Abstract
Background and Purpose
Many dementia patients exhibit behavioural and psychological symptoms (BPSD) that include psychosis, aggressivity, depression and anxiety. Antipsychotic drugs are frequently prescribed but fail to significantly attenuate mood deficits, may interfere with cognitive function and are associated with motor and cardiac side effects, which are problematic in elderly patients. A need therefore exists for drugs that are better suited for the treatment of BPSD.
Experimental Approach
We used in vitro cellular and in vivo behavioural tests to characterize ADN-1184, a novel arylsulfonamide ligand with potential utility for treatment of BPSD.
Key Results
ADN-1184 exhibits substantial 5-HT6/5-HT7/5-HT2A/D2 receptor affinity and antagonist properties in vitro. In tests of antipsychotic-like activity, it reversed MK-801-induced hyperactivity and stereotypies and inhibited conditioned avoidance response (MED = 3 mg·kg−1 i.p.). Remarkably, ADN-1184 also reduced immobility time in the forced swim test at low doses (0.3 and 1 mg·kg−1 i.p.; higher doses were not significantly active). Notably, up to 30 mg·kg−1 ADN-1184 did not impair memory performance in the passive avoidance test or elicit significant catalepsy and only modestly inhibited spontaneous locomotor activity (MED = 30 mg·kg−1 i.p.).
Conclusions and Implications
ADN-1184 combines antipsychotic-like with antidepressant-like properties without interfering with memory function or locomotion. This profile is better than that of commonly used atypical antipsychotics tested under the same conditions and suggests that it is feasible to identify drugs that improve BPSD, without exacerbating cognitive deficit or movement impairment, which are of particular concern in patients with dementia.
Keywords: ADN-1184, dementia, BPSD, antipsychotics, depression, cognition, behavioural pharmacology
Introduction
Behavioural and psychological symptoms of dementia (BPSD) constitute a substantial medical challenge among elderly patients. Indeed, the progressive worsening of symptoms including hallucinations, delusions and mood deficits is the major reason for patient institutionalization, and decreased quality of life of both patients and caregivers (Liperoti et al., 2008). Overall, 25–50% of patients with dementia show symptoms of psychosis (Jeste et al., 2008) and 40–60% experience significant depressive symptoms at some stage of the disease (Hersch and Falzgraf, 2007).
BPSD are usually treated with psychotropic drugs, often second-generation antipsychotics (SGA) that produce less extrapyramidal symptoms (EPS) and have better efficacy against negative symptoms than classical ones (Liperoti et al., 2008). However, a recent meta-analysis based on 14 placebo-controlled trials of elderly patients with BPSD revealed only modest effects for three SGA, that is, risperidone, aripiprazole and olanzapine (Maher et al., 2011). In addition, antipsychotics may produce adverse effects including EPS, and cardiovascular and metabolic side effects (Nobili et al., 2009; Schulze et al., 2013). Moreover, antipsychotic drugs may worsen cognitive functioning, which can be a substantial drawback in the case of elderly patients who already suffer from cognitive deficits (Jeste et al., 2008; Vigen et al., 2011).
It should be noted that psychosis in dementia may have a different neurobiological substrate from that in schizophrenia. Indeed, psychotic Alzheimer patients often experience visual hallucinations and misidentifications of caregivers – symptoms that are not commonly found in schizophrenia patients. Conversely, bizarre or complex delusions that occur frequently in patients with schizophrenia are not often observed in dementia patients (Jeste and Finkel, 2000). The distinct nature of psychotic symptoms in dementia suggests that different neurobiological mechanisms are at play. In particular, serotonergic systems may be involved because hallucinations in dementia are similar to those caused by serotonergic agonists such as mescaline or lysergic acid (Marsh, 1979). Strong visual hallucinations can be also evoked by NMDA receptor antagonists such as ketamine or phencyclidine (Siegel, 2013) but are less frequently evoked by dopaminomimetics such as amphetamine or cocaine, which are widely used in preclinical screening of new drugs for schizophrenia (Jones et al., 2011). Currently available antipsychotics were selected primarily for their capacity to oppose the effects of dopaminomimetics, and, therefore, may potentially provide suboptimal therapeutic efficacy in the treatment of BPSD. In particular, they were not selected to provide alleviation of mood deficits or to avoid accentuating cognitive deficits in elderly patients. It may be surmised that novel drugs should be identified which are specifically optimized for treatment of BPSD.
There are substantial data supporting the importance of the serotonin system in the development of BPSD. For example, serotonin receptor gene polymorphisms are associated with visual and auditory hallucinations in patients with Alzheimer's disease (AD) (Holmes et al., 1998). A genetic polymorphism of the serotonin transporter promoter region (L/L genotype) has been associated with aggressive behaviour (Sukonick et al., 2001). Other studies show involvement of 5HT2A and 5HT6 receptors in the pathogenesis of AD (Lorke et al., 2006) as well as association of 5-HT6 receptors with psychotic symptoms in patients with AD (Marcos et al., 2008; receptor nomenclature follows Alexander et al., 2013). 5-HT6 receptor antagonists are also active in a range of models of cognition relevant to psychotic disorders (Loiseau et al., 2008; Rodefer et al., 2008; Arnt and Olsen, 2011) as well as in tests of antidepressant-like and anxiolytic activity (Wesołowska, 2007; Carr et al., 2011). Accordingly, a selective 5-HT6 antagonist, LuAE58054, improved cognitive performance of AD patients, in combination with the acetylcholinesterase inhibitor, donepezil (H. Lundbeck A/S, 2012). Finally, some authors proposed that an improved antipsychotic profile may be achieved by combining 5-HT6 antagonism with an absence of anti-muscarinic activity, as is observed for sertindole, but not clozapine or olanzapine (Rodefer et al., 2008). The possibility of clinical evaluation of sertindole, also with respect to BPSD, was however significantly hampered by its arrhythmogenic potential caused by potent hERG channel inhibition.
Based on the above considerations, we designed a series of novel arylsulfonamide derivatives displaying high affinity for 5-HT6, 5-HT7 and 5-HT2A receptor subtypes, as well as dopamine D2 receptors, and devoid of significant interaction with muscarinic receptors or hERG channels (Kołaczkowski et al., 2012). The compounds were screened in vitro and in vivo as potential treatments for BPSD. As no animal model of BPSD is currently available, studies included measures of antipsychotic-like efficacy [MK-801-induced locomotion and conditioned avoidance response (CAR)], antidepressant-like efficacy [forced swimming test (FST)] and cognitive interference [passive avoidance (PA) test]. We recently used these tests to compare eight antipsychotics (haloperidol, chloropromazine, clozapine, olanzapine, risperidone, aripiprazole, lurasidone and asenapine) that are commonly used for treatment of BPSD. None of the drugs presented an optimal profile, i.e., activity in models of both antipsychotic-like and antidepressant-like activities, at doses that produce no or minimal detrimental effects on cognitive or motor performance (Kołaczkowski et al., 2013). In the present study, we describe the profile of one of our lead compounds, ADN-1184 (Figure 1), in a series of preclinical models (in vitro ligand binding and receptor antagonism and in vivo tests) and discuss its potential relevance to treatment of BPSD.
