and PMEE on MDCK and A375 cells’ viability, MTT assay was used. Figure 3 shows that the addition of PMME and PMEE (500, 1000, 2000, and 4000 μg/ml, respectively) in MDCK culture medium could increase the cell proliferation rate in a dose-dependent manner, in comparison to the control group (P < 0.05).
Figure 3.
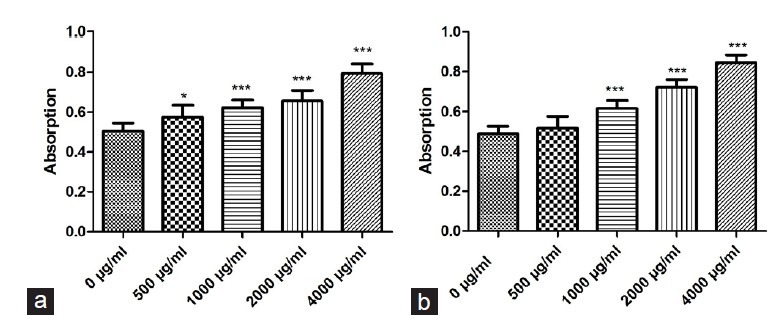
. Effects of PMME and PMEE on MDCK cell survival rates, determined by using MTT assay: (a) PMME; (b) PMEE (*P < 0.05, ***P < 0.0001, compared with control)
It can be observed from the results [Figure 4] that compared to the control group, PMME at concentrations of 2000 and 4000 μg/ml showed better inhibition rate of melanoma cell proliferation. This finding indicates that PMME possesses anti-proliferation properties against melanoma cells. Similarly, PMEE at concentrations of 1000, 2000, and 4000 μg/ml, compared to the control group, also showed stronger suppression of melanoma cell survival.
Figure 4.
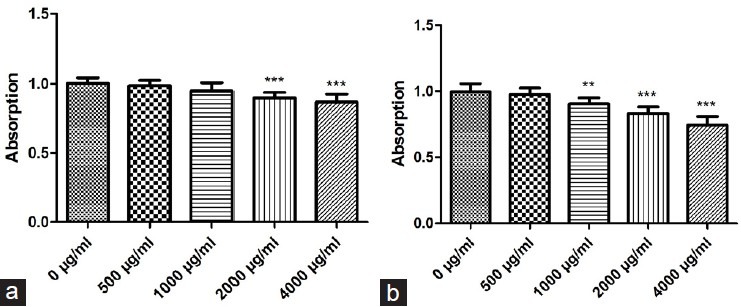
Effects of PMME and PMEE on A375 (melanoma cell line) cell survival rates, determined by using MTT assay: (a) PMME; (b) PMEE (*P < 0.01, ***P < 0.0001, compared with control)
PMEE and PMME were found to reduce the viability of A375 cells and increase the viability of MDCK cells markedly.
Assay of SOD and CAT activities in MDCK and A375 cells
Figure 5 shows the impact of PMEE on the SOD and CAT activities of MDCK cells treated with different concentrations of PMEE. Treatment with 500, 1000, and 2000 μg/ml concentrations of PMEE led to higher SOD and CAT activities than those obtained for the control group. This finding suggests that PMEE could substantially elevate the enzymatic activity of SOD and CAT in MDCK cells.
Figure 5.
