It has been observed that full restoration of myocardial structure and function may be achievable with a combination of ongoing research, creativity, and perseverance. This report summarizes the importance of skeletal muscle stem cells and how they can play a key role to surpass current results in the future and enhance the efficacious implementation of regenerative cell therapy for heart failure.
Keywords: Adult stem cells, Cell transplantation, Tissue-specific stem cells, Progenitor cells, Cardiac
Abstract
Valuable and ample resources have been spent over the last two decades in pursuit of interventional strategies to treat the unmet demand of heart failure patients to restore myocardial structure and function. At present, it is clear that full restoration of myocardial structure and function is outside our reach from both clinical and basic research studies, but it may be achievable with a combination of ongoing research, creativity, and perseverance. Since the 1990s, skeletal myoblasts have been extensively investigated for cardiac cell therapy of congestive heart failure. Whereas the Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial revealed that transplanted skeletal myoblasts did not integrate into the host myocardium and also did not transdifferentiate into cardiomyocytes despite some beneficial effects on recipient myocardial function, recent studies suggest that skeletal muscle-derived stem cells have the ability to adopt a cardiomyocyte phenotype in vitro and in vivo. This brief review endeavors to summarize the importance of skeletal muscle stem cells and how they can play a key role to surpass current results in the future and enhance the efficacious implementation of regenerative cell therapy for heart failure.
Introduction
In the United States, an estimated 15.4 million people have coronary heart disease, of whom 7.6 million are affected by myocardial infarction and 2.1 million by congestive heart failure [1]. In the last two decades, the classic concept of the heart as an organ with extremely limited regenerative capacity has been analyzed. The cell therapy approaches were limited at the turn of the new millennium to the transfer of noncardiac cell types such as smooth muscle cells, embryonic stem cells, mesenchymal bone marrow stromal cells, hematopoietic stem cells [2], and skeletal myoblasts [3] into an injured heart. The idea of these engraftments was to stimulate repair and regeneration of the injured myocardium [4–6]. The field of cardiac regenerative medicine was brought into greater focus with the recognition of cardiac stem cells in 2001 [7] and the ability to antigenically select cardiac progenitor cells experimentally [8]. The capacity to generate cardiomyocytes in the adult human heart is 1% at the age of 25, and it decreases to 0.45% at the age of 75 [9]. This rationale, together with published research reports and experimental studies [10], indicated that the heart is in fact capable of regeneration, but therapeutic strategies are needed to stimulate this process in cardiac pathologies. The use of fetal cardiac cells poses ethical, logistical, and technical issues, and there still is no means for effectively mobilizing a hypothetical pool of resident stem cells. Long-term functional improvement requires stem cells with true cardiomyogenic properties and angiogenic potential. Presently, it is not clear whether such a perfect stem cell exists. However, a number of stem cell types show promise for autologous cardiomyoplasty, including multipotent stem cells isolated from skeletal muscle, which we highlight in this review.
Stem Cells Are a Promising Source for Cardiomyoplasty
Unspecialized cells that are capable of continuous self-renewal, while maintaining the ability to differentiate into multiple different cell types, are defined as stem cells. There are, broadly, three major categories of stem cells: embryonic stem cells, adult stem cells, and induced pluripotent stem (iPS) cells. Embryonic stem cells are undifferentiated cells, which are present during embryonic development and possess pluripotent differentiation capacity. Adult stem cells can be isolated from postnatal tissues and are multipotent. Sim et al. in 2002 [3] and Murry et al. in 2004 [11] concluded that none of the adult stem cell sources convincingly demonstrated a potential for significant long-term engraftment and differentiation into functional cardiomyocytes. The technique developed by Takahashi and Yamanaka in 2006 [12] demonstrated that the introduction of a quartet of transcription factors, Oct4, Sox2, Klf4, and c-Myc, into terminally differentiated cells (e.g., skin fibroblasts) changed these cells into an embryonic stem cell-like state known as iPS cells. Human iPS cells are similar to human embryonic cells in morphology, proliferation, surface antigens, gene expression, telomerase activity, epigenetic status of pluripotent cell-specific genes, and cardiac potential [13]. However, the maturation of iPS cell-derived cardiomyocytes remains nonuniform [14, 15]. Human implantation of embryonic and iPS cell-derived cardiomyocytes is limited by significant safety and ethical issues. Studies have revealed significant genetic and epigenetic abnormalities in iPS cells, higher than embryonic stem cells or fibroblasts. The mutation rates are estimated to be 10 times as high as those of fibroblasts [16]. Chromosome p12 has been found to be over-represented in iPS cell cultures, a characteristic associated with testicular germ cell tumors [16]. Other mutations are also associated with cell cycle regulation and oncogenesis, which raises the risk of teratogenesis in vivo. High-throughput functional genomics approaches are needed to better understand the nature and consequences of these mutations. iPS technology avoids the ethical dilemma of embryo destruction related to embryonic stem cells, but other ethical issues, including informed consent and genetic anonymity of donors, must still be carefully considered. Although iPS technology holds great promise for the regeneration of the heart and other organs, clinical use of iPS technology in ischemic heart disease remains untested. From this point of view, adult stem cells remain a more practical option at the present time.
Significant efforts in the field of cardiac cell therapy have focused on bone marrow-derived cells, especially bone marrow mononuclear cells such as hematopoietic and mesenchymal stem cells. Mesenchymal stem cells have numerous desirable characteristics, including positive paracrine effects, angiogenic potential, and an immunoprivileged profile. Studies have shown that mesenchymal stem cells, but not hematopoietic stem cells, can differentiate into cardiomyocytes in vivo [17, 18], with slightly to significantly positive results in clinical trials [19, 20]. However, bone marrow mesenchymal stem cells do not differentiate into cardiomyocytes in significant numbers in vitro, suggesting that their therapeutic benefit is predominantly through paracrine effects rather than through transdifferentiation [21]. Challenges include expanding these stem cells in clinically relevant numbers and developing safe methods for cardiomyocyte differentiation. (5-Azacytidine used for in vitro differentiation of mesenchymal stem cells into cardiomyocytes is toxic [17].) More recent work has focused on cardiac stem cells, which are found in niches within the heart and can be isolated by myocardial biopsies. Studies using immunosuppressed rats have shown that human cardiac stem cells can give rise to myocardium with improved function [22]. In light of these results, clinical trials have been initiated [23, 24]. Whereas initial results of the SCIPIO trial are promising, the CADUCEUS trial showed no difference in ventricular function compared with control despite a reduction of infarct size. Isolation of cardiac stem cells is also invasive and carries higher risk compared with isolation from other tissues. Further investigation is needed to safely and effectively isolate, expand, differentiate, and deliver these stem cells.
