Abstract
In developing implantable tissues based on cellular remodeling of a fibrin scaffold, a key indicator of success is high collagen content. Cellular collagen synthesis is stimulated by cyclic stretching but is limited by cellular adaptation. Adaptation is mediated by deactivation of extracellular signal-regulated kinase (ERK); therefore inhibition of ERK deactivation should improve mechanically stimulated collagen production and accelerate the development of strong engineered tissues. The hypothesis of this study is that p38 mitogen activated protein kinase (p38) activation by stretching limits ERK activation and that chemical inhibition of p38/isoforms with SB203580 will increase stretching-induced ERK activation and collagen production. Both p38 and ERK were activated by 15 minutes of stretching but only p38 remained active after 1 hour. After an effective dose of inhibitor was identified using cell monolayers, 5 M SB203580 was found to increase ERK activation by two-fold in cyclically stretched fibrin-based tissue constructs. When 5 M SB203580 was added to the culture medium of constructs exposed to three weeks of incremental amplitude cyclic stretch, 2.6 fold higher stretching-induced total collagen was obtained. In conclusion, SB203580 circumvents adaptation to stretching induced collagen production and may be useful in engineering tissues where mechanical strength is a priority.
Key Terms: Cell signaling, Collagen, Fibrin, Fibroblast, Inhibition, Mechanical signaling, Mitogen-activated protein kinase, p38
Introduction
Fibrin is an attractive biomaterial for a variety of engineered tissues, including arteries, valves, and myocardium, because it is fully degradable, can be derived from the patient’s own protein and cells, and can be cast in the desired geometry. Fibrin scaffolds also align in response to cell traction forces, resulting in the alignment of cell-produced extracellular matrix such as type I collagen2, 9, 10, 19, 31. Collagen content is correlated with the ultimate tensile strength and modulus of fibrin-based tissue constructs and is therefore a strong indicator of the tissue’s durability in response to relevant mechanical forces (e.g. pulse pressure for the case of engineered vessels)25. A variety of stimuli have been used to improve the collagen content of fibrin-based constructs, including changing the initial cell content10, adding growth factors to the medium25, and applying cyclic stretching using a bioreactor38.
Current attempts at optimization of mechanically-induced collagen production have been thwarted by the phenomenon of adaptation. Adaptation can be mediated by receptor internalization and/or degradation, as is seen with the epidermal growth factor receptor7, negative feedback via protein inhibitors, as is the case for Smad7 and TGF- signaling24, 36, or via transcriptional repression, as in the circadian clock12. Strategies of incremental and intermittent cyclic stretching have increased collagen production in response to mechanical stimulation28, 32, 38 but have failed to address the fundamental problem of adaptation by pharmacologically targeting the signaling pathway(s) responsible for the effect.
The mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) has been previously implicated as a key signaling species involved in adaptation38. ERK mediates type I collagen expression in response to stimulation by mechanical force13, 16 as well as in response to treatment with TGF-23, 29, the cytokines IL-4 and IL-133, endothelin-I30, and angiotensin II39, 45. Another MAPK activated in response to stretching, p38, has an antagonistic effect on ERK activation4, 14, 15, 26, 41. In the context of engineered connective tissue, TGF- -induced p38 signaling limits stretching-induced collagen production37; however, the contribution of p38 MAPK signaling to collagen expression in the absence of exogenous TGF- has been insufficiently explored.
The hypothesis of this study was that chemical inhibition of p38 during an incremental amplitude cyclic stretching regime would lead to increased collagen content in the final tissue construct. To address this hypothesis, the stretching-activated cellular response was monitored from the early step of ERK activation to the final collagen content.
Methods
Cell Culture
Neonatal human dermal fibroblasts (Invitrogen) were maintained in DMEM/F12 supplemented with 10% fetal bovine serum (Hyclone), 100 units/ml penicillin, and 100 g/ml streptomycin. Cells were passaged near 100% confluence and harvested for use from passages 7–9. For cell stretching experiments, cells were plated on fibronectin-coated silicone membranes in 24-well high throughput BioFlex plates (FlexCell International), and were stretched once they reached confluence.
Tissue Construct Fabrication and Culture
A cell-seeded fibrin gel was molded by mixing cells suspended in DMEM into a solution of bovine fibrinogen (Sigma) in 20 mM HEPES-buffered saline. A mixture of bovine thrombin (Sigma) and CaCl2 in DMEM was then added to the suspension. All components were on ice prior to mixing. Final concentrations were 6.7 mg/ml fibrinogen, 0.8 units/ml thrombin, 5 mM CaCl2, and 500,000 cells/ml. Suspensions were mixed well by trituration and pipetted (1.5 ml/well) into circular tissue-train 6-well plates (FlexCell International), making sure to incorporate the gel into the circumferential foam mesh. The initial gelation was performed for 30 minutes in a tissue culture incubator, then 4 mL of culture medium was added to each well. Subsequent media changes used 3 mL/well. Constructs were maintained in static culture for 1 week before stretching.
