Abstract
Background and Purpose
The GABAB receptor agonist baclofen reduces urethral resistance and detrusor overactivity in patients with spasticity. However, baclofen's side effects limit its use for the treatment of overactive bladder (OAB). Here, we tested a novel GABAB positive allosteric modulator (PAM) ADX71441 in models of OAB in mice and guinea pigs.
Experimental Approach
Mice were left untreated or given (p.o.) vehicle (1= CMC), ADX71441 (1, 3, 10 mg kg−1) or oxybutynin (100 mg kg−1; Experiment 1) or vehicle (1= CMC), baclofen (1, 3, 6 mg kg−1) or oxybutynin (Experiment 2). Treated mice were then overhydrated with water, challenged with furosemide, before being placed into micturition chambers and monitored for urinary parameters. In anaesthetized guinea pigs, intravesical infusion of acetic acid was used to induce OAB and the effects of ADX71441 (1, 3 mg kg−1) or baclofen (1 mg kg−1), administered i.v., on cystometric parameters were monitored.
Key Results
In mice, 10 mg kg−1 ADX71441 increased urinary latencies, reduced the number of urinary events and the total and average urinary volumes. In guinea pigs, ADX71441 (1 and 3 mg kg−1) increased the intercontraction interval (ICI) and bladder capacity (BC), and reduced micturition frequency (MF) compared to vehicle. At 3 mg kg−1 ADX71441 completely inhibited the micturition reflex and induced overflow incontinence in five out of 10 animals. Baclofen slightly increased ICI and BC and reduced MF.
Conclusion and Implications
Our findings demonstrate, for the first time, that a GABAB PAM has potential as a novel approach for the treatment of OAB.
Keywords: allosteric modulation, GABAB, urinary bladder, micturition, overactive bladder, baclofen, oxybutynin
Introduction
Overactive bladder (OAB) is a common, chronic and debilitating disorder characterized by urinary urgency often accompanied by incontinence (involuntary urine leakage), frequency (≥8 micturition in 24 h) and nocturia (≥1 awakening per night to void; Lam and Hilas, 2007). OAB has a significant negative effect on the patient's quality of life and emotional well-being. It leads to withdrawal from social activities and isolation, increased risks of falls and fractures (especially in elderly patients), depression, sleep disturbances and associated fatigue (Ouslander, 2004; Chu and Dmochowski, 2006).
OAB is abnormal and involuntary contraction of the detrusor muscle in the bladder during the filling phase. Its pathophysiology appears to be complex and believed to involve peripheral as well as central mechanisms. The first-line pharmacotherapy for OAB involves several muscarinic receptor antagonists, including oxybutynin and tolterodine (Lam and Hilas, 2007). While these drugs have been shown to produce some improvement in OAB symptoms, they are also linked to significant side effects, including dry mouth, constipation, tachycardia, sedation, impaired cognitive function and blurred vision that cause non-compliance and discontinuation of treatment (Erdem and Chu, 2006). Thus, alternative pharmacological strategies are needed to deliver more effective and better tolerated compounds compared to those available at present.
There is a wealth of experimental, preclinical and clinical evidence suggesting that the GABAB receptor is a viable target for the discovery of new pharmacotherapies for OAB. GABAB receptors play an important role in the regulation of bladder activity at several sites in the CNS and periphery (Santicioli et al., 1984; Maggi et al., 1985; Chen et al., 1992; Malcangio and Bowery, 1996; Coggeshall and Carlton, 1997). In several animal models of OAB, baclofen, the orthosteric agonist of the GABAB receptor, reduced signs of bladder overactivity. For example, intrathecal baclofen abolished bladder overactivity induced in female rats by intravesical oxyhaemoglobin administration (Pehrson et al., 2002). Also, baclofen dose-dependently inhibited bladder contractions and decreased micturition pressure in female rats showing bladder overactivity as a result of spinal cord injury (Miyazato et al., 2008). Importantly, baclofen has been shown to alleviate the symptoms of OAB in patients with idiopathic detrusor instability (Taylor and Bates, 1979), neurogenic voiding disturbances (Haubensak, 1977) and non-neurogenic dysfunctional voiding (Xu et al., 2007). However, while the efficacy of baclofen in OAB has been shown in several clinical studies, the drug is not widely prescribed due to its short half-life and long list of side effects.
Here, we evaluated the effects of a novel GABAB positive allosteric modulator (PAM), ADX71441, in experimental models of OAB. GABAB PAMs have long been recognized as a powerful pharmacological approach to achieve activity-dependent efficacy in drug action while offering the potential of being devoid of side effects associated with GABAB receptor agonists (Pin and Prézeau, 2007). ADX71441 was discovered through the lead optimization of a chemical series acting as PAMs, which was identified following a high throughput screening campaign of the corporate chemical library of Addex Therapeutics (L. Tang et al. unpubli. obs.). A series of in vitro and in vivo studies identified ADX71441 as a potent, selective, reversible and orally bioavailable GABAB PAM, which recently received approval for Phase I clinical studies (Addex Therapeutics Web page). In rodents, ADX71441 showed efficacy in the marble burying and elevated plus maze tests relevant for anxiety-like reactivity (M. Kalinichev et al., unpubl. data), supporting and expanding earlier evidence of the broad anxiolytic-like profiles of GABAB PAMs CGP7930 and GS39783 (Cryan et al., 2004; Mombereau et al., 2004; Jacobson and Cryan, 2008). Acute ADX71441 reduced visceral pain in the acetic acid (AA) writhing test and also reduced chronic pain in the monosodium iodoacetate (MIA)-induced model of osteoarthritis, after acute and subchronic administration regimens (M. Kalinichev, unpubl. obs.). Also, ADX71441 was found to markedly and specifically reduce alcohol consumption in mouse models of binge drinking and chronic alcoholism (Hwa et al., 2013). Here, we tested the effects of ADX71441 and baclofen in two rodent models of OAB: (i) bladder overactivity in conscious mice undergoing water overload and (ii) overactivity induced by intravesicle administration of diluted AA in anaesthetized guinea pigs. ADX71441 reduced the signs of bladder overactivity in both models of OAB, suggesting that the GABAB PAM approach has potential for the pharmacological treatment of OAB.
Methods
Animals
Unless otherwise indicated below, studies used adult male C57Bl6/J mice (24–30 g), purchased from Charles River Laboratories (Wilmington, MA, USA) and group-housed (3–5 per cage) in the animal facility of Melior Discovery (Exton, PA, USA), as well as adult female Dunkin Hartley guinea pigs (290–370 g) purchased from Charles River (L'Arbresle, France) and group-housed (four per cage) in the animal facility of UROsphere (Toulouse, France) under standard conditions. Animals were kept on a 12-h light/dark cycle and were provided with food and water ad libitum.
