The use of hypoxia was investigated as a preconditioning agent and in differentiating cultures to enhance mesenchymal stem cell (MSC) function. Findings suggest that hypoxic preconditioning may blunt the differentiation potential of MSCs, compromising their utility for regenerative tissue engineering. Exposure to hypoxia during differentiation (post-normoxic expansion), however, appears to result in a greater quantity of functional osteoblasts and chondrocytes and ultimately a larger quantity of high-quality differentiated tissue.
Keywords: Mesenchymal stem cells, Hypoxia, Colony-forming units assay, Chondrogenesis, Osteogenesis, Adipogenesis
Abstract
Stem cells are promising candidate cells for regenerative applications because they possess high proliferative capacity and the potential to differentiate into other cell types. Mesenchymal stem cells (MSCs) are easily sourced but do not retain their proliferative and multilineage differentiative capabilities after prolonged ex vivo propagation. We investigated the use of hypoxia as a preconditioning agent and in differentiating cultures to enhance MSC function. Culture in 5% ambient O2 consistently enhanced clonogenic potential of primary MSCs from all donors tested. We determined that enhanced clonogenicity was attributable to increased proliferation, increased vascular endothelial growth factor secretion, and increased matrix turnover. Hypoxia did not impact the incidence of cell death. Application of hypoxia to osteogenic cultures resulted in enhanced total mineral deposition, although this effect was detected only in MSCs preconditioned in normoxic conditions. Osteogenesis-associated genes were upregulated in hypoxia, and alkaline phosphatase activity was enhanced. Adipogenic differentiation was inhibited by exposure to hypoxia during differentiation. Chondrogenesis in three-dimensional pellet cultures was inhibited by preconditioning with hypoxia. However, in cultures expanded under normoxia, hypoxia applied during subsequent pellet culture enhanced chondrogenesis. Whereas hypoxic preconditioning appears to be an excellent way to expand a highly clonogenic progenitor pool, our findings suggest that it may blunt the differentiation potential of MSCs, compromising their utility for regenerative tissue engineering. Exposure to hypoxia during differentiation (post-normoxic expansion), however, appears to result in a greater quantity of functional osteoblasts and chondrocytes and ultimately a larger quantity of high-quality differentiated tissue.
Introduction
Adult mesenchymal stem cells (MSCs) are considered highly promising candidate cells for regenerative applications because they possess a high proliferative capacity and the potential to differentiate into other cell types [1–4]. MSCs are also actively investigated for clinical use as gene delivery agents to enhance tissue regeneration, destroy cancer cells, and regenerate cartilage and bone [5].
It has been suggested that the high plasticity of adult MSCs, which makes them an attractive candidate for cell-based therapies, is the result of low-level expression of a variety of gene families that characterize differentiated progeny, endowing them with a state of readiness to differentiate along one direction or another, depending on external cues [6]. Hypoxia is a critical external cue in mesenchymal progenitor fate starting as early as embryogenesis. A notable example of this is formation of the early skeleton, in which chondrogenic differentiation of limb mesenchyma is initiated by hypoxic niches formed when embryonic blood vessels regress from sites of mesenchymal cell condensation [7–11]. MSCs are hypothesized to persist in the adult for tissue repair and remodeling, among other functions, in perivascular niches throughout the body [12].
One in vivo niche for MSCs is the bone marrow, in which oxygen concentration has been reported to vary from 7% to less than 1% [13–15]. However, bone marrow-derived MSCs are frequently cultured at atmospheric oxygen (20%–21% O2) in the laboratory. The evolved defenses of MSCs against oxidative stress may be overwhelmed by the level of free radicals generated in these culture conditions, leading to decreased utility of these cells after expansion in vitro [15]. Many studies have thus explored the effects of low oxygen on MSCs relative to atmospheric oxygen, with highly variable results. Definitions of hypoxia vary in these studies, with different groups defining hypoxia at oxygen concentrations ranging from less than 1% O2 to 8% O2 [16]. MSCs are cultured under widely varying conditions, with different laboratories using different isolation methods, selection markers, culture media, supplements, oxygen tensions, and differentiation induction strategies [17]. Compounding this lack of consistency in the field is the heterogeneous nature of MSCs themselves, which perhaps reflects their high plasticity, or perhaps reflects multiple related cell types. Many studies on the effect of oxygen concentration on MSC differentiation investigate only one lineage, such as adipogenesis. Given the lack of consistent methodology, it is difficult to compare these studies with one other.
Learning how to appropriately tune contextual cues for differentiation in synchrony, of which oxygen tension is only one, to recapitulate microenvironments present during development may yield a more desirable tissue-engineered product than empiric manipulation of one variable in response to a single or limited set of outcome measures. With this motivation we undertook a comprehensive characterization of the effects of hypoxia on MSC differentiation along three commonly studied lineages as well as on their phenotype and other cell functions. This would allow us to observe the effects of hypoxia on various measures of potency and stemness from the same starting conditions. Because our primary interest is cartilage tissue engineering, we selected 5% ambient oxygen for our hypoxic condition, as one study that examined MSC chondrogenic differentiation at 0%–35% O2 found 5% O2 to be optimal [9].
In human MSCs the effects of hypoxia have been tested extensively with varying results. There is general agreement that hypoxia enhances proliferation of human MSCs; this has been shown at oxygen tensions varying from 1% to 5% [18–23]. However, there are studies that have shown decreased proliferation at low oxygen levels. In one such study, platelet lysate and fresh frozen plasma were used as culture supplements in lieu of serum, and MSCs were isolated from pediatric patients, whereas most studies of hypoxia in human MSCs have been performed with adult-derived cells [24].
In terms of human MSC differentiation, some studies have demonstrated inhibition of differentiation along adipogenic and osteogenic lineages under hypoxic conditions (1%–3% O2) [13, 24–27], and others found adipogenic and osteogenic differentiation potential in hypoxic conditions (1.5%–3% O2) comparable to normoxia [24, 28]. With regard to chondrogenic differentiation potential, several studies showed that hypoxia as a preconditioning agent results in preservation of chondrogenic differentiation potential or enrichment for chondrogenic progenitors during ex vivo expansion, but there are fewer studies detailing the isolated effects of hypoxia on MSCs during induction of chondrogenic differentiation [29]. Some studies have reported that hypoxia (1%–5% O2) enhanced chondrogenesis in pellet cultures of MSCs relative to 21% O2 [25, 28, 30–32]; however, hypoxia did not enhance chondrogenesis in pellet cultures in other studies, with optimal chondrogenic conditions achieved at higher oxygen concentrations (15%–20%) [33, 34]. The variability in results from human MSCs is frequently attributed to interindividual differences in patient samples.
Our study originally arose from curiosity about why colony-forming unit fibroblasts (CFU-Fs) arise in greater numbers when MSCs are cultured under hypoxic conditions in our and other laboratories. Hypoxia-mediated increase of MSC colony formation has been shown to be hypoxia-inducible factor (HIF)-independent [35]. Numerous factors contribute to the size and number of colonies in a 14-day CFU-F assay, among them (a) cell proliferation, (b) cell death, (c) cell migration, (d) growth factor secretion (which in turn affects cell proliferation), and (e) matrix turnover (which in turn affects cell migration). In this study, we sought to address each of these factors in MSCs cultured under hypoxic (5% O2) and normoxic (21% O2) conditions.
We then investigated the effects of steady-state hypoxia on differentiation. Many studies have addressed the use of hypoxia in preconditioning before differentiating MSCs under normoxic conditions. Preconditioning with hypoxia would be an easy, inexpensive, and theoretically benign approach for maximizing expansion of a progenitor pool before differentiation, expanding the clinical use of autologous MSCs, provided differentiation potential is preserved during hypoxic preconditioning. Our objective in this study was to address this question of whether differentiation potential is preserved if MSCs are cultured (preconditioned) under hypoxic conditions, and then compare this with MSCs cultured in normoxic conditions and to do so in osteogenic, adipogenic, and chondrogenic lineages within the same study. Additionally, we wanted to address the effect of hypoxia during the differentiation stage.
Several studies have demonstrated the negative impact of hypoxia on osteogenic potential of MSCs [24, 26, 35–38]. One study using an approach similar to ours demonstrated enhanced chondrogenesis under normoxic conditions as a result of hypoxic preconditioning (5% O2); however, this study was performed using ovine, not human, MSCs and examined differentiation to only one lineage [39]. The same group published another study demonstrating this enhancement held true in three-dimensional (3D) collagen hydrogel cultures as well as pellet cultures [40]. Similar results for chondrogenesis have been obtained in 3D micromass cultures with murine adipose-derived mesenchymal stem cells; in that study, osteogenesis was inhibited by hypoxic preconditioning [29]. Adipogenic differentiation potential has been shown to be enhanced by hypoxic preconditioning (2% O2) in murine MSCs [41].
Materials and Methods
Cell Culture
MSCs were harvested from consenting arthroplasty patients with Institutional Review Board approval, as described by Baksh et al. [42]. Adherent passage 0 MSCs were cultured in growth medium consisting of high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco/Invitrogen, Grand Island, NY, http://www.lifetechnologies.com/ipac/en/home/brands/gibco.html) with antibiotic-antimycotic (Gibco/Invitrogen) and 10% MSC-qualified fetal bovine serum (Gibco/Invitrogen) until they reached 80% confluence, and then passaged using 0.25% trypsin and replated at a density of 6.0 × 103 cells per cm2 as passage 1 MSCs. At 80% confluence passage 1 MSCs were trypsinized, cryopreserved in commercially available freeze medium (Invitrogen), and stored in liquid nitrogen. Upon recovery from cryopreservation, passage 2 MSCs were cultured in separate closed incubators purged with either 95% air and 5% CO2 (21% O2 or normoxia) or 5% oxygen, 5% CO2, and 90% nitrogen (5% O2 or hypoxia).