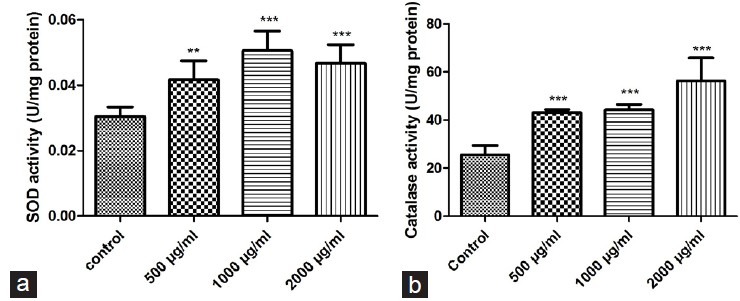
Effects on antioxidant enzymes in MDCK cells after 48 h treatment with different doses of PMEE: (a) SOD; (b) CAT (**P < 0.01, ***P < 0.0001, compared with control)
Figure 6 shows the impact of different concentrations of PMEE on the antioxidant activities of SOD and CAT in malignant melanoma cells. The results show that the addition of 500 μg/ml of PMEE did not affect the antioxidant activity of SOD in malignant melanoma cells and no significant difference between the test sample and control group was observed. However, in the presence of 1000 and 2000 μg/ml of PMEE, the SOD activity in malignant melanoma cells was substantially lower than that found in the control group. Treatment with 500 or 1000 μg/ml of PMEE did not lead to significantly different CAT activity from that of the control group. In contrast, treatment with 2000 μg/ml of PMEE led to a significantly reduced CAT activity in comparison to that of the control group. Therefore, it can be concluded that the addition of PMEE at a concentration of 2000 μg/ml could effectively reduce the activities of SOD and CAT in melanoma cells.
Figure 6.
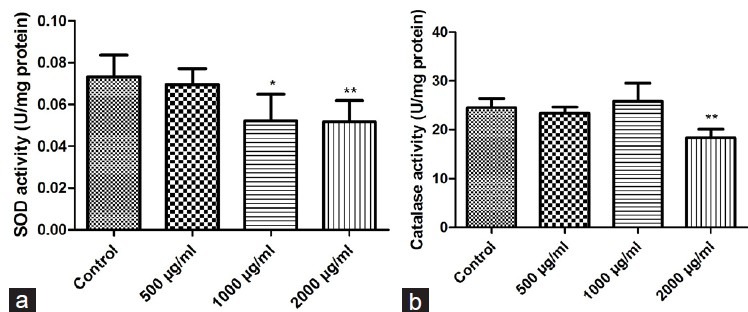
Effects on antioxidant enzymes in A375 cells after 48 h treatment with different doses of PMEE: (a) SOD; (b) CAT (**P < 0.01, ***P < 0.0001, compared with control)
In vivo antioxidant activity determination
Body weight profiles
The effects of orally administered P. mungo at different doses on the weight of mice were investigated. The weights of mice were evaluated once per week, and the results show that P. mungo did not have a significant influence [Figure 7].
Figure 7.
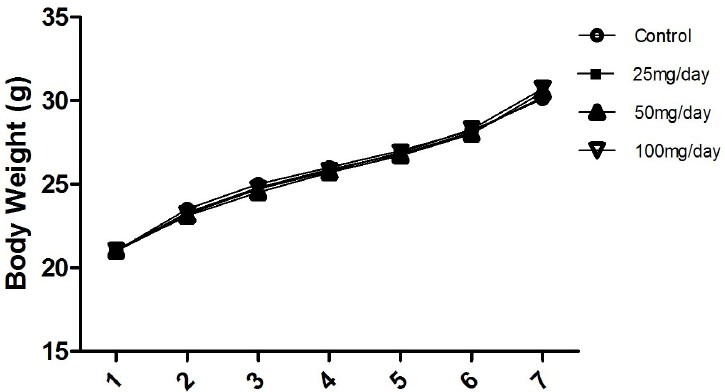
The weight change profiles of mice after administration of different doses of Pracparatum mungo for 7 weeks
Antioxidant enzyme activities in the liver
In vivo testing was performed to investigate the influence of P. mungo on the activity of liver antioxidant enzymes in mice. Different doses of P. mungo were fed to mice and the impact of P. mungo on the activity of the antioxidant enzymes, SOD, CAT, and GPx, in livers was evaluated.
The results show that P. mungo, when fed in amounts of 25, 50, and 100 mg/day, could effectively increase CAT and GPx activities in mice livers, and these activities were significantly different from those found in the control group mice [Figure 8]. Administration of 50 and 100 mg/day of P. mungo resulted in a significantly higher SOD activity than in the control group mice. The experimental results show that P. mungo significantly increases not only the activities of SOD and CAT in the epithelial cells but also the activities of SOD, CAT, and GPx in the mice livers.
Figure 8.
