This study reports the clinical course of two patients with osteogenesis imperfecta who received prenatal human fetal mesenchymal stem cell (MSC) transplantation and postnatal boosting with same-donor MSCs. Findings suggest that prenatal transplantation of allogeneic human fetal MSCs in osteogenesis imperfecta appears safe and is of likely clinical benefit and that retransplantation with same-donor cells is feasible. Further studies are required.
Keywords: Mesenchymal stem cells, Mesenchymal stromal cells, Cell therapy, Osteogenesis imperfecta, Prenatal transplantation, In utero transplantation
Abstract
Osteogenesis imperfecta (OI) can be recognized prenatally with ultrasound. Transplantation of mesenchymal stem cells (MSCs) has the potential to ameliorate skeletal damage. We report the clinical course of two patients with OI who received prenatal human fetal MSC (hfMSC) transplantation and postnatal boosting with same-donor MSCs. We have previously reported on prenatal transplantation for OI type III. This patient was retransplanted with 2.8 × 106 same-donor MSCs per kilogram at 8 years of age, resulting in low-level engraftment in bone and improved linear growth, mobility, and fracture incidence. An infant with an identical mutation who did not receive MSC therapy succumbed at 5 months despite postnatal bisphosphonate therapy. A second fetus with OI type IV was also transplanted with 30 × 106 hfMSCs per kilogram at 31 weeks of gestation and did not suffer any new fractures for the remainder of the pregnancy or during infancy. The patient followed her normal growth velocity until 13 months of age, at which time longitudinal length plateaued. A postnatal infusion of 10 × 106 MSCs per kilogram from the same donor was performed at 19 months of age, resulting in resumption of her growth trajectory. Neither patient demonstrated alloreactivity toward the donor hfMSCs or manifested any evidence of toxicities after transplantation. Our findings suggest that prenatal transplantation of allogeneic hfMSCs in OI appears safe and is of likely clinical benefit and that retransplantation with same-donor cells is feasible. However, the limited experience to date means that it is not possible to be conclusive and that further studies are required.
Introduction
Osteogenesis imperfecta (OI) is a genetically and clinically heterogeneous disorder in which the major clinical manifestations are atypical skeletal development, osteopenia, multiple painful fractures, and short stature [1]. In more than 90% of affected individuals, the causative mutations lie in the two genes that encode the chains of the major protein of the bone, type I collagen, COL1A1 and COL1A2. In the most severely affected individuals, the mutations result in production of abnormal type I collagen molecules [1]. The effects of these changes begin during fetal life and often present with abnormal fetal skeletal development, shortened long bones, and multiple fractures that can be identified during routine prenatal ultrasonography. The prevalence of OI is approximately 6–7 per 100,000 [2]. OI type I and IV, the two mildest forms, account for more than half of all recognized affected individuals. OI type III, the most severe form in children who survive the neonatal period, has a prevalence of approximately 1–2 per 100,000 and is usually caused by de novo mutations [2] in type I collagen genes; however, phenocopies occur among those with some recessively inherited forms of OI. Currently, there is no cure for OI, although use of bisphosphonates has some symptomatic but not curative therapeutic effect, and the longer-term effects of early intervention remain undetermined [3, 4].
Mesenchymal stem cells (MSCs) are multipotent cells that are readily isolated and culture expanded and can be induced to differentiate into osteoblasts, chondrocytes, or adipocytes [5]. MSCs have a low immunogenic profile and typically are not rejected during allogeneic transplantation paradigms [6, 7], although engraftment may not be long lived [8]. MSCs are generally considered safe for transplantation when grown under normal culture conditions, and there are no reports of malignant transformation or generation of ectopic tissues [9–12]. The first clinical transplantation of MSCs was performed more than 15 years ago, and adult MSCs have now been used clinically in hundreds of patients without adverse effect [13, 14].
The potential of MSC transplantation for OI was demonstrated initially in the oim mouse, in which allogeneic transplanted wild-type donor MSCs homed to the bones, where they contributed to the osteoprogenitor pool, with improvement in collagen content and mineralization [15]. This was followed by a clinical trial in which children with severe OI type III were treated with an infusion of allogeneic MSCs. This was reported to result in increased skeletal mineralization and growth velocity, despite a low level of engraftment (∼1%) [8]. The mechanisms by which such a small population of cells could confer benefit [16, 17] were uncertain, and an effect of nonadherent bone marrow cells cannot be excluded [18].
Stem cells derived from the fetus present some favorable characteristics over adult sources. Although human fetal MSCs (hfMSCs) are phenotypically similar and nonimmunogenic [19–21] like their adult counterparts, they have higher proliferative capacity and longer telomeres and differentiate more readily into bone, skeletal muscle, and oligodendrocytes [22–26]. These characteristics suggest that they may have a greater utility in cellular therapy.
Because the damage in severe OI begins early in fetal life and may lead to perinatal lethality, a good case can be made for prenatal MSC transplantation, to treat the fetus before additional pathology occurs. Other arguments that favor a fetal approach include higher engraftment rates reported after prenatal fetal stem cell transplantation; the small size of the fetus, which allows higher proportionate cell doses to be delivered; the right-to-left heart shunting that enhances systemic distribution of the cells; and the relative naiveté of the immune system, which permits the development of immune tolerance toward donor cells [27–30]. We have previously found that prenatal transplantation in the oim mouse with hfMSCs resulted in significant engraftment to bone (∼5%), with restitution of bone histology toward normal, reduction in fracture frequency [31], and measurable expression of normal human type I collagen [32]. Recently, Panaroni et al. demonstrated that prenatal allogeneic bone marrow transplantation in a perinatally lethal, dominant model of OI resulted in increased perinatal survival, bone mineralization, and architecture. In this model, donor cells accounted for 20% of the total collagen I content of bone, despite an engraftment rate of only 2% [33].
Ten years ago, we reported that prenatal hfMSC transplantation in a fetus with OI type III resulted in engraftment in bone that could be detected at 9 months of age [34]. We now describe the same patient's long-term clinical course with a secondary transplantation of same-donor hfMSCs at 8 years of age. We also present a case with the same mutation as this patient but without transplantation. In addition, we describe an infant with OI type IV that underwent prenatal and postnatal hfMSC transplantation.