Colony-Forming Unit-Fibroblast Assay
CFU-F assays were performed as described by Baksh and Tuan [43]. One hundred passage 2 MSCs were plated on 10-cm tissue culture-treated polystyrene dishes in triplicate in growth medium and cultured for 14 days with medium changes every 3 days. At the end of 14 days, colonies were rinsed with phosphate-buffered saline (PBS), fixed with crystal violet dye in methanol, and again rinsed with PBS to remove residual dye. Differences in colony number were evaluated by a two-tailed one-sample t test to block for variability between individual human subjects.
Cell Proliferation Assays
The Click-iT 5-ethynyl-2′-deoxyuridine (EdU) Alexa Fluor 647 Cell Proliferation kit (Molecular Probes, Eugene, OR, http://probes.invitrogen.com) was used, according to the manufacturer’s protocol. MSCs were incubated with 10 μM Click-iT EdU for 16 hours, fixed, permeabilized, and labeled, and EdU was detected via flow cytometry using a FACSAria cytometer and FACSDiva software (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). Data were analyzed using FlowJo (Tree Star, Ashland, OR, http://www.treestar.com). Differences in EdU incorporation were evaluated by a paired two-tailed t test.
Proliferation was also assessed with Ki67 immunostaining. MSCs cultured on Laboratory-Tek Permanox chamber slides (Nunc, Rochester, NY, htpp://www.nuncbrand.com) at a density of 6.0 × 103 cells per cm2 for 48 hours were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with a mouse anti-Ki67 antibody (Abcam, Cambridge, U.K., http://www.abcam.com; clone PP-67) overnight at 4°C, a fluorescein isothiocyanate-conjugated goat anti-mouse secondary (Abcam), and then counterstained with Vectashield 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). Differences in number of Ki67-positive cells were evaluated by a paired two-tailed t test.
Metabolic Activity Assay
AlamarBlue (Invitrogen) was used to quantify MSC metabolic activity, according to the manufacturer’s protocol. MSCs from each condition were plated in triplicate at a density of 3.0 × 103 cells per well in a 96-well plate and incubated with 10% AlamarBlue in culture medium for 3 hours. Fluorescence was measured on a BioTek microplate reader. AlamarBlue solution from an empty well and AlamarBlue solution incubated with cells overnight were used to determine the lower and upper bounds of the assay, respectively. Differences in metabolic activity were evaluated by a two-tailed one-sample t test at 24- and 96-hour time points.
Cell Death Assays
Cell death was measured using a fluorescent LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen), according to the manufacturer’s protocol. MSCs from each condition were plated in triplicate at a density of 3.0 × 103 cells per well in a 96-well plate and incubated for 48 hours before staining. Fluorescence of each population was measured on a BioTek microplate reader, and differences in live:dead ratios were determined using a paired two-tailed t test.
The DeadEnd fluorometric terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) system (Promega, Madison, WI, http://www.promega.com) was used to quantify apoptosis, according to the manufacturer’s protocol. Briefly, MSCs were plated on Laboratory-Tek Permanox chamber slides (Nunc), cultured under normoxic or hypoxic conditions, fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100. A positive control sample was prepared by applying DNase to the fixed, permeabilized cells. TdT solution was applied to fluorescently label nick ends of DNA fragments, and differences in the number of apoptotic cells were determined using a two-tailed one-sample t test.
Immunophenotyping
MSCs were stained after 14 days of culture, according to the methods described in Nesti et al. [44]. A panel of standard positive and negative MSC markers was interrogated, including CD34 (clone 563), CD44 (clone 515), CD45 (clone TU116), CD73 (clone AD2), CD90 (clone 5E10), CD105 (clone 266), CD146 (clone P1H12) (BD Biosciences), and Stro-1 (clone STRO-1) (BioLegend, San Diego, CA, http://www.biolegend.com), as well as the stem cell markers octamer-binding transcription factor 4 (Oct4) (clone 40/Oct-3), SRY (sex determining region Y)-box 2 (Sox2) (clone 245610), homeobox transcription factor Nanog (clone N31-355) (BD Biosciences), and c-Myc (clone 9E10) (Abcam). Marker expression was measured using a FACSAria cytometer and FACSDiva software (BD Biosciences). Isotype controls for each marker were used to calculate Δ mean fluorescence intensity for the population. Data were analyzed using FlowJo (Tree Star), and two-tailed one-sample t tests were used to test for differences between hypoxic and normoxic cultures for each surface marker.
Gene Expression Assay
Reverse-transcriptase polymerase chain reaction (PCR) SuperArrays (SABiosciences/Qiagen, Hilden, Germany, http://www.qiagen.com) were used, according to the manufacturer’s protocol. RNA was isolated from MSCs using the RNeasy Mini-Prep Kit (Qiagen) and then amplified using the RT2 profiler first-strand kit (SABiosciences/Qiagen). RT2 profiler SYBR Green master mix with 6-Carboxyl-X-Rhodamine (ROX) (SABiosciences/Qiagen) was used to amplify cDNA on SuperArray plates with prealiquoted primers using an ABI 7900HT. All data were analyzed using SDS 2.3 software and the company’s web-based PCR Array Data Analysis platform, as well as Excel (Microsoft, Redmond, WA, http://www.microsoft.com), Prism (GraphPad Software, San Diego, CA, http://www.graphpad.com), and SPSS (IBM, Armonk, NY, http://www.ibm.com). Fold regulation was determined for hypoxia, extracellular matrix and adhesion molecules, stem cells, osteogenesis, and apoptosis pathways at 2 days and for hypoxia, extracellular matrix and adhesion molecules, cell cycle, and apoptosis pathways at 10 days, and differences in Δ CTs were detected for each gene using two-tailed t tests with blocking for patients where appropriate.
Enzyme-Linked Immunosorbent Assay
Secreted levels of growth factors, fibroblast growth factor 2 (FGF2), and vascular endothelial growth factor (VEGF) in culture media, as well as tumor necrosis factor α (TNFα), were quantified by enzyme-linked immunosorbent assay (ELISA), according to the manufacturers’ protocols. FGF2 (Abnova, Tapei City, Taiwan, http://www.abnova.com), VEGF, and TNFα (Pierce/ThermoFisher, Rockford, IL, http://www.piercenet.com) ELISAs were performed on 1-day and 3-day culture supernatants applied to precoated microtiter plates and read colorimetrically on a BioTek microplate reader. All samples and standards were measured in duplicate. Results were normalized to DNA content of each culture as determined by Picogreen assay (Invitrogen), and data were analyzed using repeated-measures two-way analysis of variance (ANOVA), with matched subjects serving as their own controls and Bonferroni post tests to detect differences between group means.
Matrix Metalloproteinase Activity Assay
Matrix metalloproteinase (MMP) activity was assayed using 520 MMP Fret Substrate XI (sequence 5-FAM-P-Cha-G-Nva-HA-Dap[QXL 520]-NH2) (AnaSpec, Fremont, CA, http://www.anaspec.com). MSCs were plated in triplicate at a density of 3.0 × 103 cells per well in a black 96-well plate and cultured for 3 days under serum-free conditions. To measure secreted MMP activity, two experimental medium replicates were collected without pooling from independent cultures of cells for each patient sample and condition. After addition of the substrate, fluorescence was measured on a BioTek microplate reader every 5 minutes over 60 minutes, and a linear fit model was used to determine the rate of the cleavage reaction. To measure activity of retained MMPs, 520 MMP Fret Substrate XI solution was applied to the cells remaining after collection of conditioned media, and MMP activity was measured in a similar fashion and then normalized to the DNA content of each culture. Differences between hypoxic and normoxic cultures were detected using two-tailed one-sample t tests.
Differentiation Assays
Osteogenic differentiation was induced and assayed according to the methods described in Boland et al. [45]. Osteogenesis was induced in monolayer MSC cultures plated at a density of 1.0 × 104 cells per cm2 in 6-well and 24-well plates with osteogenic medium consisting of DMEM supplemented with 10% fetal bovine serum (FBS), 50 μg/ml L-ascorbate-2-phosphate, 0.1 μM dexamethasone, 10 mM β-glycerophosphate, and 10 nM 1α,25-(OH)2 vitamin D3 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). On day 14, alkaline phosphatase activity was detected histochemically in paraformaldehyde/methanol-fixed cultures using the Leukocyte Alkaline Phosphatase Kit (Sigma-Aldrich), according to the manufacturer's protocol. On day 21, osteogenic cultures were rinsed with PBS, fixed in 60% isopropanol, and stained in a 1% Alizarin Red solution (Rowley Biochemical, Danvers, MA, http://www.rowleybio.com) to detect matrix mineralization. For assessment of gene expression in osteogenic cultures, RNA was collected at day 21. For preconditioning assays, MSCs were cultured at either 5% or 21% O2 for 2 weeks in growth medium, then trypsinized, counted, replated at 1.0 × 104 cells per cm2 in 24-well tissue culture plates, and cultured in osteogenic medium, as described above, at either the same or the opposite oxygen tension. This resulted in four groups: preconditioned and differentiated at 5% O2 (5%–5%), preconditioned at 5% O2 and differentiated at 21% O2 (5%–21%), preconditioned at 21% O2 and differentiated at 5% O2 (21%–5%), or preconditioned and differentiated at 21% O2 (21%–21%). To determine the contribution of preconditioning oxygen tension versus differentiation oxygen tension, as well as the interaction of these two variables, to the observed variance of each dependent variable tested, we used two-way ANOVA. Bonferroni corrections were used to compare the means of the two pairs of treatment groups with one another for each dependent variable tested. This approach was used for analysis of all experiments with preconditioning.
