Abstract
Hepatocellular carcinoma (HCC) has long been one of the most important causes of cancer mortality in the world. Many natural products and traditional herbal medicines have been used to treat HCC in Asian countries such as Japan, Korea, Taiwan, and China. The present review aims to describe the anticancer properties and apoptotic mechanisms of cinnamaldehyde, the bioactive ingredient isolated from cinnamon trees, and the herbal prescription Huang-Lian-Jie-Du-Tang (黃連解毒湯 Huáng Lián Jiě Dú Tang; HLJDT) against human hepatoma cells in vitro and in vivo. Implication of their treatment for the development of targeted therapy against HCC is discussed.
Keywords: Anticancer, Apoptosis, Cinnamaldehyde, Hepatoma, Huang-Lian-Jie-Du-Tang
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm with more than 750,000 new cases diagnosed every year and is the third leading cause of cancer-related mortality worldwide.[1,2] The normal hepatocytes may transform into liver tumor cells by risk factors such as viral hepatitis, alcohol consumption, fatty liver disease, dietary exposure to aflatoxin B1, smoking, obesity, and diabetes.[3,4,5] Viral hepatitis is the major causative factor of HCC and approximately 80% of HCC cases are associated with persistent infection by either hepatitis B virus (HBV) or hepatitis C virus (HCV).[6] Globally, chronic hepatitis B alone is responsible for about 50% of the underlying etiologies for the development of HCC.[7] With hepatitis C, it is estimated that the HCV-infected individuals are associated with 3-5% of HCC incidence worldwide.[8] More than 70% of all newly diagnosed liver cancers occur in Asia, a region which accounts for 75% of all those chronically infected with HBV in the world.[9] About 55% of global HCC cases occur in China.[10]
Surgery, liver transplantation, radiotherapy, chemotherapy, immunotherapy, and newer pharmaco/biological treatments are currently used for the management of HCC. Chemotherapy is one of the major conventional HCC therapies, but the associated strong side effects and the development of drug resistance often affect the treatment outcome. Development of safe and effective chemopreventive agents is therefore necessary to better improve liver cancer morbidity and mortality. Both natural products and herbal medicines have also been used to prevent and treat liver diseases including hepatitis, liver cirrhosis, and HCC. They are still extensively adopted, particularly in Asian countries, due to their efficacy, availability/accessibility, lesser side effects, and improved quality of life. Ongoing research continues to explore their bioactivities against various cancers as well as characterize their underlying mechanism (s), which could lead to novel methods of treating cancers. In this review, we summarize the anti-HCC properties and mechanism (s) of action of the natural product cinnamaldehyde, as well as the herbal prescription Huang-Lian-Jie-Du-Tang (黃連解毒湯 Huáng Lián Jiě Dú Tāng; HLJDT) from recent studies.
CINNAMALDEHYDE
Cinnamaldehyde (CIN) [Figure 1] is an active constituent isolated from the stem bark of cinnamon trees such as Cinnamomum cassia Presl. (肉桂 Ròu Guì) (Lauraceae). This aromatic aldehyde has been widely investigated for its biological and pharmacological properties, including anticancer, antioxidative, anti-inflammatory, anti-diabetic, anti-mutagenic, and immunomodulatory activities.[11,12,13,14,15,16] CIN is the major component of cinnamon bark essential oil that is also widely used as a fragrance ingredient and as an antibacterial agent in the food industry.[17,18] Results from our study as well as other studies have shown that CIN can exert antiproliferative activity against various types of human cancer cells, including those derived from HCC such as PLC/PRF/5 and HepG2 cells.[19,20,21,22,23,24,25]
Figure 1.