Cellular cardiomyoplasty of autologous skeletal muscle cells into the myocardium to reinforce its structure and function can be used as an alternative to heart transplantation [25]. Transdifferentiation directly converts a specialized cell type to another specialized cell type, bypassing a pluripotent state. In the 1980s, it was shown that fibroblasts can be converted into skeletal muscle cells with the use of the MyoD transcription factor [26]. In recent years, direct reprogramming of cardiac fibroblasts into functional cardiomyocytes by transcription factors GATA4, Tbx5, and Mef2c both in vitro and in vivo has been reported [15, 27]. However, the low reprogramming efficiency of this approach and the use of viral vectors are undesirable characteristics. It is interesting to speculate that direct reprogramming to cardiomyocytes might be more effective in skeletal muscle stem cells, which are pre-endowed with myogenic potential. Lymphocytes were converted into macrophages by using a transcription factor called C/EBPa [28]. But once they are in one committed lineage, it is a challenge to make them convert to another unrelated cell type, and transdifferentiation efficiency is often low. Szabo et al. [29] also proved that blood cells can be generated from fibroblasts, making it likely that transcription factors can induce large jumps between distantly related cell types. This opened up the prospect that any desired specialized cell could be generated from essentially any other cell type. Because skeletal and cardiac muscle both arise from myogenic, mesodermal lineages and share some characteristics, transdifferentiation from skeletal muscle progenitors to cardiac muscle would require less comprehensive alteration compared with distantly related cell types, at least in theory. Cells are considered ideal if they are derived from relatively easy to obtain tissue, readily grown in large numbers, available off the shelf for acute indications, nonimmunogenic, nonarrhythmogenic, and able to regenerate healthy myocardium [30]. Although the skeletal muscle stem cell isolation procedure is invasive compared with isolation of cells from blood or urine, skeletal muscle comprises the largest percentage of total body mass [31]. These desirable characteristics and developmental similarity to cardiac muscle, described below, make skeletal muscle-derived cells a strong candidate to repopulate damaged myocardium.
Skeletal Muscle and Cardiac Muscle: Difference and Similarity During Development
It is a widely accepted notion that terminally differentiated mature cardiac muscle does not express proteins that are specific to skeletal muscle. However, developing skeletal muscle has been proven to be similar to cardiac muscle by various studies, suggesting that they have a similar cell lineage. Different isoforms of myosin heavy chain (MHC) are present in different muscle fiber types to meet their functional needs. There are four major MHC isoforms in rat skeletal muscle. I/β is a slow type, and IIa, IIx/IId, and IIb are fast types. These are equivalent to skeletal muscle-specific fast MHC (sk-fMHC) [32–34]. In the adult myocardium, α-MHC and β-MHC are present, with the latter being identical to I/β-MHC found in skeletal muscle. Noncardiac MHCs play a major role during the early myofibrillogenesis of nascent cardiomyocytes in assembling sarcomere structure [35]. Recently, it was found that sk-fMHC was expressed in developing rat myocardium, and its expression was attenuated in postnatal life [36].
The thin filament system in skeletal and cardiac muscle is regulated by a family of proteins known as troponins, which form part of the troponin complex. Troponin I is encoded by three different genes. Cardiac troponin I was previously shown to be exclusively expressed in the myocardium in adults [37], but recently, it was revealed that it is also expressed in developing skeletal muscle [36]. It is distinct from the fast and slow forms in skeletal muscle [37]. Cardiac troponin T is a component of the troponin complex. It is encoded by the gene TNNT2 and allows actomyosin interaction and contraction to occur in response to Ca2+. TNNT2 is a common mutation in familial hypertrophic cardiomyopathy, but surprisingly, it has been found that distinct TNNT2 mutations also lead to dilated cardiomyopathy [38]. It is also expressed in skeletal muscle during injury. Apart from this, skeletal muscle-specific troponins are transiently present in the immature heart [39]. In the early phases of myogenesis in skeletal muscle, cardiac-like excitation-contraction coupling mechanisms dominate, whereas skeletal muscle-like excitation-contraction coupling dominates in more mature muscle [40, 41]. Thus, between cardiac and skeletal muscle, there is a strong overlap in the genes encoding key proteins responsible for contractility, which is a hallmark of striated muscle.
Cardiac and skeletal muscles also share common metabolic regulatory proteins. Fatty acid-binding protein 3 is a member of a family of binding proteins and is mainly expressed in cardiac and skeletal muscle cells, and it has been linked to fatty acid metabolism, trafficking, and signaling [42]. UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) is involved in the early development of both cardiac and smooth muscle. GNE-deficient cardiac cells degraded very soon, and their beating capacity decayed rapidly. Skeletal muscle-committed cells were identified in the GNE-deficient embryonic stem cell cultures by the expression of Pax7, MyoD, and MHC markers. GNE is strongly involved in cardiac tissue and skeletal muscle early survival and organization, as shown by these results [43].
A common finding in certain types of muscular dystrophy, including Emery-Dreifuss, Duchenne, and Becker type dystrophies, is chronic cardiac diseases [44]. Some types are caused by mutations in the β-sarcoglycan-associated proteins [45], which are expressed both in cardiac and skeletal muscle. Limb-girdle muscular dystrophy type 2E is caused by mutations in Sgcb gene, and Sgcb-deficient mice develop severe cardiomyopathy and severe muscular dystrophy, suggesting that cardiac and skeletal muscle pathologies arise from a common origin [46]. All of these studies indicate how closely cardiac and skeletal muscle are interlinked, especially in their immature phase. Recently, our group found that skeletal and cardiac muscle share similar transcription factors and structural proteins during development [36]. However, the molecular mechanisms that regulate the fate determination between these two lineages of striated muscle remain poorly understood.
Skeletal Myoblasts: An Early Candidate for Cellular Cardiomyoplasty
Given their phenotypic similarity to cardiac muscle, ease of isolation/expansion, and relative resilience to hypoxia, skeletal myoblasts were considered an appealing cell source for cellular cardiomyoplasty [47–54]. Skeletal myoblasts have shown promise for ischemic heart regeneration, making them potential candidates for early cardiomyoplasty studies [55]. Skeletal myoblasts were the first cell type to enter the clinical arena of heart regeneration. Lavine and Upcott in 1937 [56] performed the first of its kind surgery in which their team treated myocardial ischemia by using a skeletal muscle graft to the heart. The patient was typically acromegalic. Before the procedure, he could not walk 5 yards without resting, but after the surgery, he was able to walk 140 yards without any shortness of breath. Early animal studies conducted in porcine animal models showed that myoblast-derived muscle grafts remained functionally isolated from the surrounding myocardium. The outcome did not reveal an increased frequency in ventricular tachycardia or fibrillation [57]. Menasché et al. [49] in 2001 transplanted autologous skeletal myoblasts into the postinfarction scar during coronary artery bypass grafting of remote myocardial areas. However, in the distinct case of skeletal myoblasts, the results of several preclinical and clinical studies have shown improved functional outcomes following transplantation of these myogenic progenitors into postinfarction scar.