Figure 1.
Chemical structure of ADN-1184.
Methods
In vitro ligand binding and receptor activation
Profiling of ADN-1184 on a series of in vitro competition binding and functional experiments was outsourced to Cerep (Le Bois l'Evêque, Poitiers, France). An outline of methodologies is shown in Tables 1 and 2. Further methodological details are available on the company website (http://www.cerep.fr). Concentration-response experiments were carried out in duplicate and, unless stated otherwise, repeated three times. Data from all experiments were analysed using non-linear curve fitting programs and results are given as Ki values for binding affinity or Kb values for antagonist potency. The activity of ADN-1184 was also determined on hERG-mediated potassium currents. Concentration-response experiments were carried out by ChanTest (Cleveland, OH, USA) and expressed as IC50 values (Table 1).
Table 1.
Receptor binding profile of ADN-1184
| Receptor | Cell line | Radioligand or readout | Reference ligand (Ki, nM) | ADN-1184 Ki ± SEM (nM) |
|---|---|---|---|---|
| h 5-HT6 | CHO cells | [3H]LSD | 5-HT (33) | 16 ± 13 |
| h 5-HT7 | CHO cells | [3H]LSD | 5-HT (0.8) | 0.50 ± 0.27 |
| h 5-HT2A | HEK-293 cells | [3H]ketanserin | Ketanserin (0.3) | 2.0 ± 1.0 |
| h 5-HT1A | HEK-293 cells | [3H]8-OH-DPAT | 8-OH-DPAT (0.4) | 173 ± 85 |
| h 5-HT2C | HEK-293 cells | [3H]mesulergine | RS102221 (0.6) | 630 ± 370 |
| h D2S | HEK-293 cells | [3H]methyl-spiperone | (+)butaclamol (0.04) | 18 ± 6 |
| h D3 | CHO cells | [3H]methyl-spiperone | (+)butaclamol (0.04) | 20 ± 11 |
| h D4 | CHO cells | [3H]methyl-spiperone | Clozapine (17) | 17 ± 7 |
| h D1 | CHO cells | [3H]SCH23390 | SCH23390 (0.1) | 30 ± 13 |
| h α1A | CHO cells | [3H]prazosin | Prazosin (0.04) | 0.84 ± 0.58 |
| h α2C | CHO cells | [3H]RX 821002 | Yohimbine (0.5) | 8.5 ± 5.7 |
| h H1 | HEK-293 cells | [3H]pyrilamine | Pyrilamine (1.7) | 116 ± 60 |
| h M1 | CHO cells | [3H]pirenzepine | Pirenzepine (16) | >1000a |
| h M2 | CHO cells | [3H]AF-DX 384 | Methoctramine (27) | >1000a |
| h M3 | CHO cells | [3H]4-DAMP | 4-DAMP (0.44) | >1000a |
| h M4 | CHO cells | [3H]4-DAMP | 4-DAMP (0.88) | >1000a |
| h M5 | CHO cells | [3H]4-DAMP | 4-DAMP (0.48) | >1000a |
| hERG | CHO cells | Inhibition of K + current | – | 2080 ± 550b |
Unless indicated, all experiments were carried out by Cerep.
Less than 10% inhibition of binding observed at a concentration of 1 μM (n = 2).
IC50 value. hERG channel current experiments were carried out by ChanTest.
h, human recombinant.
Table 2.
Receptor activation profile of ADN-1184
| Receptor | Functional test | Agonist | Kb ± SEM |
|---|---|---|---|
| h 5-HT6 | cAMP | 5-HT (300 nM) | 24 ± 13 |
| h 5-HT7 | cAMP | 5-HT (300 nM) | 0.11 ± 0.04 |
| h 5-HT2A | IP1 | 5-HT (100 nM) | 3.4 ± 1.0 |
| h 5-HT1A | Cellular dielectric spectroscopy | 8-OH-DPAT (100 nM) | 116 ± 43 |
| h 5-HT2C | IP1 | 5-HT (10 nM) | >1000 |
| h D2S | cAMP | Dopamine (300 nM) | 2.4 ± 1.1 |
| h D3 | cAMP | Dopamine (10 nM) | 3.9 ± 1.1 |
| h D4 | cAMP | Dopamine (100 nM) | 22 ± 4 |
| h D1 | cAMP | Dopamine (300 nM) | 140 ± 10a |
| h α1A | Intracellular Ca2+ release | Adrenaline (3 nM) | 0.16 ± 0.07 |
When tested alone, ADN-1184 did not show agonist properties in any of the assays. The cell lines used are the same as those shown in Table 2009. Experiments were carried out by Cerep. For further methodological information, see the company's online catalogue (http://www.cerep.fr). KB values of antagonists for inhibition of agonist action were calculated according to Lazareno and Birdsall (1993): [KB = IC50: 1 + (Agonist/EC50)], where IC50 = inhibitory concentration50 of antagonist, agonist = concentration of agonist in the test, and EC50 = effective concentration50 of agonist.
Mean ± range.
h, human recombinant; IP1, inositol phosphate.
Subjects
All animal care and experimental procedures complied with the standards laid down in Polish regulations and the International European Guidelines (Directive No. 86/609/EEC), and were reviewed and approved by the institutional ethics committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).A total of 313 animals were used in the experiments described here.
Drug-naive male Wistar rats (Charles River, Sulzfeld, Germany) weighing 200–225 g on arrival were used for behavioural experiments. Animals were supplied by the breeder 2–3 weeks before the experiments. Rats were housed 4 per plastic cage and kept in a room with constant environmental conditions (22 ± 1°C, relative humidity 60%, a 12:12 light-dark cycle with lights on at 07:00 h). During this time, the subjects were weighed and handled several times. Tap water and standard lab chow (Labofeed H; WPIK, Kcynia, Poland) were available ad libitum. All tests were carried out in a sound-attenuated experimental room between 09:00 h and 15:00 h. A total of 273 animals were used in the behavioural experiments.
The plasma and brain exposure experiments were carried out by ITR Laboratories (Baie-D'Urfe, Quebec, Canada). The study plan was approved by the Animal Care Committee of ITR and animals were cared for in accordance with the Guide to the Care and Use of Experimental Animals as published by the Canadian Council on Animal Care, and the Guide for the Care and Use of Laboratory Animals, an NIH publication. Male Sprague–Dawley rats (Charles River Canada Inc., St. Constant, Quebec, Canada) were used, weighing 200–264 g at time of experimentation. Housing conditions were similar to those outlined above except that rats were housed individually in stainless steel wire mesh bottom rodent cages equipped with an automatic watering system and that animals were offered non-dietary items (i.e. Nylabone®, Bio-Serv, Frenchtown, NJ, USA) as part of the ITR environmental enrichment program. A total of 40 animals were used in the exposure studies.