Materials and Methods
Ethics and Institutional Research Board Approval
Derivation of good manufacturing practice MSCs from human first trimester fetal liver and its prenatal and postnatal transplantation was approved by the ethical review boards in Stockholm, Sweden (428/01, 2006/308-31/2, 2005/867-31/3) and Singapore (D/10/247). Informed written consent was obtained from all participants in this study.
Patients
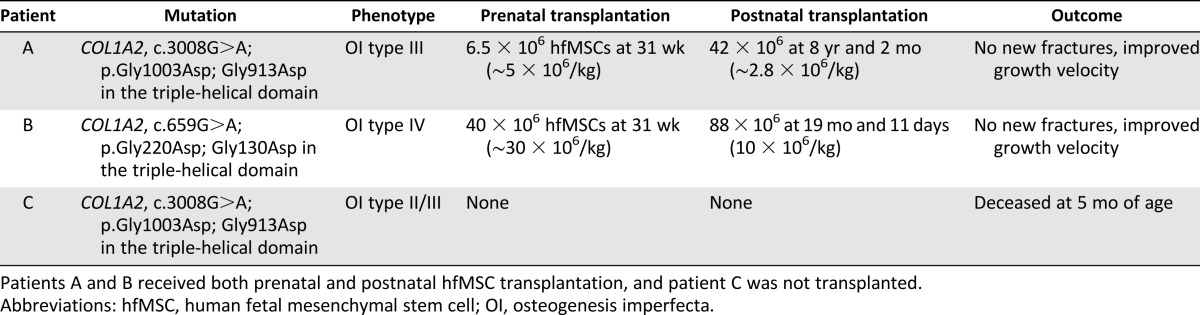
This paper presents two infants in whom prenatal and postnatal transplantation were performed; patients A and B were transplanted in Sweden and Singapore, respectively (Table 1). In addition, we present patient C from Canada, with a mutation identical to that of patient A but who had not received a transplantation.
Table 1.
Details of the three cases of osteogenesis imperfecta
MSC Isolation and Culture
MSCs were isolated from two male fetal livers (from 7 weeks and 3 days and 10 weeks of gestation). Cells were analyzed for HIV antigen, human T-lymphotropic virus types 1 and 2, and hepatitis B and C and were cultured as described previously in good manufacturing practice-qualified premises at Karolinska Institutet [21]. Briefly, the fetal liver was mechanically disrupted by passage through a 100-μm nylon filter, and the cell suspension diluted to a final concentration of 107 cells per milliliter. Mononuclear cells were collected by gradient centrifugation and suspended in Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG; Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 10% fetal bovine serum (FBS; Hyclone; Invitrogen) approved for clinical cultures of MSCs and 100 IU/ml penicillin and 100 μg/ml streptomycin (Invitrogen). Cells were plated at 1.6 × 105 cells per cm2 in culture flasks and maintained at 37°C in a humidified environment containing 5% carbon dioxide. After 3 days, nonadherent cells were discarded, and the medium was replaced every 3–4 days thereafter. Before confluence, the cells were detached by treatment with 0.05% trypsin and 0.53 mM ethylenediaminetetraacetic acid (Invitrogen) and replated at a density of 4 × 103 cells per square centimeter in culture flasks. After subsequent passaging, the cells were frozen in DMEM-LG, 10% AB plasma, 2 IU/ml heparin (Leo Pharma A/S, Ballerup, Denmark, http://www.leo-pharma.com), 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% dimethyl sulfoxide (WAK-Chemie Medical GmbH, Steinbach, Germany, http://www.wak-chemie.net), and aliquots were assayed for sterility, mycoplasma, and cell characteristics (surface expression of proteins, trilineage differentiation ability, and karyotype).
Enzyme-Linked Immunosorbent Assay for Detection of Antibodies Against FBS
Enzyme-linked immunosorbent assay was performed to detect antibodies directed against FBS used in the expansion of hfMSCs, as described previously [35]. Briefly, 96-well plates were coated with 1% FBS, washed and incubated with recipient serum, and washed and then incubated with alkaline phosphatase-conjugated polyclonal anti-human IgG antibodies (DakoCytomation, Glostrup, Denmark, http://www.dakocytomation.com). Diethanolamine with addition of p-nitrophenol phosphate (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) was used as the color development substrate solution. Plates were assayed at 405 nm in a spectrophotometer. The positive control was serum from a male with known high titers of anti-FBS antibodies, and the negative control consisted of PBS-Tween. Optical density values of more than 1.0 were deemed positive.
Transplantation Patient A
The prenatal transplantation procedure was reported previously [34]. For the postnatal transplantation of hfMSCs in Sweden (patient A), male hfMSCs derived from 10-week gestation fetal liver from the same donor as the first transplantation were thawed and cultured as described previously for one passage, followed by 2 days in 10% human AB plasma with 2 IU/ml heparin in DMEM-LG and 100 IU/ml penicillin and 100 μg/ml streptomycin. The cells were then frozen, and sterility and hfMSC characteristics were analyzed as described. On the day of infusion, the cells were thawed and counted, and viability was evaluated. The syringe used for infusion was heparinized with 100 IU/ml heparin. Then, 42 × 106 hfMSCs (2.8 × 106 per kilogram) at passage 5 in 21 ml saline containing 10% human AB plasma was slowly infused in patient A through her intravenous line 5 days after surgery for bilateral femoral osteotomy and replacement of rods at 8 years and 2 months of age.
Transplantation Patient B
For transplantation of hfMSCs in Singapore (patient B), male hfMSCs (7 weeks and 3 days of gestation) were thawed and cultured at 1 × 104 cells/cm2 for one to three passages in 10% FBS (Sigma-Aldrich) in DMEM-LG and harvested as described previously. The hfMSCs were obtained from the tissue bank for fetal transplantation at Karolinska. 40 × 106 hfMSCs (∼30 × 106 hfMSCs per kilogram of fetal weight) at passage 7 suspended in 8 ml of warmed saline were used for the prenatal transplantation, and 88 × 106 of the same donor’s hfMSCs (10 × 106 MSCs per kilogram) at passage 9 suspended in 15 ml of warmed saline supplemented with 500 IU heparin were used for the postnatal transplantation.