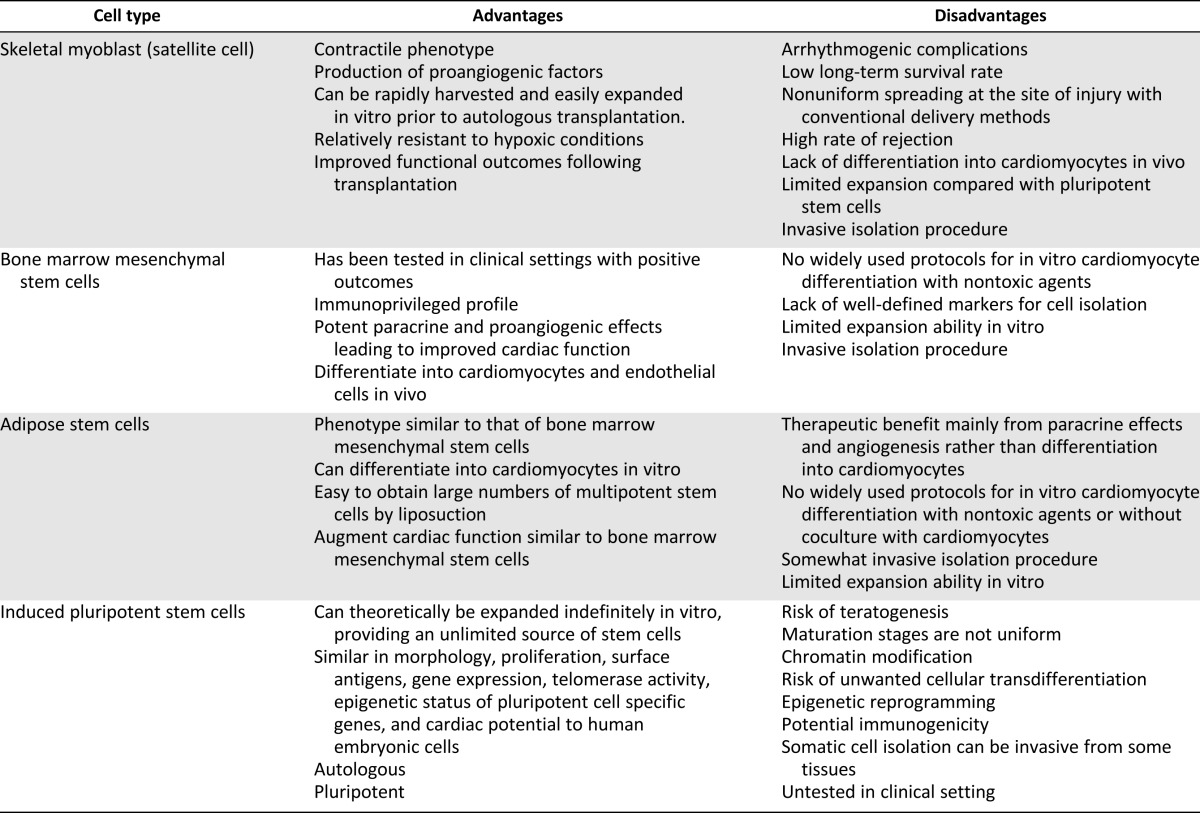
Table 1 summarizes the potential advantages and disadvantages of using various stem cell types for cardiac repair. Arrhythmogenic complications have raised concern over the safety of adoptive transfer of skeletal muscle cell therapy [3, 11, 46–48, 58]. There is a risk of ventricular arrhythmias [49, 59–62]. The fact remains that myoblast transplantation has its limitations, as implanted cells have limited survival and do not spread uniformly at the site of injury [48]. Skeletal myoblasts do not differentiate into cardiomyocytes but rather into multinucleated myotubes after injection into the heart. These myotubes form islands of conduction block in the heart and lack gap junctions. This results in electrical inhomogeneities that slow conduction velocity and predispose patients to re-entrant ventricular arrhythmias [63]. Whereas skeletal myoblasts (and other skeletal muscle-derived cells) show promise of improved myocardial contractile function, risk of arrhythmia is an issue that needs to be addressed to advance this therapy.
Table 1.
Advantages and disadvantages of various stem cell sources
Cardiomyocyte Differentiation Potential From Skeletal Muscle-Derived Cells
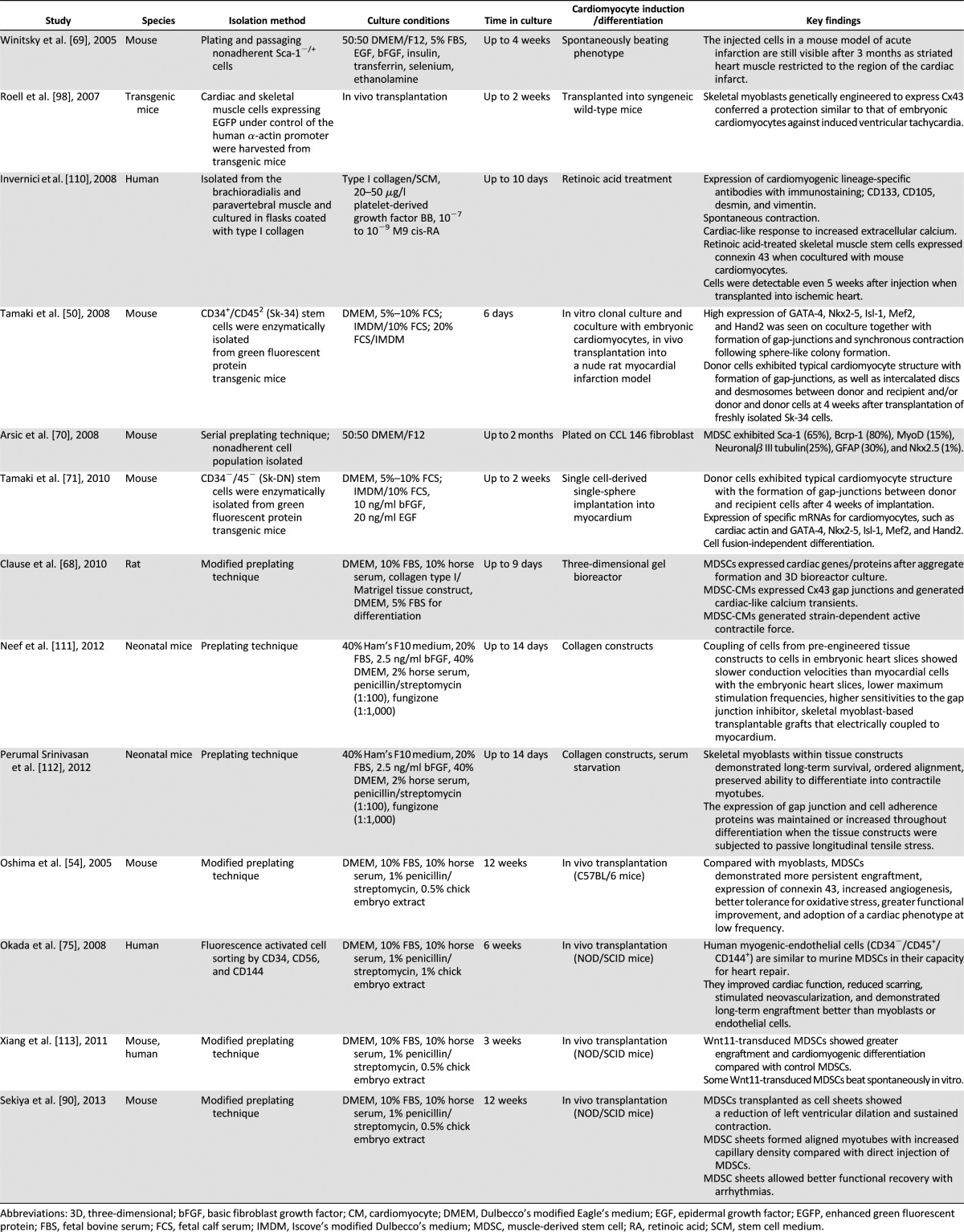
Since skeletal myoblasts do not differentiate into functioning cardiomyocytes in vivo, efforts have been made to isolate cells from skeletal muscle that can differentiate into cardiomyocytes in vitro or in vivo. The skeletal muscle-derived stem cell (MDSC) is a somatic stem cell that is different from skeletal myoblasts (satellite cell) in its multipotent properties [64–66]. Table 2 summarizes the differences between skeletal myoblasts and MDSCs. MDSCs are obtained from skeletal muscle via a preplate method or fluorescent activated cell sorting of surface markers and are easily expanded under in vitro culture environment as an autologous cell source [54, 65, 66]. MDSCs differentiate into skeletal muscle, bone, tendon, nerve, endothelial, hematopoietic, and smooth muscle cells [54, 64–67]. They have long-term proliferation capacity and enhanced resistance to hypoxia. Several studies have shown that MDSCs can differentiate into functioning cardiomyocytes using various strategies. Clause et al. [68] demonstrated that rat MDSCs could differentiate into cardiomyocyte-like cells expressing cardiac-specific genes and proteins by combining cell aggregation with 3D culture in a collagen bioreactor. Winitsky et al. [69] isolated nonadherent cells, skeletal-based precursors of cardiomyocyte (SPOC) cells, from adult murine skeletal muscle that become beating floating cells. They are CD34−/CD45−/c-kit−. The muscle-derived side population cells express the surface marker Sca-1 (65%), and the beating cells develop out of the Sca-1− pool, which makes up 20%–40% of the total isolated cells. Cells expressing the cardiac-specific marker Nkx2.5 and spontaneously contracting in culture were detected in reverse transcription-polymerase chain reaction analysis. The presence of specific mRNA coding markers for skeletal (myogenin, Myf5) and cardiac muscle (GATA4) and smooth muscle actin was seen in differentiated cells. Interestingly, all the above-mentioned markers were also detected in floating proliferating progenitor cells. This supports the idea that stem cells can express markers of multiple lineages in their differentiating state [70]. Skeletal muscle interstitium-derived multipotent stem cells (Sk-34 and Sk-DN cells) differentiated into cardiomyocytes in a cardiac environment [50, 71]. After 4 weeks, the transplanted cells became