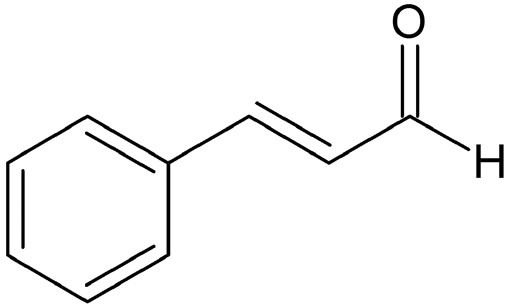
Chemical structure of cinnamaldehyde
Effect of CIN on apoptosis modulated by the mitochondria and the Bcl-2 family members
Apoptosis is a major physiological process of the cell involved in the development of multicellular organism and the regulation of cellular homeostasis. Deregulation of the apoptotic program is linked to the pathogenesis of many diseases including cancer, autoimmune diseases, stroke, and neurodegenerative disorders.[26,27] The mitochondria are known to occupy a key position in the induction of apoptosis mediated by various apoptotic stimuli, including chemotherapeutic drugs, DNA damage, UV irradiation, reactive oxygen species (ROS), and other cellular stress factors.[28,29] Mitochondrial apoptosis is triggered by the collapse of mitochondrial membrane potential (ΔΨm) and generation of ROS, which are modulated by Bcl-2 family of proteins including pro-apoptotic (Bax, Bak, Bid, and Bad) and anti-apoptotic (Bcl-2, Bcl-xL, Bcl-w, and Mcl-1) molecules.[30] In cancer treatment, apoptosis induced by many chemotherapeutic agents involves the cleavage of Bid to its truncated form (t-Bid) by caspase (CASP)-8. This event, in conjunction with a favorable ratio of pro-apoptotic to anti-apoptotic Bcl-2 family members, causes the release of cytochrome c from the mitochondria into the cytosol; cytochrome c, upon forming a complex with the apoptotic protease activating factor 1 (Apaf-1), leads to the activation of CASP-9 and the downstream CASP-3, eventually resulting in cell death.[31] The second mitochondria-derived activator of caspase (Smac/DIABLO) and/or Omi/HtrA2 are the factors released from the mitochondria, along with cytochrome c during apoptosis. These molecules function to promote caspase activation by eliminating the negative effect mediated by the inhibitor of apoptosis (IAP) family of proteins.[32,33]
Apoptosis due to CIN treatment has been shown to involve the mitochondria and Bcl-2 family of proteins in HCC cells. Specifically, treatment with CIN induces the PLC/PRF/5 hepatoma cells to accumulate in S phase, which is associated with loss of ΔΨm and up-regulation of ROS formation and Bax expression.[21,25] An increased cytochrome c leakage from the mitochondria to the cytosol is also observed with the activation of CASP-8 and CASP-3, and with the resulting cleavage of targets such as Bid and poly (ADP-ribose) polymerase (PARP), respectively. Levels of Bcl-2, Mcl-1, and X-linked inhibitor of apoptosis protein (XIAP) expression are also down-regulated in hepatoma cells treated with CIN [Figure 2].[21,25] Furthermore, these mitochondria-related apoptotic effects triggered by CIN can be blocked by pretreatment with the mitochondrial permeability transition (MPT) pore inhibitor, cyclosporin A (CsA), and the general caspase inhibitor z-VAD-fmk, suggesting the involvement of the mitochondria in CIN-induced apoptosis.[22] It is also noteworthy that CIN treatment in conjunction with an antioxidant such as vitamin E has been observed to suppress the release of apoptotic factors from the mitochondria in the hepatoma cells.[25]
Figure 2.