Cyclic Stretch Conditioning of Tissue Constructs
Prior to stretching experiments, all constructs were changed to media supplemented with insulin (2 g/ml, Sigma) and ascorbic acid (50 g/ml, Sigma); SB203580 (5 M, Sigma) was also added to the indicated samples. Cyclic stretching was performed by placing the plates on the FX-5000 Tension System (FlexCell International), using 25 mm circular loading posts and applying a cyclic stretch regime (duty cycle of 15% and frequency of 0.5 Hz). The stretching amplitude was either held constant, as indicated in the figure captions, or varied from a set point of 2.5 to 9.5% during the long-term studies; a 1% increment in amplitude and fresh SB203580 was added during each media change (every 48–72 hours).
Protein Extraction
At harvest, each construct was rinsed with cold phosphate buffered saline (PBS) and frozen at −80 C until lysis. The tissue was then disrupted by sonication in ice-cold lysis buffer [25 mM NaF, 0.5% Nonidet P-40, 1 mM EDTA, HALT phosphatase inhibitor cocktail (Pierce), 1 g/ml aprotinin, pepstatin, and leupeptin (Sigma) in PBS]. The soluble lysate was isolated by centrifugation, and the protein concentration was determined by bicinchoninic assay (Pierce). For cyclically stretched monolayers, cells in each well of the 24-well plate were lysed in 100 L of SDS-PAGE sample buffer.
Western Blot
20 g of total protein/well (or 5 L of cell lysate from monolayers/well) was separated by reducing SDS-PAGE, transferred to a nitrocellulose membrane (Whatman), and immunoblotted for beta-actin (Sigma #A5441), phosphorylated ERK (#9101), total ERK (#9102), phosphorylated p38 (#9211), or total p38 (#9212, all ERK and p38 primary antibodies were from Cell Signaling Technologies). Briefly, the blots were blocked for 1 hour (block was 5% dry milk, 0.1% Tween-20 in PBS), incubated 1 hour with primary antibody (1:2000 in block for ERK, 1:2000 in TBS-T (Tris buffered saline + 0.1% Tween-20) + 5% bovine serum albumin for p38), washed in TBS-T, incubated 1 hour in secondary antibody (1:2000 HRP-conjugated anti-rabbit IgG, Amersham, in block), washed in TBS-T and PBS, and developed by enhanced chemiluminescence. Films were digitized using ScanJet G4050 set to transparency mode (Hewlett Packard) and quantified for band density using ImageJ (NIH). On a given blot, normalization was performed by first dividing the phosphoprotein intensity by the total protein intensity (e.g. pERK/tERK) and then dividing all sample data by the untreated static control value. Static values were therefore always equal to 1 (dashed line in figures).
Quantification of Collagen and Cellularity
Collagen content was measured with the hydroxyproline assay of Stegemann and Stalder35, with collagen per sample calculated using a conversion factor of 7.46 mg of collagen per mg of 4-hydroxyproline6. Values were then normalized to the drug-matched static controls. Total protein was measured with the modified ninhydrin assay19, 34. Cellularity was measured with a modified Hoechst assay43.
Statistical Analysis
Unless otherwise noted, all numerical data indicates the mean standard error with n=3 or greater in each sample group. Significance was assigned by Student’s t-test between paired sample groups, and p<0.05 was reported as significant. With the exception of the cellularity data and any comparisons marked “n.s.”, all values for cyclically stretched samples were significantly higher than paired static controls.
Results
Cyclic stretching induces activation of both p38 and ERK MAPK in fibrin-based tissue constructs
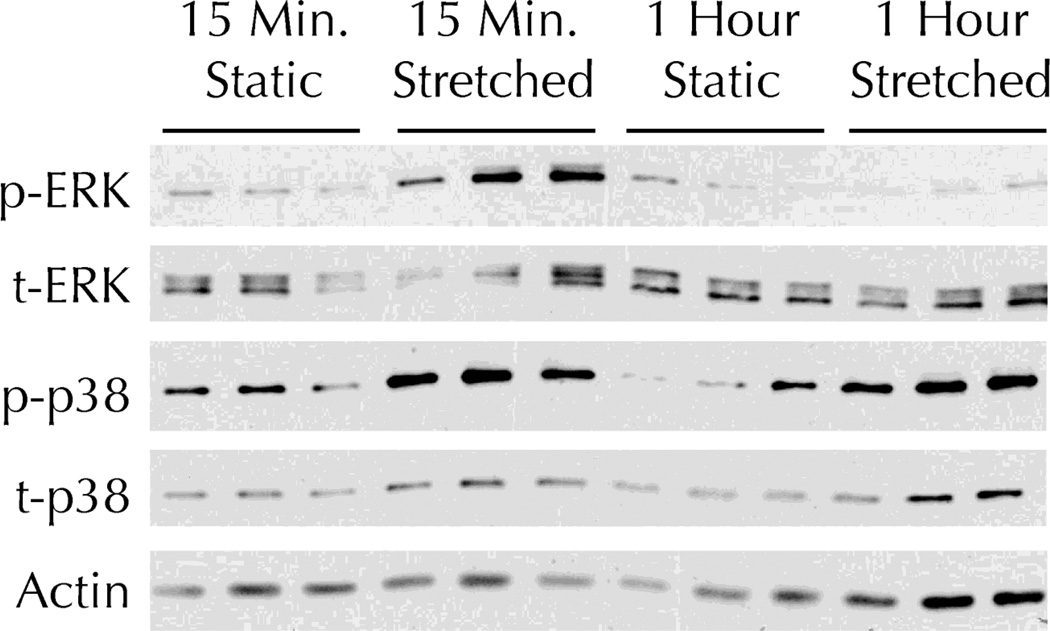
Fibrin-based tissue constructs were fabricated in 6-well plates where the fibrin was physically anchored by a foam mesh around the circumference of each well. After one week of static incubation, the FX-5000 Tension System (FlexCell International) was used to expose the cells embedded within the constructs to constant amplitude cyclic stretching. 15 minutes of stretching induced both p38 and ERK activation in the tissue constructs, relative to static controls (Figure 1). After one hour of stretching, p38 activation remained elevated while ERK activation was reduced to static control levels.