Adipogenic and chondrogenic differentiation were induced and assayed, as described previously by Baksh et al. [46]. Preconditioning was carried out, as stated above, for osteogenic differentiation. Adipogenesis was induced in monolayer MSCs plated at a density of 1.0 × 104 cells per cm2 in 6-well and 24-well plates with adipogenic medium consisting of DMEM supplemented with 10% FBS, 1 μM dexamethasone, 1 μg/ml insulin, and 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich). On day 21, hypoxic and normoxic adipogenic cultures were stained with Oil Red O stain (Sigma-Aldrich), and dye content was quantified by calculating area of positive staining in each culture across three to four photomicrograph fields. We used the analytical approach described in the previous paragraph to determine the contribution of preconditioning versus differentiation oxygen tension and any interaction.
To induce chondrogenesis, MSCs were grown as high-density pellets (2.5 × 105 cells) in serum-free DMEM supplemented with ITS Premix (BD Biosciences), 50 μg/ml ascorbic acid (Sigma-Aldrich), 40 μg/ml l-proline (Sigma-Aldrich), 100 μg/ml sodium pyruvate (Gibco/Invitrogen), 0.1 μM dexamethasone (Sigma-Aldrich), and 10 ng/ml recombinant human transforming growth factor (TGF)-β3 (R&D Systems, Minneapolis, MN, http://www.rndsystems.com). On day 28, pellets were harvested for histology to detect total proteoglycan content via Safranin O/Fast Green staining (Rowley) and sulfated glycosaminoglycan (sGAG) specifically via Alcian Blue staining (Rowley). sGAG content was quantified using the Blyscan sGAG assay (Accurate Chemical & Scientific Corporation, Westbury, NY, http://www.accuratechemical.com) and normalized to DNA content of each pellet. We developed a modified scoring system for cartilage histology (supplemental online Table 1) based on the system described in Im et al. [47]. Duplicate pellets from each patient and condition were independently scored by two investigators, and an average quality score was generated. We used the two-way ANOVA approach described above to determine the contribution of preconditioning versus differentiation oxygen tension and any interaction on cartilage quality score and sulfated glycosaminoglycan (GAG) content of pellets.
Results
Hypoxia Enhances MSC Colony Formation, Proliferation, and Metabolic Activity
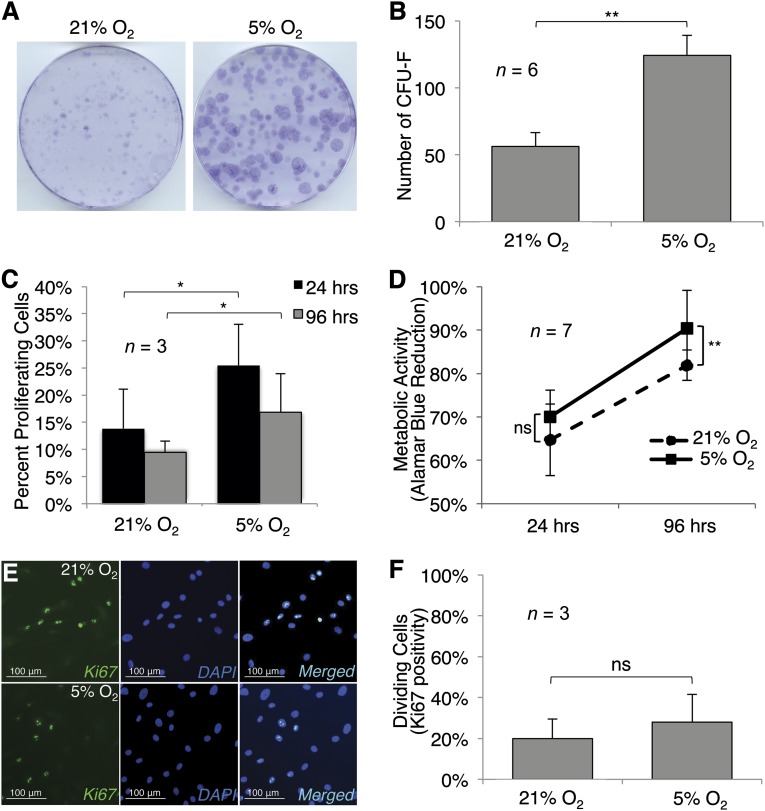
CFU-F assays performed on MSCs cultured in hypoxic or normoxic conditions showed that hypoxia enhanced colony formation (p < .01). Hypoxic conditions increased proliferation, as shown by EdU incorporation by (p < .05); Ki67 staining was not significantly enhanced by hypoxic culture. Normalized AlamarBlue assays showed no difference in metabolic activity after 24 hours, but higher metabolic activity in hypoxic cultures after 96 hours (Fig. 1) (p < .01).
Figure 1.
Hypoxia enhances clonogenicity, proliferation, and metabolic activity of human mesenchymal stem cells (MSCs). (A–C, E, F): MSC colony size (A), colony number (B), and cell proliferation as measured by 5-ethynyl-2′-deoxyuridine (EdU) incorporation (C) and Ki67 labeling (E) and quantified (F). AlamarBlue staining showed increased reduction of the substrate in hypoxic cultures, with a statistically significant difference observed in the 4-day cultures (D). Photomicrographs were captured at ×10; scale bars = 100 μm. Significant differences of relevant comparisons are indicated: ∗, ∗∗, p values of <.05 and <.01, respectively. Abbreviations: CFU-F, colony-forming unit-fibroblast; DAPI, 4′,6-diamidino-2-phenylindole; ns, nonsignificant.
Hypoxia Does Not Alter Incidence of MSC Death
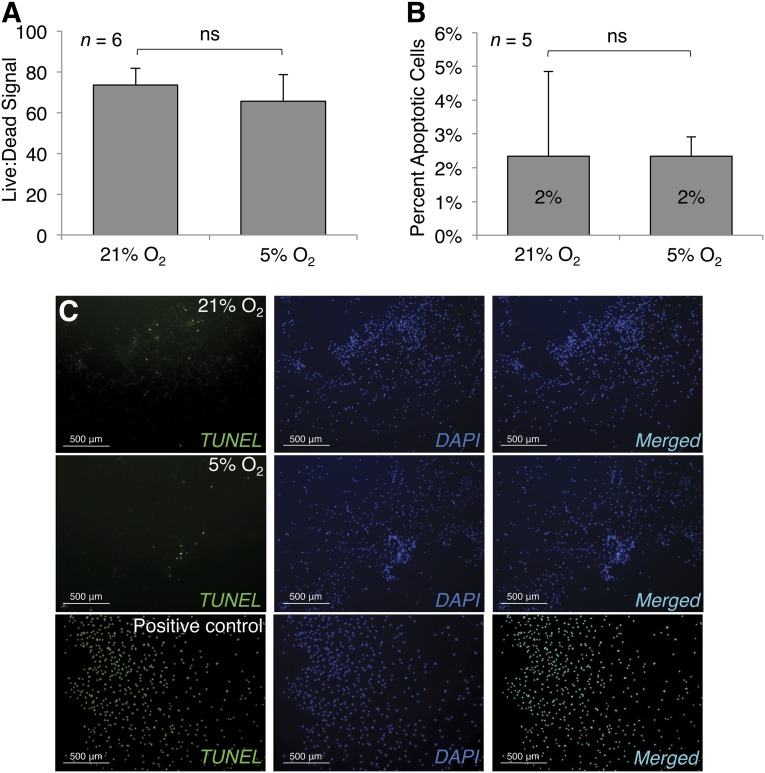
LIVE/DEAD assays to quantify nonspecific cell death showed no difference in the ratio of viable to dead cells between MSCs cultured in hypoxic conditions and MSCs cultured in normoxic conditions. To determine whether there was any difference in cell death because of apoptosis specifically, we performed TUNEL staining, which showed a very low level of apoptosis in all MSC cultures, and no difference in level of apoptosis between MSCs cultured in hypoxia versus normoxia (Fig. 2).
Figure 2.
Hypoxia does not impact mesenchymal stem cell death and apoptosis rates. Both normoxic and hypoxic conditions generated similar rates of cell death, as measured by LIVE/DEAD (A), and apoptosis, as measured by TUNEL staining (C), and quantified (B). Photomicrographs were captured at ×4; scale bars = 500 μm. Significant differences of relevant comparisons are indicated. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ns, nonsignificant; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
Differential Gene Expression in Hypoxic MSC Cultures
We performed PCR arrays representing a number of pathways of interest to screen for genes differentially regulated under hypoxic versus normoxic conditions both short-term (2 days) and in the steady state (10 days) (supplemental online Tables 2, 3).