MK-801-induced hyperlocomotion
Antipsychotic-like activity was assessed by inhibition of hyperactivity elicited by the NMDA receptor antagonist, MK-801 (Andiné et al., 1999). Briefly, groups of drug-naive rats (n = 7–8) were transferred in their home cages to the experimental room 24 h prior to testing and allowed to habituate for 60 min and returned to the colony room. The next day, locomotor activity was assessed in black octagonal open fields (80 cm in diameter, 30 cm high) under dim light and continuous white noise (65 dB). Each animal was placed in the centre of the open field and allowed to explore the whole area for 30 min. Subjects did not have visual contact with other rats. Forward locomotion (cm per 30 min) was recorded and analysed with the aid of the computerized video tracking system (Videomot; TSE, Bad Homburg, Germany).
Rats were given drug or vehicle (i.p. or s.c.), 60 min before the start of the locomotor activity test. Fifteen minutes before the start of the test, rats were given MK-801 (0.3 mg·kg−1 i.p.). When assessing spontaneous locomotor activity, rats were given saline 15 min before the test.
MK-801-induced stereotypies
Administration of a higher dose of MK-801 elicits a different pattern of behaviours in rodents, notably including stereotypical behaviour. The effects of ADN-1184 on these behaviours were examined as follows. On the day before the test, rats were individually habituated to glass observation cages (25 × 25 × 40 cm, W × H × L) with wood chip bedding on the floor for 20 min. On the test day, animals were injected with vehicle or drugs 60 min i.p. or s.c. before behavioural observation. Rats were injected with MK-801 (1.2 mg·kg−1) and placed in the observation cages, which were screened from view of other rats. Ten minutes later, head weaving and circling induced by MK-801 was recorded by a trained observer for 300 s.
CAR
The antipsychotic-like activity of AND-1184 was assessed as described (Arnt, 1982). Briefly, the two-way active avoidance apparatus consisted of six identical stainless steel shuttle boxes (PACS-30 system; Columbus Instruments, Columbus, OH, USA). Each box consisted of two identical compartments (23 × 23 × 23 cm), with black Plexiglas covers and a central separation fitted with an opening that allowed the rat to pass between the compartments. A stimulus light was attached to the cover of each compartment. The shuttle box was equipped with a tone generator and subjects were placed on an electrified grid that spanned the entire floor area of the compartments. Photocells detected the position of the rat within the box.
Training sessions started with a 3 min habituation period in darkness, followed by 50 trials presented on a 15 s variable interval schedule (range: 0–30 s). Each trial consisted of a 10 s warning tone and light stimulus (conditioned stimulus, CS) followed by a 10 s foot shock generated using an EACS-30 shock generator from Columbus Instruments (0.5 mA constant current; unconditioned stimulus, UCS) delivered through the grid floor on the side where the rat was located. The light was off during delivery of the shock. An avoidance response (CAR), that is, crossing through the opening to the other compartment during the initial 10 s of the trial, terminated the CS and prevented shock delivery.
There were 14–18 training sessions with one session performed per day. Rats with stable CAR (>80% avoidance responses in the last two training sessions) were used in further tests.
Rats were given drug, or its vehicle, 60 min i.p. or s.c. before the start of the test session. A 7 day washout period was maintained between test sessions. Two non-drug training sessions were conducted during the washout period in order to maintain stable CAR performance. Performance criterion had to be fulfilled during the interspersed sessions.
The number of CS-UCS trials during the test and non-drug sessions was reduced from 50 to 30; for further proceedings, only rats with stable CAR response (see above) were included.
FST
The procedure used to determine antidepressant-like activity was based on the technique described previously (Porsolt et al., 1978). Briefly, rats were individually placed in glass cylinders (40 cm in height, 17 cm in diameter) filled with water (temperature: 23 ± 1°C) at a height that made it impossible to reach the bottom with hind paws (25 cm). There were two swimming sessions separated by 24 h: an initial 15 min pre-test and a 5 min test. The duration of immobility (s) in the test session was recorded by an observer, unaware of the treatments, located in an adjacent room with the aid of a video camera. A rat was considered immobile when it floated without moving except to keep its head above the water surface. Animals were injected with vehicle or drugs 60 min i.p. or s.c. before the test.
Step-through PA test
Effects of antipsychotics on memory function were evaluated as described (Ishiyama et al., 2007). Briefly, the passive avoidance apparatus (PACS-30; Columbus Instruments) comprised four identical stainless steel cages with black Plexiglas covers. Each cage consisted of a lighted and a dark compartment (23 × 23 × 23 cm) and a stainless steel grid floor. The compartments were separated by an automated sliding door. In the training (acquisition) session, animals were individually placed in the lighted compartment and allowed to explore it for 10 s. The sliding door was then opened, and the step-through latency for animals to enter the dark compartment was measured (300 s cut-off time). As soon as the animals entered the dark compartment, the door was closed. An inescapable foot shock (0.5 mA pulse constant current for 3 s) was delivered 3 s later through the grid floor with a constant current shock generator (EACS-30; Columbus Instruments). The test compound, or its vehicle, was administered 60 min before the start of the training session. All vehicle-treated animals entered the dark compartment during the training session and received a foot shock. Drug-treated animals that did not enter the dark compartment in the training session were not subjected to the test session. In the present study, all the tested animals entered the dark compartment, except those that had been treated with haloperidol at doses of 0.3 and 1.0 mg·kg−1. At these doses, 50% of the rats entered the dark compartment.
The test session was performed 24 h after the training session using the same paradigm but without the foot shock (Ishiyama et al., 2007). Step-through latencies for animals to enter the dark compartment were measured with a 300 s cut-off time. Drug-induced decreases in step-through latencies to enter the dark compartment in the test session were taken as a measure of drug's ‘amnesic’ effects.
Catalepsy bar test
Catalepsy was assessed using the bar test. Each rat was placed on a clean, smooth table with a wooden bar (2 × 3 × 25 cm, H × W × L) suspended 10 cm above the working surface. The animal's hind limbs were freely placed on the table, the tail laid out to the back, and the forelimbs gently placed over the bar. The length of time the animal touched the bar with both front paws was measured up to a preset cut-off time of 180 s. Results of each trial were scored as follows: 0 for holding the position for <15 s, 1 for holding it for 15–29.9 s, 2 for holding it for 30–59.9 s, and a maximum score of 3 for staying on the bar for >60 s. The minimum cataleptogenic dose was defined as the lowest dose inducing a mean catalepsy score of >1 (Ogren et al., 1986). Catalepsy was scored 30, 60 and 120 min after administration of vehicle or a test drug.
Plasma and brain exposure determination
Experiments were carried out by ITR Laboratories. Rats were treated with ADN-1184 at a dose of 10 mg·kg−1 i.p.; this dose was chosen because it produces an almost maximal effect in the ‘therapeutic’ behavioural tests. Blood samples (0.4 mL) were taken by jugular venepuncture and collected into polypropylene tubes containing K2EDTA and placed immediately on wet ice. Samples were centrifuged at 4°C for 10 min at 1341.6 × g and the resulting plasma was stored frozen (−20°C). For determination of ADN-1184 levels in brain tissue, subjects were anaesthetized with isoflurane, and the brains rapidly collected, weighed, then stored frozen (−80°C).
Samples were analysed using an LCMS analytical method developed at ITR. The lower limit of quantification of ADN-1184 was 1.0 ng·mL−1 in plasma and 10 ng·g−1 in brain tissue.