Figure 1. Cyclic stretching induces activation of both p38 and ERK MAPK in fibrin-based tissue constructs.
Fibrin-based tissue constructs were exposed to the indicated duration of 8% amplitude cyclic stretch and then analyzed by Western Blot. A representative blot for phosphorylated (p-) and total (t-) ERK, p-p38, t-p38, and beta-actin (as a loading control) is shown.
Stretching-induced ERK activation is enhanced with p38/inhibition
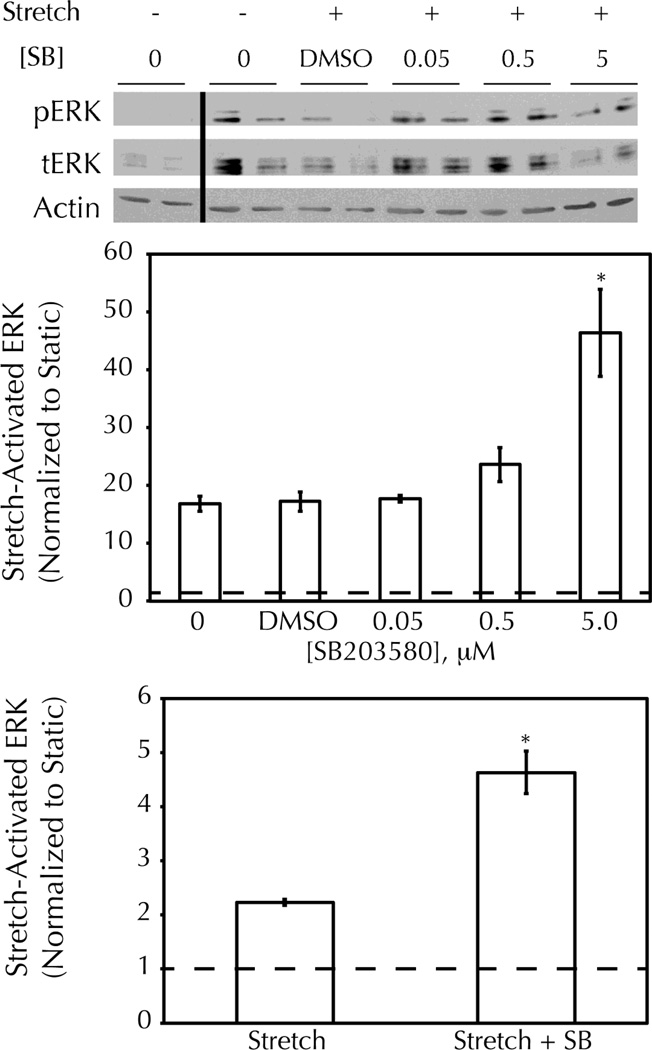
Since p38 is activated by cyclic stretching for the entire duration of ERK activation (Figure 1) and negatively regulates ERK signaling4, 14, 15, 26, 41, the effect of the p38/antagonist SB203580 on stretching-induced ERK activation was evaluated. Cells grown on pronectin-coated silicone membranes (FlexCell International) were treated with a range of SB203580 concentrations from 0.05 to 5 M prior to exposure to 15 minutes of cyclic stretching. This membrane coating was chosen to mimic the fibronectin-rich environment of the remodeled three-dimensional fibrin scaffold. While the vehicle control had no effect on stretching-induced ERK activation, a 5 M concentration of SB203580 showed a significant benefit (Figure 2A,B).
Figure 2. Stretching-induced ERK activation is enhanced with p38/inhibition.
Cells were exposed to 15 minutes of 5% amplitude cyclic stretch following pretreatment with the indicated concentration of SB203580 (SB) or DMSO (1%) and then analyzed by Western Blot for phosphorylated (p) and total (t) ERK. A) Representative Western Blot showing ERK activation with stretching, and increased activation with 5.0 M SB pretreatment. B) Semi-quantitative analysis of Western Blot data for multiple SB doses, normalized to the value for untreated static controls. C) Semi-quantitative analysis of Western Blot data for fibrin-based tissue constructs exposed to 15 minutes of 5% amplitude cyclic stretch +/− 5 M SB. * = p<0.05 with student’s t-test (n=3 per group).