All experimental procedures and conditions were performed in full compliance with the Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes, the French National Committee (décret 87/848) for the care and use of laboratory animals, the National Institutes of Health and the Declaration of Helsinki. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Model of OAB in mice
Equipment
Micturition chambers consisted of two clear cylindrical Plexiglas observation tubes (20 cm length × 13 cm diameter), where the top tube was designed to hold the animal, while the bottom one was equipped with the urine collection and detection system comprised of omega load sensors with a plastic cup on top to collect urine. Data were collected using pDAQ data collection box and Dataquest pDAQ programme every 5 s post-furosemide injection. Urination events were determined by calculating the differences between subsequent load sensor readings. A difference of 5 mg or more for 60 continuous seconds was recorded as an event, total volume equals final reading minus initial reading and the average volume per event was calculated as the total volume divided by the number of events. The time to the first event was also determined by calculating the amount of time from furosemide injection to the first event.
Experimental procedure
The mouse stress incontinence model was modified from the procedure described previously in rats (Harada et al., 1992; Harada and Constantinou, 1993). In a pilot study in mice, oxybutynin, administered p.o. (via gavage), at doses between 10 and 100 mg kg−1, induced maximal effects on the OAB variables. Also, according to Yoshida et al., (2010), in mice, 30 mg kg−1 oxybutynin resulted in 50= binding on bladder tissue. Thus, in the present study, 100 mg kg−1 oxybutynin was used as a positive control.
Mice (n = 8 per group) were deprived of food for 24 h prior to the study, and were either left untreated (although handled to imitate dosing) or were administered p.o. (via gavage), vehicle [1= carboxymethyl cellulose (CMC)], ADX71441 (1, 3, 10 mg kg−1) or oxybutynin (100 mg kg−1; Experiment 1). In Experiment 2, mice (n = 8 per group), were either untreated or received (p.o.) vehicle (1= CMC), baclofen (1, 3, 6 mg kg−1) or oxybutynin (100 mg kg−1). The doses of ADX71441 and baclofen in this study were chosen to avoid robust and potentially confounding effects on locomotor activity. Earlier, we observed that at 10 mg kg−1, the effects of ADX71441 on locomotor activity in mice, if any, were mild, while at the same dose, baclofen causes near-complete suppression of locomotor activity (M. Kalinichev et al., unpubl. data). Fifty-five minutes after administration of vehicle/ADX71441/baclofen or 5 min after administration of oxybutynin, the treated animals were administered 1 mL of water s.c. Within 5 min after water administration, furosemide (10 mg mL−1; dissolved in water) was administered i.p. to all animals except the untreated controls. Immediately following the furosemide injection, mice were individually placed in micturition chambers for 90 min. Terminal blood samples were collected from all animals treated with ADX71441 at the end of the experiment, approximately 150 min following treatment, and plasma was analysed as described for pharmacokinetic studies. The following variables were assessed: total urinary events (the event being defined as production of at least 100 μL of urine in less than 1 min), latency to the first urinary event (s), total urinary volume (mL) and average urinary volume per event (mL). All variables (except the latency) were analysed as totals for the first (0–45 min) and the second (45–90 min) 45-min periods. The data were analysed with one-way ANOVA, followed by Dunnett's test with comparison of treatment groups to vehicle treatment and to the control group. P < 0.05 was accepted as being statistically significant.
Pharmacokinetic study in the guinea pig using the i.v. route
Adult male Dunkin Hartley guinea pigs were purchased from Vital River Laboratories (Beijing, China) and kept in the animal facility of WuXi (Shanghai, China) under standard conditions. Experimental animals (n=3) were anaesthetized with pentobarbital (60 mg kg-1, i.p.) before being surgically implanted with catheters in the carotid artery and the jugular vein using polyethylene tubing and heparin (50 IU mL−1)/glucose (50=) solutions as the lumen lock solution. Squeezing the animal's foot with forceps and observing its breathing was used to assess the depth of anaesthesia.
Three to five days of recovery were allowed after the surgery before starting the pharmacokinetic experiment. ADX71441 (1 mg kg−1, i.v.), dissolved in PEG400/saline (50/50) and was administered at a volume of 3 mL kg−1. Blood was collected from the carotid artery (approximately 200 μL per sample) via a catheter at 0.083, 0.17, 0.25, 0.5, 1, 2, 4, 8 and 24 h post dosing. Blood samples (taken on K2-EDTA) were kept on ice (up to 30 min) until centrifugation for approximately 15 min at 2–8°C at approximately 3000x g. Plasma samples were stored at −80°C, until analysis. In order to precipitate proteins, 160 μL of 0.1= formic acid in 50= acetonitrile/methanol (containing 100 ng mL−1 of diclofenac and 200 ng mL−1 tolbutamide as internal standards) were added to either 40 μL of plasma for unknown samples or to plasma containing ADX71441 for calibration and QC samples. After vortex mixing, centrifugation (15 min, 4°C, 10347x g), an aliquot (80 μL) was removed and mixed with 240 μL 0.1= formic acid in 95= water/acetonitrile, vortexed for 10 min and centrifuged again at 980x g. Five microlitres of the supernatant was injected into the UPLC system (Waters, Milford, MA, USA) coupled with a mass spectrometer (API 4000, Applied Biosystems, Lucerne, Switzerland): the samples were assessed as electrospray positive in MRM mode. The calibration curve was set between 2 and 3000 ng mL−1 (linear 1/X2), with four levels of QC samples at 3, 50, 800 and 2400 ng mL−1.
Calculation of pharmacokinetic parameters was performed using WinNonLin® Pro 5.2 (Pharsight Corporation, Sunnyvale, CA, USA). A non-compartmental analysis was performed using the linear trapezoidal method. The area under the concentration-time curve (AUClast and AUCo-∞) was calculated by linear trapezoidal rule. The clearance (CL) and volume of distribution (Vdss) were calculated for the drug after it had been administered i.v. The elimination rate constant value (k) was obtained by linear regression of the log-linear terminal phase of the concentration-time profile using at least three declining concentrations in terminal phase. The terminal half-life value (t1/2) was calculated using the equation In2/k. For the calculation of the pharmacokinetic parameters, the theoretical sampling times were used.
Model of OAB in guinea pigs
Surgical procedures
Guinea pigs were anaesthetized with urethane (1.2 g kg−1, i.p.). Body temperature of the animal was maintained at 37 ± 2°C with the aid of TCAT-2LV controller heating pads (Physitemp, ADInstruments Pty Ltd, Castle Hill, Australia) throughout the experiment. After tracheotomy, a plastic tube was inserted into the trachea. After a midline incision of the abdomen, a polyethylene catheter was inserted into the bladder dome and secured with a purse-string suture. Another catheter was inserted into a jugular vein for administration of test substances. The intravesical catheter was connected via a T-tube to a strain gauge MX 860 Novatrans III Gold (Medex Medical, Nantes-Carquefou, France) and another one was connected to a syringe pump (Model II plus, Harvard Apparatus, Les Ulis, France). Intravesical pressure was recorded continuously using a PowerLab interface (ADInstruments Pty Ltd) and Chart® software running on a PC.