Data analysis
Analysis of results was carried out by one-way anova followed by post hoc tests. The choice of tests depended on whether the data generated were normally distributed. Accordingly, for analysis of normally distributed data (such as those generated in most of the above tests), anova was followed by the Newman–Keuls post hoc test. For non-normally distributed variables (such as those generated in the PA test and for MK-801-induced stereotypies), the Kruskal–Wallis test was followed by the post hoc Mann–Whitney U-test. For comparison of two groups (e.g. vehicle group vs. MK-801-treated group for hyperlocomotion), the Student's t-test was used. P values <0.05 were considered significant. The Statistica 8.0 software package for Windows (StatSoft, Tulsa, OK, USA) was used to analyse all data.
Materials
MK-801 (Sigma-Aldrich, Poznan, Poland) was dissolved in sterile physiological saline (0.9% NaCl; Baxter, Warsaw, Poland) and administered i.p. in a volume of 1.0 mL·kg−1. For in vitro experiments, ADN-1184 (hydrochloride salt; provided by Adamed Ltd.) was dissolved in DMSO at a concentration of 1 mM and diluted appropriately with distilled water. For in vivo experiments, ADN-1184 was suspended in 1.5% Tween 80 (Sigma-Aldrich) and administered i.p. in a volume of 2.0 mL·kg−1. All solutions were prepared immediately prior to use and protected from light.
Results
In vitro ligand binding and functional tests
ADN-1184 exhibited marked affinity (nanomolar Ki values) for receptors associated with antipsychotic and antidepressant activities, including h5-HT2A, h5-HT6 and h5-HT7 receptors and dopamine receptors (hD2, hD3 and hD4). ADN-1184 also showed a marked affinity for α1 and α2C adrenoceptors, as shown in Table 1. In contrast, ADN-1184 showed lower affinity at other serotonin receptor subtypes including h5-HT1A and h5-HT2C. ADN-1184 showed low affinity at histamine hH1 and muscarinic hM1 to hM5 receptors (Table 1) and for hERG channels, targets that are associated with side effects such as metabolic dysfunction, sedation and arrhythmia.
In cell-based functional tests, ADN-1184 exhibited antagonist properties at all the receptors tested, blocking agonist-stimulated receptor activation (Table 2). Thus, ADN-1184 potently antagonized 5-HT2A, 5-HT6, 5-HT7, hD2S, hD3 and α1 adrenoceptors. ADN-1184 did not elicit any stimulation when tested alone.
MK-801-induced hyperlocomotion
ADN-1184 dose-dependently inhibited MK-801-induced hyperlocomotion, F(3, 28) = 6.9, P < 0.01 (Figure 2A). The post hoc Newman–Keuls test revealed significant effects at doses of 3 (P < 0.05), 10 and 30 mg·kg−1 (P < 0.01), but not 1 mg·kg−1. The MED was therefore 3.0 mg·kg−1.
Figure 2.
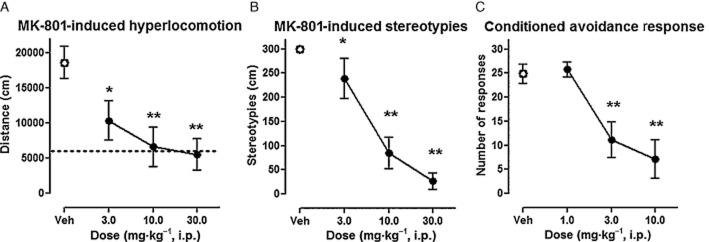
Effects of ADN-1184 in tests related to antipsychotic-like activity. (A) Effects of ADN-1184 on hyperlocomotor activity induced by MK-801 (0.3 mg·kg−1). Each symbol represents mean ± SEM of the distance travelled by rats in the 16–45 min period after MK-801 administration (n = 8 per group). Drug or vehicle was administered 45 min before MK-801. *P < 0.05, **P < 0.01, compared with MK-801-treated group using Newman–Keuls post hoc test, following significant anova. Baseline activity is shown by the dotted line. (B) Effects of ADN-1184 on stereotypies induced by MK-801 (1.2 mg·kg−1). Each symbol represents mean ± SEM of the duration of stereotyped behaviour in the 11–15 min period after MK-801 administration (n = 8 per group). *P < 0.05, **P < 0.01, compared with vehicle-treated group using Mann–Whitney U-test, following significant Kruskal–Wallis anova. (C) Effects of ADN-1184 on conditioned avoidance. Each symbol represents mean ± SEM number of CAR recorded during a session with 30 trials, 60 min after drug administration (n = 7 per group). *P < 0.05, **P < 0.01, CAR compared with vehicle-treated group using Newman–Keuls post hoc test, following significant anova.
MK-801-induced stereotypies
ADN-1184 dose-dependently reduced MK-801-induced stereotyped behaviour (Figure 2B). Kruskal–Wallis anova revealed a significant difference between tested groups, H(3) = 21.2, P < 0.01. Post hoc Mann–Whitney U-test revealed significant effects at doses of 3 (P < 0.05), 10 and 30 mg·kg−1 (P < 0.01); the MED was 3.0 mg·kg−1.
CAR
Control animals injected with vehicle showed a high level of avoidance while ADN-1184 dose-dependently suppressed CAR, F(3, 24) = 10.0, P < 0.01 (Figure 2C). The post hoc Newman–Keuls test revealed that significant CAR suppression was present at the dose of 3.0 (MED) and 10.0 mg·kg−1 (P < 0.01).
FST
ADN-1184 reduced the immobility time, F(5, 50) = 5,8, P < 0.01. The post hoc Newman–Keuls test revealed that significant reduction in immobility, in comparison to the control group, was present at two tested doses, that is, 0.3 and 1.0 mg·kg−1 (P < 0.01), MED = 0.3 mg·kg−1. The higher doses of the drug (>3.0 mg·kg−1) were inactive (Figure 3).
Figure 3.
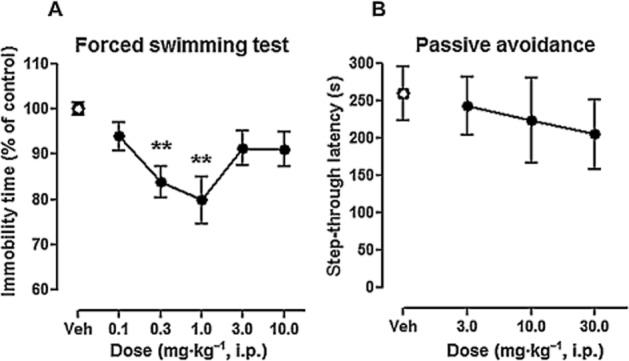
Effects of ADN-1184 in tests related to antidepressant-like activity and impairment of memory. (A) Effects of ADN-1184 on forced swimming test. Each symbol represents mean ± SEM immobility time during 5 min forced swimming session. Drug or vehicle was administered 1 h before the test (n = 16 for vehicle group, n = 8 per drug group). **P < 0.01, compared with vehicle-injected control group using Newman–Keuls post hoc test, following significant anova. (B) Effects of ADN-1184 on step-through latency performed 1 day after training. Animals were given drug or vehicle 1 h before training session. Each symbol represents mean ± SEM latency to enter the dark compartment (n = 8 per group). **P < 0.01, compared with vehicle group using Mann–Whitney U-test, following significant results of Kruskal–Wallis anova.