Hypoxic Culture Minimally Alters MSC Immunophenotype
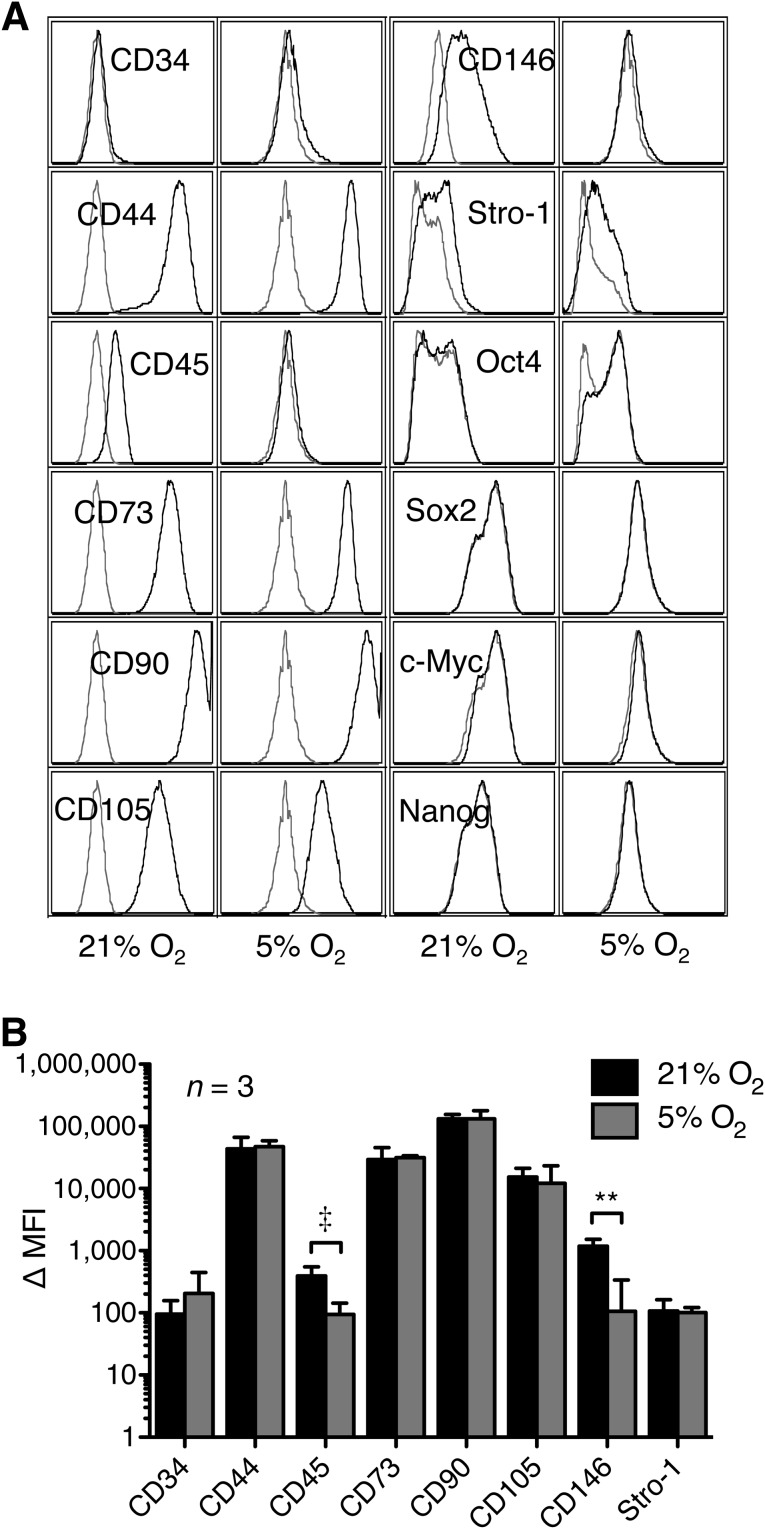
Flow cytometric immunophenotyping of MSCs cultured under normoxic conditions for 2 weeks showed the cells to be CD34−, CD44+, CD45dim, CD73+, CD90++, CD105+, CD146dim, and Stro-1dim relative to isotype controls. This differed slightly from the immunophenotype of MSCs cultured under hypoxic conditions, which showed no detectable CD45 expression (p = .06) or CD146 expression (p < .01) (Fig. 3). We also performed intracellular staining for the transcription factors Oct4, Sox2, c-Myc, and Nanog. Both normoxic and hypoxic cultures were negative for all four of these transcription factors in the samples tested.
Figure 3.
Effect of hypoxia on mesenchymal stem cell immunophenotype. (A): Immunophenotype under normoxic (left) and hypoxic (right) conditions. Histograms are from one representative donor; isotype controls are represented as gray curves, stained samples as black curves. (B): Quantitation of surface marker expression, shown as Δ MFI. Data plotted are from three distinct donors; error bars represent SD. Significant differences of relevant comparisons are indicated: ‡, ∗∗, p values of .06 and <.01, respectively. Surface marker designations are as defined by Human Cell Differentiation Molecule/Human Leukocyte Differentiation Antigens nomenclature, and intracellular markers are listed as defined by the HUGO Gene Nomenclature Committee. Abbreviation: MFI, mean fluorescence intensity.
Effects of Hypoxic Culture on MSC Growth Factor and Cytokine Secretion
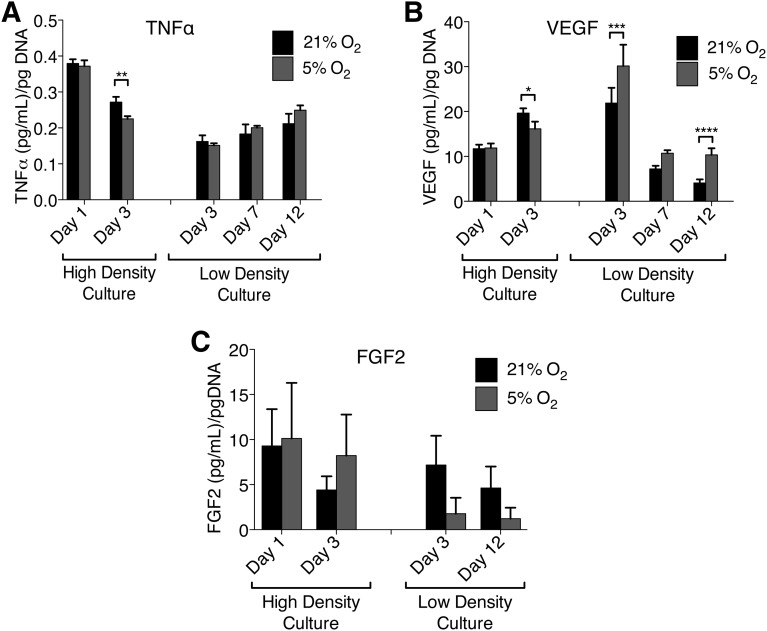
We assayed hypoxic versus normoxic cultures for differences in growth factor secretion and TNFα secretion. ELISAs for VEGF showed increasing VEGF secretion from day 1 to day 3 in high-density cultures (6.0 × 103 cells per cm2), with slightly higher overall VEGF levels in hypoxic cultures but lower VEGF production per cell in hypoxic cultures after normalization to cell number. In low-density cultures analogous to the conditions experienced by MSCs in colony-forming assays, VEGF secretion normalized for cell number was initially undetectable, quite high by day 3, and then decreased over time, but it was maintained at higher levels in hypoxic cultures (oxygen tension p < .0001; repeated-measures two-way ANOVA, matched subjects serving as their own controls). ELISAs for TNFα showed the opposite: TNFα secretion was initially high in high-density cultures and dropped by day 3, with higher TNFα secretion in normoxic cultures. In low-density cultures, there was no difference in TNFα secretion between hypoxic and normoxic cultures, and TNF levels at day 3 were low compared with high-density cultures (when normalized to cell number) but increased as cell density increased, with a slight lead in hypoxic cultures by day 12. ELISAs for FGF2 showed that FGF2 secretion varied significantly between samples from different patients for high-density cultures, with a greater spread in levels of FGF2 secretion in hypoxic cultures. In low-density cultures FGF2 secretion decreased over time, but overall there was no observable effect of oxygen tension on FGF2 secretion (Fig. 4).
Figure 4.
TNF and growth factor secretion by mesenchymal stem cells in normoxic and hypoxic cultures. (A–C): TNFα secretion (A), VEGF secretion (B), and FGF2 secretion (C) in high- and low-density cultures. All data are normalized to cell number. Error bars represent SEM computed on three to five biologic replicates (distinct donors). Significant differences of relevant comparisons are indicated: ∗, ∗∗, ∗∗∗, ∗∗∗∗, p values of <.05, <.01, <.001, and <.0001, respectively. Abbreviations: FGF2, basic fibroblast growth factor; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
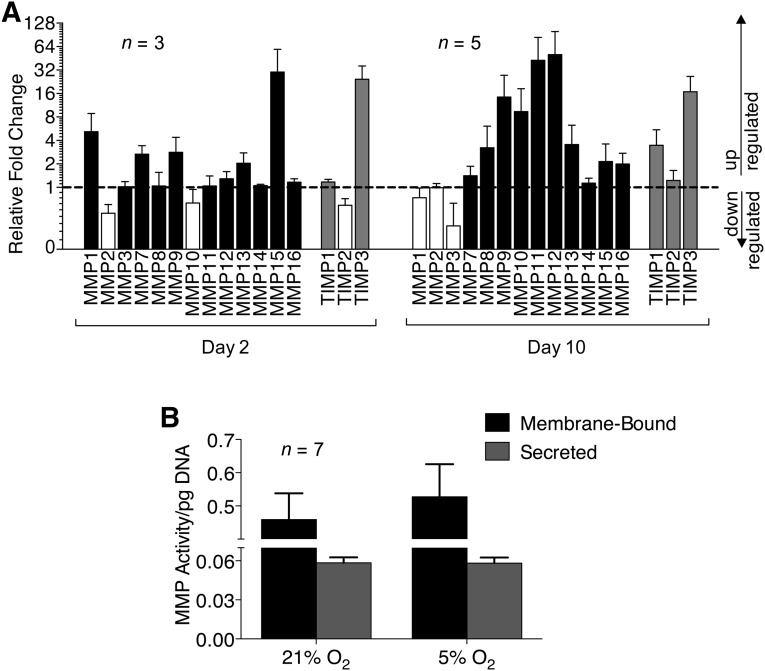
Hypoxic Culture Enhances MMP Expression in MSCs
Analysis of gene expression of MMPs showed upregulation at day 2 of several MMP genes under hypoxic conditions relative to normoxic conditions, most notably MMP15, with upregulation of several others by day 10, especially MMP9, MMP10, MMP11, and MMP12. This was accompanied by an increase in overall tissue inhibitor of metalloproteinases (TIMP) gene expression in response to hypoxic culture. TIMP1 expression in hypoxic cultures was initially the same as normoxic cultures (at day 2), but increased more than threefold compared with normoxic cultures by day 10. TIMP3 expression at day 2 of culture was 24-fold higher in hypoxic cultures than normoxic cultures and remained highly elevated at day 10. These results led us to test MMP activity in hypoxic and normoxic cultures using a broad-spectrum activity assay. There were no significant differences observed in membrane-bound or secreted MMP activity between hypoxic and normoxic cultures when the data were normalized for cell number; however, there was a nonsignificant trend toward greater membrane-bound MMP activity in hypoxic cultures (Fig. 5).