Genotyping
The COL1A2 mutation in patient A was determined previously [34]. Analysis of the sequence of the COL1A1 and COL1A2 genes in DNA from patients B and C was performed following amplification of 16 segments of genomic DNA that encompassed all of the coding sequences and flanking intronic domains of COL1A1 and 23 fragments of the larger COL1A2 gene. Amplification primers and conditions and sequencing primers and conditions are available on request to the authors (Collagen Diagnostic Laboratory, Seattle, WA, http://www.pathology.washington.edu/clinical/collagen/).
Immune Response Assay
Blood samples for mixed lymphocyte cultures were collected from patients A and B before pre- and postnatal transplantation. Briefly, lymphocytes from umbilical cord blood or peripheral blood were prepared by centrifugation on a Ficoll gradient (Lymphoprep; Nycomed Pharma, Asker, Norway, http://www.nycomed.no). To investigate whether the donor hfMSCs induced an allograft reaction in the patient, triplicate samples of 1 × 105 lymphocytes were cultured with 10,000 irradiated donor or control MSCs in 0.2 ml RPMI 1640 medium supplemented with 20 mM HEPES, 100 IU/ml penicillin, 100 μg/ml streptomycin, 20 mM L-glutamine (Invitrogen), and 10% pooled human AB serum in 96-well plates. The cultures were incubated at 37°C in humidified 5% carbon dioxide air for 6 days. On day 5, 1 μCi/ml [3][H]-thymidine (GE Healthcare Life Sciences, Little Chalfont, U.K., http://www.gelifesciences.com) was added. After 24 hours, the cells were harvested on a glass-fiber filter (PerkinElmer, Waltham, MA, http://www.perkinelmer.com), using a semiautomatic harvesting machine (Harvester 96; Tomtec, Hamden, CT, http://www.tomtec.com/). Radioactivity was determined as counts per minute with an Intertechnique β-counter (PerkinElmer).
Fluorescence In Situ Hybridization
Fluorescence in situ hybridization (FISH) was performed on specimens obtained before and after postnatal transplantation, as previously described [5]. At 6 years and 1 month of age, when patient A underwent osteotomy to reposition the femurs and to replace her intramedullary rods, opportunistic samples of bone (proximal femur), muscle, and skin were taken. Another section of discarded bone (bone overgrowth, trochanter major, ventral rim of the proximal plate of the hip) was acquired 9 months after postnatal transplantation, at 8 years and 11 months of age, in a procedure to remove the plates used in the previous surgery.
Samples were fixed in 4% formalin, embedded in paraffin, sectioned in 4-μm slices, and dewaxed before preparation for FISH with a tissue pretreatment kit (MP Biomedicals, Illkirch Cedex, France, http://www.mpbio.com). Several sets of FISH probes were used: CEPH satellite probes for the centromeric regions of X and Y (Abbott-Vysis, Abbott Park, IL, http://www.abbottmolecular.com), X/Y Poseidon SE probes (BioNordikaBergman AS, Oslo, Norway, http://www.bionordika.no), or probes for the total human genome X (red) and Y (green) (Abbott-Vysis). The DNA in the slides and in the probes were simultaneously denatured in a HYBrite (Abbott-Vysis) at 72°C and hybridized at 37°C for 16 hours. After washing, the slides were counterstained with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) to detect cell nuclei and then scanned in a Nikon microscope (Tokyo, Japan, http://www.nikon.com) and documented with the CytoVision image system (Leica, Heerbrugg, Switzerland, http://www.leica.com), and XY and XX cells were counted.
Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (PCR) analysis of tissues acquired from patient A at 6 years and 1 month of age was performed. DNA was extracted from the bone using the protocol described by Svensson et al. [36] and from the skin, muscle, and MSCs isolated from the bone marrow and bone using the salting-out method [37]. Chimerism analysis was performed using a biallelic genetic system to distinguish the patient from the donor [38]. PCR analysis was performed on the ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) using TaqMan technology. The sensitivity of the method to detect a minor population is 1 in 10,000.
Results
The details of genetic mutation, type of OI, donor cells transplanted, response, and outcome for the three cases of OI are summarized in Table 1.
MSC Characteristics
Harvested hfMSCs composed a single phenotypic population by flow cytometry, being positive for CD29, CD44, CD73, CD105, and CD166; intermediate for human leukocyte antigen (HLA) class I antigens; and negative for HLA class II antigens and for the hematopoietic and endothelial markers CD14, CD31, CD34, and CD45 (data not shown). The cells differentiated along the adipogenic, chondrogenic, and osteogenic lineages, as described previously (data not shown) [21]. The cell cultures were negative for HIV antigen, human T-lymphotropic virus types 1 and 2, hepatitis B and C, anaerobic and aerobic bacteria, and mycoplasma and had a normal karyotype (data not shown).
Clinical Course and Experimental Findings: Patient A
We describe the initial outcome of the prenatal transplantation performed for patient A [5], demonstrating evidence of osteoblastic differentiation of donor cells in a bone biopsy at 9 months of age. Although the molecular analysis predicted a severe OI phenotype based on the nature and location of the mutation in the COL1A2 gene (c.3008G>A; p.Gly1003Asp; Gly913Asp in the triple-helical domain) (Table 1), she had a better-than-expected clinical course. Patient A grew along her own growth velocity curves for weight and height, albeit at 5 standard deviations (SD) below the mean, and had three fractures but no complications or pain for the first 2 years of life, as previously reported [34]. She was treated with intermittent intravenous pamidronate infusion beginning at 4 months of age for postnatally acquired vertebral compression fractures.