cardiomyocytes with desmosomes and intercalated discs associated with gap junctions [50]. Myotube formation was not observed. Sk-34 and Sk-DN cell differentiation is based on a cardiac environment. Table 3 lists the studies done over the years showing cardiomyocyte differentiation and cardiac repair potential from skeletal muscle-derived stem/progenitor cells. Taken together, these studies emphasize the importance of isolation techniques and culture conditions in obtaining the desired target cell. For example, Parker et al. [72] showed that activation of Notch signaling during ex vivo expansion of muscle-derived cells can significantly improve their engraftment.
Table 2.
Comparison of MDSCs and skeletal myoblasts
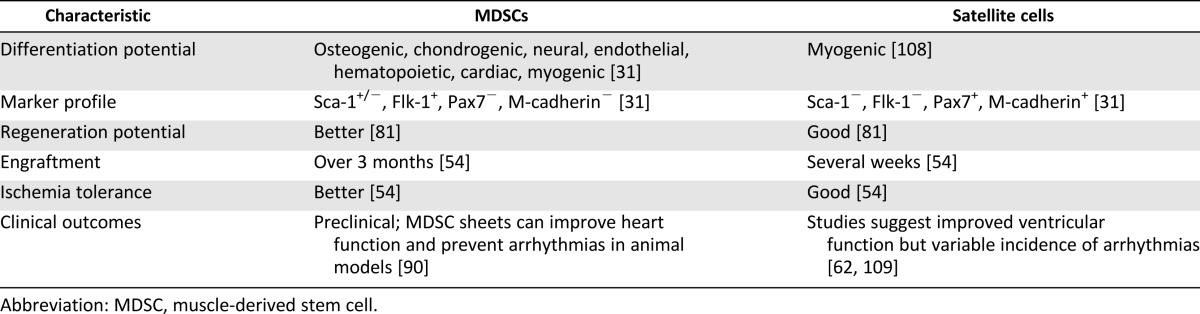
Table 3.
Studies of cardiac differentiation/repair from skeletal muscle stem cells
There are a number of problems faced during isolation of these cells. Cell surface markers define muscle-derived stem and progenitor cell populations. Some of these markers are restricted to these populations, whereas other markers are shared with other cell populations. The marker profile-based isolation methods exclusively depend on the expression of cell surface markers that are variable and may change under in vitro cell culture conditions. Thus, these methods have their limitations. Presently, the most prevalent method for isolating MDSCs is the modified preplate technique. It is a marker profile-independent technique that is based on variable adherence of freshly dissociated muscle cells to collagen-coated flasks. However, the isolated populations are heterogeneous [73]. Multiple non-satellite stem cell fractions reside in skeletal muscle, although it remains unclear how these different populations are related. Okada et al. [74] demonstrated that slowly adhering cells showed greater regenerative potential and oxidative/inflammatory stress tolerance compared with rapidly adhering cells. Myogenic-endothelial cells, cells that express both myogenic and endothelial markers (CD56, CD34, and CD144), can be isolated from skeletal muscle and have been shown to have superior regenerative properties and stress tolerance compared with myoblasts [75, 76]. The blood vessels of skeletal muscle also contain stem cells, perivascular stem cells, which demonstrate myogenic and multipotent potential [77]. They are isolated on the basis of CD146 expression and lack of CD34, CD45, CD144, and CD56 expression.
In vitro generated MDSC-derived cardiomyocytes are more similar functionally and biochemically to fetal cardiomyocytes [78]. However, the rate of cardiomyocyte differentiation alone is likely not sufficient to explain the functional improvements gained from MDSC transplantation. Another possible mechanism by which MDSCs exert therapeutic benefit is by acting as a reservoir to secrete paracrine factors that promote angiogenesis and cell survival within the injured microenvironment [54]. Transplanted undifferentiated human skeletal myoblasts (CD56+) in an immunodeficient nude rat model demonstrated expression of human-specific matrix metalloproteinase 2 (MMP-2) TNNI3, CNN3, proangiogenic factors (PGF), TNNT2, PAX7, transforming growth factor-β (TGF-β), and insulin-like growth factor-1 (IGF-1) 1 month after transplant. Gene expression showed upregulation of PGF, antiapoptotics (BAG-1, BCL-2), heart development (TNNT2, TNNC1), and extracellular matrix remodeling (MMP-2, MMP-7) in skeletal myoblasts. The data suggested that myoblast-secreted factors may contribute to the beneficial effects of myogenic cell transplantation in infarcted myocardium [79].