Cystometric experiment
The design of the protocol is shown in Figure 1. Fifteen minutes after surgery, physiological saline (at room temperature) was infused into the bladder at a constant flow rate (12 mL h−1) for 30–45 min and then switched to 0.2= AA in saline. Thirty minutes after the first micturition, ADX71441 (1 or 3 mg kg−1, i.v.) or its vehicle (PEG400/saline, i.v.) were administered (n = 10 per group). Additional groups (n = 10 per group) received baclofen (1 mg kg−1, i.v.) or its vehicle (saline, i.v.). Vesical pressure was recorded continuously for 1 h post-administration. The cystometric parameters measured were the following: intercontraction interval (ICI), micturition frequency (MF), bladder capacity (BC), calculated by ICI (s) × infusion rate (mL s−1), threshold pressure (ThP), the intravesical pressure before micturition (mmHg) and basal pressure (BP; mmHg).
Figure 1.
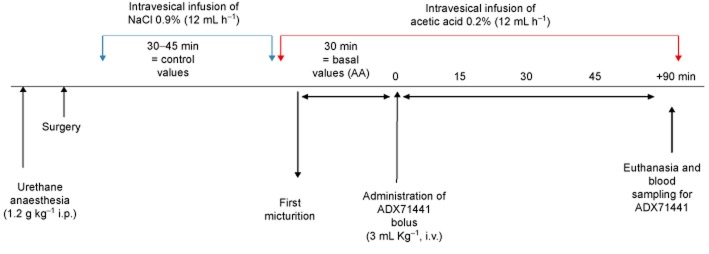
The design of the guinea pig OAB model.
Data analysis
For each guinea pig, control values were calculated as the mean of two to four micturition cycles during physiological saline infusion. Basal values for each parameter were calculated as a mean of three micturitions observed during the period of 30 min of 0.2= AA infusion, before test substance or vehicle administration. Each cystometric parameter was analysed post-administration and presented as means of values per 15 min periods. The effect of ADX71441 was analysed by two-way repeated measures ANOVA followed by one-way ANOVA and Dunnett's test at each time point. The effect of baclofen was analysed by two-way repeated measures ANOVA followed by one-way ANOVA and Student's t-test at each time point. P < 0.05 was accepted as being statistically significant.
Plasma analysis
Terminal blood samples were taken from mice and guinea pigs treated with ADX71441 at the end of the study. They were collected in EDTA (K3E) tubes from vena cava or cardiac puncture after pentobarbital administration. Samples were stored at 4°C before centrifugation at 177x g (110 mm diameter of the rotor), for 12 min at 4°C. Plasma samples were then transferred in Eppendorf tubes and stored at −20°C until analysis at Addex Therapeutics using the same method as that used for the above-mentioned independent pharmacokinetic study.
Materials
ADX71441 was synthesized at Addex Therapeutics. Baclofen (R-baclofen), AA, furosemide, oxybutynin and urethane were supplied by Sigma-Aldrich (St. Quentin Fallavier, France). PEG400 was purchased from VWR (Fontenay-Sous-Bois, France). Physiological saline and pentobarbital were purchased from Centravet (Lapalisse, France). AA was freshly diluted in physiological saline to a concentration of 0.2= (v v-1) each experimental day.
In the OAB study in mice, ADX71441, baclofen and oxybutynin were suspended in 1= CMC in saline and administered at a volume of 10 mL kg−1. In the OAB study in guinea pigs, preliminary tests on solubility of ADX71441 in saline showed that it was incomplete, which could have potentially interfered with the i.v. route of administration. Therefore, PEG400/saline (50/50) was chosen as a vehicle for ADX71441, while baclofen was dissolved in saline, for consistency with historical data. PEG400/saline (50/50) and saline were used as vehicle controls for ADX71441 and baclofen respectively. In guinea pigs, all drugs were administered in a volume of 3 mL kg−1.
Nomenclature
The nomenclature regarding receptors fully conforms to that of The British Journal of Pharmacology's Concise Guide to PHARMACOLOGY (Alexander et al., 2013).
Results
In both the experiments involving the evaluation of the effects of ADX71441 and baclofen in the mouse OAB model, signs of bladder overactivity, induced by overhydration and furosemide challenge, were robust during the first 45-min period (0–45 min; see below), but were virtually absent during the second 45-min period (45–90 min; data not included). Therefore, analysis of the total activity during the first 45-min period only is presented for both mouse OAB experiments below.
Model of OAB in mice
In Experiment 1, during the first 45-min period, animals overhydrated with water and challenged with furosemide (i.e. Reference group) showed 70= reduction (P < 0.01) in latency to the first urinary event, an eightfold increase (P < 0.001) in the number of urinary events, as well as five-(P < 0.001) and threefold increases (P < 0.05) in the total and average urinary volumes, respectively (Figure 2A–D).
Figure 2.
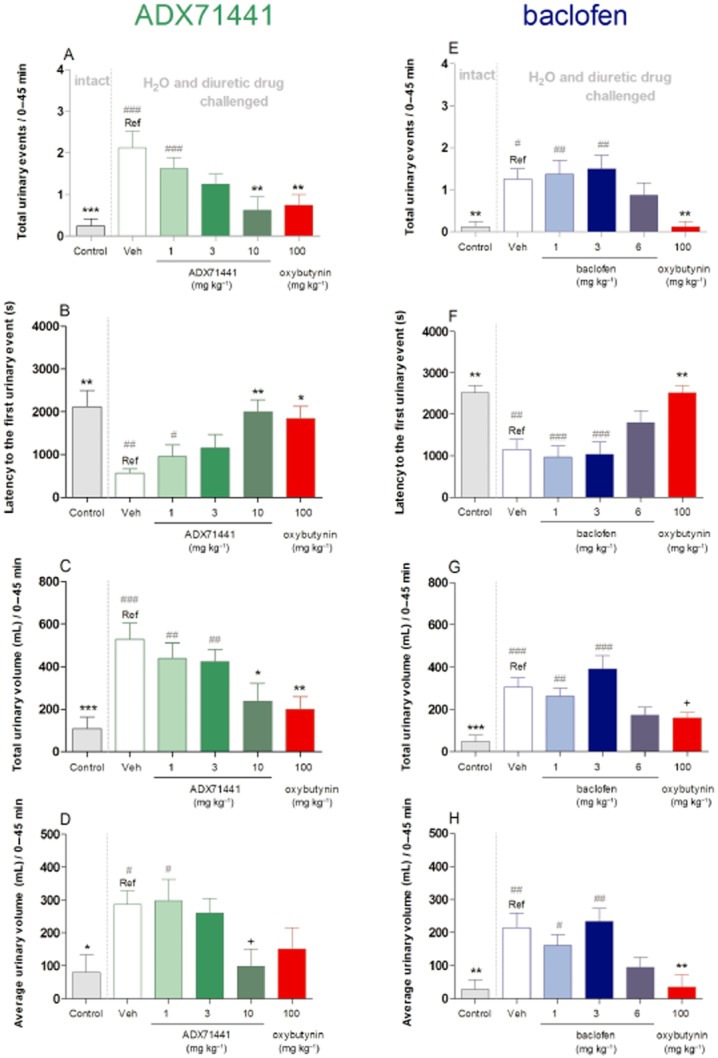
Micturition variables in C57Bl6/J mice (n = 8 per group) that were left untreated (intact; control groups) or received (p.o.) vehicle (1= CMC), ADX71441 (1, 3, 10 mg kg−1), oxybutynin (100 mg kg−1) in Experiment 1 (A–D) or vehicle (1= CMC), baclofen (1, 3, 6 mg kg−1), oxybutynin (100 mg kg−1) in Experiment 2 (E–H). All animals, except controls, were subsequently overhydrated with water before receiving the diuretic drug, furosemide and monitored for micturition (see text). The variables assessed included number of urinary events (A, E), latency to the first urinary event (s; B, F), total urinary volume (mL; C, G) and the average urinary volume (mL; D, H). Each point represents the observed mean (+ SEM) for 0–45 min of the 90-min period. +P = 0.07, * P < 0.05, ** P < 0.01 compared with the corresponding Reference (Ref) group. #P < 0.05, ## P < 0.01, ### P < 0.001 compared with the corresponding control group.