From these data, and consistent with other published work27, 37, 5 M SB203580 was selected as a suitable concentration for subsequent studies using fibrin-based tissue constructs. 15 minutes of cyclic stretching induced ERK activation by cells in this 3D system; pretreatment with 5 M SB203580 augmented the stretching-induced ERK activation by 207 18% (Figure 2C).
p38/inhibition increases the final collagen content of cyclically stretched fibrin-based tissue constructs and offsets a stretching-induced decrease in cell proliferation
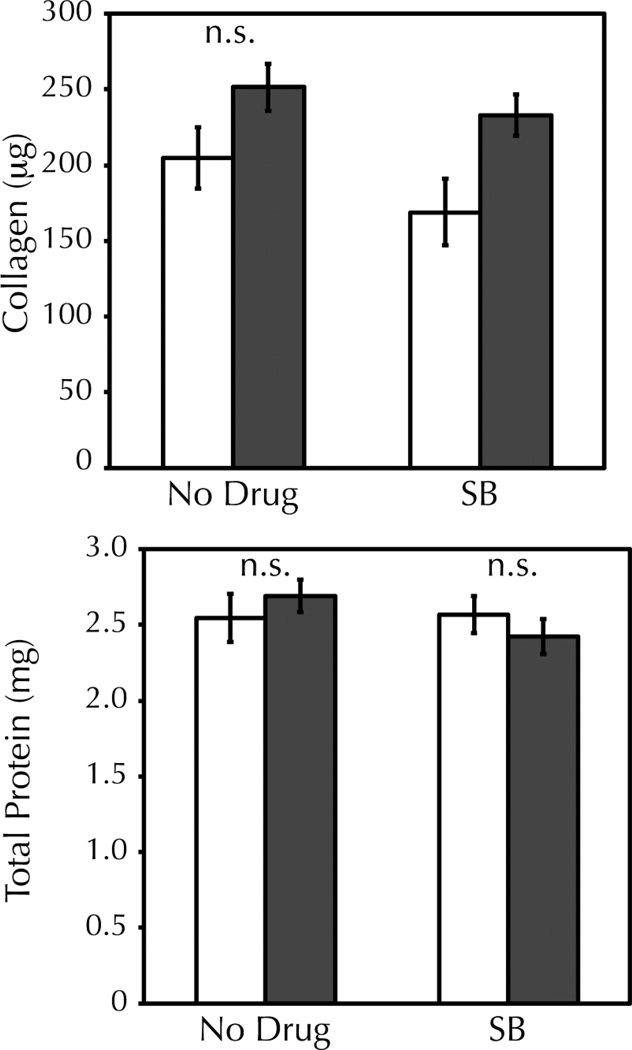
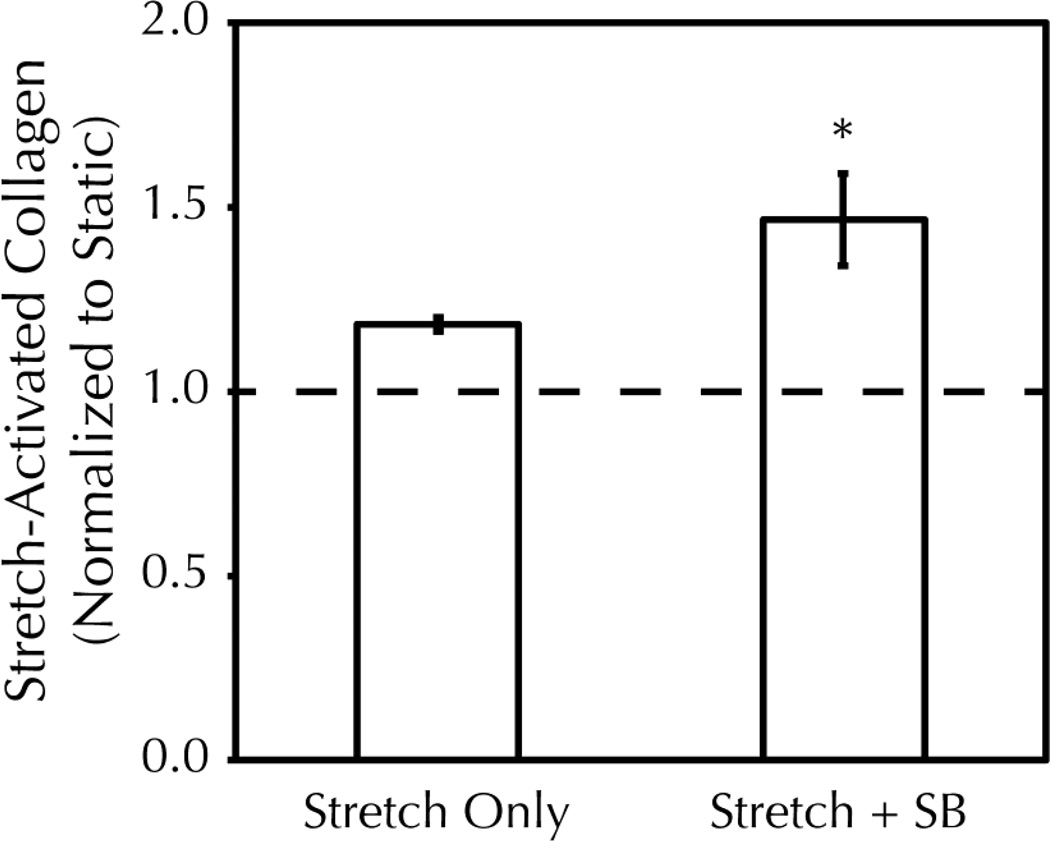
Following on previously published studies that used periodic strain incrementation to combat adaptation38, an incremental amplitude cyclic stretch regime was employed for long-term stretching of tissue constructs. At each increment in strain amplitude, stretching-induced signaling should re-occur33; therefore, the culture medium was changed prior to each incrementation step with a fresh 5 M dose of SB203580. After an eight step incrementation regime from 2.5–9.5% strain amplitude over three weeks, the final collagen content of the tissue constructs was determined. Incremental amplitude stretching led to an increase in collagen content over static controls as expected (Figure 3A). Notably, 5 M SB203580 augmented the stretching-induced collagen content 2.6 fold higher than that obtainable by stretching alone (1.5 0.13 fold increase in collagen over drug-treated static controls compared to a 1.2 0.02 fold increase in collagen over static with no drug). In the absence of stretching, 5 M SB203580 had no effect on collagen content (Figure 3B). Total protein content was constant regardless of stretching or drug treatment (Figure 3C).