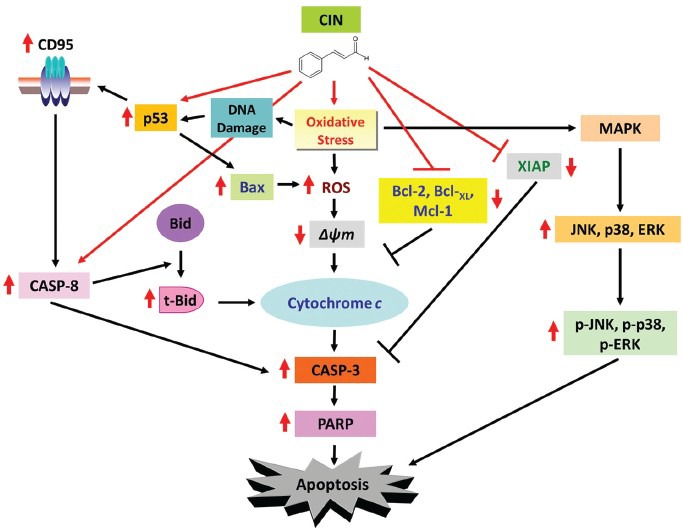
Model for the molecular mechanisms of CIN-induced apoptosis in hepatoma cells. Red arrows indicate the effects of CIN
Effect of CIN on the MAPK-mediated apoptosis
In mammalian cells, the mitogen-activated protein kinases (MAPKs) are a superfamily of proline-directed serine/threonine protein kinases that include the c-Jun N-terminal kinases (JNKs), extracellular signal-regulated kinases (ERKs), and p38.[34] Downstream targets of MAPKs can include mitogenic/pro-inflammatory enzymes and nuclear transcription factors, and thus, the MAPKs play a pivotal role in inflammation, cell proliferation, cell differentiation, and cell death. The activation of JNK and p38 has been associated with apoptosis, whereas ERK activation has been observed to enhance cell growth and differentiation.[35,36,37]
The apoptosis-inducing effect of many natural products involves MAPKs. It has been reported that the RRR-α-tocopheryl succinate induces cell death in human breast cancer cells through ERK activation.[38] JNK, p38, and ERK have also been shown to be activated via phosphorylation by natural anticancer agents such as caffeic acid phenethyl ester, epigallocatechin-3-gallate, and phenyethyl isothiocyanate in many cancer cell types.[39,40,41]
Treatment with CIN has been demonstrated to significantly induce apoptosis by activating JNK, p38, and ERK kinases, as observed with their phosphorylated status in the human hepatoma PLC/PRF/5 cells [Figure 2].[21] In addition, the use of MAPK inhibitors such as the JNK inhibitor (SP600125) and the p38 inhibitor (SB203580) remarkably protects the PLC/PRF/5 cells against CIN-induced apoptosis, whereas treatment with ERK inhibitor (PD98059) has a less profound impact in rescuing the CIN-induced hepatoma cell death.[21]
Effect of CIN on the death receptor-mediated apoptosis
The CD95 (APO-1/Fas) receptor/ligand system is an important signaling pathway involved in the regulation of apoptosis in different cell types, particularly those of the immune system and in the liver.[42,43] Belonging to the tumor necrosis factor receptor (TNF-R) superfamily, CD95 is a type I transmembrane receptor expressed on activated lymphocytes and in a variety of tissues of lymphoid or non-lymphoid origin, as well as on tumor cells. The death receptor pathway is initiated by the cross-linking of CD95 to its mature ligand, CD95L, followed by the formation of the death-inducing signaling complex (DISC), which then induces the orderly triggering of activator caspases (CASP-8 and -10), executioner caspases (CASP-3, -6, and -7), and the production of death substrates (cleavage of PARP), thereby ultimately leading to cell death.[44] The CD95/CD95L–CASP-8 signaling pathway has been shown to be involved in apoptosis induced by several naturally occurring anticancer agents.[45,46,47]
The tumor suppressor protein p53 directly targets the promoter of the CD95 gene in response to DNA damage by anticancer agents. The up-regulation of the CD95 death receptor is observed in cells with wild-type p53 (HepG2), but not in cells with mutant (PLC/PRF/5) or null p53 (Hep3B).[48,49] The activation of p53 is known to alter the transcription of a variety of genes including those involved in cellular metabolism, cell cycle regulation, and apoptosis. Pifithrin-alpha (PFTα), a p53 inhibitor, is able to suppress p53-mediated transactivation[50] and can significantly decrease p53 expression in wild-type p53 cells, but not in mutant p53 or p53-deficient cells.[51]
CIN inhibits the proliferation of HepG2 cells in a dose- and time-dependent fashion.[23] Treatment with CIN results in down-regulated expression of Bcl-XL and up-regulated levels of CD95, p53, and Bax proteins, with downstream cleavage of PARP in a time-dependent pattern [Figure 2]. These effects are reversed upon treatment with PFTα, which protects against CIN-induced apoptosis of HepG2 cells and results in decreased levels of p53, CD95, Bax, and PARP cleavage.[23]
HUANG-LIAN-JIE-DU-TANG
Huang-Lian-Jie-Du-Tang (黃連解毒湯 Huáng Lián Jiě Dú Tāng; HLJDT) is a traditional Chinese medicine with anti-inflammatory functions. This herbal prescription is widely used for the treatment of dermatitis, gastritis, and liver injuries, and also to stop bleeding of the intestines and uterus. HLJDT is prepared from boiled water extracts of four medicinal herbs in equal ratio, namely, Coptis chinensis Franch (黃連 Huáng Lián), Scutellaria baicalensis Georgi (黃芩 Huáng Qín), Phellodendron amurense Ruprecht (黃柏 Huáng Bǎi), and Gardenia jasminoides Ellis (山黃槴 Shān Huáng Huā) [Table 1]. Molecular constituents of HLJDT have been shown to possess antitumor properties, and bioactive compounds such as berberine, genipin, baicalein, and wogonin have been associated with inhibition of cancer cell growth, regulation of cell cycle, as well as induction of apoptosis.[52,53,54,55,56,57,58] Our previous study has shown that HLJDT can inhibit the human hepatoma HepG2 and PLC/PRF/5 cell proliferation in vitro and restrict the hepatoma cell-induced tumor growth in nude mice.[59]
Table 1.