The paracrine effect exerted by MDSCs and other stem cells has a positive effect within the injured myocardium. Bioactive molecules such as basic fibroblast factor (bFGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor, SDF-1, hepatocyte growth factor (HGF), and IGF-1 confer cardioprotective effects. In addition, secretion of molecules such as VEGF, angiopoietin, and bFGF increase blood vessel formation in the ischemic region, improving perfusion and function. HGF and IGF-1 also activate resident stem cells and promote endogenous repair mechanisms. Additional factors such as TGF-β, MMPs, and tissue inhibitor of metalloproteinase reduce fibroblast proliferation, fibrosis, and left ventricular dilation [80, 81]. Okada et al. [82] showed that a subpopulation of slowly adhering MDSCs showed enhanced paracrine factor secretion, increased stress tolerance, engraftment, and angiogenesis compared with rapidly adhering MDSCs. They also found increased cardiomyocyte proliferation within the infarct region, suggesting the enhancement of endogenous repair mechanisms. The selective enrichment of certain types of MDSCs may improve their regenerative effects through enhancement of paracrine effects. Studies of MDSC transplantation have shown that only a small fraction of donor cells demonstrates long-term engraftment, and the majority of cells within the regenerating tissue come from the host via the chemoattraction of postnatal stem cells from the vasculature [83]. Thus, the paracrine or bystander effect of MDSCs may be as important as, if not more important than, myogenic differentiation in contributing to cardiac repair.
Tissue-Engineering Approaches to Cardiac Repair
Transvascular methods of cell delivery are commonly used to treat recently infarcted myocardium. Intracoronary artery infusion can deliver a high concentration of cells homogeneously to the site of injury. Direct needle injection into the ventricular wall is preferred for patients late in the disease process when occlusion of the coronary artery prevents transvascular delivery [84]. However, it often results in poor engraftment, poor survival, and further damage by needle injury. Tissue-engineering approaches may provide a more effective means of cell transplantation. Two main engineered tissue transplantation approaches for cardiac tissue are three-dimensional scaffolds and cell sheets. One of the most challenging issues in tissue engineering is the reconstruction of myocardium in three dimensions. Three-dimensional (3D) scaffolds are now available [85, 86]. Cell sheets are used for treating advanced heart failure. Myocardial cell sheets decrease fibrosis and increase vascularization in the injured heart [87–90]. In 2006, Zimmerman et al. [91] produced convincing evidence that a heart tissue can be produced with enough contractile force to support a failing heart. Neonatal rat heart cells were seeded with liquid collagen type I and Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) in molds. The rings were grafted onto the infarcted rat heart and after 1 month showed positive results. Further dilation of the heart was prevented, and systolic wall thickness increased. In 2011, Fujimoto et al. also proved that engineered cardiac tissue from immature cardiomyocytes can functionally integrate into damaged myocardium. In this study, engineered fetal cardiac tissue proved to be more successful than neonatal tissue. Engineered fetal cardiac tissue implantation increased left ventricle contraction and showed higher cardiomyocyte proliferation than engineered neonatal cardiac tissue [92].
The major issues concerning the therapeutic application of stem cells remain unresolved because of three primary factors: poor survival, marginal proliferation, and limited functional engraftment/commitment within the host tissue. Furthermore, the cells should be prepared to combat against apoptotic, necrotic, and hypoxic conditions prevalent within the damaged tissue. Finally, if cells manage to proliferate, they can be functionally incapable of appropriate lineage commitment and can end in an oncogenic transformation [93].
The in vivo studies with MDSCs show promising results for meeting some of these challenges. Repair of the infarcted heart with MDSCs is more effective than conventional myoblasts [54]. MDSCs injected into the myocardium of an infarcted heart showed outstanding survival and engraftment, promoted angiogenesis, and increased left ventricular function in a more effective manner in comparison with the transplantation of skeletal myoblasts [94]. In a postinfarcted myocardium, transplanted MDSCs sustained its contractile function, preventing left ventricular chamber remodeling. Some transplanted donor MDSCs differentiated into both sk-fMHC- and/or cardiac troponin I-positive muscle cells [54, 75]. Clause et al. [68] demonstrated that MDSCs cultured within a three-dimensional engineered tissue construct differentiated into an immature functioning cardiomyocyte phenotype. Transplantation of tissue-engineered cell sheets has negated some of the limitations by eliminating use of trypsin, direct needle injury to the heart, and by covering a larger infarcted area [95, 96]. Reduction in left ventricle (LV) dilation and sustained LV contraction were displayed with the implanted MDSC sheet. It yielded better functional recovery of chronic infarcted myocardium compared with direct MDSC injection without any significant arrhythmic events, indicating that this cell sheet delivery system could markedly improve the myocardial regenerative potential of the MDSCs [90]. These studies suggest that tissue-engineering approaches may provide a means to further improve upon the functional benefits derived from MDSC transplantation.
Tissue-engineering approaches help to address one of the major limitations of current delivery modalities—the immediate and massive attrition of naked cells that occurs after cell delivery, which leads to low retention of implanted cells even 24 hours after the procedure. However, current tissue-engineering approaches focus on building complex 3D structures, which precludes catheter-based delivery and necessitates surgery. Using the cell patch approach, transplanted cells are initially localized to the epicardial region. A recent development, which improves cell retention, is the delivery of hydrogel-cell liquid suspensions via catheter [97]. The hydrogel polymerizes en route and traps cells in the target tissue. If successful, this method can combine the demonstrated safety of catheter-based delivery with the ability of a tissue-engineered scaffold to improve engraftment.
Future Challenges and Opportunities
Although skeletal muscle stem/progenitor cells have benefits, a large number of injected cells undergo early death. The first prospective randomized placebo-controlled phase II skeletal myoblast trial (MAGIC trial) exhibited lack of efficacy and was discontinued prematurely [48]. The trial’s disappointing results were due to several reasons: a low rate of initial cell retention, a high rate of subsequent cell death, and the inability of engrafted myoblasts to establish functional electromechanical connections with the host cardiomyocytes [3]. In addition, a trend toward excess arrhythmias was observed in myoblast-treated patients despite the use of the prophylactic antiarrhythmic drug amiodarone. This raised safety concerns that had already been raised by earlier phase 1 trials. In contrast, SEISMIC trial [63] argued that injection of autologous skeletal myoblasts in heart failure patients is safe and may provide symptomatic relief, as a trend toward increased exercise tolerance was observed in the cell-treated group. Nevertheless, no significant effect on global left ventricular ejection fraction was detected. All this taken together indicates that more work needs to be done to bridge the gap between preclinical animal models and clinical trials.