Figure 3. p38/inhibition increases the final collagen content of cyclically stretched fibrin-based tissue constructs and offsets a stretching-induced decrease in cell proliferation.
Fibrin-based tissue constructs were exposed to three weeks of incremental amplitude cyclic stretch (filled bars) +/− 5 M SB203580 (SB) or were maintained in static culture (empty bars). A, B) Total collagen was determined by the biochemical quantification of hydroxyproline content and normalized to the collagen content of drug-matched static controls. There was no change in the collagen content of static constructs with drug treatment, but stretching-induced collagen was increased 2.6 fold further with SB treatment. * = p<0.05 with student’s t-test (n=19 per group). C) Total protein content of constructs was unchanged with stretching or SB treatment. D) Stretching led to a decrease in cellularity at harvest, as measured by Hoechst assay. SB treatment partially offset the stretching effect but did not prevent it entirely. * = p<0.05 with student’s t-test (n=3 per group).
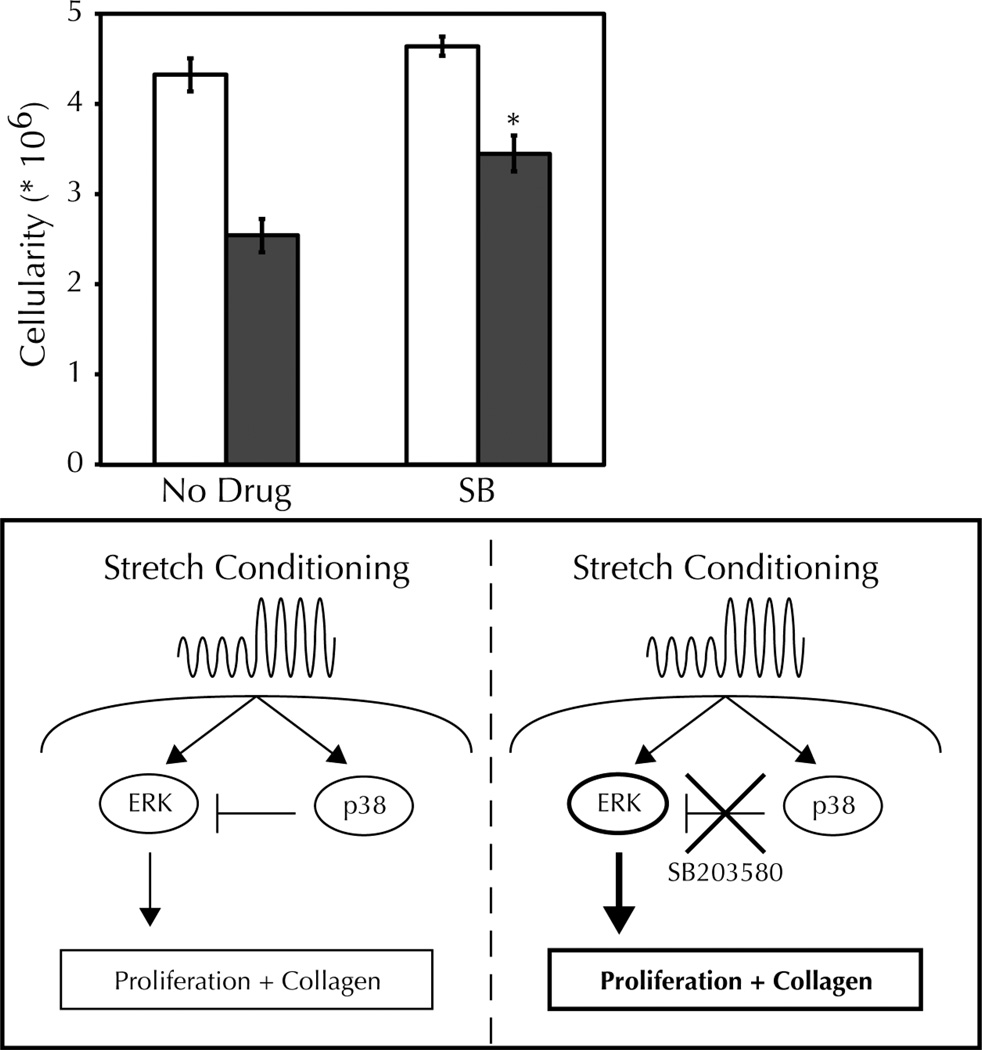
Inhibition of p38 with SB203580 can promote the proliferation of differentiated cells8. Therefore, we characterized the effect of stretching and SB203580 on the final cellularity of our constructs (Figure 3D). Stretching alone decreased the final cellularity regardless of drug treatment; since the final cellularity was greater than at fabrication (0.75 million cells), we consider this a stretching-mediated inhibition of proliferation. SB203580 treatment of static constructs had no effect on cellularity (4.32 0.18 × 106 cells vs. 4.64 0.11 × 106 cells for untreated vs. 5 M SB, p > 0.2). However, SB treatment appeared to ameliorate the stretching-induced decrease in proliferation we typically observe, with an increase in cellularity with drug treatment when comparing the stretching groups (2.54 0.19 × 106 cells vs. 3.45 0.20 × 106 cells for untreated vs. 5 M SB, p < 0.01). Stated differently, the stretching-induced decrease in cellularity was less in the presence of SB (41 4% vs. 26 4% for untreated vs. 5 M SB, p < 0.05).
Discussion
To produce a collagen-rich tissue from the cell-mediated remodeling of a fibrin gel, it is essential to consider the cell’s biomechanical context. Stretching activates MAPK signaling downstream of both stretch-sensitive ion channels17 and focal adhesion kinase20; also, changes in stiffness can dynamically alter fibroblast phenotype40. Notably, the current study shows that deactivation kinetics can vary by MAPK type; while both ERK and p38 are strongly activated 15 minutes after the onset of stretching, only p38 remains activated after 1 hour. Since p38 signaling has known antagonistic effects towards ERK activity, this suggests that the co-activation of ERK and p38 in response to stretching acts to dampen the ERK response. Uncontrolled ERK signaling can be dangerous to the organism; in many cancers Ras/MAPK signaling is constitutively activated leading to the proliferation hallmark of tumor cells. To this end ERK signaling is highly regulated; in addition to p38, Akt signaling18 and the activity of dephosphorylases such as DUSP622 can deactivate the ERK signal. In the context of tissue engineering, however, cyclic stretching is a common and convenient strategy to promote ERK-mediated collagen production so the negative p38 feedback is problematic.