Between the ages of 2 years and 8 years and 2 months, patient A suffered multiple complications; 9 fractures (5 femoral, 2 clavicular, 1 shoulder, and 1 skull) and 11 vertebral compression fractures have been confirmed, and another 4 long-bone fractures and 1 vertebral compression fracture were suspected clinically. She developed scoliosis and was treated with a brace beginning at 6 years of age. During surgery at 6 years and 1 month of age for rod replacement in her long bones, opportunistic biopsies of the bone, bone marrow, muscle, skin, and isolated MSCs from the bone and bone marrow showed no evidence of donor cells by either XY FISH or SRY by quantitative PCR (data not shown).
A decision to give a second transplant of hfMSCs from the same donor to patient A was made on the following basis: (a) the limited duration of the beneficial effects of MSC infusion that have been shown in the postnatal setting [8], (b) the absence of donor cells in the bone at 6 years of age, and (c) the continued occurrence of spontaneous fractures and reduced linear growth (−5 SD at 2 years of age to −6.5 SD at 8 years of age). A pretransplantation workup showed the absence of antibodies directed toward HLA class I and II, IgG and IgM, or FBS (data not shown). There was no alloreactivity of the patient’s lymphocytes toward the donor hfMSCs (Fig. 1A). Five days after bilateral femur osteotomy and rerodding, 42 × 106 male hfMSCs (2.8 × 106 per kilogram) were infused intravenously without any adverse early or late reactions.
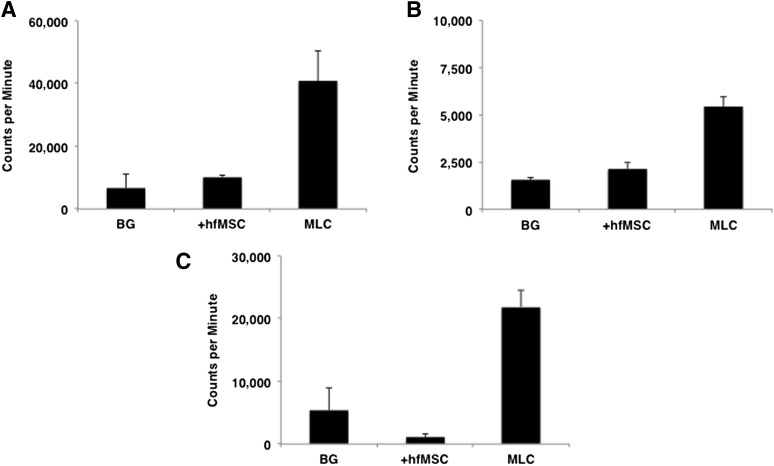
Figure 1.
Immunological reaction toward donor cells. MLC was performed to evaluate patient A’s and patient B’s immunological reactions toward donor cells. Overall, 10,000 hfMSCs or 100,000 allogeneic peripheral blood lymphocytes were cocultured with 100,000 patient peripheral blood lymphocytes. (A): Patient A before postnatal transplantation, 8 years after prenatal transplantation. (B): Patient B before prenatal transplantation. (C): Patient B at birth, 7 weeks after prenatal transplantation. In all cases, an immune response was detected against allogeneic lymphocytes (MLC) but not against donor hfMSCs. Data are reported as mean ± SD of triplicate experiments. Abbreviations: BG, background; hfMSC, human fetal mesenchymal stem cell; MLC, mixed lymphocyte culture.
During the 2 years after the postnatal transplantation, patient A did not develop any new fractures. Her linear growth followed her previous growth (from −6.5 SD to −6 SD; Fig. 2A). Dual-energy x-ray absorptiometry (DXA; QDR 4500 system; Hologic, Bedford, MA, http://www.hologic.com) revealed a lumbar spine (L1–L4) skeletal mineralization of 0.539 g/cm2 (Z score of −0.3 of age-matched controls before retransplantation at 7 years and 9 months of age), 0.558 g/cm2 (Z score of −0.4 of age-matched controls 1 year after retransplantation at 9 years of age), and 0.584 g/cm2 (Z score of −0.5 of age-matched controls at 10 years of age), using Hologic Discovery A system. Her ability to walk improved, and she is able to walk for 1,000 meters without difficulties. She was able to start dance classes, increased her participation in gymnastics at school, and could play modified indoor hockey. Analysis of a bone sample acquired 9 months after the postnatal transplantation at 8 years and 11 months of age showed low levels (0.003%) of donor cell engraftment by Y-chromosomal FISH (Fig. 3).
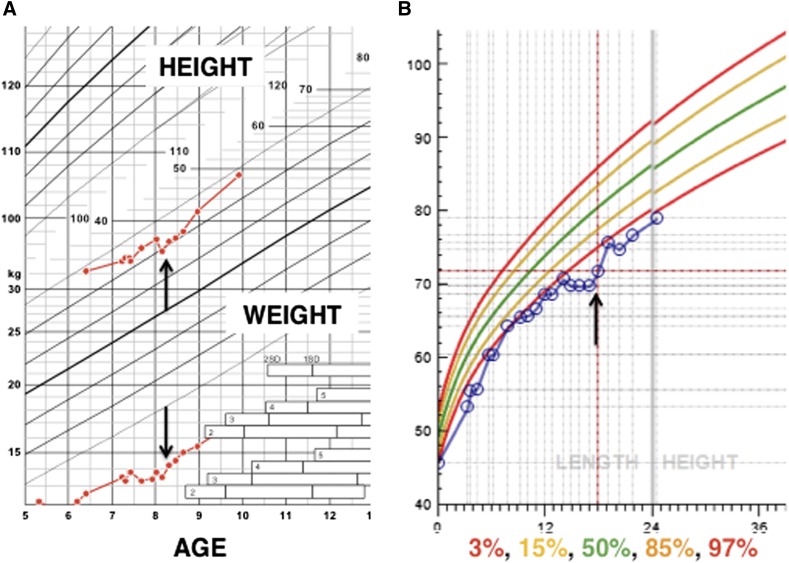
Figure 2.