During the first 45 min, acute ADX71441 (1, 3, 10 mg kg−1, p.o.) dose-dependently reduced the total number of urinary events in overhydrated mice [F(5, 42) = 5.99, P < 0.001], 70= reduction (P < 0.01) was observed at the highest dose (10 mg kg−1) in comparison with vehicle-treated mice (Figure 2A). Also, in OAB mice treated with 3 and 10 mg kg−1 ADX71441, the number of urinary events was not significantly different from that of untreated (intact) controls. The total number of urinary events in oxybutynin-treated animals was 60= (P < 0.01) lower in comparison to those of vehicle-treated animals and similar to untreated (intact) controls (Figure 2A).
ADX71441 also dose-dependently increased the latency to the first urinary event [F(5, 42) = 4.80, P < 0.01], by more than threefold (P < 0.01) at the highest dose used (10 mg kg−1) in comparison with vehicle treatment (Figure 2B). Also, at 3 and 10 mg kg−1 ADX71441, the latencies to the first urinary event were not significantly different from those of the untreated (intact) controls. The latencies in the oxybutynin-treated animals were threefold higher (P < 0.05) compared to those in vehicle-treated animals and similar to those of intact controls (Figure 2B).
ADX71441 dose-dependently decreased the total urinary volume [F(5, 42) = 5.69, P < 0.001], achieving 55= (P < 0.05) reductions at 10 mg kg−1 in comparison with vehicle treatment (Figure 2C). Also, at 10 mg kg−1 ADX71441, the total urinary volume was not significantly different from that of intact controls. The total urinary volume in oxybutynin-treated animals was approximately 60= (P < 0.01) lower than that in vehicle-treated animals and similar to intact controls (Figure 2C).
ADX71441 dose-dependently reduced the average urinary volume [F(5, 42) = 3.21, P < 0.05] achieving approximately 65= reduction (P = 0.07) at 10 mg kg−1 in comparison with vehicle treatment (Figure 2D). At 3 and 10 mg kg−1 ADX71441, the average urinary volume was not significantly different from that of intact controls. The average urinary volume of the oxybutynin-treated animals did not differ from that of vehicle-treated OAB animals or from intact controls (Figure 2D).
The corresponding plasma concentrations of ADX71441 in mice treated with 1, 3 and 10 mg kg−1 of this drug were 101, 208 and 652 ng mL−1 respectively (Table 1). These plasma concentrations of ADX71441 resulted in unbound plasma concentration over IC50 values of 0.36, 0.74 and 2.31 respectively (Table 1).
Table 1.
Plasma, unbound plasma concentrations and unbound plasma concentration/IC50 (in vitro) of ADX71441 at the end of the experiment in mice
| Treatment | Route | Dose (mg kg−1) | n | Plasma exposure (ng mL−1) | Plasma exposure (nM) | Unbound conc. (ng mL−1) | Unbound conc. (nM) | Unbound conc. EC50 (in vitro) |
|---|---|---|---|---|---|---|---|---|
| Vehicle | p.o. | 8 | ||||||
| ADX71441 | p.o. | 1 | 8 | 101 | 231 | 6 | 14 | 0.36 |
| ADX71441 | p.o. | 3 | 8 | 208 | 476 | 13 | 30 | 0.74 |
| ADX71441 | p.o. | 10 | 8 | 652 | 1493 | 40 | 93 | 2.31 |
| Oxybutynin | p.o. | 100 | 8 |
Unbound concentrations of ADX71441 were calculated from total plasma concentration using measured plasma protein binding in the mouse (fraction unbound = 6.2=).
In Experiment 2, during the first 45-min period, animals overhydrated with water and challenged with furosemide (i.e. Reference group) showed approximately 55= reduction (P < 0.01) in latency to the first urinary event, 10-fold increases (P < 0.05) in the total number of urinary events, as well as six-(P < 0.001) and sevenfold (P < 0.01) increases in the total and average urinary volumes respectively (Figure 2E–H).
There was a significant overall effect of treatment on the total urinary events [F(5, 42) = 5.82, P < 0.001] during the first 45 min. While baclofen had no effect on this variable, oxybutynin (100 mg kg−1, p.o.) induced an almost 90= reduction (P < 0.05) in the total number of urinary events in comparison with vehicle (Figure 2E). Also, the total number of urinary events in the 6 mg kg−1 baclofen-and oxybutynin-treated animals did not differ significantly from that of the intact controls (Figure 2E).
There was a significant effect of treatment on the latency to the first urinary event [F(5, 42) = 8.82, P < 0.001]. While 6 mg kg−1 baclofen-treated animals showed a trend of increased urinary latencies, oxybutynin-treated animals showed more than twofold (P < 0.01) increases in comparison to those of vehicle-treated, overhydrated animals (Figure 2F). Also, the urinary latencies of the 6 mg kg−1 baclofen-and oxybutynin-treated animals did not differ significantly from that of intact controls (Figure 2F).
There was significant effect of treatment on the total urinary volume [F(5, 42) = 8.85, P < 0.001]. While 6 mg kg−1 baclofen-treated animals showed a trend of reduced urinary volume, oxybutynin-treated animals showed an almost 50= reduction (P = 0.07) in total urinary volume in comparison with overhydrated animals (Figure 2G). Also, the total urinary volumes of the 6 mg kg−1 baclofen-and oxybutynin-treated animals did not differ significantly from that of the intact controls (Figure 2G).
There was a significant effect of treatment on the average urinary volume [F(5, 42) = 6.07, P < 0.001]. While 6 mg kg−1 baclofen-treated animals showed reduced average urinary volume as a trend, oxybutynin-treated animals showed more than 80= reduction (P < 0.01) in this measure in comparison with vehicle-treated, overhydrated animals (Figure 2H). Also, the average urinary volume of the 6 mg kg−1 baclofen-and oxybutynin-treated animals did not differ significantly from that of the intact control animals (Figure 2H).