Application of SB203580, which inhibits the and isoforms of p381, increased ERK activation in response to stretching (Figure 2). It is unlikely that the positive effects of SB203580 would have been identified in a typical drug screen, since the benefits were limited to the context of stretching. High-throughput stretching systems are emerging that could be useful for identifying additional targets21, 44. SB203580 has a known off-target effect as an activator of Raf protein kinase and downstream ERK signaling at high doses11; however, at the 5 M dose used in this study it did not activate ERK in static culture. An alternative explanation for the positive effects of SB203580 involves the antibiotic streptomycin, which was present in our culture media. Streptomycin can block stretch-sensitive ion channels5; potentially, SB203580 acts to relieve the streptomycin block, enabling stronger stretchinginduced signaling. To address this, we measured p38 and ERK activation in cell monolayers after 15 minutes of stretching in the presence and absence of streptomycin. We found no difference in MAPK activation due to the antibiotic (data not shown).
During three weeks of incremental amplitude cyclic stretching, 5 M SB203580 supplementation in the culture medium provided a significant benefit for stretching-induced collagen content. This was remarkable for two reasons. First, the drug enhanced collagen production beyond that obtained with an eight-step incremental amplitude cyclic stretching regime; incremental and intermittent stretching regimes are the current best practices for increasing collagen production28, 38. Second, SB203580 has been reported to have a half life on the order of 4 hours42, which is much smaller than the 2–3 day intervals between drug replenishment. The half-life of the drug is long enough to inhibit p38 during the first few hours of stretching, which appears to be the critical period for stimulating cellular collagen production.
An additional benefit of augmenting stretching-induced ERK signaling in the tissue engineering context is increased cellular proliferation. Since the sole source of collagen in remodeled fibrin-based tissue constructs is the entrapped cells, cell proliferation is desirable. Stretching inhibits proliferation, and therefore can have a negative impact on total matrix production. By prolonging ERK activation with SB203580, we achieved higher final cellularity in our stretched tissue constructs. This benefit on proliferation was limited to stretched constructs since the drug had no effect on the cellularity of static constructs. The likely mechanism for beneficial SB203580 activity is thus a combination of cellular proliferation and collagen synthesis downstream of prolonged ERK activation (Figure 4).
Figure 4. Schematic model for the SB203580-mediated relief of adaptation to stretching investigated in this manuscript.