Growth curves for patients A and B. (A): Over the 2 years following postnatal transplantation (arrows), patient A’s linear growth improved from −6.5 to −6 standard deviations, and she is almost following her own growth velocity centiles. (B): Patient B’s growth followed a line just under the 3rd centile until 12 months of age, where it plateaued. Her growth resumed at just under the 3rd centile after postnatal transplantation of same-donor human fetal mesenchymal stem cells (arrow). Length is given in centimeters, weight in kilograms.
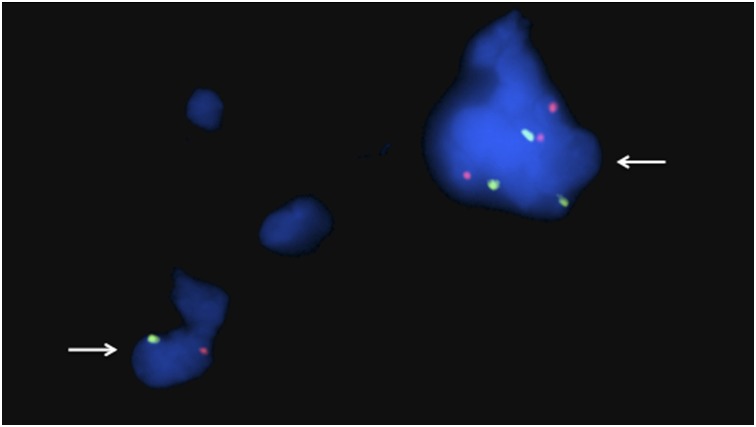
Figure 3.
Detection of male cells in sections from a bone biopsy taken after postnatal transplantation. Fluorescence in situ hybridization analysis of 4-μm single sections for X and Y chromosomes using α-satellite probes for the centromeric regions. The bone sample was taken 9 months after postnatal transplantation at 8 years and 11 months of age from patient A. X chromosomes (red dots) and Y chromosomes (green dots) can be recognized in 4′,6-diamidino-2-phenylindole-stained cell nuclei (blue). In total, 4 Y-chromosome-positive cells were detected among 60,000 cells. The arrows indicate cells containing Y chromosomes. Original magnification, ×100.
Clinical Course and Experimental Findings: Patient B
The second patient was a female fetus identified at 26 weeks of gestation in a 31-year-old woman. The fetus’s short long bones (<5th centile) and multiple fresh and healing fractures were identified at the Chang Gung Memorial Hospital in Taiwan. The mother was referred to the National University Hospital in Singapore for an allogeneic prenatal transplantation at 31 weeks of gestation, and repeat ultrasound confirmed the presence of short long bones and multiple fresh healing and healed fractures (Fig. 4A).
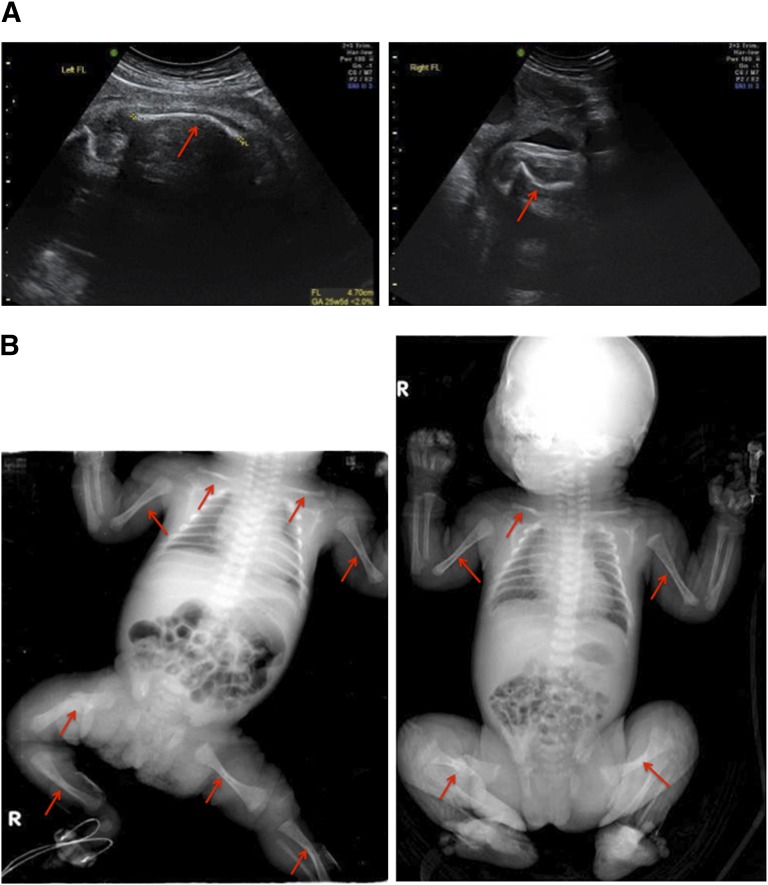
Figure 4.
Skeletal examination of patient B. (A): Fetal ultrasound examination at 26 weeks showed the presence of short long bones and multiple fresh healing and healed fractures (arrows). (B): A full-body radiological skeletal survey at birth confirmed the occurrence of healed fractures, indicated by arrows.
The fetus was visualized on ultrasound, and the placenta and intrahepatic vein were mapped. After administering terbutaline to the mother for uterine relaxation, a 22G needle was used to deliver a dose of intrahepatic fetal pancuronium (0.18 mg), followed by cannulation of the intrahepatic vein. Eight hundred microliters of fetal blood was aspirated before 40 × 106 male hfMSCs in 8 ml of warmed saline were infused over 1 minute under direct ultrasound visualization (∼30 × 106 hfMSCs per kilogram). The fetal heart rate and umbilical artery Doppler waveforms were normal before and 5 minutes after transplantation. Similarly, cardiographic monitoring before and 1 hour after infusion was uneventful. Testing for alloreactivity toward donor hfMSCs performed on peripheral blood lymphocytes retrieved from fetal blood prior to transplantation was negative (Fig. 1B).