In light of the mixed results from clinical trials, further work has gone into eliminating the heterogeneity of transplanted cells and improving electromechanical coupling between transplanted and host myocytes. Transplantation of myogenic-endothelial cells that express CD56, CD146, and Ulex europaeus agglutin I (UEA-I), purified from human skeletal muscle into the ischemic heart, drastically improved left ventricular function, reduced scar tissue, and promoted angiogenesis [54]. Connexin-43 is the predominant gap junction of the ventricular myocardium. Skeletal myoblasts lack connexin-43 after fusion into elongated contractile myotubes. In cellular monolayers, conduction velocity was slowed and re-entry-induced arrhythmias were promoted when skeletal myoblasts were cocultured with neonatal cardiomyocytes in vitro and studied with high-resolution optical mapping. The proarrhythmic effect was reduced when engineered cells overexpressed connexin-43 [98]. The findings were later tested in an animal model [11]. Methods to improve electromechanical compatibility between engrafted muscle and host myocardium are currently under investigation.
Issues related to electromechanical compatibility between cardiac and skeletal muscle tissue could be ameliorated by generating cells from MDSCs that have a cardiomyocyte-like phenotype. The heart also contains resident stem cells. Oh et al. [99] identified in 2003 an independent population of Sca-1+ cardiac stem cells as a subgroup of cells (constituting ∼14%) isolated in the noncardiomyocyte cell fraction of the adult mouse heart in a whole heart digestion. Sca-1+ cells coexpressed CD31 and CD38 and lacked c-Kit, CD34, and CD45 when freshly isolated. Ninety-three percent of the side population was Sca-1+. Freshly isolated Sca-1+ cells did express the early cardiac-specific transcription factors GATA4, Mef2C, and Tef-1 but not Nkx2.5 or genes encoding cardiac sarcomeric proteins. Sca-1+ cells engrafted at a much higher rate than Sca-1− cells in a mouse model of ischemia-reperfusion after 2 weeks and could be found forming new cardiomyocytes. Cardiac stem cells in bulk culture upregulated GATA-4 expression resulting in enhanced cardiomyocyte differentiation, suggesting that the GATA-4 high c-kit+ cardiac stem cells have potent cardiac regenerative potential. The study also demonstrated spontaneous differentiation into skeletal myocytes [100]. Hasan et al. [101] established cardiac pluripotent stem cell-like cells from the left atrium of adult rat hearts that could differentiate into beating cardiomyocytes in the methylcellulose-based medium containing interleukin-3 and stem cell factor, which contributed to the differentiation into cardiac troponin I-positive cells. Distinctly small populations of pluripotent stem cell-like cells from the left atrium coexpressed GATA4 and myogenin, which are markers specific to cardiomyocytes and skeletal myocytes, respectively. These could differentiate into both cardiac and skeletal myocytes. These studies suggest the possibility that cardiac and skeletal muscle can arise from a common myogenic progenitor, and stem cells purified from skeletal muscle may have similar differentiation potential, as demonstrated by studies of cardiomyocyte differentiation from MDSCs. However, the pathways that determine whether a cell differentiates into a cardiomyocyte or skeletal muscle cell are only beginning to be unraveled.
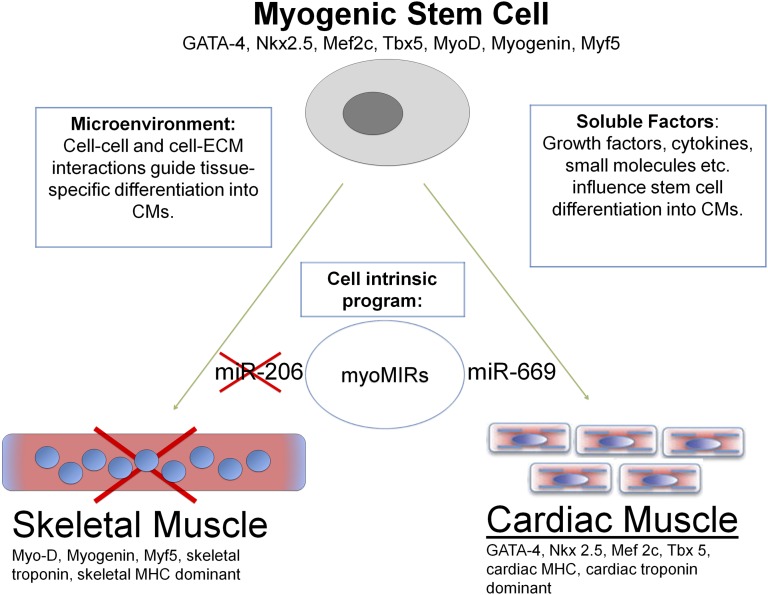
A hypothesis is presented in Figure 1 showing how skeletal muscle stem/progenitor cells can be induced to become cardiac muscle with post-transcriptional modification. Microribonucleic acids (micro-RNAs, miRs) are post-transcriptional regulators of cardiac and skeletal myogenesis, including miR206, which specifically promotes skeletal myogenesis [102–104] as part of an intrinsic cell-regulatory program. Crippa et al. [105] isolated cardiac progenitors from neonatal sarcoglycan-null mouse hearts affected by dilated cardiomyopathy, and they spontaneously differentiated into skeletal muscle fibers both in vitro and when transplanted into regenerating muscles or infarcted hearts. The absence of expression of miR669q and with downregulation of miR669a was associated with differentiation potential. Skeletal myogenesis was prevented by miR669a and miR669q acting upstream of myogenic regulatory factors by directly targeting the MyoD untranslated region. Successful conversion of cardiac cells into skeletal muscle fibers opened a huge area of discussion if the reverse is possible. MDSCs have genes similar to those of cardiac and skeletal muscle, and cardiac and skeletal muscles are interchangeable because of miR669. The physical environment may also present important environmental cues [106]. Three-dimensional cultures and cardiac-specific extracellular matrix have been shown to promote cardiomyocyte induction [36, 68, 107].
Figure 1.
MicroRNAs post-transcriptionally regulate myogenesis. miR-206 specifically promotes skeletal muscle differentiation, whereas miR-669 promotes cardiac differentiation by inhibiting MyoD, a skeletal muscle transcription factor. By modulating expression of these and other muscle-specific microRNAs, stem cells can be induced to differentiate into cardiac or skeletal muscle. The local microenvironment and soluble factors also play important roles in stem cell differentiation. Abbreviations: CM, cardiomyocyte; ECM, extracellular matrix; MHC, myosin heavy chain; MIR, microRNA.
Conclusion
In any endeavor as complex and perplexing as stem cell research, controversies and disagreements are to be expected. Whereas early clinical trials of skeletal myoblast transplantation yielded mixed results and did not meet initial expectations, more recent works have shown that improved isolation methods, novel tissue-engineering approaches, and differentiation strategies may improve the efficacy of myogenic stem cell transplantation. Skeletal muscle-derived stem cells remain the only stem cell that can differentiate into a muscle phenotype and can be isolated easily in abundant numbers. Cardiovascular regenerative medicine is still in its early stages, but recent studies show renewed promise. The field of regenerative medicine has been quite progressive, as a search on PubMed for the key words “cardiac, stem cell, heart,” yields more than 9,000 references as of today with almost 2,000 review articles to summarize all current understanding. This field was nonexistent a little over a decade ago.