Cyclic stretch conditioning activates cellular mechanotransduction pathways, including ERK and p38 MAPK. Activated p38 exerts an inhibitory effect on ERK, thereby limiting downstream proliferation and synthesis of type I collagen. With application of the p38 inhibitor SB203580, stretching-induced ERK signaling is enhanced, as are proliferation and downstream collagen production (bold).
Conclusions
Cyclic stretching activates both ERK and p38 MAPK signaling in fibrin-based tissue constructs. By inhibiting p38/in the context of cyclic stretching, higher ERK activation is achieved, leading to the production of a tissue starting from a fibrin gel with increased cellularity and superior collagen content.
Acknowledgements
The authors thank Naomi Ferguson for assistance with maintaining cells and tissue constructs. This study was funded by a National Science Foundation Graduate Research Fellowship (to J.B.S) and National Institutes of Health/National Heart, Lung, and Blood Institute awards HL104768 (to J.S.W.), HL083880, and HL107572 (to R.T.T.).
Abbreviations used in this manuscript
- DMSO
dimethylsulfoxide
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- PBS
phosphate buffered saline
- SB
SB203580
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TBS
tris buffered saline
Footnotes
Conflict of Interest Statement
The authors declare that no benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: A further update. Biochem J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barocas VH, Girton TS, Tranquillo RT. Engineered alignment in media equivalents: Magnetic prealignment and mandrel compaction. J Biomech Eng. 1998;120(5):660–666. doi: 10.1115/1.2834759. [DOI] [PubMed] [Google Scholar]
- 3.Bhogal RK, Bona CA. Regulatory effect of extracellular signal-regulated kinases (erk) on type i collagen synthesis in human dermal fibroblasts stimulated by il-4 and il-13. Int Rev Immunol. 2008;27(6):472–496. doi: 10.1080/08830180802430974. [DOI] [PubMed] [Google Scholar]
- 4.Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. The p38 map kinase inhibitor sb203580 enhances nuclear factor-kappa b transcriptional activity by a non-specific effect upon the erk pathway. Br J Pharmacol. 2000;131(1):99–107. doi: 10.1038/sj.bjp.0703534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birla RK, Huang YC, Dennis RG. Effect of streptomycin on the active force of bioengineered heart muscle in response to controlled stretch. In Vitro Cell Dev Biol Anim. 2008;44(7):253–260. doi: 10.1007/s11626-008-9114-0. [DOI] [PubMed] [Google Scholar]
- 6.Dombi GW, Haut RC, Sullivan WG. Correlation of high-speed tensile strength with collagen content in control and lathyritic rat skin. J Surg Res. 1993;54(1):21–28. doi: 10.1006/jsre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 7.Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278(31):28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- 8.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. P38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19(10):1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-i collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60(4):607–612. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 10.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A. 2003;66(3):550–561. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 11.Hall-Jackson CA, Goedert M, Hedge P, Cohen P. Effect of sb 203580 on the activity of c-raf in vitro and in vivo. Oncogene. 1999;18(12):2047–2054. doi: 10.1038/sj.onc.1202603. [DOI] [PubMed] [Google Scholar]
- 12.Isojima Y, Okumura N, Nagai K. Molecular mechanism of mammalian circadian clock. J Biochem. 2003;134(6):777–784. doi: 10.1093/jb/mvg219. [DOI] [PubMed] [Google Scholar]
- 13.Jeon YM, Kook SH, Son YO, Kim EM, Park SS, Kim JG, Lee JC. Role of mapk in mechanical force-induced up-regulation of type i collagen and osteopontin in human gingival fibroblasts. Mol Cell Biochem. 2009;320(1–2):45–52. doi: 10.1007/s11010-008-9897-z. [DOI] [PubMed] [Google Scholar]
- 14.Kakisis JD, Pradhan S, Cordova A, Liapis CD, Sumpio BE. The role of stat-3 in the mediation of smooth muscle cell response to cyclic strain. Int J Biochem Cell Biol. 2005;37(7):1396–1406. doi: 10.1016/j.biocel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Kalmes A, Deou J, Clowes AW, Daum G. Raf-1 is activated by the p38 mitogen-activated protein kinase inhibitor, sb203580. FEBS Lett. 1999;444(1):71–74. doi: 10.1016/s0014-5793(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 16.Kook SH, Hwang JM, Park JS, Kim EM, Heo JS, Jeon YM, Lee JC. Mechanical force induces type i collagen expression in human periodontal ligament fibroblasts through activation of erk/jnk and ap-1. J Cell Biochem. 2009;106(6):1060–1067. doi: 10.1002/jcb.22085. [DOI] [PubMed] [Google Scholar]
- 17.Kushida N, Kabuyama Y, Yamaguchi O, Homma Y. Essential role for extracellular ca(2+) in jnk activation by mechanical stretch in bladder smooth muscle cells. Am J Physiol Cell Physiol. 2001;281(4):C1165–C1172. doi: 10.1152/ajpcell.2001.281.4.C1165. [DOI] [PubMed] [Google Scholar]
- 18.Lee JT, Steelman LS, Chappell WH, McCubrey JA. Akt inactivates erk causing decreased response to chemotherapeutic drugs in advanced cap cells. Cell Cycle. 2008;7(5):631–636. doi: 10.4161/cc.7.5.5416. [DOI] [PubMed] [Google Scholar]