Figure 5.
Effect of hypoxia on MMP expression and activity. (A): Fold regulation of MMP and TIMP gene expression in mesenchymal stem cells under hypoxic conditions relative to normoxic conditions. Black: upregulated MMPs. Gray: upregulated TIMPs. White: downregulated MMPs and TIMPs. Dashed line indicates a fold change of 1. (B): Membrane-bound and secreted MMP activity. Error bars represent SEM. Abbreviations: MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases.
Hypoxia During Differentiation but Not Preconditioning Enhances Osteogenic Matrix Mineralization
Hypoxia enhanced alkaline phosphatase staining at 14 days of osteogenic induction, and quantitative PCR showed increased gene expression of osteocalcin, collagen type I, and runt-related transcription factor 2 (RUNX2) in hypoxic osteogenic cultures relative to normoxic osteogenic cultures, in addition to many other markers of osteogenesis, with the exception of cathepsin K, which was significantly downregulated (p = .01) (Fig. 6F). Two-way ANOVA analyses were performed to examine the effect of preconditioning oxygen tension and differentiation oxygen tension on both total matrix mineralization and matrix mineralization normalized to cell number. A significant interaction between the effects of preconditioning oxygen tension and differentiation oxygen tension on total matrix mineralization could not be detected (F = 2.078, p = .16). There was a significant main effect of differentiation oxygen tension on total mineralization (F = 7.506, p = .01) but not of preconditioning oxygen tension (F = 0.968, p = .333). Post hoc tests (Bonferroni) of the differentiation oxygen tension effect showed that cultures differentiated at 5% O2 yielded higher total mineral content (p < .05), but this effect was only apparent in cultures preconditioned at 21% O2 (Fig. 6G). After normalization of mineralization to DNA content in each culture, no significant effects of oxygen tension could be detected (Fig. 6H).
Figure 6.
Effects of hypoxia on mesenchymal stem cell osteogenesis. (A, C, D): Alizarin Red (A), staining quantified (C), and normalized to DNA (D). (B): Alkaline phosphatase. (E, F): Expression of osteogenic markers (E) and osteogenesis-associated genes (F). Black line indicates a fold regulation of 1; pink lines define fold regulation of −3 to 3. Effect of hypoxic preconditioning: Alizarin Red quantitation (G), normalized to DNA (H). Significant differences of relevant Bonferroni comparisons are indicated: ∗, p value of <.05. Images of the cultures were obtained at ×1 and are representative of three donors tested. Error bars represent SEM. Gene abbreviations are as defined by the HUGO Gene Nomenclature Committee. Abbreviations: Alk Phos, alkaline phosphatase; diff, differentiation culture oxygen tension; pre, preconditioning oxygen tension.
Adipogenesis of MSCs Is Inhibited by Hypoxia
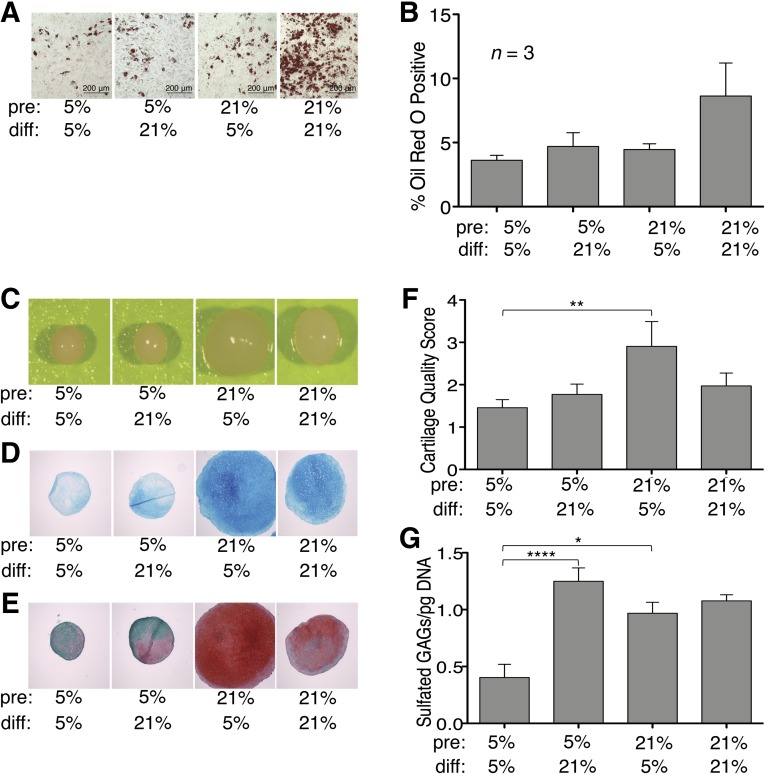
A two-way ANOVA performed to determine the effects of preconditioning oxygen tension and differentiation oxygen tension on adipogenesis revealed no significant interaction or main effect of either variable, but there was a trend for hypoxia applied during differentiation to inhibit adipogenesis relative to normoxia. When we examined the data to look at the response of individual patient samples, we observed that some samples exhibited modest adipogenic differentiation under all experimental conditions (apparent differentiation relative to noninduction medium controls but lack of responsiveness to changing oxygen tensions). If we excluded these subjects, the observed effect of differentiation oxygen tension was statistically significant in cultures preconditioned in normoxia (p < .05) (Fig. 7).
Figure 7.
Hypoxia inhibits adipogenesis but enhances chondrogenesis. Adipogenesis: Oil Red O-stained accumulated intracellular lipid droplets (A), and staining quantified (B). Photomicrographs were captured at ×10 . Chondrogenesis: wet pellet (C), Alcian Blue (D), and Safranin-O/Fast Green (E). Photomicrographs were captured at ×4; scale bars = 200 μm. (F, G): Effect of hypoxic preconditioning on cartilage quality score (F) and content of sulfated GAGs (G). Significant differences of relevant Bonferroni comparisons are indicated: ∗, ∗∗, ∗∗∗∗, p values of <.05, <.01, and <.0001, respectively. Abbreviations: diff, differentiation culture oxygen tension; GAG, glycosaminoglycan; pre, preconditioning oxygen tension.
Chondrogenesis Is Enhanced in MSCs Expanded in Normoxia and Then Differentiated in Hypoxia
Overall, hypoxic preconditioning decreased chondrogenesis of MSCs in pellet cultures accompanied by lower cartilage quality scores on histologic analysis. The 5%–5% condition generated extremely low quantities of sulfated GAGs per cell number compared with other conditions tested. MSCs preconditioned in normoxic conditions differentiated robustly in pellet cultures under both hypoxic and normoxic conditions (21%–5% and 21%–21%). The 21%–5% condition showed enhanced chondrogenesis compared with the 21%–21% condition, as demonstrated by wet pellet size, Alcian Blue staining, and Safranin-O/Fast Green staining (Fig. 7). Two-way ANOVAs were performed to examine the effect of oxygen tension during preconditioning and during differentiation on both cartilage quality score and sulfated GAG content of pellets. There was a significant interaction between the effects of preconditioning O2 and differentiation O2 on cartilage quality score (F = 5.367, p = .023). There was a significant main effect of preconditioning O2 on cartilage quality score (F = 4.110, p = .046) but not of differentiation O2 (F = 0.827, p = .366). Post hoc tests of the preconditioning effect showed that MSCs preconditioned at 21% O2 generated pellets with higher cartilage quality scores when differentiated at 5% O2 (p < .01), but the positive effect of preconditioning at 21% O2 was lost when the pellets were differentiated at 21% O2 (p > .05) (Fig. 7F). There was also a significant interaction between the effects of preconditioning O2 and differentiation O2 on sulfated GAG content of the pellets (F = 8.642, p = .006). There was a significant main effect of differentiation O2 on sulfated GAG content (F = 14.50, p = .0006) but not of preconditioning O2 (F = 2.458, p = .126). Post hoc tests of the differentiation O2 effect showed that MSCs preconditioned at 5% O2 produced significantly more sulfated GAGs when subsequently differentiated at 21% O2 (p < .0001), whereas MSCs preconditioned at 21% O2 were able to generate a large quantity of sulfated GAGs whether they were differentiated at 5% O2 or 21% O2 (p > .05) (Fig. 7G). In monolayer chondrogenic culture, hypoxia had no discernible effect (supplemental online Fig. 1).
Discussion
We observed hypoxia-enhanced MSC clonogenicity in all samples tested. Hypoxia-enhanced proliferation of MSCs was accompanied by no detectable change in cell death. Of note, all of our proliferation experiments were conducted in high-glucose culture medium supplemented with serum. MSCs have been previously reported to be more sensitive to oxygen deprivation in the face of concurrent nutrient deprivation [48, 49].