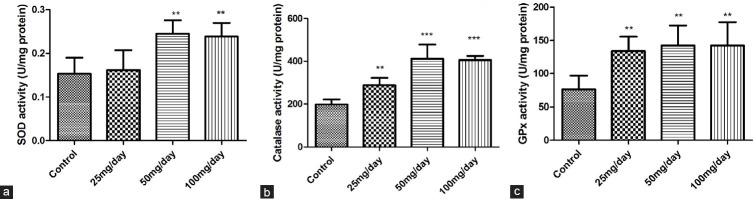
The effects of oral administration of different doses of P. mungo on liver antioxidant enzymes in mice: (a) SOD; (b) CAT; (c) GPx (**P < 0.01, ***P < 0.0001, compared with control)
DISCUSSION
Mung beans (Lǜ Dòu) transform into P. mungo (Lu-Do Huang) upon natural fermentation, processing, mixing with various Chinese herbs, and drying. Accordingly, this study evaluated the efficacy of P. mungo. It was first discovered in the in vitro experiment that PMME and PMEE possess anti-tyrosinase effects, and that both types of extracts can eliminate DPPH radicals. In the ex vivo experiment, which made use of MDCK cells and A375 cells to conduct MTT assay, it was discovered that PMME and PMEE can promote the growth of MDCK cells and prevent the growth of A375 cells. In addition, the best results were obtained with PMEE, which is why PMEE was used in the subsequent experiments. PMEE can promote the enzymatic activities of SOD and CAT in MDCK cells and suppress the activities of SOD and CAT in A375 cells. The in vivo experiment revealed that PMEE can increase the enzymatic activities of SOD, CAT, and GPx in the internal organs of mice. Therefore, it is deduced that P. mungo can suppress the growth of A375 cells, as well as enhance the antioxidant capabilities of the internal organs and skin cells of mice by increasing the enzymatic activities of SOD and CAT.
Tyrosinase is a form of oxidant enzyme that consists of bronze, which is multifunctional and plays an important role in the preliminary procedures of melanin biosynthesis.[5,30] The function of tyrosinase in melanocytes is to stimulate the transformation of tyrosine into L-dopa which is in turn converted into dopaquinone. Dopaquinone is a form of substance with high reactivity that becomes melanin after a series of oxidative polymerization reactions.[31,32,33] However, excessive accumulation of melanin would lead to various kinds of skin diseases and melanin pigmentation which affects the aesthetics of human skin negatively. Earlier studies have shown that substances capable of suppressing the activity of tyrosinase can lead to healthier skin and reduce the spots.[7] There have been many discoveries made regarding how anti-hyperpigmentation skincare products can help to suppress tyrosinase activity.[34,35] For example, ginger leaves and stems extracts,[36,37] polysaccharides in Punica granatum,[38] quercetin in fungus,[39] resveratrol in red wine,[40] nobiletin and hesperidin in orange peel extracts,[41] anisic acid (p-methoxybenzoic acid) in fennel, and others are found to possess functions that suppress tyrosinase activity, and can therefore promote skin whitening. The results showed that PMME and PMEE have the same tyrosinase suppressing functions as the above-mentioned components and can thus promote skin whitening through reduction of melanin and preventing melanin hyperpigmentation.
High oxidative stress and UV rays would lead to excessive oxidative harm. Research has discovered that excessive accumulation of radicals has a high correlation with skin diseases and that antioxidants are beneficial for skin health.[16] ROS radicals play an important regulating role in the growth of melanocytes and melatonin, and thus radical inhibitors and scavenging agents reduce tyrosinase activity. This reduces melanin and thus decreases hyperpigmentation and melanogenesis caused by UV rays.[17] It was also discovered that high oxidative stress would increase the mRNA concentration of tyrosinase and produce melanin, leading to severe skin and health problems.[42] Therefore, consuming health products with radical scavenging ability can help to promote skin health and suppress the growth of melanocytes. Previously studies have shown that many constituents of plants have strong free radical cleaning ability, such as the diterpenoid alkaloids in Aconitum; total phenolics, geranium fruticosa present in Potentilla fruticosa and Salvia sclarea; total flavonoids found in Geranium macrorrhizum, Rhaponticum carthamoides, Salvia glutinosa, and Juglans regia;[43,44,45] polysaccharides in Longans;[46] feruloylaloesin and p-coumaroylaloesin from catechins[46] and Aloe vera extracts in green tea;[47] and gallic acid and catechin components from O. fragrans acetonic extract (OFE), thereby reducing the oxidative damage to cells. The present study showed that PMME and PMEE have DPPH radical scavenging activity functions similar to the above-mentioned components and are capable of suppressing tyrosinase enzyme activities. Therefore, PMME and PMEE are found to possess antioxidant functions that can suppress the growth of melanocytes, thus improving skin health and whitening.