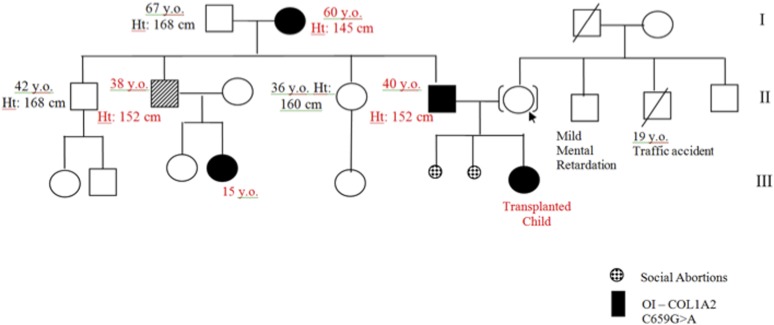
Genotyping of the fetus (patient B) identified a mutation in the COL1A2 gene at exon 14 (c.659G>A; p.Gly220Asp, Gly130Asp in the triple-helical domain) consistent with OI type IV. Because there was a familial history of an affected first cousin with a presumptive diagnosis of OI and both the patient’s father and paternal grandmother had short stature and a history of multiple fractures, genotyping of her family pedigree showed the same mutation in her father, her paternal uncle and first cousin, and her paternal grandmother, consistent with an autosomal dominant mode of inheritance (Fig. 5).
Figure 5.
Pedigree tree of patient B’s family history of mutation indicating osteogenesis imperfecta. Genotyping of patient B’s family showed the same mutation in the patient’s father, first uncle, first cousin, and paternal grandmother, suggesting an autosomal dominant mode of inheritance. Abbreviations: Ht, height; y.o., years old.
After prenatal transplantation, the fetus was followed up by serial weekly ultrasound at a tertiary maternal-fetal medicine unit, and no new fractures were identified over the ensuing 7 weeks. The child was delivered at 38 weeks and 3 days of gestation by elective cesarean section. She weighed 2.54 kg and was in good condition. Quantitative PCR of umbilical cord blood, umbilical cord, and placenta did not yield any Y-chromosome signals (sensitivity of 1 in 500; data not shown). There was no alloreactivity of patient B’s umbilical cord blood-derived lymphocytes toward the donor cells at the time of birth (Fig. 1C).
A full-body skeletal survey at birth confirmed the presence of multiple healed fractures (Fig. 4B), and the neonate was started on bisphosphonate therapy from 1 month of age. A DXA scan of the lumbar spine confirmed poor mineralization, with a Z score of –7.3 (0.245 g/cm2) neonatally, which gradually increased to –0.9 (0.365 g/cm2) at 13 months of age. Over the course of her first 12 months, she grew at just below the 3rd centile for length along her own centile lines, with no new fractures detected. Because her linear growth stopped just after 12 months of age, a decision was reached to perform a postnatal hfMSC transplantation.
At 19 months and 11 days of age, patient B underwent a postnatal intravenous infusion of 88 × 106 same-donor hfMSCs (10 × 106 hfMSCs per kilogram) without any acute adverse event. The child was assessed and then discharged from the hospital the following day. Thereafter, her growth velocity improved, she continued to grow just below the 3rd centile (Fig. 2B), and she started to walk shortly after the transplantation.
Clinical Course and Experimental Findings: Patient C
Patient C was born at 38 weeks of gestation in 2009 to a healthy 22-year-old woman in Canada. The newborn boy weighed 2155 g (<5 SD) with a length of 39 cm (<5 SD). Physical examination revealed the presence of a very large anterior fontanelle, soft skull, bitemporal narrowing, blue sclera, superiorly narrow chest with reduced internipple distance (-2 SD), bilateral inguinal hernias, bilateral hydroceles, short limbs with notable rhizomelic involvement, positional abnormalities of the limbs (elbows, hips, knees, and feet were all held in flexion), bowed femurs, adducted thumbs, and bilateral talipes equinovarus. Skeletal survey demonstrated severe and diffuse osteopenia, prominent fontanelles, multiple Wormian bones, multiple bilateral healing rib fractures sustained prenatally, and compression fractures of T11, T12, and L1 with a related thoracolumbar kyphosis. The boy suffered from multiple fractures of his humeri, femurs, tibias and fibulas, and left ulna and shortening, widening, and deformity of both humeri, with all his lower-extremity long bones having the typical accordion appearance of severe OI.
The infant was started on calcium/vitamin D3 and zoledronic acid (0.0125 mg/kg) treatment on day 6 of life. At day 9, a DXA scan of the lumbar spine at L2–L4 was 0.133 g/cm2, and whole-body bone mineral density was 0.450 g/cm2. New fractures of the left humerus resulting in a widened, irregular, and accordion-like shape secondary to multiple fractures, together with fractures of the left radius, left acromion, and right tibia, and compression fractures of T2, T3, T6, and L2 were seen on serial radiology performed on days 25, 27, and 37 of life.
He received a second dose of zoledronate (0.025 mg/kg) at 12 weeks of age, and at 16 weeks, lumbar spine bone mineral density at L2–L4 assessed by a DXA scan had improved to 0.146 g/cm2 (an increase of 14%) and whole-body bone mineral density had improved to 0.458 g/cm2 (an increase of 1%–2%). The infant was discharged home following this treatment but was readmitted at 19 weeks of age when he succumbed 1 week later to respiratory failure secondary to pneumonia. Postmortem examination was declined.
Genotyping of patient C identified the same dominant mutation seen in patient A in COL1A2 (c.3008G>A; p.Gly1003Asp; Gly913Asp in the triple-helical domain), which confirmed the diagnosis of severe OI (Table 1). Analysis of the type I collagen produced by cultured fibroblasts from a skin biopsy (Collagen Diagnostic Laboratory) was compatible with the mutation and demonstrated that two populations of molecules were produced. One population was normal and the other contained markedly overmodified chains with slow mobility, and the molecules were poorly secreted (data not shown).