Components of the herbal prescription Huang-Lian-Jie-Du-Tang

Effect of HLJDT on cell cycle distribution
In mammalian cells, the cell cycle progression includes the sequential activation of cyclin-dependent kinases (CDKs), whose activation is reliant on their binding of regulatory subunits called cyclins.[60] CDK1 (also called CDC2), a major kinase for driving the cell cycle, associates with cyclin B1 to form a complex, the mitosis-promoting factor (MPF), that regulates cell entry into mitosis. The phosphorylation of Thr14/Tyr15 on CDK1 by WEE1 and myelin transcription factor 1 (MYT1) kinases inhibits the activity of CDK1/cyclin B1 kinase complex, whereas dephosphorylation of these residues by CDC25 phosphatases promotes activation.[60,61] Phosphorylation of CDC25 by checkpoint kinases renders it inactive and initiates cell cycle checkpoints.[62] Cyclin A, which associates with CDK1 or CDK2 kinases, promotes activation and stabilization of CDK1/cyclin B1 complex; down-regulation of cyclin B1, cyclin A, and CDK1 hinders G2/M transition.[62,63,64]
Treatment with HLJDT causes cycle arrest of HepG2 and PLC/PRF/5 cells in S–G2/M phase [Figure 3].[59] This event is accompanied by decreased expression of cyclin B1, cyclin A, CDK1, and CDC25C, and an increased level of inactive phospho-CDK1 and phospho-CDC25C.
Figure 3.
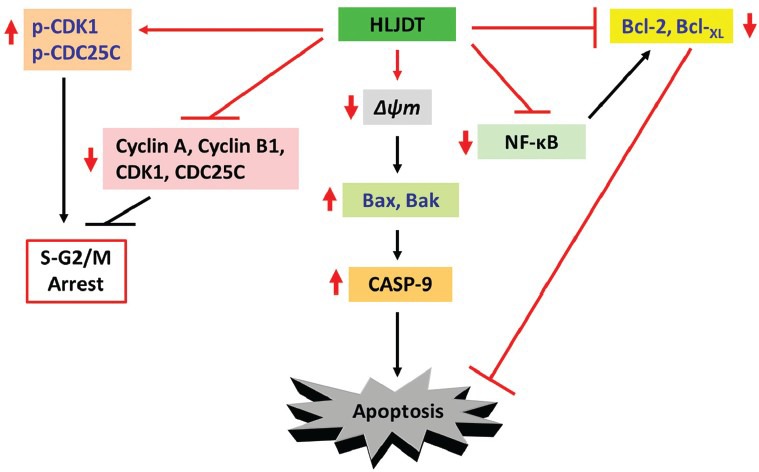
Model for the molecular mechanisms of HLJDT-induced apoptosis in hepatoma cells. Red arrows indicate the effects of HLJDT
Effect of HLJDT on mitochondria-mediated apoptosis
As described earlier, the mitochondrial apoptotic pathway is regulated through Bcl-2 family proteins such as the anti-apoptotic molecules Bcl-2 and Bcl-XL, as well as pro-apoptotic members including Bax and Bak, leading to destabilization of the mitochondrial membrane and activation of caspases. Treatment with HLJDT triggers the loss of mitochondrial membrane potential in both HepG2 and PLC/PRF/5 cells with concomitant up-regulation of Bax and Bak expression, down-regulation of Bcl-2 and Bcl-XL levels, and activation of CASP-9 [Figure 3].[59]
Effect of HLJDT on the NF-κB pathway
The pro-inflammatory microenvironment preset by continuously elevated expression of nuclear factor kappa B (NF-κB) in the liver tissue due to HBV and HCV infections, bacterial infections, and exposure to hepatotoxic chemicals can promote the development of HCC.[65] NF-κB is associated with a wide array of pro-inflammatory and regulatory gene expression, including those involved in cell survival/death such as the Bcl-2 family of proteins. Upon degradation of the inhibitor of NF-κB (IκB) proteins, the freed NF-κB translocates to the nucleus and initiates gene transcription. Activation of NF-κB in hepatocytes can lead to secretion of pro-inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), IL-6, and vascular endothelial growth factor (VEGF), increase of cyclin D and cell cycle progression, as well as maintenance of adequate JNK activation for stimulation of hepatocyte proliferation.[66,67,68,69] Many studies have revealed NF-κB as an important target for anticancer treatment, whereby inhibition of NF-κB can be used as a targeted approach in combination with chemotherapy or radiotherapy for treating a variety of carcinomas.[70,71,72]