The ex vivo study involved MTT assay by making use of MDCK cells and A375 cells. Results from the MTT assay using MDCK cells showed that PMME and PMEE led to better cell survival rates. In the case of an injury, the links between single layer cells would be broken, leading to a wound. Cell proliferation and migration would then occur at the edge of the skin wound healing process due to an increase in concentration of growth factors and healing of the wound.[48,49,50] Our results showed that PMME and PMEE enhance the growth of MDCK cells. Therefore, we deduced that PMME and PMEE can help to promote the wound healing process. The MTT assay of A375 cells showed that PMME and PMEE are capable of inhibiting the growth of A375 cells and reducing melatonin. Thus, PMME and PMEE were found to be capable of promoting wound healing and inhibiting the growth of A375 cells, and are therefore suitable for skin protective functions.
The skin, the human organ with the largest surface area, is a highly metabolic tissue and serves as a protective layer for the human body.[51] The human skin has a higher probability of sustaining ROS injury as compared to the other issues due to two reasons. Firstly, the skin has sufficient blood flow with a large portion of surface area exposed in the air; secondly, the skin is an organ that is sensitive to light rays and this would result in more severe oxidation damage.[52] As the antioxidant enzymes SOD and CAT play the most important role in preventing skin damage caused by oxidation,[53] extracting the components that can enhance SOD and CAT enzyme activities would lead to better skincare effects. For example, the squid skin gelatin hydrolysate can promote the oxidative enzyme activities of SOD and GPx, as well as reduce the amount of malondialdehyde (MDA), an indicator of damage due to fat oxidation.[54] Extract of vegetable flavones in bamboo leaves (Ebl), catechin and epicatechin in acai (Euterpe oleracea Mart.), as well as oligomeric procyanidins can promote SOD enzyme activities.[55] Figures 5 and 6 show that PMME and PMEE extracts can promote the enzyme activities of SOD and CAT on MDCK cells, implying that PMME and PMEE probably have similar constituents as the above-mentioned components and can be used for skincare purposes. Due to its ability to reduce oxidative stress and inhibit tyrosinase activity, it can also be used for cell protection and prevention of melonocytes’ growth. The suppression of SOD and CAT enzyme activities in A375 cells in turn inhibits the proliferation of the latter. The design of therapeutic strategies to preferentially kill the malignant cells while minimizing the harmful effects to normal cells depends on our understanding of the biological differences between cancer and normal cells. The increased oxidative stress in cancer cells forces these cells to rely more on antioxidant enzymes such as SOD for O2 elimination, thus making the malignant cells more vulnerable to SOD inhibition than normal cells. It was previously demonstrated that certain agents generating ROS, such as 2-methoxyestradiol (2-ME), preferentially kill human leukemia cells without exhibiting significant cytotoxicity in normal lymphocytes.[56,57] Our results showed that PME increased the antioxidant activities (SOD, CAT) in MDCK cells, but decreased the antioxidant activities in A375 cells. It is possible to take advantage of the biological differences between cancer cells and normal cells to selectively kill the malignant cells. The disparities in ROS generation and metabolism in cancer cells versus normal cells provide a biochemical basis to develop new therapeutic strategies to preferentially increase ROS to a toxic level in cancer cells.