- 19.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22(4):339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 20.Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci. 2012;125(Pt 13):3061–3073. doi: 10.1242/jcs.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez JP, Legant W, Lam V, Cayemberg A, Elson E, Wakatsuki T. High-throughput measurements of hydrogel tissue construct mechanics. Tissue Eng Part C Methods. 2009;15(2):181–190. doi: 10.1089/ten.tec.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina G, Vogt A, Bakan A, Dai W, Queiroz de Oliveira P, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, Tsang M. Zebrafish chemical screening reveals an inhibitor of dusp6 that expands cardiac cell lineages. Nat Chem Biol. 2009;5(9):680–687. doi: 10.1038/nchembio.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small gtp-binding protein, rac, contribute to the effects of transforming growth factor-beta1 on gene expression. J Biol Chem. 1996;271(28):16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 24.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of smad7, a tgfbeta-inducible antagonist of tgf-beta signalling. Nature. 1997;389(6651):631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 25.Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with tgf-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23(17):3717–3731. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 26.Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, Chun JS. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275(8):5613–5619. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- 27.Papakrivopoulou J, Lindahl GE, Bishop JE, Laurent GJ. Differential roles of extracellular signal-regulated kinase 1/2 and p38mapk in mechanical load-induced procollagen alpha1(i) gene expression in cardiac fibroblasts. Cardiovasc Res. 2004;61(4):736–744. doi: 10.1016/j.cardiores.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Paxton JZ, Hagerty P, Andrick JJ, Baar K. Optimizing an intermittent stretch paradigm using erk1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012;18(3–4):277–284. doi: 10.1089/ten.tea.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: Mapk-dependent transcriptional activation of type i collagen. Arthritis Rheum. 2009;60(7):2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza JJ, Egido J. Endothelin-1, via eta receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res. 2005;97(2):125–134. doi: 10.1161/01.RES.0000174614.74469.83. [DOI] [PubMed] [Google Scholar]
- 31.Ross JJ, Tranquillo RT. Ecm gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22(6):477–490. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 32.Rubbens MP, Mol A, Boerboom RA, Bank RA, Baaijens FP, Bouten CV. Intermittent straining accelerates the development of tissue properties in engineered heart valve tissue. Tissue Eng Part A. 2009;15(5):999–1008. doi: 10.1089/ten.tea.2007.0396. [DOI] [PubMed] [Google Scholar]
- 33.Shi F, Chiu YJ, Cho Y, Bullard TA, Sokabe M, Fujiwara K. Down-regulation of erk but not mek phosphorylation in cultured endothelial cells by repeated changes in cyclic stretch. Cardiovasc Res. 2007;73(4):813–822. doi: 10.1016/j.cardiores.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292(1):125–129. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 35.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 36.Stopa M, Anhuf D, Terstegen L, Gatsios P, Gressner AM, Dooley S. Participation of smad2, smad3, and smad4 in transforming growth factor beta (tgf-beta)-induced activation of smad7. The tgf-beta response element of the promoter requires functional smad binding element and e-box sequences for transcriptional regulation. J Biol Chem. 2000;275(38):29308–29317. doi: 10.1074/jbc.M003282200. [DOI] [PubMed] [Google Scholar]
- 37.Syedain ZH, Tranquillo RT. Tgf-beta1 diminishes collagen production during long-term cyclic stretching of engineered connective tissue: Implication of decreased erk signaling. J Biomech. 2011;44(5):848–855. doi: 10.1016/j.jbiomech.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: Evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105(18):6537–6542. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC. Angiotensin ii activates collagen i gene through a mechanism involving the map/er kinase pathway. Hypertension. 2000;36(3):330–336. doi: 10.1161/01.hyp.36.3.330. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One. 2012;7(7):e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Rao J, Studzinski GP. Inhibition of p38 map kinase activity up-regulates multiple map kinase pathways and potentiates 1,25-dihydroxyvitamin d(3)-induced differentiation of human leukemia hl60 cells. Exp Cell Res. 2000;258(2):425–437. doi: 10.1006/excr.2000.4939. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between smad, erk1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic atdc5 cells. J Biol Chem. 2001;276(17):14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 43.Williams C, Johnson SL, Robinson PS, Tranquillo RT. Cell sourcing and culture conditions for fibrin-based valve constructs. Tissue Eng. 2006;12(6):1489–1502. doi: 10.1089/ten.2006.12.1489. [DOI] [PubMed] [Google Scholar]
- 44.Wu MH, Wang HY, Liu HL, Wang SS, Liu YT, Chen YM, Tsai SW, Lin CL. Development of high-throughput perfusion-based microbioreactor platform capable of providing tunable dynamic tensile loading to cells and its application for the study of bovine articular chondrocytes. Biomed Microdevices. 2011;13(4):789–798. doi: 10.1007/s10544-011-9549-z. [DOI] [PubMed] [Google Scholar]
- 45.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin ii induces connective tissue growth factor and collagen i expression via transforming growth factor-beta-dependent and -independent smad pathways: The role of smad3. Hypertension. 2009;54(4):877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]