PA
ADN-1184 did not alter step-through latencies to enter the dark compartment Kruskal–Wallis anova, H(3) = 0.4, P > 0.05. At the dose range tested, ADN-1184 did not demonstrate significant amnesic effects (MED > 30.0 mg·kg−1 i.p.) (Figure 3).
Catalepsy bar test
ADN-1184 induced weak cataleptogenic responses (see Figure 4A). A Kruskal–Wallis anova revealed non-significant effects in rats tested 30 and 120 min after injection, Hs < 7.4, P > 0.05. There was a weak cataleptogenic effect observed 60 min after ADN-1184 injection, H(3) = 13.3, P < 0.05. Up to a dose of 30 mg·kg−1, the catalepsy was below cut-off threshold as measured with the applied scoring system (MED > 30 mg·kg−1). The Mann–Whitney U-test revealed a significant effect at the highest tested dose – 30 mg·kg−1 (P < 0.01).
Figure 4.
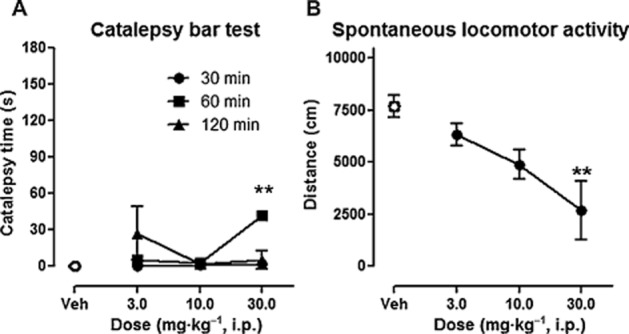
Effects of ADN-1184 in tests of motor interference. (A) Catalepsy induced by ADN-1184. Each symbol represents mean ± SEM, catalepsy was measured 30, 60 and 120 min after drug administration (n = 8 per group). **P < 0.01, compared with vehicle-injected control group using Mann–Whitney U-test, following significant Kruskal–Wallis anova. (B) Effects of ADN-1184 on spontaneous locomotor activity. Distance travelled by rats was recorded 61–90 min after substance injection (n = 8 per group). **P < 0.01, compared with vehicle-injected control group using Newman–Keuls post hoc test, following significant anova.
Spontaneous locomotor activity
ADN-1184 modestly reduced spontaneous locomotor activity, F(3, 28) = 5.9, P < 0.01 (see Figure 4B). The post hoc Newman–Keuls test revealed that significant locomotor impairment occurred only at the highest tested dose (MED = 30.0 mg·kg−1 i.p., P < 0.01).
Plasma and brain exposure determination
Substantial plasma exposure of ADN-1184 was observed at 1 h after dosing (the time point used for behavioural observations). Exposure then decreased to near the limit of quantitation by 6 h post dosing. Similarly, marked brain ADN-1184 concentrations were noted at 1 h post dose, following which ADN-1184 levels decreased to low residual levels by 6 h after dosing (see Table 3).
Table 3.
Plasma and brain exposure in rat with ADN-1184
| Time (h) | Plasma (ng·mL−1) | Brain (ng·g−1) |
|---|---|---|
| 1 | 210 ± 35 | 656 ± 155 |
| 2 | 148 ± 23 | n.d. |
| 4 | 20 ± 4 | n.d. |
| 6 | 3 ± 1 | 30 ± 1 |
ADN-1184 was administered at a dose of 10 mg·kg−1 i.p. Blood and brain samples were collected at different time points and drug concentrations determined by LCMS.
n.d., not determined.
Discussion
In view of the ageing population of many developed countries, BPSD is an increasingly challenging problem, generating onerous social and economic costs for the care of elderly patients. Although atypical antipsychotics are commonly used to treat BPSD, they have substantial limitations due to their propensity to elicit memory deficits, EPS, adverse cardiovascular events and metabolic dysfunction. It is therefore desirable to identify novel treatments that are capable of controlling both psychosis and depressive symptoms without exacerbating cognitive impairment or inducing motor disruption (Jeste et al., 2008).
The present study suggests that ADN-1184 possesses a preclinical profile of activity that corresponds to these criteria. Unlike commercially available antipsychotics, ADN-1184 was active in a series of tests related to both psychotic symptoms and mood deficits without disrupting motor control or memory performance. This favourable in vivo profile is likely mediated by ADN-1184's potent antagonism of 5-HT6, 5-HT7 and 5-HT2A receptors with more modest antagonism of dopamine D2 and D3 receptors.
ADN-1184 is potently active in models of psychosis
In tests related to antipsychotic properties, ADN-1184 showed activity consistent with marked capacity to control psychosis.
Firstly, ADN-1184 reversed hyperactivity induced by the NMDA receptor antagonist, MK-801 (0.3 mg·kg−1). This test is particularly relevant to dementia patients who suffer from psychoses of glutamatergic origin (see Introduction) which may be controlled by potent antagonism of 5-HT2A receptors (Millan et al., 1999).
The potency of ADN-1184 was similar to that of clozapine, olanzapine and lurasidone, as previously tested in the same conditions (Kołaczkowski et al., 2013). In contrast, ADN-1184 was more efficacious in this test than aripiprazole, which only partially reversed MK-801-induced hyperactivity (Kołaczkowski et al., 2013) and did not ameliorate psychotic symptoms in patients with AD (De Deyn et al., 2005). D2 receptor partial agonists may therefore be less ‘incisive’ in controlling NMDA receptor hypofunction-elicited psychosis, as previously noted in other studies (Bardin et al., 2007).
Secondly, ADN-1184 also abolished stereotypies induced by a higher dose of MK-801 (1.2 mg·kg−1). Under these conditions, head weaving and circling behaviour is observed in a complex pattern (Wu et al., 2005). Antipsychotics such as olanzapine or risperidone are capable of completely inhibiting MK-801-induced behaviours (Bradford et al., 2010). The fact that ADN-1184 achieved full reversal of the stereotypies therefore suggests that it has potent ‘antipsychotic-like’ properties.
Finally, ADN-1184 inhibited CAR, an effect typical of antipsychotics (Wadenberg, 2010), in a dose range similar to that of its effects against MK-801-induced hyperlocomotion (MED 3 mg·kg−1; Figure 2). The CAR response was completely abolished, similar to the effect achieved with haloperidol and asenapine under the same conditions, but unlike lurasidone or aripiprazole that exhibited only partial effects (Kołaczkowski et al., 2013).
Taken together, the present results suggest that ADN-1184 possesses incisive antipsychotic activity that is compatible with control of psychotic symptoms such as those observed in BPSD. In particular, glutamatergic dysfunction has been associated with psychotic symptoms in elderly patients suffering from dementia (Scheuer et al., 1996; Olivares et al., 2012).
ADN-1184 is active in models of mood deficits
ADN-1184 was active in a classic model of antidepressant-like activity (Porsolt et al., 1978), thus combining incisive antipsychotic-like activity (see above) with a favourable profile on a test of mood deficit.