We used PCR arrays to screen for signaling pathways related to colony formation, which might be perturbed by hypoxia. We observed small but significant changes in several genes, many with interesting connections to regulation of Wnt/β-catenin signaling, which is critical for maintenance of stemness in adult and embryonic stem cell populations [50]. After 2 days of hypoxic culture, among those transcripts differentially regulated were the following upregulated transcripts: glucose-6-phosphate isomerase/autocrine motility factor (GPI) and MMP8, and downregulated transcripts: CDH1 (E-cadherin), CTNNA1 (α-catenin), APC, SMAD2, and SMAD3, all of which are connected in regulation of epithelial-to-mesenchymal transition and canonical Wnt signaling through modulation β-catenin trafficking and signaling, either directly or through release or increase of cell adhesions. GPI has been shown to promote degradation of β-catenin but also to enhance its expression and nuclear translocation [51]. Downregulation of E-cadherin, α-catenin, and APC, all known to inhibit nuclear translocation and signaling of β-catenin [52–55], also suggests enhanced Wnt/β-catenin activity at this time point. Downregulation of SMAD2 and SMAD3, both of which enable β-catenin coactivation in response to TGFβ signaling and vice versa [56–60], suggests that cross-talk between TGFβ and Wnt signaling pathways is playing a less important role in directing cell fate after brief (2-day) exposure of undifferentiated MSCs to hypoxia. After 10 days of hypoxic culture, none of these specific genes continued to be significantly differentially regulated in hypoxic versus normoxic cultures, suggesting they are differentially regulated as the cell re-establishes homeostasis. In the case of MSCs, this is most likely achieved through metabolic conversion to a largely glycolytic phenotype; induction of this phenomenon, known in cancer biology as the Warburg effect, can be mediated through Wnt or β-catenin activation, either in combination or with other signaling partners [61–63]. At that point, however, when the cells are at or approaching a steady state, we observed differential regulation of different actors shown to impact the same signaling pathway, namely the following upregulated transcripts: IGFBP1, an inhibitor of β-catenin, VEGFA, CDC2, SPARC, and downregulated transcripts: HIF1A, TNFRSF21 (DR6), CDKN1A, CUL1, and HIF1AN. HIF1A was downregulated in long-term hypoxic culture relative to normoxia; regulation of HIF1A in hypoxia has been shown to occur as a result of altered redox status of the cell [64–66]. This would suggest that in the absence of caloric restriction, MSCs readily adapt to hypoxic conditions and are able to establish redox homeostasis.
In longer-term cultures we also observed significant upregulation of leptin. Hypoxia has been shown to induce leptin expression in multiple cell types, including preadipocytes and MSCs [67, 68]. Leptin has a somewhat cryptic role in influencing MSC fate, as a result of its distinct systemic versus local activities and disparate effects on different mesenchymal cell types, but is generally thought to serve as a regulatory link between fat and bone, with secretion of leptin by fat serving to protect bone through maintenance of mineralization [69]. Leptin has been shown to maintain peripheral mesenchymal progenitors in an undifferentiated state but to also promote mineralization in differentiated osteoblasts [70].
Analysis of the effect of hypoxia on immunophenotype of MSCs showed low-level CD45 expression on MSCs cultured in normoxic conditions, whereas hypoxia appeared to maintain MSCs in culture with no CD45 expression. MSC fractions have been shown to have low CD45 and low CD146 expression directly after harvest from bone marrow [71, 72], and MSCs can be induced to express CD45 after epigenetic modification using 5-aza-2′-deoxycytidine [73]. MSCs cultured under normoxic conditions expressed CD146, as expected; however, MSCs cultured under hypoxic conditions were negative for CD146, a known pericyte marker. This finding has been observed by others, with CD146 expression in CFU-Fs correlating with in situ localization to the endosteal versus perivascular space and induction of CD146 expression upon ex vivo culture in normoxic conditions [72]. Taken together, these findings regarding CD45 and CD146 expression suggest that during in vitro culture hypoxia maintains an MSC immunophenotype more akin to what would be observed in vivo in the bone marrow or in avascular cartilaginous tissues than does normoxia. CD146 negativity has been associated with increased chondrogenic potential in adult stem cells [74] and other chondrogenic progenitor populations [75].
Differential expression of genes related to growth factor signaling and inflammatory signaling prompted our investigation of growth factor and TNF levels in the medium. In low-density cultures recapitulating the environment of a colony-forming assay, we observed higher VEGF levels in hypoxic cultures over time, which most likely contributed directly to enhanced colony formation in hypoxia through its regulation of proliferation. In high-density cultures, we observed slightly higher overall VEGF levels in hypoxic cultures but lower VEGF production per cell in hypoxic cultures after normalization to cell number of the culture, suggesting that VEGF secretion in MSC culture is tightly regulated beyond a critical threshold. VEGF secretion in MSCs was enhanced by hypoxia in several studies [76], and hypoxia has been shown both to induce expression of and increase the half-life of VEGF mRNA [77]. VEGF secretion has been shown to be attenuated in hypoxic cultures by the presence of high glucose concentrations [49], which may explain why the observed effect in this study was not more pronounced.
Although we observed increased expression of MMP genes and TIMP genes with hypoxia, there was no increase in MMP activity in supernatants from hypoxic cultures. When we looked at MMPs retained on the cell surface and in the extracellular matrix, slightly higher overall MMP activity was observed in hypoxic cultures. This would suggest that MSCs are in a matrix-stabilizing state in normoxic cultures and that hypoxia may be a stimulus for matrix turnover, enabling cell migration and contributing to colony expansion but with continued tight regulation of matrix metalloproteinase activity.
Application of hypoxia to osteogenic cultures during differentiation yielded more mineralized tissue but no more mineral deposition per cell in the tissue. This would suggest that hypoxia is useful for tissue engineering of bone ex vivo but from a cell biology perspective does not necessarily enhance the osteogenic potential of an individual MSC. It is possible that alkaline phosphatase activity was higher in hypoxic cultures as an indication of preserved stemness or of enhanced osteogenic differentiation; other groups have shown that levels of alkaline phosphatase activity are not proportional to observed mineralization levels in in vitro osteogenesis assays [78]. When we looked closely at gene expression data from osteogenic cultures, we noticed that osteogenic induction with supplemented medium upregulates cathepsin K expression relative to undifferentiated MSCs. This effect is nullified by the addition of hypoxia, which downregulated cathepsin K expression in all patient samples tested to the level of undifferentiated cultures (Fig. 6). Boskey et al. [79] reported that in a chick limb-bud mesenchymal micromass culture system, decreased mineral deposition was seen upon pharmacological inhibition of cathepsin K during early stages of matrix mineralization. They proposed a mechanism whereby reduced proteoglycan catabolism in the extracellular matrix in the setting of cathepsin K inhibition results in inhibited hydroxyapatite formation.
Hypoxic preconditioning did not enhance osteogenesis, as indicated by Alizarin Red quantification of matrix mineralization, either in total or when normalized to DNA content. Analysis of the differentiation oxygen tension revealed that our hypoxic cultures were mineralized to the same degree as our normoxic cultures (per cell), but with a higher number of cells present. This would suggest that matrix mineralization either starts later in hypoxic cultures or is less efficient per cell. The fact that this effect was significant only after preconditioning in normoxia suggests that there is in fact an interaction effect of preconditioning and differentiation oxygen tensions but that the effect was not great enough to be detected in this study. We conclude that expanding cells in normoxic conditions and then differentiating in hypoxia generates the highest total mineral content, although this result must be interpreted with caution because an increased rate of cell death can also enhance mineral deposition in two-dimensional (2D) cultures [80].
It is likely that 2D in vitro assays of osteogenesis give an incomplete picture of MSC osteogenic potential. Multiple studies have demonstrated that hypoxia enhances osteogenesis in 3D in vitro culture, including one study in which the authors directly compared 3D with 2D cultures and found no enhancement with hypoxia in 2D [81, 82]. However, another recent study evaluated the effect of hypoxia on osteogenesis in 3D culture and found no enhancement [83]. Increased oxygenation by delivery with perfluorotributylamine has been shown to enhance osteogenesis in a 3D in vivo model [84], yet another study examining in vivo osteogenesis in hypoxic (13%), normoxic (21%), or hyperoxic (50%) conditions found that no condition significantly improved early osteogenic or chondrogenic differentiation in a fracture-healing model [85]. Preconditioning MSCs in hypoxia ex vivo before implantation into a rabbit calvarial defect model resulted in improved bone formation compared with normoxic preconditioning [86]. Similar results have been achieved using hypoxic preconditioning before Achilles tendon repair with MSCs in a rat model [87]. It is difficult to determine the effect of ex vivo hypoxic preconditioning using in vivo studies of differentiation because of the enhanced survival and proliferation conferred on MSCs by hypoxic preconditioning, which may account for their enhanced function in critical defects and ischemia models, rather than increased differentiation potential per se.
It is also possible that conflicting reports exist in the literature regarding molecular events associated with hypoxia and osteogenesis because of inappropriate selection of housekeeping genes/proteins. GAPDH, for example, is commonly used as a housekeeping gene in these studies despite the fact that it is differentially regulated in hypoxic versus normoxic conditions [88].
There was a trend for adipogenesis to be inhibited under hypoxic conditions, which is consistent with the findings of most other groups. The HIF-1α-regulated gene DEC1(STRA13) has been shown to repress PPARG, thereby transducing oxygen sensing as a signal for suppression of adipogenesis [89, 90]. Preconditioning with hypoxia also appeared to suppress adipogenesis even when MSC cultures were switched to normoxia for differentiation. However, it should be noted that the effects of oxygen tension on adipogenesis were patient sample-dependent and, as a result, not statistically significant in our study.