Earlier studies have discovered that PMME can be used for liver protective purposes,[58] with components such as berberine and licorice (Glycyrrhiza glabra). This research used high-performance liquid chromatography (HPLC) to analyze the content of berberine and licorice (G. glabra) in PMEE (data not shown), which were 175.43 μg/g and 34.58 μg/g, respectively. Berberine is a form of isoquinoline alkaloid with antibacterial and antipyretic functions.[59] Licorice, a form of triterpene glycoside, is a conjugated compound comprising enoxolone and glucuronic acid that has anti-inflammatory, anti-virus, and antioxidant activities, prevents liver tumors, regulates the immune system, and protects the liver.[60,61] Huang, et al., found that on oral feeding of ACH and ECH to mice with paw edema symptom, ECH could effectively enhance the antioxidant enzyme activities of SOD, CAT, and GPx in the liver and reduce inflammatory conditions in the paw edema of mice.[62] The in vivo result also showed that PMEE can promote the SOD, CAT, and GPx enzyme activities in the liver of the experimental mice. Therefore, we propose that PMEE reduces skin inflammatory conditions by causing increase in SOD, CAT, and GPx enzyme activities in the liver and achieves the purpose of cell protection.
In summary, this study shows that P. mungo possesses antioxidant and melanogenesis inhibitory activities that support skin protection. In Chinese medicine, P. mungo as long been used to treat various skin problems, including eczema, skin eruptions, and pimples. At the same time, no side effects have been reported. Therefore, P. mungo can function as a natural protective agent for human skin, which is more beneficial than chemical treatment.
REFERENCES
- 1.Hearing VJ. Biogenesis of pigment granules: A sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16:101–10. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 3.Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: Recent prospects. J Agric Food Chem. 2003;51:2837–53. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- 4.Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of α-Tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13:170–4. doi: 10.1111/j.0893-5785.2000.130830.x. [DOI] [PubMed] [Google Scholar]
- 5.Martinez M, Whitaker JR. The biochemistry and control of enzymatic browning. Trends Food Sci Technol. 1995;6:195–200. [Google Scholar]
- 6.Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, et al. Survey and mechanism of skin depigmenting and lightening agents. Phytother Res. 2006;20:921–34. doi: 10.1002/ptr.1954. [DOI] [PubMed] [Google Scholar]
- 7.Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res. 2007;21:805–16. doi: 10.1002/ptr.2184. [DOI] [PubMed] [Google Scholar]
- 8.Moysan A, Marquis I, Gaboriau F, Santus R, Dubertret L, Morlière P. Ultraviolet A: Induced lipid peroxidation and antioxidant defense systems in cultured human skin fibroblasts. J Invest Dermatol. 1993;100:692–8. doi: 10.1111/1523-1747.ep12472352. [DOI] [PubMed] [Google Scholar]
- 9.Soriani M, Hejmadi V, Tyrrell RM. Modulation of c-jun and c-fos Transcription by UVB and UVA radiations in human dermal fibroblasts and KB Cells. Photochem Photobiol. 2000;71:551–8. doi: 10.1562/0031-8655(2000)071<0551:mocjac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Shindo Y, Witt E, Han D, Packer L. Dose-response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J Invest Dermatol. 1994;102:470–5. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- 11.Connor MJ, Wheeler LA. Depletion of cutaneous glutathione by ultraviolet radiation. Photochem Photobiol. 2008;46:239–45. doi: 10.1111/j.1751-1097.1987.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 12.Shindo Y, Witt E, Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J Invest Dermatol. 1993;100:260–5. doi: 10.1111/1523-1747.ep12469048. [DOI] [PubMed] [Google Scholar]