Pharmacokinetic profile of i.v. ADX71441 in guinea pigs
The plasma concentrations of ADX71441 in male guinea pigs, obtained in an independent pharmacokinetic study, are presented in Figure 3. Following an i.v. bolus of 1 mg kg−1 of ADX71441 in PEG400/saline (50/50) to three guinea pigs, plasma concentrations of ADX71441 were measured over a 24-h period. From maximum concentration extrapolated at time zero (C0 = 342 ng mL−1), a rather fast decay occurred, as concentrations were reduced to 63 and 45= of C0 at 15 min and 30 min respectively. The volume of distribution was large, around 7 L kg−1, clearance was high (35 mL min−1 kg−1 i.e. 78= of the normal liver blood flow) and terminal half-life was approximately 3 h (Table 2). It should be noted that there was a minimal interindividual variability at each time point.
Figure 3.
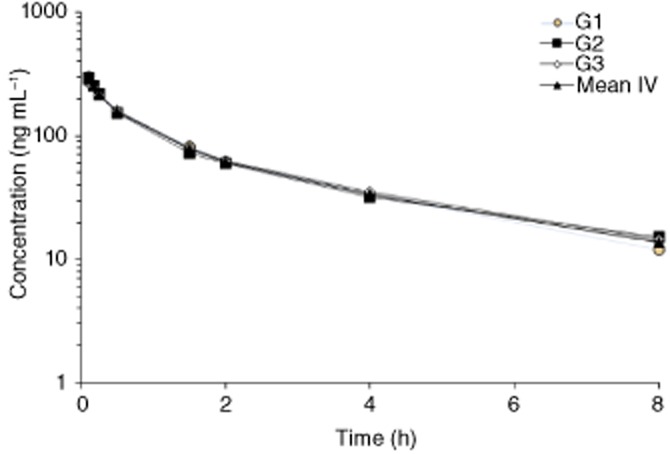
Plasma concentration (ng mL−1)-time profiles of ADX71441 following i.v. administration of 1 mg kg−1 ADX71441 over 5 min in three male guinea pigs (G1–3).
Table 2.
Mean pharmacokinetic parameters following i.v bolus (1 mg kg−1 over 5 min) of ADX71441 in three male guinea pigs (G1–3)
| G1 | G2 | G3 | Mean | SD | |
|---|---|---|---|---|---|
| C0 (ng mL−1) | 367 | 366 | 293 | 342 | 34.6 |
| t1/2 (h) | 2.57 | 3.11 | 2.88 | 2.85 | 0.3 |
| Vdds (L kg−1) | 6.6 | 7.5 | 7.0 | 7.0 | 0.5 |
| Cl (mL min−1 kg−1) | 35.7 | 34.3 | 33.5 | 34.5 | 1.1 |
Model of OAB in guinea pigs
Representative cystometric recordings in guinea pigs treated with ADX71441 1 and 3 mg kg−1 are illustrated in Figure 4. When cystometric values obtained during intravesical saline infusion (control values) were compared to the corresponding values obtained during intravesical AA infusion (basal values) in the same animal, it was evident that AA elicited significant decreases in ICI, BC and ThP as well as increases in MF values in all groups (Figure 5). A slight, but significant decrease in ThP was also observed (Figure 5D,I), whereas AA had no effect on the BP (Figure 5E,J). In five out of 10 animals, 3 mg kg−1 ADX71441 completely inhibited the micturition reflex and induced overflow incontinence (Figure 4B). Moreover, for one animal in both ADX71441-treated groups (1 and 3 mg kg−1), micturition did not occur during the first 15 min post-administration. Consequently, the results presented here were obtained from n = 9 and n = 4 for ADX71441 at 1 and 3 mg kg−1 respectively.
Figure 4.
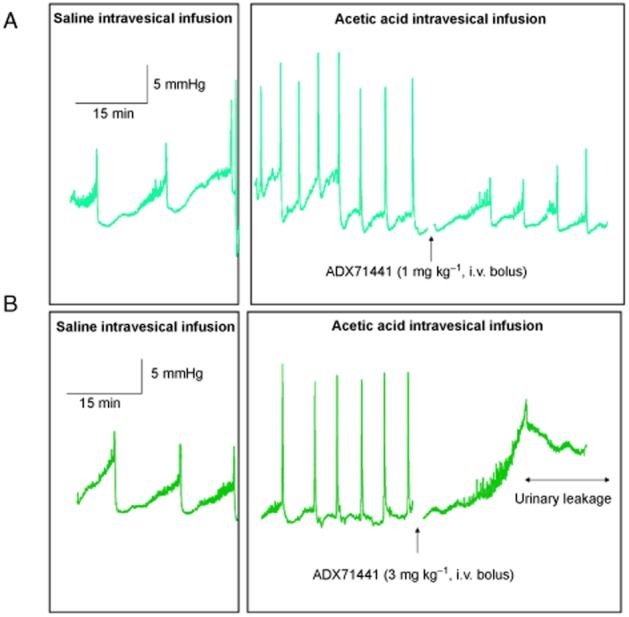
Representative cystometric recordings from the anaesthetized female guinea pig bladder in response to saline followed by acetic acid infusion. Animals were treated i.v. with ADX71441 at 1 mg kg−1 (A) or 3 mg kg−1 (B).
Figure 5.