Firstly, at low doses, ADN-1184 significantly decreased immobility duration, a measure of behavioural despair, with higher doses not showing significant effects. Risperidone, aripiprazole and lurasidone also exhibited U-shaped effects at low doses (Kołaczkowski et al., 2013) but ADN-1184 was active at two doses (0.3 and 1.0 mg·kg−1; Figure 3) whereas other antipsychotics were only active at a single dose (Kołaczkowski et al., 2013). Nevertheless, clozapine was a notable exception, showing activity in the FST at two doses, similar to ADN-1184 here. These data suggest that ADN-1184 may have promising activity for alleviation of depressive symptoms.
Secondly, ADN-1184 reduced immobility time in the FST by about 20% compared with that of vehicle-treated subjects. In contrast, other antipsychotics, including clozapine and lurasidone, reduced immobility times by about 10–15% when tested under the same conditions. Insofar as an acute effect in this animal model is predictive of clinical efficacy, this suggests that ADN-1184 may have more pronounced effects on mood deficits. It should be noted that the tricyclic antidepressant, imipramine 10 mg·kg−1, decreased immobility times by about 25%, relative to control values (Kołaczkowski et al., 2013), an effect only slightly greater than that of ADN-1184. Thus, the properties of ADN-1184 observed herein are of the same order of magnitude as those of an established antidepressant.
Thirdly, the active dose range of ADN-1184 in the FST is somewhat lower than in tests of antipsychotic-like activity. A similar pattern was observed for other antipsychotics, although not with clozapine. It may be speculated that, for drugs such as ADN-1184, clinical dosing may be adapted according to the symptoms exhibited by BPSD patients: those with depressive symptoms would only need to receive low doses whereas those exhibiting psychotic episodes would warrant increased dosing.
ADN-1184 does not impair performance in a memory test
In view of the fact that BPSD patients experience the full spectrum of cognitive disturbance found in dementia (Hersch and Falzgraf, 2007), it would be desirable to avoid treating them with drugs that elicit or accentuate cognitive impairment.
ADN-1184 did not impair the PA response across a broad dose range that covers activity in the FST, CAR and MK-801-induced behaviours (Figure 3). This contrasts sharply with the effects of clinically used antipsychotics: under the same conditions, all the drugs elicited dose-dependent disruption in the PA test. In the case of clozapine and olanzapine, the doses that impaired performance were lower than those that inhibited the CAR response or MK-801-induced hyperlocomotion (Kołaczkowski et al., 2013), indicating that antipsychotic-like effects are accompanied by interference with memory function. In contrast, ADN-1184 shows therapeutic-like activity without impairing memory performance, suggesting that it is possible to identify drug candidates that are more compatible with treatment of patients with dementia.
ADN-1184 does not elicit motor interference at ‘therapeutic’ doses
While being active in models of psychosis and mood deficit, ADN-1184 did not elicit catalepsy, except weakly at a high dose (30 mg·kg−1; Figure 4). This differentiates ADN-1184 from other antipsychotics, including risperidone or haloperidol, which are still used in agitated patients (Carson et al., 2006). These drugs elicit dose-dependent EPS (Rummel-Kluge et al., 2012), a side effect that may be especially disruptive in elderly patients who already suffer from motor difficulties.
A related measure of motor impairment is inhibition of spontaneous locomotor activity as determined by the distance travelled by the subject in an open field. Even antipsychotics that do not elicit catalepsy, such as clozapine, may potently decrease locomotor activity because of their sedative properties (Miller, 2000). In fact, decreased locomotor activity was observed for reference drugs over a similar dose range as that which elicits antipsychotic-like activity (Kołaczkowski et al., 2013). In contrast, ADN-1184 only began to decrease locomotor activity at doses (MED 30 mg·kg−1) that were significantly greater than those active in the ‘therapeutic’ tests.
ADN-1184 exhibits a novel profile of receptor interaction
A distinctive in vitro profile may underlie the differentiation of ADN-1184 from current atypical antipsychotics. The latter generally interact with many different receptor targets, including those that may elicit some side effects and/or limit their therapeutic efficacy. For example, these drugs also bind potently to muscarinic and histamine receptors which are associated with cholinergic interference and sedation respectively. In addition, they act as potent 5-HT2C receptor antagonists, a property that is a risk factor for metabolic dysfunction (Reynolds and Kirk, 2010; Bai et al., 2011). Aripiprazole is well tolerated but its partial agonist activity at dopamine D2 receptors may limit its efficacy for control of psychotic symptoms in elderly patients (De Deyn et al., 2005).
In contrast to these drugs, ADN-1184 combines dopamine D2 and D3 receptor antagonism with potent blockade of 5-HT6, 5-HT7 and 5-HT2A receptors in the absence of anticholinergic effects (Tables 1 and 2). This profile may underlie its unusual combination of antipsychotic-like and antidepressant-like activities without cognitive or motor impairment. Indeed, as described in the Introduction, 5-HT6 receptor antagonism is associated with beneficial effects on mood and cognitive parameters (Geldenhuys and Van der Schyf, 2009; Hirano et al., 2009). In the case of ADN-1184, its affinity for 5-HT6 receptors is of the same order of magnitude as that for D2 receptors and likely to be expressed at antipsychotic doses. Nevertheless, it is possible that a different balance of 5-HT6/D2 affinity could yield a pharmacological profile that shows improved activity.
In the case of 5-HT7 receptors, a growing body of work indicates that blockade of these sites mediates antidepressant and pro-cognitive properties (Galici et al., 2008; Hedlund, 2009; Bonaventure et al., 2011). In addition, 5-HT7 receptor antagonism induces antipsychotic-like activity in animal models (Galici et al., 2008) and may underlie some therapeutic properties of lurasidone (Ishibashi et al., 2010). It should also be noted that amisulpride, which shows clinical antidepressant properties in addition to antipsychotic activity, possesses 5-HT7 receptor antagonist activity (Abbas et al., 2009; Ishibashi et al., 2010).
ADN-1184 also interacts with α1 adrenoceptors. Antagonism at these receptors is a prominent feature of the receptor profile of many antipsychotics including clozapine, risperidone and quetiapine (Arnt et al., 2008; Newman-Tancredi and Kleven, 2011) and may contribute to their therapeutic activity. Indeed, in combination with modest dopamine D2 receptor occupancy, α1 adrenoceptor blockade might improve antipsychotic efficacy and widen the therapeutic window with regard to EPS (Wadenberg et al., 2000). In addition, α1 adrenoceptors are highly co-expressed with 5-HT2A receptors in prefrontal cortex (PFC), suggesting that combined blockade of both these targets, as found in ADN-1184, may provide more robust control of PFC function (Santana et al., 2013). Furthermore, in AD patients, (i) deregulation of α1 adrenoceptors has been reported in frontal cortex of post mortem brain (Szot et al., 2006); and (ii) treatment with the α1 adrenoceptor antagonist, prazosin, reduced agitation and aggressive behaviour in a pilot clinical trial (Wang et al., 2009). While these observations suggest that α1 adrenoceptor antagonism may be desirable in drugs targeted at BPSD, antagonism of peripheral α1 adrenoceptors is associated with cardiovascular effects, notably hypotensive activity, which would need to be addressed in elderly patients with potentially fragile cardiac function.