- 13.Herrling T, Jung K, Fuchs J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:840–5. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Wlaschek M, Tantcheva-Poór I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, et al. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B. 2001;63:41–51. doi: 10.1016/s1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 15.Black HS. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem Photobiol. 2008;46:213–21. doi: 10.1111/j.1751-1097.1987.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 16.Yasui H, Sakurai H. Age-dependent generation of reactive oxygen species in the skin of live hairless rats exposed to UVA light. Exp Dermatol. 2003;12:655–61. doi: 10.1034/j.1600-0625.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, et al. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003;16:629–38. doi: 10.1046/j.1600-0749.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 18.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–12. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 19.Li GH, Le GW, Liu H, Shi YH. Mung-bean protein hydrolysates obtained with alcalase exhibit angiotensin I-converting enzyme inhibitory activity. Food Sci Technol Int. 2005;11:281–7. [Google Scholar]
- 20.Huang SC, Chan HY, Liao CM, Liao LC, Hwang SM. Fermented composition of mung bean hulls, method for forming thereof, and anti-oxidation and anti-inflammation composition using the same. In: Google Patents. 2009 [Google Scholar]
- 21.Somashekaraiah B, Padmaja K, Prasad A. Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): Involvement of lipid peroxides in chlorphyll degradation. Physiol Plant. 2006;85:85–9. [Google Scholar]
- 22.Duh PD, Du PC, Yen GC. Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem Toxicol. 1999;37:1055–61. doi: 10.1016/s0278-6915(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 23.Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, et al. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek) Environ Exp Bot. 2008;62:153–9. [Google Scholar]
- 24.Cao D, Li H, Yi J, Zhang J, Che H, Cao J, et al. Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS One. 2011;6:e21071. doi: 10.1371/journal.pone.0021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabir M, Rizkalla SW, Champ M, Luo J, Boillot J, Bruzzo F, et al. Dietary amylose-amylopectin starch content affects glucose and lipid metabolism in adipocytes of normal and diabetic rats. J Nutr. 1998;128:35–43. doi: 10.1093/jn/128.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Yao Y, Cheng X, Wang L, Wang S, Ren G. Biological potential of sixteen legumes in China. Int J Mol Sci. 2011;12:7048–58. doi: 10.3390/ijms12107048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng X, Zheng Z, Cheng KW, Shan F, Ren GX, Chen F, et al. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008;106:475–81. [Google Scholar]
- 28.Espín JC, Soler-Rivas C, Wichers HJ. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem. 2000;48:648–56. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- 29.Food U, Administrtion D. Guidance for Industry, Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research, USFDA, US Department of Health and Human Services. 2005 [Google Scholar]
- 30.Strothkamp K, Jolley R, Mason H. Quaternary structure of mushroom tyrosinase. Biochem Biophys Res Commun. 1976;70:519–24. doi: 10.1016/0006-291x(76)91077-9. [DOI] [PubMed] [Google Scholar]
- 31.Olivares C, Jiménez-Cervantes C, Lozano JA, Solano F, García-Borrón JC. The 5, 6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem J. 2001;354:131–9. doi: 10.1042/0264-6021:3540131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason HS. The chemistry of melanin III.Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J Biol Chem. 1948;172:83–99. [PubMed] [Google Scholar]
- 33.Lerner AB, Fitzpatrick TB, Calkins E, Summerson WH. Mammalian tyrosinase: Preparation and properties. J Biol Chem. 1949;178:185–95. [PubMed] [Google Scholar]
- 34.Maeda K, Fukuda M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J Soc Cosmet Chem. 1991;42:361–8. [Google Scholar]
- 35.Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, et al. Inhibition of melanosome transfer results in skin lightening 1. J Invest Dermatol. 2000;115:162–7. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 36.Chan EWC, Lim YY, Wong LF, Lianto FS, Wong SK, Lim KK, et al. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477–83. [Google Scholar]
- 37.Lim T, Lim Y, Yule C. Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chem. 2009;114:594–9. [Google Scholar]
- 38.Rout S, Banerjee R. Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide fraction isolated from the rind from Punica granatum. Bioresour Technol. 2007;98:3159–63. doi: 10.1016/j.biortech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Chen QX, Kubo I. Kinetics of mushroom tyrosinase inhibition by quercetin. J Agric Food Chem. 2002;50:4108–12. doi: 10.1021/jf011378z. [DOI] [PubMed] [Google Scholar]