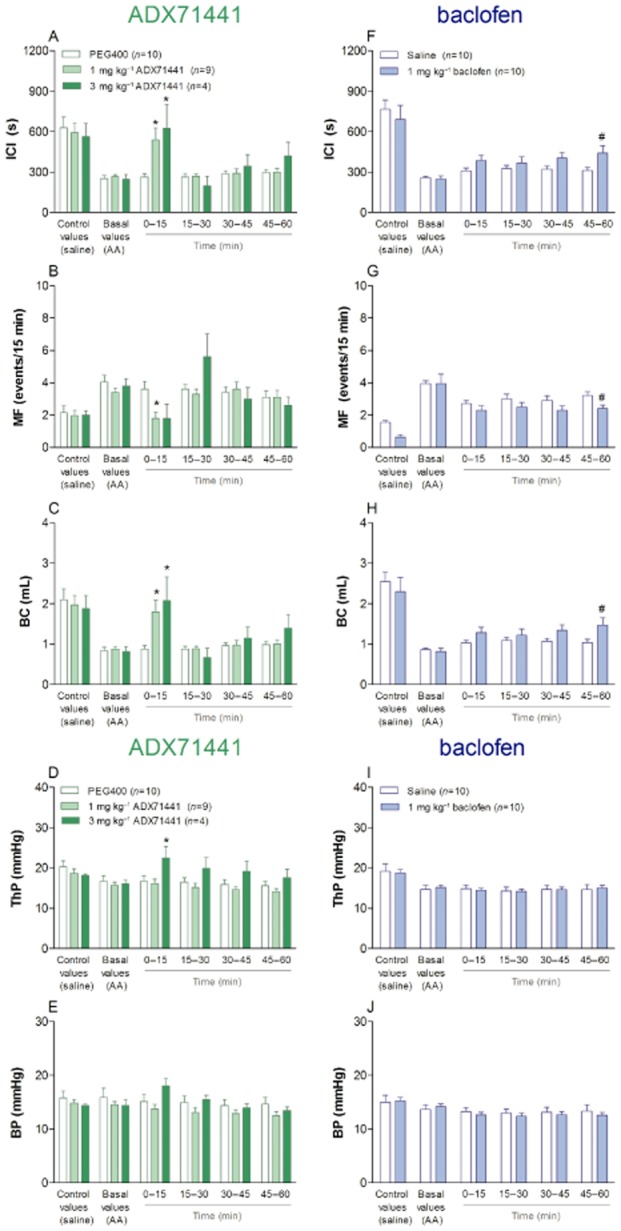
Cystometry variables in anaesthetized female guinea pigs after infusion of saline (control values) and acetic acid (AA; basal values) followed by i.v. administration of PEG400 (n = 10), 1 mg kg−1 (n = 9) or 3 mg kg−1ADX71441 (n = 4; A–E). Additional groups were administered i.v. saline (n = 10) or 1 mg kg−1 baclofen (n = 10; F-J). After the treatment, micturition was monitored for 60 min (see text). The variables assessed included intercontraction interval (ICI; A, F), micturition frequency (MF; B, G), bladder capacity (BC; mL; C, H), threshold pressure (ThP; mmHg; D, I) and bladder pressure (BP; mmHg; E, J). Each point represents the observed mean (+ SEM) *P < 0.05 compared with PEG400, #P < 0.05 compared with saline.
ADX71441, 1 or 3 mg kg−1 i.v., significantly increased ICI compared with vehicle (P < 0.05) 0–15 min post-administration (Figure 5A). Baclofen significantly increased ICI compared with vehicle (P < 0.05) 45–60 min post-administration (Figure 5F). ADX71441 (1 mg kg−1, i.v.) significantly decreased MF (P < 0.05) compared with vehicle 0–15 min post-administration, while at 3 mg kg−1 a similar trend was seen (Figure 5B). Baclofen significantly decreased MF compared to vehicle (P < 0.05) 45–60 min post-administration (Figure 5G). ADX71441, 1 or 3 mg kg−1 i.v., significantly increased BC (P < 0.05) compared with vehicle 0–15 min post-administration (Figure 5C). Baclofen significantly increased BC compared to vehicle (P < 0.05) 45–60 min post-administration (Figure 5H). ADX71441 at 3 mg kg−1 significantly increased ThP (P < 0.05) compared to vehicle 0–15 min post-administration (Figure 5D), while baclofen had no effect on this variable (Figure 5I). BP was unaltered in all the treated animals (Figure 5E,J).
The corresponding plasma concentrations of ADX71441 in guinea pigs treated with 1 and 3 mg kg−1 (i.v.) at the end of the experiment were 67 and 195 ng mL−1 respectively ( Table 3). These plasma concentrations resulted in unbound plasma concentrations over IC50 values of 0.3 and 0.9 respectively (Table 3).
Table 3.
Total plasma concentration, calculated unbound plasma concentrations and unbound plasma concentration/IC50 (in vitro) of ADX71441 at the end of the experiment in female guinea pigs
| Treatment | Route | Dose (mg kg−1) | n | Total plasma exposure (ng mL−1) | Total plasma exposure (nM) | Unbound conc. (ng mL−1) | Unbound conc. (nM) | Unbound conc./EC50 (in vitro) |
|---|---|---|---|---|---|---|---|---|
| Vehicle | i.v. | 10 | ||||||
| ADX71441 | i.v. | 1 | 10 | 67 | 153 | 5.3 | 12.1 | 0.3 |
| ADX71441 | i.v. | 3 | 10 | 195 | 446 | 15.4 | 35.3 | 0.9 |
| Baclofen | i.v. | 6 | 10 |
Unbound plasma concentrations of ADX71441 were calculated from total plasma concentration using measured plasma protein binding in the guinea pig (fraction unbound = 7.9=).
Discussion and conclusions
This is the first study in which a GABAB PAM was tested in models of OAB. Previously, baclofen, a GABAB orthosteric agonist, showed efficacy in rodent models of OAB (Pehrson et al., 2002; Miyazato et al., 2008) as well as in several clinical studies (Haubensak, 1977; Taylor and Bates, 1979; Xu et al., 2007). Here, ADX71441, a novel, selective and orally bioavailable GABAB PAM, showed a robust and dose-dependent ability to reverse the signs of OAB in conscious mice and anaesthetized guinea pigs.
The diuretic stress-induced overactive bladder model in conscious mice has been used to evaluate the efficacy of potential therapeutics for OAB. This model has been used increasingly as an in vivo screening assay, as it shows sensitivity to cholinergic antagonists, such as oxybutynin (Yoshida et al., 2010). Here, ADX71441 reduced the number of urinary events, total and average urinary volumes, normalizing the OAB values to those seen in untreated, intact controls. ADX71441 also increased urinary latency, restoring it to that seen in intact controls. The in vitro effects of ADX71441 on urinary parameters showed a good correlation with those in vivo, indicative of a target-related, OAB-reducing efficacy. The magnitudes of the changes in micturition parameters seen in animals treated with 10 mg kg−1 ADX71441 were similar to those seen in oxybutynin-treated animals. In fact, ADX71441 was greater than 10-fold more potent than oxybutynin. Among the micturition parameters monitored in the study, urinary latency and the number of urinary events are likely to be the most clinically relevant and translatable variables. Baclofen was less active in the mouse OAB model, showing only trends of activity at the highest dose administered (6 mg kg−1). It is unlikely that somewhat smaller absolute bladder activity values shown by the reference group in this experiment made it difficult to detect the effect of baclofen, as in all urinary parameters the relative changes in comparison to intact controls were either comparable to the study with ADX71441 (urinary latency) or even larger (number of urinary events, total and average urinary volumes).
As a follow-up study, ADX71441 and baclofen were tested in a model of OAB in anaesthetized guinea pigs. The guinea pig has been considered as one of the most appropriate species to study urinary bladder function since its bladder physiology and neural control are similar to those in humans (McMurray et al., 2006). Moreover, as in humans, bladder voiding in urethane-anaesthetized guinea pigs is largely dependent on cholinergic neurotransmission (Maggi et al., 1988). Recently, a model of AA-induced bladder overactivity in anaesthetized guinea pigs was validated using an antimuscarinic drug, tolterodine, and an NK1 receptor antagonist, netupitant (Palea et al., 2010).