In comparison, ADN-1184 has only moderate or low affinity for other receptor targets, including hERG channels associated with cardiovascular risk (Table 1) or muscarinic and histamine receptors. Antagonism of the latter sites is associated with cognitive interference and sedative properties, respectively, so the low affinity of ADN-1184 at these sites is likely to underlie its freedom from impairment of performance in the PA test and only modest inhibition of spontaneous locomotor activity.
Conclusions and perspectives
The present study shows that ADN-1184 (i) is potently active in tests of antipsychotic-like activity; (ii) exhibits antidepressant-like activity; and (iii) does not disrupt performance in a memory test or in motor coordination. This profile of in vivo activity is promising in the context of identifying novel treatments for BPSD. Indeed, current antipsychotics do not possess such a favourable profile and there is an unmet need for novel pharmacotherapeutic agents that are better suited to elderly patients.
However, the in vivo results described here were all obtained upon acute administration and it is necessary to determine the effects of the compound following repeated administration. Indeed, antidepressant-like effects may become more accentuated upon chronic treatment (Morley-Fletcher et al., 2004), possibly increasing the dose range that is active in the FST. Further, it would be interesting to determine the capacity of ADN-1184 to reverse memory deficits elicited by a muscarinic antagonist such as scopolamine. Indeed, we would expect the pronounced 5-HT6 antagonist properties of ADN-1184 to favour cholinergic transmission and improve memory function in elderly patients (Da Silva Costa-Aze et al., 2012; H. Lundbeck A/S, 2012).
Taken together, the present data indicate that it is possible to identify drug candidates that exhibit an in vivo profile of action that is consistent with potentially improved management of BPSD. Indeed, ADN-1184 is an example of an investigational drug that exhibits activity in animal models of psychosis and mood deficit without disrupting memory or eliciting motor side effects.
Conflict of interest
MK is an employee of Adamed Ltd. AN-T has received consulting and/or speaker honoraria from Adamed, AstraZeneca, BioWin Consortium, Bristol-Myers Squibb, ESC Corp., Sunovion, and UBC, and is Chief Scientific Officer and stockholder at Neurolixis Inc. All other authors declare that they have no conflicts of interest.
Acknowledgments
This study was financially supported by Adamed Ltd. and the National Centre for Research and Development (NCBR), Grant No. KB/88/12655/IT1-C/U/08.
Glossary
- AD
Alzheimer's disease
- BPSD
behavioural and psychological symptoms of dementia
- CAR
conditioned avoidance response
- CS
conditioned stimulus
- EPS
extrapyramidal symptoms
- FST
forced swimming test
- PA
passive avoidance
- SGA
second-generation antipsychotics
- UCS
unconditioned stimulus
References
- Abbas AI, Hedlund PB, Huang X-P, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl) 2009;205:119–128. doi: 10.1007/s00213-009-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ, Collaborators CGTP. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. [Google Scholar]
- Andiné P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Mårtensson E, et al. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Arnt J. Pharmacological specificity of conditioned avoidance response inhibition in rats: inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol (Copenh) 1982;51:321–329. doi: 10.1111/j.1600-0773.1982.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Arnt J, Olsen CK. 5-HT6 receptor ligands and their antipsychotic potential. Int Rev Neurobiol. 2011;96:141–161. doi: 10.1016/B978-0-12-385902-0.00006-1. [DOI] [PubMed] [Google Scholar]
- Arnt J, Bang-Andersen B, Dias R, Bøgesø KP. Strategies for pharmacotherapy of schizophrenia. Drugs Future. 2008;33:777–791. [Google Scholar]
- Bai YM, Chen T-T, Liou Y-J, Hong C-J, Tsai S-J. Association between HTR2C polymorphisms and metabolic syndrome in patients with schizophrenia treated with atypical antipsychotics. Schizophr Res. 2011;125:179–186. doi: 10.1016/j.schres.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Bardin L, Auclair A, Kleven MS, Prinssen EPM, Koek W, Newman-Tancredi A, et al. Pharmacological profiles in rats of novel antipsychotics with combined dopamine D2/serotonin 5-HT1A activity: comparison with typical and atypical conventional antipsychotics. Behav Pharmacol. 2007;18:103–118. doi: 10.1097/FBP.0b013e3280ae6c96. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Aluisio L, Shoblock J, Boggs JD, Fraser IC, Lord B, et al. Pharmacological blockade of serotonin 5-HT7 receptor reverses working memory deficits in rats by normalizing cortical glutamate neurotransmission. PLoS ONE. 2011;6:e20210. doi: 10.1371/journal.pone.0020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AM, Savage KM, Jones DNC, Kalinichev M. Validation and pharmacological characterisation of MK-801-induced locomotor hyperactivity in BALB/C mice as an assay for detection of novel antipsychotics. Psychopharmacology (Berl) 2010;212:155–170. doi: 10.1007/s00213-010-1938-0. [DOI] [PubMed] [Google Scholar]
- Carr GV, Schechter LE, Lucki I. Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology (Berl) 2011;213:499–507. doi: 10.1007/s00213-010-1798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson S, McDonagh MS, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc. 2006;54:354–361. doi: 10.1111/j.1532-5415.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- Da Silva Costa-Aze V, Quiedeville A, Boulouard M, Dauphin F. 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology (Berl) 2012;222:99–115. doi: 10.1007/s00213-011-2627-3. [DOI] [PubMed] [Google Scholar]
- De Deyn P, Jeste DV, Swanink R, Kostic D, Breder C, Carson WH, et al. Aripiprazole for the treatment of psychosis in patients with Alzheimer's disease: a randomized, placebo-controlled study. J Clin Psychopharmacol. 2005;25:463–467. doi: 10.1097/01.jcp.0000178415.22309.8f. [DOI] [PubMed] [Google Scholar]
- Galici R, Boggs JD, Miller KL, Bonaventure P, Atack JR. Effects of SB-269970, a 5-HT7 receptor antagonist, in mouse models predictive of antipsychotic-like activity. Behav Pharmacol. 2008;19:153–159. doi: 10.1097/FBP.0b013e3282f62d8c. [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Van der Schyf CJ. The serotonin 5-HT6 receptor: a viable drug target for treating cognitive deficits in Alzheimer's disease. Expert Rev Neurother. 2009;9:1073–1085. doi: 10.1586/ern.09.51. [DOI] [PubMed] [Google Scholar]
- H. Lundbeck A/S. Lundbeck's Lu AE58054 meets primary endpoint in large placebo-controlled clinical proof of concept study in people with Alzheimer's disease. Corp. Release. 2012;472:1–4. Available at: http://investor.lundbeck.com/releasedetail.cfm?ReleaseID=677436 (accessed 23/10/2012) [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch EC, Falzgraf S. Management of the behavioral and psychological symptoms of dementia. Clin Interv Aging. 2007;2:611–621. doi: 10.2147/cia.s1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Piers TM, Searle KL, Miller ND, Rutter AR, Chapman PF. Procognitive 5-HT6 antagonists in the rat forced swimming test: potential therapeutic utility in mood disorders associated with Alzheimer's disease. Life Sci. 2009;84:558–562. doi: 10.1016/j.lfs.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer's disease. Hum Mol Genet. 1998;7:1507–1509. doi: 10.1093/hmg/7.9.1507. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334:171–181. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol. 2007;572:160–170. doi: 10.1016/j.ejphar.2007.06.058. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Finkel SI. Psychosis of Alzheimer's disease and related dementias. Diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33:957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołaczkowski M, Kowalski P, Jaśkowska J, Marcinkowska M, Mitka K, Bucki A, et al. Aryl sulfonamides for the treatment of CNS diseases. 2012. PCT Int Appl WO 2012035123.