We have previously identified that HLJDT treatment of HepG2 and PLC/PRF/5 cells inhibits NF-κB translocation to the nucleus and, therefore, its subsequent activity.[59] This is observed with a concomitant increase in IκBα expression in the cytoplasm, suggesting that HLJDT's apoptotic function against HepG2 and PLC/PRF/5 cells may be linked to its effect on NF-κB.
DISCUSSION
Based on the studies presented, it is clear that both CIN and HLJDT may induce apoptotic cell death in HCC cells through multiple mechanisms. Depending on the inducible level of CD95, CIN appears to be capable of engaging both intrinsic (mitochondria-mediated) and extrinsic (death receptor–mediated) apoptosis, as shown in the CD95-inducible (HepG2) and non-inducible (PLC/PRF/5) hepatoma cells.[23] Stimulation of the CD95 substrate CASP-8 is, however, still observed in apoptosis of the PLC/PRF/5 cells under CIN treatment.[21] This observation suggests that additional mechanism (s) may be involved in the activation of CASP-8/t-Bid pathway by CIN in a CD95-independent manner, an event which has been similarly noted in response to certain apoptotic stimuli and treatment with anticancer agents.[73,74,75,76] More importantly, targeting of the MAPK signaling, induction of ROS production, and disruption of the ΔΨm appear to play a pivotal role in CIN-induced apoptosis of the liver cancer cells.[21,22,24,25] These findings suggest that CIN could be further explored as a specific inhibitor alone or in combination with other anticancer therapies for targeting these individual pathways to induce apoptotic cell death in the hepatoma.
With regards to HLJDT, the herbal prescription effectively causes hepatoma cell cycle arrest and stimulates mitochondria-mediated apoptosis while dampening the activity of NF-κB. Some of these anticancer effects have been recapitulated with its bioactive constituents including berberine, genipin, baicalein, and wogonin.[53,54,56,77,78] The multi-specific anti-hepatoma effect of HLJDT could therefore provide insight to combinatorial strategies in inhibiting several specific pathways concurrently to suppress hepatoma cell growth.
CONCLUSION
Given their anticancer efficacy, both CIN and HLJDT have the potential to be developed into promising anticancer treatments against HCC. Further studies are encouraged for exploring the anti-hepatoma effect of CIN in vivo and for evaluating the treatment effect of HLJDT against liver cancer in clinical setting.