In all experimental groups, AA elicited bladder overactivity characterized by increases in MF paralleled by marked decreases in ICI, BC and a similar trend in ThP. These results are in agreement with previous data obtained in our laboratories using the same experimental model of OAB (Palea et al., 2010). In the present study, 1 and 3 mg kg−1 ADX71441 had a similar effect, increasing ICI and BC and reducing MF, normalizing them to the levels seen following saline treatment (i.e. control values). As the effects of 1 and 3 mg kg−1 on all variables were near maximal (approaching control values), the minimal effective dose of ADX71441 in this model is likely to be less than 1 mg kg−1 (i.v.). All the effects of ADX71441 on the cystometry measures were seen during the first 15 min after its administration, while they were absent at later time points. In an independent pharmacokinetic experiment, plasma concentrations of ADX71441 declined rapidly after i.v. administration of 1 mg kg−1 to guinea pigs. Although the pharmacokinetic data were obtained in conscious animals, plasma concentrations measured in this experiment and the plasma concentrations evaluated at the end of the cystometric measurements were comparable, indicating that pharmacokinetic data can be used in support of the interpretation of the time course of efficacy. The short-lived effect of ADX71441 in the cystometric guinea pig model can therefore be explained by the fast decline in plasma concentrations observed after its i.v. administration, suggesting that concentrations sufficient to produce an effect were present only during the first 15 min, but not later. Indeed, concentrations of ADX71441 at the end of the test, 67 and 195 ng mL−1 (at 1 and 3 mg kg−1, respectively), were below the minimal concentration (∼350 ng mL−1) needed to obtain an in vivo effect (M. Kalinichev et al., unpubl. data). It should be noted that the use of distinct vehicles in the guinea pig OAB study (PEG400/saline for ADX71441 and saline for baclofen) could have contributed to the differences observed for the time profiles of the two drugs.
In five out of 10 animals, 3 mg kg−1 ADX71441 completely blocked the micturition reflex and induced dribbling incontinence almost immediately after its administration. In addition, ADX71441 (3 mg kg−1, i.v.) significantly increased ThP, which accords with the finding that 50= of the animals tested presented urinary leakage, characterized by a strong increase in intravesical pressure during bladder filling as well as dribbling incontinence. These results support and expand those obtained previously, which showed that intrathecal baclofen (0.5 μg) produced dribbling incontinence in 58= of rats (Watanabe et al., 1997). Whether or not ADX71441 at certain doses will induce in incontinence in human patients with OAB remains to be seen.
In guinea pigs, baclofen had modest, albeit significant effects on ICI, MF, BC, while having no effect on ThP and BP. In accord with this, administration of racemic baclofen, 8–16 mg kg−1, to conscious rats resulted in progressive increases in BC, as assessed by cystometry, while causing dribbling incontinence in some animals (Igawa et al., 1993). In another study, baclofen 4 mg kg−1, i.v., attenuated bladder overactivity induced by intravesical administration of oxyhaemoglobin in conscious rats (Pehrson et al., 2002), but was devoid of effect on BC in normal, conscious rats at 1 mg kg−1, i.v. (Kontani and Ueda, 2005). Therefore, modest effects of 1 mg kg−1 baclofen (i.v.) on cystometric parameters were anticipated. The weak activity of baclofen suggests that direct GABAB agonism might be less effective than positive modulation in the models of OAB used in the present study. Indeed, these data indicate that, unlike spinal injury models, diuretic-induced stress models do not elicit changes in GABAergic transmission that can be overcome by direct stimulation of the GABAB receptor. However, enhancing GABAergic transmission by increasing GABAB receptor responsiveness and thus enhancing the effects of normal levels of GABA through the use of PAMs, such as ADX71441, may be effective in this model of urinary incontinence. Alternatively, baclofen may be more effective in guinea pigs than in mice because of the different pharmacokinetic profiles of the compound in the two species. Finally, urinary incontinence induced by AA may be more responsive to GABAB agonism than diuretic-induced stress.
At this point, we can only speculate about the site of action of ADX71441 as various GABAB receptor populations localized on different central and peripheral sites have been implicated in the control of voiding in mammals. From results obtained in previous investigations it was concluded that GABA inhibits the micturition reflex by acting on GABAB receptors localized in several distinct sites, including the pontine micturition centre in the brain, the sacral parasympathetic neurons in the spinal cord, the pelvic ganglions and the urinary bladder (Maggi et al., 1988). Since ADX71441 can easily cross the blood-brain barrier and has a balanced central-peripheral distribution (M. Kalinichev et al., unpubl. data), the effects we observed in the mouse and guinea pig studies could involve any or all of the sites described above.
We hypothesize that the inhibitory effects of ADX71441 in models of OAB are mediated through inhibition of cholinergic neurotransmission, at the central and maybe also the peripheral level, since activation of GABAB receptors was reported to inhibit electrical field stimulation-induced contractions in the guinea pig isolated urinary bladder (Maggi et al., 1985). In the clinic, administration of baclofen is associated with improvements in OAB symptoms in patients with idiopathic detrusor instability (Taylor and Bates, 1979), neurogenic voiding disturbances (Haubensak, 1977) and non-neurogenic dysfunctional voiding (Xu et al., 2007). Taken together, these findings confirm the role of the GABAB system in controlling the micturition reflex and for the first time demonstrate that GABAB receptor PAMs may represent a novel approach to treat OAB. However, to confirm this hypothesis, further studies in conscious animals, using a validated model of bladder overactivity, like rats with chronic bladder outlet obstruction (Igawa et al., 1993), are needed to further support the therapeutic potential of ADX71441 for the treatment of OAB.
The possibility that the muscle-relaxant properties of ADX71441 contributed to its efficacy in our models of OAB in mice and guinea pigs cannot be excluded from the present results. In mice, the muscle-relaxant effects of ADX71441 were probably mild, as it has previously been found that 10 mg kg−1 causes a mild reduction in the rotarod activity in mice in an accelerated setup, while being inactive in a constant speed setup (M. Kalinichev et al., unpubl. data). A small reduction in rotarod activity was also seen in rats with 10 mg kg−1 ADX71441 (M. Kalinichev et al., unpubl. data). In addition, ADX71441 has been shown to dose-dependently reduce spontaneous locomotor activity (sLMA) in mice and rats. These effects may not be centrally-mediated sedative effects of the compound per se, as ADX71441 and pentobarbital have very distinct sleep/wake EEG and neck muscle EMG profiles, while resulting in similar reductions in sLMA in rats (M. Kalinichev, unpubl. data). Also, ADX71441 can dose-dependently and transiently reduce body temperature in mice and rats (M. Kalinichev et al., unpubl. data). However, it is unlikely that the hypothermic effects of the compound had any effect on its efficacy in models of OAB. In guinea pigs, the hypothermic effects can be fully excluded, as the body temperature of the anaesthetized animals was maintained at 37°C by automated heating pads. In mice, the hypothermic effects of ADX71441, if any, were probably mild, as no shivers or piloerection were noticed in the treated animals.