Footnotes
Contributed equally as first authors.
Author Contributions
N.H. and J.T.: conception and design, manuscript writing; K.T.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.MacLellan RW. Mending broken hearts one cell at a time. J Mol Cell Cardiol. 2002;34:87–89. doi: 10.1006/jmcc.2001.1509. [DOI] [PubMed] [Google Scholar]
- 3.Sim EK, Jiang S, Ye L, et al. Skeletal myoblast transplant in heart failure. J Card Surg. 2003;18:319–327. doi: 10.1046/j.1540-8191.2003.02033.x. [DOI] [PubMed] [Google Scholar]
- 4.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 5.Olivares EL, Ribeiro VP, Werneck de Castro JP, et al. Bone marrow stromal cells improve cardiac performance in healed infarcted rat hearts. Am J Physiol Heart Circ Physiol. 2004;287:H464–H470. doi: 10.1152/ajpheart.01141.2003. [DOI] [PubMed] [Google Scholar]
- 6.Xu M, Wani M, Dai YS, et al. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 8.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajstura J, Urbanek K, Perl S, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–2806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes. Circ Res. 2006;98:1002–1013. doi: 10.1161/01.RES.0000218272.18669.6e. [DOI] [PubMed] [Google Scholar]
- 18.Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 19.Assmus B, Schächinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 20.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Rendon E, Sweeney D, Lu F, et al. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008;95:137–148. doi: 10.1111/j.1423-0410.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 22.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajnoch C, Chachques JC, Berrebi A, et al. Cellular therapy reverses myocardial dysfunction. J Thorac Cardiovasc Surg. 2001;121:871–878. doi: 10.1067/mtc.2001.112937. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Costa ML, Mermelstein CS, et al. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci USA. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laiosa CV, Stadtfeld M, Xie H, et al. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Szabo E, Rampalli S, Risueño RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 30.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 31.Ūsas A, Mačiulaitis J, Mačiulaitis R, et al. Skeletal muscle-derived stem cells: Implications for cell-mediated therapies. Medicina. 2011;47:469–479. [PubMed] [Google Scholar]
- 32.Schiaffino S, Gorza L, Sartore S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 33.Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- 34.Bortolotto SK, Cellini M, Stephenson DG, et al. MHC isoform composition and Ca(2+)- or Sr(2+)-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;279:C1564–C1577. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- 35.Du A, Sanger JM, Sanger JW. Cardiac myofibrillogenesis inside intact embryonic hearts. Dev Biol. 2008;318:236–246. doi: 10.1016/j.ydbio.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clause KC, Tchao J, Powell MC, et al. Developing cardiac and skeletal muscle share fast-skeletal myosin heavy chain and cardiac troponin-I expression. PLoS One. 2012;7:e40725. doi: 10.1371/journal.pone.0040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apple FS. Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clin Chim Acta. 1999;284:151–159. doi: 10.1016/s0009-8981(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 38.Sehnert AJ, Huq A, Weinstein BM, et al. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- 39.Saggin L, Gorza L, Ausoni S, et al. Troponin I switching in the developing heart. J Biol Chem. 1989;264:16299–16302. [PubMed] [Google Scholar]
- 40.Cognard C, Rivet-Bastide M, Constantin B, et al. Progressive predominance of ‘skeletal’ versus ‘cardiac’ types of excitation-contraction coupling during in vitro skeletal myogenesis. Pflugers Arch. 1992;422:207–209. doi: 10.1007/BF00370424. [DOI] [PubMed] [Google Scholar]
- 41.Haufe V, Camacho JA, Dumaine R, et al. Expression pattern of neuronal and skeletal muscle voltage-gated Na+ channels in the developing mouse heart. J Physiol. 2005;564:683–696. doi: 10.1113/jphysiol.2004.079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu C, Hu DL, Liu YQ, et al. Fabp3 inhibits proliferation and promotes apoptosis of embryonic myocardial cells. Cell Biochem Biophys. 2011;60:259–266. doi: 10.1007/s12013-010-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milman Krentsis I, Sela I, Eiges R, et al. GNE is involved in the early development of skeletal and cardiac muscle. PLoS One. 2011;6:e21389. doi: 10.1371/journal.pone.0021389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muntoni F. Cardiomyopathy in muscular dystrophies. Curr Opin Neurol. 2003;16:577–583. doi: 10.1097/01.wco.0000093100.34793.81. [DOI] [PubMed] [Google Scholar]
- 45.Barresi R, Di Blasi C, Negri T, et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J Med Genet. 2000;37:102–107. doi: 10.1136/jmg.37.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durbeej M, Cohn RD, Hrstka RF, et al. Disruption of the beta-sarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E. Mol Cell. 2000;5:141–151. doi: 10.1016/s1097-2765(00)80410-4. [DOI] [PubMed] [Google Scholar]
- 47.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 48.Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 49.Menasché P, Hagège AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 50.Tamaki T, Akatsuka A, Okada Y, et al. Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium. PLoS One. 2008;3:e1789. doi: 10.1371/journal.pone.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kessler PD, Byrne BJ. Myoblast cell grafting into heart muscle: Cellular biology and potential applications. Annu Rev Physiol. 1999;61:219–242. doi: 10.1146/annurev.physiol.61.1.219. [DOI] [PubMed] [Google Scholar]
- 52.Hansson EM, Lindsay ME, Chien KR. Regeneration next: Toward heart stem cell therapeutics. Cell Stem Cell. 2009;5:364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 54.Oshima H, Payne TR, Urish KL, et al. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130–1141. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 55.Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavine L, Upcott H. Myocardial ischaemia treated by graft of skeletal muscle to the heart. Proc R Soc Med. 1937;30:772. [PMC free article] [PubMed] [Google Scholar]
- 57.Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159–167. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 58.Marban E, Cingolani E. Heart to heart: Cardiospheres for myocardial regeneration. Heart Rhythm. 2012;9:1727–1731. doi: 10.1016/j.hrthm.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Hagège AA, Marolleau JP, Vilquin JT, et al. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114:I108–I113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 60.Gavira JJ, Perez-Ilzarbe M, Abizanda G, et al. A comparison between percutaneous and surgical transplantation of autologous skeletal myoblasts in a swine model of chronic myocardial infarction. Cardiovasc Res. 2006;71:744–753. doi: 10.1016/j.cardiores.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Gavira JJ, Herreros J, Perez A, et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J Thorac Cardiovasc Surg. 2006;131:799–804. doi: 10.1016/j.jtcvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Menasché P, Hagège AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 63.Duckers HJ, Houtgraaf J, Hehrlein C, et al. Final results of a phase IIa, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: The SEISMIC trial. EuroIntervention. 2011;6:805–812. doi: 10.4244/EIJV6I7A139. [DOI] [PubMed] [Google Scholar]
- 64.Sato K, Li Y, Foster W, et al. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- 65.Peng H, Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol. 2004;12:311–319. doi: 10.1016/j.trim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Jankowski RJ, Deasy BM, Cao B, et al. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115:4361–4374. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- 67.Peng H, Usas A, Gearhart B, et al. Converse relationship between in vitro osteogenic differentiation and in vivo bone healing elicited by different populations of muscle-derived cells genetically engineered to express BMP4. J Bone Miner Res. 2004;19:630–641. doi: 10.1359/JBMR.040102. [DOI] [PubMed] [Google Scholar]