- Kołaczkowski M, Mierzejewski P, Bienkowski P, Wesolowska A, Newman-Tancredi A. Targeting behavioural and psychological symptoms of dementia: comparison of antipsychotics and the novel compound ADN-1184 in rat models. European Neuropsychopharmacology. 2013;23(Supplement 2) Poster P.5.a.003. [Google Scholar]
- Lazareno S, Birdsall NJ. Estimation of competitive antagonist affinity from functional inhibition curves using the Gaddum, Schild and Cheng-Prusoff equations. Br J Pharmacol. 1993;109:1110–1119. doi: 10.1111/j.1476-5381.1993.tb13737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liperoti R, Pedone C, Corsonello A. Antipsychotics for the treatment of behavioral and psychological symptoms of dementia (BPSD) Curr Neuropharmacol. 2008;6:117–124. doi: 10.2174/157015908784533860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiseau F, Dekeyne A, Millan MJ. Pro-cognitive effects of 5-HT6 receptor antagonists in the social recognition procedure in rats: implication of the frontal cortex. Psychopharmacology (Berl) 2008;196:93–104. doi: 10.1007/s00213-007-0934-5. [DOI] [PubMed] [Google Scholar]
- Lorke DE, Lu G, Cho E, Yew DT. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. doi: 10.1186/1471-2202-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu J-H, Ewing B, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306:1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- Marcos B, García-Alloza M, Gil-Bea FJ, Chuang TT, Francis PT, Chen CP, et al. Involvement of an altered 5-HT-{6} receptor function in behavioral symptoms of Alzheimer's disease. J Alzheimers Dis. 2008;14:43–50. doi: 10.3233/jad-2008-14104. [DOI] [PubMed] [Google Scholar]
- Marsh A. Visual hallucinations during hallucinogenic experience and schizophrenia. Schizophr Bull. 1979;5:627–630. doi: 10.1093/schbul/5.4.627. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet J, et al. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Miller DD. Review and management of clozapine side effects. J Clin Psychiatry. 2000;61(Suppl. 8):14–17. discussion 18–19. [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudéry M, Mocaer E, Froger N, Lanfumey L, Laviola G, et al. Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT(1A) receptor mRNA in prenatally stressed rats. Neuropharmacology. 2004;47:841–847. doi: 10.1016/j.neuropharm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Kleven MS. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl) 2011;216:451–473. doi: 10.1007/s00213-011-2247-y. [DOI] [PubMed] [Google Scholar]
- Nobili A, Pasina L, Trevisan S, Riva E, Lucca U, Tettamanti M, et al. Use and misuse of antipsychotic drugs in patients with dementia in Alzheimer special care units. Int Clin Psychopharmacol. 2009;24:97–104. doi: 10.1097/yic.0b013e328323aaf0. [DOI] [PubMed] [Google Scholar]
- Ogren SO, Hall H, Köhler C, Magnusson O, Sjöstrand SE. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology (Berl) 1986;90:287–294. doi: 10.1007/BF00179179. [DOI] [PubMed] [Google Scholar]
- Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, et al. N-methyl d-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012;9:746–758. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment – pharmacological mechanisms. Pharmacol Ther. 2010;125:169–179. doi: 10.1016/j.pharmthera.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Nguyen TN, Karlsson J-J, Arnt J. Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology. 2008;33:2657–2666. doi: 10.1038/sj.npp.1301654. [DOI] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Kissling W, et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull. 2012;38:167–177. doi: 10.1093/schbul/sbq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Expression of α1-adrenergic receptors in rat prefrontal cortex: cellular co-localization with 5-HT2A receptors. Int J Neuropsychopharmacol. 2013;16:1139–1151. doi: 10.1017/S1461145712001083. [DOI] [PubMed] [Google Scholar]
- Scheuer K, Maras A, Gattaz WF, Cairns N, Förstl H, Müller WE. Cortical NMDA receptor properties and membrane fluidity are altered in Alzheimer's disease. Dement Basel Switz. 1996;7:210–214. doi: 10.1159/000106881. [DOI] [PubMed] [Google Scholar]
- Schulze J, Bussche H, Glaeske G, Kaduszkiewicz H, Wiese B, Hoffmann F. Impact of safety warnings on antipsychotic prescriptions in dementia: nothing has changed but the years and the substances. Eur Neuropsychopharmacol. 23:1034–1042. doi: 10.1016/j.euroneuro.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Siegel RK. Phencyclidine and ketamine intoxication: a study of four populations of recreational users. NIDA Res Monogr. 1978;21:119–147. [PubMed] [Google Scholar]
- Sukonick DL, Pollock BG, Sweet RA, Mulsant BH, Rosen J, Klunk WE, et al. The 5-HTTPR*S/*L polymorphism and aggressive behavior in Alzheimer disease. Arch Neurol. 2001;58:1425–1428. doi: 10.1001/archneur.58.9.1425. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus coeruleus and hippocampus of postmortem subjects with Alzheimer's disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen CLP, Mack WJ, Keefe RSE, Sano M, Sultzer DL, Stroup TS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168:831–839. doi: 10.1176/appi.ajp.2011.08121844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg ML, Hertel P, Fernholm R, Hygge Blakeman K, Ahlenius S, Svensson TH. Enhancement of antipsychotic-like effects by combined treatment with the alpha1-adrenoceptor antagonist prazosin and the dopamine D2 receptor antagonist raclopride in rats. J Neural Transm. 2000;107:1229–1238. doi: 10.1007/s007020070036. [DOI] [PubMed] [Google Scholar]
- Wadenberg M-LG. Conditioned avoidance response in the development of new antipsychotics. Curr Pharm Des. 2010;16:358–370. doi: 10.2174/138161210790170085. [DOI] [PubMed] [Google Scholar]
- Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009;17:744–751. doi: 10.1097/JGP.0b013e3181ab8c61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesołowska A. Study into a possible mechanism responsible for the antidepressant-like activity of the selective 5-HT6 receptor antagonist SB-399885 in rats. Pharmacol Rep. 2007;59:664–671. [PubMed] [Google Scholar]
- Wu J, Zou H, Strong JA, Yu J, Zhou X, Xie Q, et al. Bimodal effects of MK-801 on locomotion and stereotypy in C57BL/6 mice. Psychopharmacology (Berl) 2005;177:256–263. doi: 10.1007/s00213-004-1944-1. [DOI] [PubMed] [Google Scholar]