Upon histological examination of chondrogenesis in pellet culture, we observed internal heterogeneity at 5% O2, with obvious cartilage formation in patches and fibrous matrix deposition lacking proteoglycans in other areas. Despite this patchy differentiation, sGAG content was still higher overall when normalized to DNA content of the pellet compared with normoxic pellets, suggesting that there was a higher rate of cell death in hypoxic pellets, but the surviving cells differentiated robustly. This is perhaps because of uneven oxygen diffusion within the 3D pellet culture. To further assess these results, we performed a monolayer screen for chondrogenic potential in hypoxic versus normoxic MSC cultures. Chondrogenesis was poor in all monolayer cultures, consistent with the findings of others [91]. Alcian Blue staining, quantified after guanidine-HCl extraction, was not different after normalization to DNA content of the culture. Markway et al. [30] have shown enhanced chondrogenesis with hypoxia in micropellet cultures, in which oxygen concentration is more consistent throughout the pellet. Although that model is excellent for addressing the problem of gas and nutrient distribution in the pellet, its obvious limitation is that for tissue-engineering purposes we would like to study larger pieces of cartilage in culture rather than smaller ones.
For chondrogenic differentiation we found that the best overall results were obtained by expanding MSCs in normoxic conditions and then performing pellet culture with chondrogenic induction medium in hypoxic conditions. This approach has been practiced in many laboratories based on the empiric finding that it works, but pellet cultures from some patients developed severely hypoxic cores (supplemental online Fig. 1). It is possible this approach could be improved with the application of a flow-perfusion bioreactor to optimize diffusion of oxygen and nutrients throughout the pellet. In all cases, preconditioning with hypoxia failed to enhance chondrogenesis and, in most subjects, significantly inhibited it. When we considered these results in the context of our MMP expression data, the possibility emerged that enhanced chondrogenesis in hypoxia is mediated, at least in part, through regulation of MMP activity. It has been established that MMP production is critical for progression through chondrogenic differentiation, both in the growth plate and in pellet culture [92–96]. Dose-dependent disruption of chondrogenic differentiation of MSCs in pellet culture has been demonstrated with application of pan-MMP inhibitors [96]. Whereas we did not directly analyze matrix turnover in cartilage pellets, a significant increase in MMP activity was not observed with hypoxia in conditioned medium, although increased MMP transcription and MMP accumulation in matrix were detected. We observed no differential regulation of ADAMTS1, 8, or 13 with hypoxic versus normoxic culture (data not shown). Overall, this suggests hypoxia-mediated protease secretion may influence matrix accumulation by modulating growth factor activity and matrix molecule processing, particularly in early chondrogenesis, when others have shown MMPs are required for differentiation. Extremely low oxygen tension may detrimentally impact matrix accumulation through unregulated high matrix protease activity leading to matrix degradation, which becomes evident later in chondrogenic pellet culture.
Interestingly, HIF1A was slightly but consistently downregulated over time in our long-term hypoxic cultures (10 days), at which time we saw more dramatic upregulation of MMP genes, as well as TIMP1 and TIMP2, in hypoxic cultures compared with normoxic cultures, than was observed at day 2. Thus, MMP activity may be tightly controlled by the concurrent hypoxia-mediated increase in protease inhibitors. Previous data from our group indicate that MSCs overall maintain a matrix-protective MMP/TIMP secretion profile even in the context of stressors like hypoxia and inflammatory cytokines, which is consistent with the findings from this study [97].
Conclusion
Mesenchymal stem cells display context-specific responsiveness to hypoxia as a differentiation cue during development and tissue repair. The existing conflicts in the literature regarding the effects of hypoxia on MSC differentiation potential most likely stem from differences in cell culture methodology between laboratories and the specific models used to test effects on differentiation, as well as how differentiation is defined and measured. Oxygen tension should be carefully considered as a variable when culturing cells for tissue-engineering applications. Our study conclusively demonstrates that expansion of MSCs in normoxic culture followed by application of hypoxia during chondrogenic differentiation is a useful technique for enhancing ex vivo chondrogenesis.
Supplementary Material
Acknowledgments
We thank the Flow Cytometry Facility at the McGowan Institute for Regenerative Medicine, Dr. Juan Taboas for discussion and comments regarding this study, Jian Tan for assistance isolating MSCs, and Dr. Paul Manner (University of Washington) for providing human tissues. This work was supported in part by the Intramural Research Program of the National Institutes of Health (Z01AR41131) and the Commonwealth of Pennsylvania Department of Health (SAP4100050913).
Author Contributions
L.B.B.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; O.A.C.: collection and assembly of data; L.G.: conception and design, collection and assembly of data, data analysis and interpretation; T.L.: collection and assembly of data, data analysis and interpretation; R.S.T.: conception and design, financial support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
O.A.C. is a compensated employee of Organovo Holdings, Inc.
References
- 1.Nöth U, Osyczka AM, Tuli R, et al. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 2.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 3.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterson EJ, Nesti LJ, Danielson KG, et al. Human marrow-derived mesenchymal progenitor cells: Isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 5.Current MSC Trials Listed in ClinicalTrials.gov. Available at http://www.clinicaltrials.gov/ct2/results?term=MSCs&Search=Search Accessed March 5, 2013.
- 6.Zipori D. Mesenchymal stem cells: Harnessing cell plasticity to tissue and organ repair. Blood Cells Mol Dis. 2004;33:211–215. doi: 10.1016/j.bcmd.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Amarilio R, Viukov SV, Sharir A, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 8.Zuscik MJ, Hilton MJ, Zhang X, et al. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 2008;118:429–438. doi: 10.1172/JCI34174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan AI, Koutroupas S. The control of muscle and cartilage development in the chick limb: The role of differential vascularization. J Embryol Exp Morphol. 1973;29:571–583. [PubMed] [Google Scholar]
- 10.Caplan AI, Syftestad G, Osdoby P. The development of embryonic bone and cartilage in tissue culture. Clin Orthop Relat Res. 1983;174:243–263. [PubMed] [Google Scholar]
- 11.Osdoby P, Caplan AI. Osteogenesis in cultures of limb mesenchymal cells. Dev Biol. 1979;73:84–102. doi: 10.1016/0012-1606(79)90140-4. [DOI] [PubMed] [Google Scholar]
- 12.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells: Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 14.Guitart AV, Debeissat C, Hermitte F, et al. Very low oxygen concentration (0.1%) reveals two FDCP-mix cell subpopulations that differ by their cell cycling, differentiation and p27KIP1 expression. Cell Death Differ. 2011;18:174–182. doi: 10.1038/cdd.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 16.Ren H, Cao Y, Zhao Q, et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 17.Ma T, Grayson WL, Fröhlich M, et al. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009;25:32–42. doi: 10.1002/btpr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, Kato T, Furu M, et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391:1471–1476. doi: 10.1016/j.bbrc.2009.12.096. [DOI] [PubMed] [Google Scholar]
- 19.Lavrentieva A, Majore I, Kasper C, et al. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun Signal. 2010;8:18. doi: 10.1186/1478-811X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Santos F, Andrade PZ, Boura JS, et al. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 21.Grayson WL, Zhao F, Bunnell B, et al. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 22.Carrancio S, López-Holgado N, Sánchez-Guijo FM, et al. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp Hematol. 2008;36:1014–1021. doi: 10.1016/j.exphem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Moussavi-Harami F, Duwayri Y, Martin JA, et al. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: Consequences for tissue engineering. Iowa Orthop J. 2004;24:15–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Holzwarth C, Vaegler M, Gieseke F, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weijers EM, Van Den Broek LJ, Waaijman T, et al. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2011;17:2675–2685. doi: 10.1089/ten.tea.2010.0661. [DOI] [PubMed] [Google Scholar]
- 26.Yang DC, Yang MH, Tsai CC, et al. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pattappa G, Thorpe SD, Jegard NC, et al. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68–79. doi: 10.1089/ten.TEC.2011.0734. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Rendon E, Hale SJ, Ryan D, et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Malladi P, Chiou M, et al. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 30.Markway BD, Tan GK, Brooke G, et al. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 31.Khan WS, Adesida AB, Tew SR, et al. Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J Orthop Res. 2010;28:834–840. doi: 10.1002/jor.21043. [DOI] [PubMed] [Google Scholar]
- 32.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilgaard L, Lund P, Duroux M, et al. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp Cell Res. 2009;315:1937–1952. doi: 10.1016/j.yexcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Pilgaard L, Lund P, Duroux M, et al. Effect of oxygen concentration, culture format and donor variability on in vitro chondrogenesis of human adipose tissue-derived stem cells. Regen Med. 2009;4:539–548. doi: 10.2217/rme.09.28. [DOI] [PubMed] [Google Scholar]
- 35.Tamama K, Kawasaki H, Kerpedjieva SS, et al. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem. 2011;112:804–817. doi: 10.1002/jcb.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkmer E, Drosse I, Otto S, et al. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A. 2008;14:1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- 37.Potier E, Ferreira E, Andriamanalijaona R, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Huang YC, Zhu HM, Cai JQ, et al. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2012;113:1407–1415. doi: 10.1002/jcb.24014. [DOI] [PubMed] [Google Scholar]