ACKNOWLEDGMENTS
LTL was supported by funding from Taipei Medical University (TMU101-AE3-Y19). CCL was supported by a research grant from the Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan of Taiwan (CCMP 95-RD-212). SJW received support from the National Science Council of Taiwan (NSC96-2313-B-041-004).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603–15. doi: 10.3748/wjg.v16.i29.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J, Xie L, Yang WS, Zhang W, Gao S, Wang J, et al. Risk factors of hepatocellular carcinoma – current status and perspectives. Asian Pac J Cancer Prev. 2012;13:743–52. doi: 10.7314/apjcp.2012.13.3.743. [DOI] [PubMed] [Google Scholar]
- 6.Thein HH, Walter SR, Gidding HF, Amin J, Law MG, George J, et al. Trends in incidence of hepatocellular carcinoma after diagnosis of hepatitis B or C infection: A population-based cohort study, 1992-2007. J Viral Hepat. 2011;18:e232–41. doi: 10.1111/j.1365-2893.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim BK, Han KH, Ahn SH. Prevention of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Oncology. 2011;81(Suppl 1):41–9. doi: 10.1159/000333258. [DOI] [PubMed] [Google Scholar]
- 8.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–14. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–94. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 11.Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, et al. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003;196:143–52. doi: 10.1016/s0304-3835(03)00238-6. [DOI] [PubMed] [Google Scholar]
- 12.Liao JC, Deng JS, Chiu CS, Hou WC, Huang SS, Shie PH, et al. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/429320. 429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaisier C, Cok A, Scott J, Opejin A, Bushhouse KT, Salie MJ, et al. Effects of cinnamaldehyde on the glucose transport activity of GLUT1. Biochimie. 2011;93:339–44. doi: 10.1016/j.biochi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaughnessy DT, Setzer RW, DeMarini DM. The antimutagenic effect of vanillin and cinnamaldehyde on spontaneous mutation in Salmonella TA104 is due to a reduction in mutations at GC but not AT sites. Mutat Res. 2001;480-481:55–69. doi: 10.1016/s0027-5107(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 15.Koh WS, Yoon SY, Kwon BM, Jeong TC, Nam KS, Han MY. Cinnamaldehyde inhibits lymphocyte proliferation and modulates T-cell differentiation. Int J Immunopharmacol. 1998;20:643–60. doi: 10.1016/s0192-0561(98)00064-2. [DOI] [PubMed] [Google Scholar]
- 16.Lin CC, Wu SJ, Chang CH, Ng LT. Antioxidant activity of Cinnamomum cassia. Phytother Res. 2003;17:726–30. doi: 10.1002/ptr.1190. [DOI] [PubMed] [Google Scholar]
- 17.Cocchiara J, Letizia CS, Lalko J, Lapczynski A, Api AM. Fragrance material review on cinnamaldehyde. Food Chem Toxicol. 2005;43:867–923. doi: 10.1016/j.fct.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Burt S. Essential oils: Their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol. 2004;94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, et al. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: Potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med. 2010;48:98–111. doi: 10.1016/j.freeradbiomed.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Nagle AA, Gan FF, Jones G, So CL, Wells G, Chew EH. Induction of tumor cell death through targeting tubulin and evoking dysregulation of cell cycle regulatory proteins by multifunctional cinnamaldehydes. PLoS One. 2012;7:e50125. doi: 10.1371/journal.pone.0050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu SJ, Ng LT, Lin CC. Cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells through activation of the proapoptotic Bcl-2 family proteins and MAPK pathway. Life Sci. 2005;77:938–51. doi: 10.1016/j.lfs.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Lin LT, Tai CJ, Chang SP, Chen JL, Wu SJ, Lin CC. Cinnamaldehyde-induced Apoptosis in Human Hepatoma PLC/PRF/5 Cells Involves the Mitochondrial Death Pathway and is Sensitive to Inhibition by Cyclosporin A and z-VAD-fmk. Anticancer Agents Med Chem. 2013 doi: 10.2174/18715206113139990144. [In press] [DOI] [PubMed] [Google Scholar]