Discussion
Short-term therapeutic effects of MSC transplantation in OI have been reported previously [39, 40]. We present the long-term follow-up after prenatal and postnatal transplantation of HLA-mismatched hfMSCs in two children with OI. The follow-up period of this study is 3–10 years after prenatal transplantation and 2–2.5 years after postnatal transplantation. We extensively investigated the patients’ responses toward the infused donor cells after prenatal transplantation and before postnatal transplantation. There was no evidence of immune reaction toward the donor cells. These data suggest that infusion of allogeneic hfMSCs does not cause any acute or chronic toxicity, underpinning their safety in a prenatal setting.
The cell dose is a critical parameter in cell transplantation because it may be related to therapeutic efficacy, but a high cell dose may cause toxicity. In our study, the cell doses infused varied between 5–30 × 106 per kilogram at prenatal transplantation and 2.8–10 × 106 per kilogram at postnatal transplantation. The cell doses were largely based on the number of cells harvested on the day of transplantation. The higher cell doses did not cause any adverse events. However, it is unclear from our data and those from others whether a higher cell dose is more efficacious. In patient A, a low engraftment rate of 0.003% in bone was found after postnatal transplantation in contrast to the 7.4% donor engraftment level seen after prenatal transplantation. There could be a number of explanations for this observation. First, systemic infusion of hfMSCs in the fetal circulation bypasses the pulmonary vasculature through the patent foramen-ovale, leading to enhanced engraftment downstream of the arterial tree. This is in contrast to postnatal infusion, for which pulmonary trapping of the cells followed by redistribution to the body has been described [41, 42]. Second, there could still be an immune response toward the infused cells, although we found no evidence of any adaptive cellular response toward the donor cells. Last, specific cellular adhesion molecules present on hfMSCs [43] may regulate more efficient homing to fetal bones than in the postnatal setting.
In patient A, the postnatal infusion was associated with the lack of new fractures over a 2-year period and improved growth velocity and mobility. In patient B, prenatal infusion was followed by the absence of new fractures in the ensuing 7 weeks of fetal and 12 months of postnatal life, and the postnatal infusion was associated with resumption of her stalled growth and improved mobility. In addition, the disparate clinical course of patient A compared with patient C, who shared an identical genetic mutation resulting in severe OI, points to at least partial efficacy of the prenatal hfMSC transplantation in ameliorating the severe OI phenotype. Although both patients A and B received concurrent bisphosphonate therapy, the improvement after a second transplantation and the poor outcome of patient C despite bisphosphonate therapy also suggest that there was a beneficial effect of hfMSC transplantation. We are aware that the genotype alone may not completely predict phenotype and severity of OI.
In patient B’s family, there was a heredity pattern consistent with an autosomal dominant form of OI. Her father, paternal cousin, and paternal grandmother were of short stature and had experienced multiple fractures during childhood. Although this pointed toward a milder OI phenotype, the decision for prenatal hfMSC transplantation was made based on the continued evolution of new fractures during antenatal surveillance and the persistent shortness of long bones. These factors were balanced against the relative safety of a prenatal transplantation in a hospital that routinely carries out similar interventions. Although we did not obtain any bone biopsies from patient B to confirm donor cell engraftment, the lack of new prenatal and postnatal fractures together with the resumption of growth velocity argues for a beneficial effect of hfMSC transplantation on skeletal development.
We previously reported that prenatal hfMSC transplantation resulted in donor cell engraftment and site-specific differentiation in bone at 9 months of age [34]. Extensive analysis of tissue samples from the same patient (patient A) showed no donor cell engraftment at 6 years of age, but at 9 months after postnatal transplantation, donor cells were detected in a bone sample. It is likely that the low level engraftment seen was from the postnatal transplantation, although it would be impossible to verify this, given the lack of additional marking of same-donor cells. The placental tissues from patient B showed no signs of donor cell engraftment, but because donor cells have been detected only in the bone of OI patients, we would not necessarily expect engraftment of donor cells in other tissues.
Clinically, the level of engraftment observed in this study has been low, between 7.4% and 0.003% in the bone after prenatal and postnatal transplantation, respectively. However, low-level engraftment of 1%–2% has been shown clinically to improve severe OI in both animal models and in patients [8, 31, 33]. The underlying understanding is that even low engraftment levels could produce mosaicism that would allow local production of normal bone and potentially convert a severe OI phenotype to one less severe (as recognized in parents who are mosaic for a mutation that produces lethal OI in their offspring). However, a recent study suggests that MSCs do not affect collagen production in OI but merely improve the growth velocity via paracrine effects independent of engraftment [18]. This may account for the transient therapeutic effects seen with MSC transplantation for OI or, indeed, for other conditions. In this sense, it might act as a complementary treatment strategy to bisphosphonate therapy because these drugs exhibit incomplete effects on longitudinal bone growth [44, 45].
Prenatal transplantation followed by postnatal transplantation appears to be both efficacious and safe in the context of OI. The lack of long-term engraftment and transient clinical improvement after transplantation suggests that further refinements in selection of the appropriate stem cell type and preparation will be required for sustained therapeutic benefits. Presently, a multiple-transplantation strategy may be required to optimize skeletal growth and development during fetal life and childhood for maximal therapeutic benefit.
Finally, fetal stem cell transplantation for OI is still a highly experimental therapy. From experience in other fields of stem cell therapy, we are aware of the importance of coordination and coherence between different centers interested in advancing this development. We advocate different research groups in this area joining forces to develop common programs that include guidelines for isolation of MSCs, transplantation strategies, and uniform measurements during the post-transplantation period. Considering the complexity of this field, we see this approach as the only realistic way to proceed if we are to accurately evaluate the potential of this treatment strategy in children with OI.
Conclusion
Prenatal and postnatal transplantation of allogeneic hfMSCs in OI is safe and probably clinically efficacious. To date, however, there is only limited clinical experience, and further studies are needed.
Acknowledgments
We acknowledge Dr. Anders Götherström for work on bone DNA extraction, Monika Jansson for fluorescence in situ hybridization, Dr. Mehmet Uzunel for polymerase chain reaction, Dr. Mark Chong and Lay Geok Tan for cell culture and expansion, Dr. Sherry Ho for polymerase chain reaction and karyotyping, and Dr. James H.P. Hui for current good manufacturing practice laboratory cell cultures. This study was financed by the Swedish Society for Medical Research and Vinnova (2010-00501). J.K.Y.C. and M.C. received salary support from the Ministry of Health’s National Medical Research Council’s Clinician Scientist Awards (CSA/009/2012, CSA/012/2009, and CSA/043/2012).