Furthermore, ADX71441 has been shown to reduce the number of buried marbles in mice [minimum effective dose [MED] 3 mg kg−1−1) and increase open arm exploration in the elevated plus maze test in mice and rats (both MED 3 mg kg−1) showing a good in vitro/in vivo correlation, indicative of target-related, anxiolytic-like efficacy (M. Kalinichev et al., unpubl. data). Also, ADX71441 reduced acute visceral pain in the AA-induced writhing test in mice (MED 3 mg kg−1) and alleviated chronic osteoarthritic-like pain in the MIA-induced osteoarthritis model in the rat (MED 15 mg kg−1) after acute and chronic treatment (M. Kalinichev, unpubl. obs.).
In conclusion, ADX71441, a novel, selective and orally bioavailable GABAB PAM, showed efficacy in two models of OAB in mice and guinea pigs with a good pharmacokinetic/pharmacodynamic relationship. These results provide strong evidence that an orally active GABAB PAM can be considered as viable approach for the treatment of various forms of OAB in humans.
Acknowledgments
We would like to thank Dr. Ray Hill for insightful and stimulating discussions of the data included in the paper.
Glossary
- AA
acetic acid
- BC
bladder capacity
- BP
basal pressure
- ICI
intercontraction interval
- i.ves
intravesical
- MF
micturition frequency
- MIA
monosodium iodoacetate
- OAB
overactive bladder
- ThP
threshold volume
Conflict of interest
M K, H H, I R-U, M S and S P are employed by Addex Therapeutics.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ, Collaborators CGTP. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. [Google Scholar]
- Chen TF, Doyle PT, Ferguson DR. Inhibitory role of gamma-amino-butyric acid in the rabbit urinary bladder. Br J Urol. 1992;69:12–16. doi: 10.1111/j.1464-410x.1992.tb15449.x. [DOI] [PubMed] [Google Scholar]
- Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med. 2006;119(Suppl):3–8. doi: 10.1016/j.amjmed.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Brain Res Rev. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (NN'-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Erdem N, Chu FM. Management of overactive bladder and urge urinary incontinence in the elderly patient. Am J Med. 2006;119:29S–36S. doi: 10.1016/j.amjmed.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Harada T, Constantinou CE. The effect of alpha 2 agonists and antagonists on the lower urinary tract of the rat. J Urol. 1993;149:159–164. doi: 10.1016/s0022-5347(17)36030-5. [DOI] [PubMed] [Google Scholar]
- Harada T, Levounis P, Constantinou CE. Rapid evaluation of the efficacy of pharmacologic agents and their analogs in enhancing bladder capacity and reducing the voiding frequency. J Pharmacol Toxicol Meth. 1992;27:119–126. doi: 10.1016/1056-8719(92)90031-u. [DOI] [PubMed] [Google Scholar]
- Haubensak K. A double-blind trial with the antispasticity drug Lioresal in 15 paraplegics with upper neuron lesions. Urol Int. 1977;132:198–201. doi: 10.1159/000280130. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Kalinichev M, Haddouk H, Poli S, Miczek KA. Reduction of excessive alcohol drinking by a novel GABAB receptor positive allosteric modulator ADX71441 in mice. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3245-z. doi: 10.1007/s00213-013-3245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa Y, Mattiasson A, Andersson K-E. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. J Urol. 1993;150:537–542. doi: 10.1016/s0022-5347(17)35542-8. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacol. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontani H, Ueda Y. A method for producing overactive bladder in the rat and investigation of the effects of GABAergic receptor agonists and glutamatergic receptor antagonists on the cystometrogram. J Urol. 2005;173:1805–1811. doi: 10.1097/01.ju.0000154345.87935.a4. [DOI] [PubMed] [Google Scholar]
- Lam S, Hilas O. Pharmacologic management of overactive bladder. Clin Interv Aging. 2007;2:337–345. [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray G, Casey JH, Naylor AM. Animal models in urological disease and sexual dysfunction. Br J Pharmacol. 2006;147(Suppl 2):S62–S79. doi: 10.1038/sj.bjp.0706630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. GABAA and GABAB receptors in detrusor strips from guinea pig isolate urinary bladder. J Auton Pharmacol. 1985;5:55–64. doi: 10.1111/j.1474-8673.1985.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Patacchini R, Geppetti P, Giuliani S, Astolfi M, et al. Regional differences in the motor responses to capsaicin in the guinea-pig urinary bladder: relative role of pre-and postjunctional factor related to neuropeptide-containing sensory nerves. Neuroscience. 1988;27:675–688. doi: 10.1016/0306-4522(88)90297-7. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci. 1996;17:457–462. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, et al. GABA-receptor activation in the lumbosacral spinal cord reduces detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety-and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Ouslander JG. Management of overactive bladder. N Eng J Med. 2004;350:786–799. doi: 10.1056/NEJMra032662. [DOI] [PubMed] [Google Scholar]
- Palea S, Guilloteau V, Guerard M, Guardia-Llorens M, Cantoreggi S, Lovati E, et al. Comparison of the effects of netupitant and tolterodine on overactive bladder induced by intravesical acetic acid infusion in anesthetized female guinea pigs. Neurourol Urodyn. 2010;29:994–995. [Google Scholar]
- Pehrson R, Lehmann A, Andersson K-E. Effects of γ-aminobutyrate B receptor modulation on normal micturition and oxyhemoglobin induced detrusor overactivity in female rats. J Urol. 2002;168:2700–2705. doi: 10.1016/S0022-5347(05)64247-4. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Prézeau L. Allosteric modulators of GABAB receptors: mechanism of action and therapeutic perspective. Curr Neuropharmacol. 2007;5:195–201. doi: 10.2174/157015907781695919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P, Maggi CA, Meli A. GABAB receptor mediated inhibition of field stimulation-induced contractions of rabbit bladder muscle in-vitro. J Pharm Pharmacol. 1984;36:378–381. doi: 10.1111/j.2042-7158.1984.tb04402.x. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Bates CP. A double-blind crossover trial of baclofen-a new treatment for the unstable bladder syndrome. Br J Urol. 1979;51:504–505. doi: 10.1111/j.1464-410x.1979.tb03588.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Perkash I, Constantinou CE. Modulation of detrusor contraction strength and micturition characteristics by intrathecal baclofen in anesthetized rats. J Urol. 1997;157:2361–2365. [PubMed] [Google Scholar]
- Xu D, Qu C, Meng H, Ren J, Zhu Y, Min Z, et al. Dysfunctional voiding confirmed by transdermal perineal electromyography, and its effective treatment with baclofen in women with lower urinary tract symptoms: a randomized double-blind placebo-controlled crossover trial. BJU Int. 2007;100:588–592. doi: 10.1111/j.1464-410X.2007.06987.x. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Fujino T, Maruyama S, Ito Y, Taki Y, Yamada S. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: bladder selectivity based on in vivo drug-receptor binding characteristics of antimuscarinic agents for treatment of overactive bladder. J Pharmacol Sci. 2010;112:142–150. doi: 10.1254/jphs.09r14fm. [DOI] [PubMed] [Google Scholar]