- 68.Clause KC, Tinney JP, Liu LJ, et al. A three-dimensional gel bioreactor for assessment of cardiomyocyte induction in skeletal muscle-derived stem cells. Tissue Eng Part C Methods. 2010;16:375–385. doi: 10.1089/ten.tec.2009.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winitsky SO, Gopal TV, Hassanzadeh S, et al. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 2005;3:e87. doi: 10.1371/journal.pbio.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arsic N, Mamaeva D, Lamb NJ, et al. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266–1280. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Tamaki T, Uchiyama Y, Okada Y, et al. Clonal differentiation of skeletal muscle-derived CD34(-)/45(-) stem cells into cardiomyocytes in vivo. Stem Cells Dev. 2010;19:503–512. doi: 10.1089/scd.2009.0179. [DOI] [PubMed] [Google Scholar]
- 72.Parker MH, Loretz C, Tyler AE, et al. Activation of Notch signaling during ex vivo expansion maintains donor muscle cell engraftment. Stem Cells. 2012;30:2212–2220. doi: 10.1002/stem.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gharaibeh B, Lu A, Tebbets J, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 74.Okada M, Payne TR, Drowley L, et al. Human skeletal muscle cells with a slow adhesion rate after isolation and an enhanced stress resistance improve function of ischemic hearts. Mol Ther. 2012;20:138–145. doi: 10.1038/mt.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okada M, Payne TR, Zheng B, et al. Myogenic endothelial cells purified from human skeletal muscle improve cardiac function after transplantation into infarcted myocardium. J Am Coll Cardiol. 2008;52:1869–1880. doi: 10.1016/j.jacc.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng B, Cao B, Crisan M, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 77.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Jacot JG, Kita-Matsuo H, Wei KA, et al. Cardiac myocyte force development during differentiation and maturation. Ann N Y Acad Sci. 2010;1188:121–127. doi: 10.1111/j.1749-6632.2009.05091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Ilzarbe M, Agbulut O, Pelacho B, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28:5401–5406. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okada M, Payne TR, Drowley L, et al. Human skeletal muscle cells with a slow adhesion rate after isolation and an enhanced stress resistance improve function of ischemic hearts. Mol Ther. 2012;20:138–145. doi: 10.1038/mt.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gharaibeh B, Lavasani M, Cummins JH, et al. Terminal differentiation is not a major determinant for the success of stem cell therapy: Cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther. 2011;2:31. doi: 10.1186/scrt72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 85.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction: A 5-year update. J Am Coll Cardiol. 2011;58:2615–2629. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 87.Shudo Y, Miyagawa S, Nakatani S, et al. Myocardial layer-specific effect of myoblast cell-sheet implantation evaluated by tissue strain imaging. Circ J. 2013;77:1063–1072. doi: 10.1253/circj.cj-12-0615. [DOI] [PubMed] [Google Scholar]
- 88.Saito S, Miyagawa S, Sakaguchi T, et al. Myoblast sheet can prevent the impairment of cardiac diastolic function and late remodeling after left ventricular restoration in ischemic cardiomyopathy. Transplantation. 2012;93:1108–1115. doi: 10.1097/TP.0b013e31824fd803. [DOI] [PubMed] [Google Scholar]
- 89.Masumoto H, Matsuo T, Yamamizu K, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–1205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
- 90.Sekiya N, Tobita K, Beckman S, et al. Muscle-derived stem cell sheets support pump function and prevent cardiac arrhythmias in a model of chronic myocardial infarction. Mol Ther. 2013;21:662–669. doi: 10.1038/mt.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 92.Fujimoto KL, Clause KC, Liu LJ, et al. Engineered fetal cardiac graft preserves its cardiomyocyte proliferation within postinfarcted myocardium and sustains cardiac function. Tissue Eng Part A. 2011;17:585–596. doi: 10.1089/ten.tea.2010.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohsin S, Siddiqi S, Collins B, et al. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Payne TR, Oshima H, Sakai T, et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264–1274. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 95.Memon IA, Sawa Y, Fukushima N, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Kondoh H, Sawa Y, Miyagawa S, et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2006;69:466–475. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Vunjak-Novakovic G, Tandon N, Godier A, et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16:169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roell W, Lewalter T, Sasse P, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 99.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyamoto S, Kawaguchi N, Ellison GM, et al. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010;19:105–116. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 101.Kamrul Hasan M, Komoike Y, Tsunesumi S, et al. Myogenic differentiation in atrium-derived adult cardiac pluripotent cells and the transcriptional regulation of GATA4 and myogenin on ANP promoter. Genes Cells. 2010;15:439–454. doi: 10.1111/j.1365-2443.2010.01394.x. [DOI] [PubMed] [Google Scholar]
- 102.McCarthy JJ. MicroRNA-206: The skeletal muscle-specific myomiR. Biochim Biophys Acta. 2008;1779:682–691. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuasa K, Hagiwara Y, Ando M, et al. MicroRNA-206 is highly expressed in newly formed muscle fibers: Implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct Funct. 2008;33:163–169. doi: 10.1247/csf.08022. [DOI] [PubMed] [Google Scholar]
- 104.Williams AH, Valdez G, Moresi V, et al. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crippa S, Cassano M, Messina G, et al. miR669a and miR669q prevent skeletal muscle differentiation in postnatal cardiac progenitors. J Cell Biol. 2011;193:1197–1212. doi: 10.1083/jcb.201011099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24:830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duan Y, Liu Z, O’Neill J, et al. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Díez Villanueva P, Sanz-Ruiz R, Núñez Garcia A, et al. Functional multipotency of stem cells: What do we need from them in the heart? Stem Cells Int. 2012;2012:817364. doi: 10.1155/2012/817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siminiak T, Kalawski R, Fiszer D, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 110.Invernici G, Cristini S, Madeddu P, et al. Human adult skeletal muscle stem cells differentiate into cardiomyocyte phenotype in vitro. Exp Cell Res. 2008;314:366–376. doi: 10.1016/j.yexcr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 111.Neef K, Choi YH, Perumal Srinivasan S, et al. Mechanical preconditioning enables electrophysiologic coupling of skeletal myoblast cells to myocardium. J Thorac Cardiovasc Surg. 2012;144:1176–1184. doi: 10.1016/j.jtcvs.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perumal Srinivasan S, Neef K, Treskes P, et al. Enhanced gap junction expression in myoblast-containing engineered tissue. Biochem Biophys Res Commun. 2012;422:462–468. doi: 10.1016/j.bbrc.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiang G, Yang Q, Wang B, et al. Lentivirus-mediated Wnt11 gene transfer enhances cardiomyogenic differentiation of skeletal muscle-derived stem cells. Mol Ther. 2011;19:790–796. doi: 10.1038/mt.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]