Author Contributions
C.G. and J.K.Y.C.: conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.W., M.C., and K.L.B.: conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript; S.W.S.S., P.-J.C., and J.-L.L.: prenatal diagnosis and antenatal care and postnatal follow-up (patient B), final approval of manuscript; E.A.: provision of study material (patient A), postnatal follow-up, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; P.H.B. and G.E.G.: provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.T. and U.E.: provision of study material (patient A), postnatal follow-up, final approval of manuscript; N.M.F.: data analysis and interpretation, manuscript writing, final approval of manuscript; A.B. and C.N.Z.M.: prenatal transplantation (patient B), final approval of manuscript; A.E.J.Y.: postnatal transplantation (patient B), final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Forlino A, Cabral WA, Barnes AM, et al. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7:540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner RD, Pepin MG, Byers PH. Osteogenesis Imperfecta. In: Pagon RA, Bird TD, Dolan CR et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle, 1993. [Google Scholar]
- 3.Glorieux FH, Bishop NJ, Plotkin H, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 4.Bishop N. Characterising and treating osteogenesis imperfecta. Early Hum Dev. 2010;86:743–746. doi: 10.1016/j.earlhumdev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Pittenger M. Mesenchymal stem cells: Progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 10.Prockop DJ. Defining the probability that a cell therapy will produce a malignancy. Mol Ther. 2010;18:1249–1250. doi: 10.1038/mt.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prockop DJ, Brenner M, Fibbe WE, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12:576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 12.von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 13.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZY, Teoh SH, Hui JH, et al. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33:2656–2672. doi: 10.1016/j.biomaterials.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Pereira RF, O’Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): Controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prockop DJ, Kota DJ, Bazhanov N, et al. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuru S, Gordon PL, Shimono K, et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood. 2012;120:1933–1941. doi: 10.1182/blood-2011-12-400085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Götherström C. Immunomodulation by multipotent mesenchymal stromal cells. Transplantation. 2007;84(suppl):S35–S37. doi: 10.1097/01.tp.0000269200.67707.c8. [DOI] [PubMed] [Google Scholar]
- 20.Götherström C, Ringdén O, Tammik C, et al. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Götherström C, Ringdén O, Westgren M, et al. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003;32:265–272. doi: 10.1038/sj.bmt.1704111. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ZY, Teoh SH, Chong MS, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 23.Chan J, Waddington SN, O’Donoghue K, et al. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25:875–884. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 24.Kennea NL, Waddington SN, Chan J, et al. Differentiation of human fetal mesenchymal stem cells into cells with an oligodendrocyte phenotype. Cell Cycle. 2009;8:1069–1079. doi: 10.4161/cc.8.7.8121. [DOI] [PubMed] [Google Scholar]
- 25.Götherström C, West A, Liden J, et al. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90:1017–1026. [PubMed] [Google Scholar]
- 26.Guillot PV, Gotherstrom C, Chan J, et al. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 27.Choolani M, Chan J, Fisk NM. Fetal therapy: 2020 and beyond. Prenat Diagn. 2010;30:699–701. doi: 10.1002/pd.2527. [DOI] [PubMed] [Google Scholar]
- 28.Tiblad E, Westgren M. Fetal stem-cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2008;22:189–201. doi: 10.1016/j.bpobgyn.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Roybal JL, Santore MT, Flake AW. Stem cell and genetic therapies for the fetus. Semin Fetal Neonatal Med. 2010;15:46–51. doi: 10.1016/j.siny.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Mattar CN, Biswas A, Choolani M, et al. The case for intrauterine stem cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2012;26:683–695. doi: 10.1016/j.bpobgyn.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Guillot PV, Abass O, Bassett JH, et al. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111:1717–1725. doi: 10.1182/blood-2007-08-105809. [DOI] [PubMed] [Google Scholar]
- 32.Vanleene M, Saldanha Z, Cloyd KL, et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood. 2011;117:1053–1060. doi: 10.1182/blood-2010-05-287565. [DOI] [PubMed] [Google Scholar]
- 33.Panaroni C, Gioia R, Lupi A, et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009;114:459–468. doi: 10.1182/blood-2008-12-195859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Blanc K, Götherström C, Ringdén O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 35.Sundin M, Ringdén O, Sundberg B, et al. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 36.Svensson EM, Anderung C, Baubliene J, et al. Tracing genetic change over time using nuclear SNPs in ancient and modern cattle. Anim Genet. 2007;38:378–383. doi: 10.1111/j.1365-2052.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 37.Miller CA, III, Martinat MA, Hyman LE. Assessment of aryl hydrocarbon receptor complex interactions using pBEVY plasmids: Expression vectors with bi-directional promoters for use in Saccharomyces cerevisiae. Nucleic Acids Res. 1998;26:3577–3583. doi: 10.1093/nar/26.15.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alizadeh M, Bernard M, Danic B, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–4625. doi: 10.1182/blood.v99.12.4618. [DOI] [PubMed] [Google Scholar]
- 39.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 41.Niyibizi C, Wang S, Mi Z, et al. The fate of mesenchymal stem cells transplanted into immunocompetent neonatal mice: Implications for skeletal gene therapy via stem cells. Mol Ther. 2004;9:955–963. doi: 10.1016/j.ymthe.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones GN, Moschidou D, Lay K, et al. Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem Cells Translational Medicine. 2012;1:70–78. doi: 10.5966/sctm.2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao SH, Evans KD, Oberbauer AM, et al. Bisphosphonate treatment in the oim mouse model alters bone modeling during growth. J Biomech. 2008;41:3371–3376. doi: 10.1016/j.jbiomech.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pataki A, Müller K, Green JR, et al. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: A comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec. 1997;249:458–468. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]