- 40.Bernard P, Berthon J. Resveratrol: An original mechanism on tyrosinase inhibition. Int J Cosmet Sci. 2000;22:219–26. doi: 10.1046/j.1467-2494.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Lu Y, Tao L, Tao X, Su X, Wei D. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. J Enzyme Inhib Med Chem. 2007;22:83–90. doi: 10.1080/14756360600953876. [DOI] [PubMed] [Google Scholar]
- 42.Jiménez-Cervantes C, Martínez-Esparza M, Pérez C, Daum N, Solano F, García-Borrón JC. Inhibition of melanogenesis in response to oxidative stress: Transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J Cell Sci. 2001;114:2335–44. doi: 10.1242/jcs.114.12.2335. [DOI] [PubMed] [Google Scholar]
- 43.Miliauskas G, Venskutonis P, Van Beek T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 44.Lu Y, Yeap Foo L. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68:81–5. [Google Scholar]
- 45.Okawa M, Kinjo J, Nohara T, Ono M. DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol Pharm Bull. 2001;24:1202–5. doi: 10.1248/bpb.24.1202. [DOI] [PubMed] [Google Scholar]
- 46.Yang B, Zhao M, Shi J, Yang N, Jiang Y. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 2008;106:685–90. [Google Scholar]
- 47.Nanjo F, Mori M, Goto K, Hara Y. Radical scavenging activity of tea catechins and their related compounds. Biosci Biotechnol Biochem. 1999;63:1621–3. doi: 10.1271/bbb.63.1621. [DOI] [PubMed] [Google Scholar]
- 48.Coomber BL, Gotlieb AI. In vitro endothelial wound repair.Interaction of cell migration and proliferation. Arteriosclerosis. 1990;10:215–22. doi: 10.1161/01.atv.10.2.215. [DOI] [PubMed] [Google Scholar]
- 49.Wong M, Gotlieb AI. The reorganization of microfilaments, centrosomes, and microtubules during in vitro small wound reendothelialization. J Cell Biol. 1988;107:1777–83. doi: 10.1083/jcb.107.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zahm JM, Kaplan H, Hérard AL, Doriot F, Pierrot D, Somelette P, et al. Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskeleton. 1997;37:33–43. doi: 10.1002/(SICI)1097-0169(1997)37:1<33::AID-CM4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 51.Rieger M, Plains M. Oxidative reactions in and on skin: Mechanism and prevention. Cosmet Toiletries. 1993;108:43–56. [Google Scholar]
- 52.Darr D, Fridovich I. Free radicals in cutaneous biology. J Invest Dermatol. 1994;102:671–5. doi: 10.1111/1523-1747.ep12374036. [DOI] [PubMed] [Google Scholar]
- 53.Shindo Y, Witt E, Han D, Epstein W, Packer L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol. 1994;102:122–4. doi: 10.1111/1523-1747.ep12371744. [DOI] [PubMed] [Google Scholar]
- 54.Lin L, Li B-f. Studies on the antioxidation activity of squid (Dosidicus eschrichitii Steenstrup) skin gelatin hydrolysate. Chin J Mar Drugs. 2006;25:48. [Google Scholar]
- 55.Ying Z. Natural functional extract of bamboo leaves-Bamboo leaf anthoxanthin. China Food Addit. 2002;3:012. [Google Scholar]
- 56.Hileman E, Liu J, Albitar M, Keating M, Huang P. Intrinsic oxidative stress in cancer cells: A biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol. 2004;53:209–19. doi: 10.1007/s00280-003-0726-5. [DOI] [PubMed] [Google Scholar]
- 57.Halliwell B. Oxidative stress and cancer: Have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 58.Kuo DH, Kang WH, Shieh PC, Chen FA, Chang CD, Tsai ML, et al. Protective effect of Pracparatum mungo extract on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. 2010;123:1007–12. [Google Scholar]
- 59.Schmeller T, Latz-Brüning B, Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry. 1997;44:257–66. doi: 10.1016/s0031-9422(96)00545-6. [DOI] [PubMed] [Google Scholar]
- 60.Kimura M, Moro T, Motegi H, Maruyama H, Sekine M, Okamoto H, et al. In vivo glycyrrhizin accelerates liver regeneration and rapidly lowers serum transaminase activities in 70% partially hepatectomized rats. Eur J Pharmacol. 2008;579:357–64. doi: 10.1016/j.ejphar.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 61.Wan XY, Luo M, Li XD, He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact. 2009;181:15–9. doi: 10.1016/j.cbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Huang MH, Huang SS, Wang BS, Wu CH, Sheu MJ, Hou WC, et al. Antioxidant and anti-inflammatory properties of Cardiospermum halicacabum and its reference compounds ex vivo and in vivo. J Ethnopharmacol. 2011;133:743–50. doi: 10.1016/j.jep.2010.11.005. [DOI] [PubMed] [Google Scholar]