- 39.Krinner A, Zscharnack M, Bader A, et al. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009;42:471–484. doi: 10.1111/j.1365-2184.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zscharnack M, Poesel C, Galle J, et al. Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel. Cells Tissues Organs. 2009;190:81–93. doi: 10.1159/000178024. [DOI] [PubMed] [Google Scholar]
- 41.Valorani MG, Germani A, Otto WR, et al. Hypoxia increases Sca-1/CD44 co-expression in murine mesenchymal stem cells and enhances their adipogenic differentiation potential. Cell Tissue Res. 2010;341:111–120. doi: 10.1007/s00441-010-0982-8. [DOI] [PubMed] [Google Scholar]
- 42.Baksh D, Boland GM, Tuan RS. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem. 2007;101:1109–1124. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- 43.Baksh D, Tuan RS. Canonical and non-canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol. 2007;212:817–826. doi: 10.1002/jcp.21080. [DOI] [PubMed] [Google Scholar]
- 44.Nesti LJ, Jackson WM, Shanti RM, et al. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90:2390–2398. doi: 10.2106/JBJS.H.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boland GM, Perkins G, Hall DJ, et al. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 46.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 47.Im GI, Kim DY, Shin JH, et al. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br. 2001;83:289–294. doi: 10.1302/0301-620x.83b2.10495. [DOI] [PubMed] [Google Scholar]
- 48.Deschepper M, Oudina K, David B, et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15:1505–1514. doi: 10.1111/j.1582-4934.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishizuka T, Hinata T, Watanabe Y. Superoxide induced by a high-glucose concentration attenuates production of angiogenic growth factors in hypoxic mouse mesenchymal stem cells. J Endocrinol. 2011;208:147–159. doi: 10.1677/JOE-10-0305. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Shen Y, Yuan X, et al. Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J Biol Chem. 2008;283:35929–35940. doi: 10.1074/jbc.M804091200. [DOI] [PubMed] [Google Scholar]
- 51.Funasaka T, Hogan V, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res. 2009;69:5349–5356. doi: 10.1158/0008-5472.CAN-09-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munemitsu S, Albert I, Souza B, et al. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inge LJ, Rajasekaran SA, Wolle D, et al. alpha-Catenin overrides Src-dependent activation of beta-catenin oncogenic signaling. Mol Cancer Ther. 2008;7:1386–1397. doi: 10.1158/1535-7163.MCT-07-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang SG, Yu SS, Ryu JH, et al. Regulation of beta-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of alpha-catenin. J Biol Chem. 2005;280:12758–12765. doi: 10.1074/jbc.M413367200. [DOI] [PubMed] [Google Scholar]
- 55.Ito K, Okamoto I, Araki N, et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- 56.Hirota M, Watanabe K, Hamada S, et al. Smad2 functions as a co-activator of canonical Wnt/beta-catenin signaling pathway independent of Smad4 through histone acetyltransferase activity of p300. Cell Signal. 2008;20:1632–1641. doi: 10.1016/j.cellsig.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clifford RL, Deacon K, Knox AJ. Novel regulation of vascular endothelial growth factor-A (VEGF-A) by transforming growth factor (beta)1: Requirement for Smads, (beta)-catenin, and GSK3(beta) J Biol Chem. 2008;283:35337–35353. doi: 10.1074/jbc.M803342200. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M, Wang M, Tan X, et al. Smad3 prevents beta-catenin degradation and facilitates beta-catenin nuclear translocation in chondrocytes. J Biol Chem. 2010;285:8703–8710. doi: 10.1074/jbc.M109.093526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian X, Zhang J, Tan TK, et al. Association of β-catenin with P-Smad3 but not LEF-1 dissociates in vitro profibrotic from anti-inflammatory effects of TGF-β1. J Cell Sci. 2013;126:67–76. doi: 10.1242/jcs.103036. [DOI] [PubMed] [Google Scholar]
- 60.Lei S, Dubeykovskiy A, Chakladar A, et al. The murine gastrin promoter is synergistically activated by transforming growth factor-beta/Smad and Wnt signaling pathways. J Biol Chem. 2004;279:42492–42502. doi: 10.1074/jbc.M404025200. [DOI] [PubMed] [Google Scholar]
- 61.Yang W, Xia Y, Ji H, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chafey P, Finzi L, Boisgard R, et al. Proteomic analysis of beta-catenin activation in mouse liver by DIGE analysis identifies glucose metabolism as a new target of the Wnt pathway. Proteomics. 2009;9:3889–3900. doi: 10.1002/pmic.200800609. [DOI] [PubMed] [Google Scholar]
- 63.Esen E, Chen J, Karner CM, et al. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17:745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin WS, Kong ZL, Shen ZF, et al. Regulation of hypoxia inducible factor-1alpha expression by the alteration of redox status in HepG2 cells. J Exp Clin Cancer Res. 2011;30:61. doi: 10.1186/1756-9966-30-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan G, Peng YJ, Reddy VD, et al. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci USA. 2013;110:E1788–E1796. doi: 10.1073/pnas.1305961110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Niecknig H, Tug S, Reyes BD, et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res. 2012;46:705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 67.Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: Differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol. 2008;198:127–134. doi: 10.1677/JOE-08-0156. [DOI] [PubMed] [Google Scholar]
- 68.Basciano L, Nemos C, Foliguet B, et al. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011;12:12. doi: 10.1186/1471-2121-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hess R, Pino AM, Ríos S, et al. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem. 2005;94:50–57. doi: 10.1002/jcb.20330. [DOI] [PubMed] [Google Scholar]
- 70.Scheller EL, Song J, Dishowitz MI, et al. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28:1071–1080. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology. 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 72.Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeh SP, Chang JG, Lo WJ, et al. Induction of CD45 expression on bone marrow-derived mesenchymal stem cells. Leukemia. 2006;20:894–896. doi: 10.1038/sj.leu.2404181. [DOI] [PubMed] [Google Scholar]
- 74.Li G, Zheng B, Meszaros LB, et al. Identification and characterization of chondrogenic progenitor cells in the fascia of postnatal skeletal muscle. J Mol Cell Biol. 2011;3:369–377. doi: 10.1093/jmcb/mjr014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.do Amaral RJ, Pedrosa CS, Kochem MC, et al. Isolation of human nasoseptal chondrogenic cells: A promise for cartilage engineering. Stem Cell Res. 2012;8:292–299. doi: 10.1016/j.scr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Annabi B, Lee YT, Turcotte S, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 77.Dibbens JA, Miller DL, Damert A, et al. Hypoxic regulation of vascular endothelial growth factor mRNA stability requires the cooperation of multiple RNA elements. Mol Biol Cell. 1999;10:907–919. doi: 10.1091/mbc.10.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol. 2009;57:318–323. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Boskey AL, Gelb BD, Pourmand E, et al. Ablation of cathepsin k activity in the young mouse causes hypermineralization of long bone and growth plates. Calcif Tissue Int. 2009;84:229–239. doi: 10.1007/s00223-008-9214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taboas JM. Mechanobiologic Regulation of Skeletal Progenitor Cell Differentiation [Ph.D. thesis] Ann Arbor, MI: University of Michigan; 2004. [Google Scholar]
- 81.Zhou Y, Guan X, Wang H, et al. Hypoxia induces osteogenic/angiogenic responses of bone marrow-derived mesenchymal stromal cells seeded on bone-derived scaffolds via ERK1/2 and p38 pathways. Biotechnol Bioeng. 2013;110:1794–1804. doi: 10.1002/bit.24827. [DOI] [PubMed] [Google Scholar]
- 82.Valorani MG, Montelatici E, Germani A, et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45:225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wise JK, Alford A, Goldstein S, et al. Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopaedic tissue engineering. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2013.0151. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benjamin S, Sheyn D, Ben-David S, et al. Oxygenated environment enhances both stem cell survival and osteogenic differentiation. Tissue Eng Part A. 2013;19:748–758. doi: 10.1089/ten.TEA.2012.0298. [DOI] [PubMed] [Google Scholar]
- 85.Lu C, Saless N, Wang X, et al. The role of oxygen during fracture healing. Bone. 2013;52:220–229. doi: 10.1016/j.bone.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yew TL, Huang TF, Ma HL, et al. Scale-up of MSC under hypoxic conditions for allogeneic transplantation and enhancing bony regeneration in a rabbit calvarial defect model. J Orthop Res. 2012;30:1213–1220. doi: 10.1002/jor.22070. [DOI] [PubMed] [Google Scholar]
- 87.Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft achilles tendon repair. Am J Sports Med. 2013;41:1117–1125. doi: 10.1177/0363546513480786. [DOI] [PubMed] [Google Scholar]
- 88.Zhong H, Simons JW. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun. 1999;259:523–526. doi: 10.1006/bbrc.1999.0815. [DOI] [PubMed] [Google Scholar]
- 89.Yun Z, Maecker HL, Johnson RS, et al. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 90.Wagegg M, Gaber T, Lohanatha FL, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One. 2012;7:e46483. doi: 10.1371/journal.pone.0046483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caron MM, Emans PJ, Coolsen MM, et al. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melton JT, Clarke NM, Roach HI. Matrix metalloproteinase-9 induces the formation of cartilage canals in the chondroepiphysis of the neonatal rabbit. J Bone Joint Surg Am. 2006;88(suppl 3):155–161. doi: 10.2106/JBJS.F.00542. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed N, Dreier R, Göpferich A, et al. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20:665–678. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 95.Borzí RM, Olivotto E, Pagani S, et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 2010;62:2370–2381. doi: 10.1002/art.27512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bertram H, Boeuf S, Wachters J, et al. Matrix metalloprotease inhibitors suppress initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev. 2009;18:881–892. doi: 10.1089/scd.2008.0306. [DOI] [PubMed] [Google Scholar]
- 97.Lozito TP, Tuan RS. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol. 2011;226:385–396. doi: 10.1002/jcp.22344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.