- 23.Ng LT, Wu SJ. Antiproliferative activity of cinnamomum cassia constituents and effects of pifithrin-alpha on their apoptotic signaling pathways in Hep G2 Cells. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/nep220. 492148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SJ, Ng LT. MAPK inhibitors and pifithrin-alpha block cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells. Food Chem Toxicol. 2007;45:2446–53. doi: 10.1016/j.fct.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Wu SJ, Ng LT, Lin CC. Effects of vitamin E on the cinnamaldehyde-induced apoptotic mechanism in human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 2004;31:770–6. doi: 10.1111/j.1440-1681.2004.04091.x. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 27.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 28.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 29.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 31.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XD, Zhang XY, Gray CP, Nguyen T, Hersey P. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of human melanoma is regulated by smac/DIABLO release from mitochondria. Cancer Res. 2001;61:7339–48. [PubMed] [Google Scholar]
- 33.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem. 2002;277:44236–43. doi: 10.1074/jbc.M207578200. [DOI] [PubMed] [Google Scholar]
- 34.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 35.Harper SJ, LoGrasso P. Signalling for survival and death in neurones: The role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Cao N, Nantajit D, Fan M, Liu Y, Li JJ. Mitogen-activated protein kinase phosphatase-1 represses c-Jun NH2-terminal kinase-mediated apoptosis via NF-kappaB regulation. J Biol Chem. 2008;283:21011–23. doi: 10.1074/jbc.M802229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–50. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 38.Yu W, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH (2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569–76. [PubMed] [Google Scholar]
- 39.Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol. 2003;66:2281–9. doi: 10.1016/j.bcp.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24:1369–78. doi: 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 41.Hu R, Kim BR, Chen C, Hebbar V, Kong AN. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis. 2003;24:1361–7. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 42.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–95. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 43.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 44.Rowinsky EK. Targeted induction of apoptosis in cancer management: The emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23:9394–407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh CC, Kuo YH, Kuo CC, Chen LT, Cheung CH, Chao TY, et al. Chamaecypanone C, a novel skeleton microtubule inhibitor, with anticancer activity by trigger caspase 8-Fas/FasL dependent apoptotic pathway in human cancer cells. Biochem Pharmacol. 2010;79:1261–71. doi: 10.1016/j.bcp.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 46.Hsu YL, Kuo PL, Lin CC. Acacetin inhibits the proliferation of Hep G2 by blocking cell cycle progression and inducing apoptosis. Biochem Pharmacol. 2004;67:823–9. doi: 10.1016/j.bcp.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 47.Singh NP, Singh UP, Hegde VL, Guan H, Hofseth L, Nagarkatti M, et al. Resveratrol (trans-3,5,4’- trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-kappaB. Mol Nutr Food Res. 2011;55:1207–18. doi: 10.1002/mnfr.201000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–45. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin EC, Shin WC, Choi Y, Kim H, Park JH, Kim SJ. Effect of interferon-gamma on the susceptibility to Fas (CD95/APO-1)-mediated cell death in human hepatoma cells. Cancer Immunol Immunother. 2001;50:23–30. doi: 10.1007/s002620000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–7. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 51.Charlot JF, Nicolier M, Pretet JL, Mougin C. Modulation of p53 transcriptional activity by PRIMA-1 and Pifithrin-alpha on staurosporine-induced apoptosis of wild-type and mutated p53 epithelial cells. Apoptosis. 2006;11:813–27. doi: 10.1007/s10495-006-5876-6. [DOI] [PubMed] [Google Scholar]
- 52.Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–9. doi: 10.1158/0008-5472.CAN-08-0511. [DOI] [PubMed] [Google Scholar]
- 53.Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, et al. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J Cell Biochem. 2010;111:1426–36. doi: 10.1002/jcb.22869. [DOI] [PubMed] [Google Scholar]
- 54.Kim BC, Kim HG, Lee SA, Lim S, Park EH, Kim SJ, et al. Genipin-induced apoptosis in hepatoma cells is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of mitochondrial pathway. Biochem Pharmacol. 2005;70:1398–407. doi: 10.1016/j.bcp.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 55.Cao H, Feng Q, Xu W, Li X, Kang Z, Ren Y, et al. Genipin induced apoptosis associated with activation of the c-Jun NH2-terminal kinase and p53 protein in HeLa cells. Biol Pharm Bull. 2010;33:1343–8. doi: 10.1248/bpb.33.1343. [DOI] [PubMed] [Google Scholar]
- 56.Ma Z, Otsuyama K, Liu S, Abroun S, Ishikawa H, Tsuyama N, et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105:3312–8. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 57.Bonham M, Posakony J, Coleman I, Montgomery B, Simon J, Nelson PS. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin Cancer Res. 2005;11:3905–14. doi: 10.1158/1078-0432.CCR-04-1974. [DOI] [PubMed] [Google Scholar]
- 58.Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, et al. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007;245:269–74. doi: 10.1016/j.canlet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Hsu YL, Kuo PL, Tzeng TF, Sung SC, Yen MH, Lin LT, et al. Huang-lian-jie-du-tang, a traditional Chinese medicine prescription, induces cell-cycle arrest and apoptosis in human liver cancer cells in vitro and in vivo. J Gastroenterol Hepatol. 2008;23:e290–9. doi: 10.1111/j.1440-1746.2008.05390.x. [DOI] [PubMed] [Google Scholar]
- 60.Stark GR, Taylor WR. Control of the G2/M transition. Mol Biotechnol. 2006;32:227–48. doi: 10.1385/MB:32:3:227. [DOI] [PubMed] [Google Scholar]
- 61.Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M. Targeting cell cycle regulation in cancer therapy. Pharmacol Ther. 2013;138:255–71. doi: 10.1016/j.pharmthera.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Perdiguero E, Nebreda AR. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle. 2004;3:733–7. [PubMed] [Google Scholar]
- 63.Poon RY, Chau MS, Yamashita K, Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–78. [PubMed] [Google Scholar]
- 64.De Boer L, Oakes V, Beamish H, Giles N, Stevens F, Somodevilla-Torres M, et al. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene. 2008;27:4261–8. doi: 10.1038/onc.2008.74. [DOI] [PubMed] [Google Scholar]
- 65.Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–44. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- 66.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 67.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–92. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 68.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 69.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP (L) turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 71.Futakuchi M, Ogawa K, Tamano S, Takahashi S, Shirai T. Suppression of metastasis by nuclear factor kappaB inhibitors in an in vivo lung metastasis model of chemically induced hepatocellular carcinoma. Cancer Sci. 2004;95:18–24. doi: 10.1111/j.1349-7006.2004.tb03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim YS, Schwabe RF, Qian T, Lemasters JJ, Brenner DA. TRAIL-mediated apoptosis requires NF-kappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology. 2002;36:1498–508. doi: 10.1053/jhep.2002.36942. [DOI] [PubMed] [Google Scholar]
- 73.Glaser T, Castro MG, Lowenstein PR, Weller M. Death receptor-independent cytochrome c release and caspase activation mediate thymidine kinase plus ganciclovir-mediated cytotoxicity in LN-18 and LN-229 human malignant glioma cells. Gene Ther. 2001;8:469–76. doi: 10.1038/sj.gt.3301415. [DOI] [PubMed] [Google Scholar]
- 74.Tang D, Lahti JM, Kidd VJ. Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. J Biol Chem. 2000;275:9303–7. doi: 10.1074/jbc.275.13.9303. [DOI] [PubMed] [Google Scholar]
- 75.Izeradjene K, Douglas L, Tillman DM, Delaney AB, Houghton JA. Reactive oxygen species regulate caspase activation in tumor necrosis factor-related apoptosis-inducing ligand-resistant human colon carcinoma cell lines. Cancer Res. 2005;65:7436–45. doi: 10.1158/0008-5472.CAN-04-2628. [DOI] [PubMed] [Google Scholar]
- 76.Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, et al. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. J Biol Chem. 2004;279:40755–61. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- 77.Chang WH, Chen CH, Lu FJ. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002;68:128–32. doi: 10.1055/s-2002-20246. [DOI] [PubMed] [Google Scholar]
- 78.Fas SC, Baumann S, Zhu JY, Giaisi M, Treiber MK, Mahlknecht U, et al. Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood. 2006;108:3700–6. doi: 10.1182/blood-2006-03-011973. [DOI] [PubMed] [Google Scholar]


